Published online Nov 12, 2013. doi: 10.5499/wjr.v3.i3.25
Revised: September 23, 2013
Accepted: October 17, 2013
Published online: November 12, 2013
Processing time: 143 Days and 17.5 Hours
Since early description of CD4/CD8 T cell duality, continuous discovery of functional T lymphocyte subsets and their related cytokines constitutes major progress in our understanding of the immune response. T-lymphocyte derived lymphokines and environmental cytokines are essential for both innate and antigen-specific immune responses to a wide variety of agents. Following immune battle and aggression overcome, cytokines may return against neighbored cells/organs, causing pathogenic hypersensitivity reactions, including autoimmune diseases. Due to their cytokine production, CD4+ T helper lymphocyte subsets may be considered as one the major players of the immune response. Among CD4+ T cell subsets, the identification of interleukin-17-producing cells (Th17) led to better understanding of coordinated cytokine involvement during inflammatory reactions together with the subsequent clarification of complex interactions between these mediators. In this review, we discuss Th17 cell differentiation, functions, and the role of this cell subset during rheumatoid arthritis pathogenesis together with therapeutic strategies to control these cells.
Core tip: identification of interleukin-17-producing cells (Th17) rise as important source of inflammatory cytokines, IL-17 in particular, with critical role during inflammatory diseases.In this paper, we reviewed the differentiation of these cells from naive lymphocytes, their role during inflammatory arthritis and therapeutic tools to control these cells.
- Citation: Boniface K, Moynet D, Mossalayi MD. Role of Th17 cells in the pathogenesis of rheumatoid arthritis. World J Rheumatol 2013; 3(3): 25-31
- URL: https://www.wjgnet.com/2220-3214/full/v3/i3/25.htm
- DOI: https://dx.doi.org/10.5499/wjr.v3.i3.25
Over 20 years ago, first description of CD4+ T helper (Th) cell diversity (Th1/Th2) pointed to distinct cytokine production capacity of these cells[1]. As in B-cell differentiation, this diversity is dependent upon cytokines and antigens that trigger CD4+ precursor cells through maturation into cells producing type 1 [interferon (IFN)-γ and interleukin (IL)-2] or type 2 (IL-4, IL-5, IL-13) lymphokines[1,2]. Two other subpopulations were subsequently identified: regulatory T cells (Treg) producing IL-10 and transforming growth factor (TGF)-β[3] and IL-17-producing CD4+ T cells (Th17)[4-6]. These two subsets play a key role during tolerance and inflammatory responses[2,7]. Recent investigations added to our knowledge through precise definition of Th17 cells subpopulations and identification of additional effector subsets, such as Th22, Th9, and follicular helper (Tfh) cells[8-10]. The role of Th17 cells during development of autoimmune diseases has largely been described over the past few years[7,11]. The involvement of Th17/IL-17 pathway during the pathogenesis of rheumatoid arthritis (RA)[12,13] led us to summarize, in this review, signals leading to the differentiation of Th17 cells, their cytokine secretion profile, in vivo correlation with other cytokines and possible targeting of IL-17 pathway as therapeutic approach in RA or other related diseases.
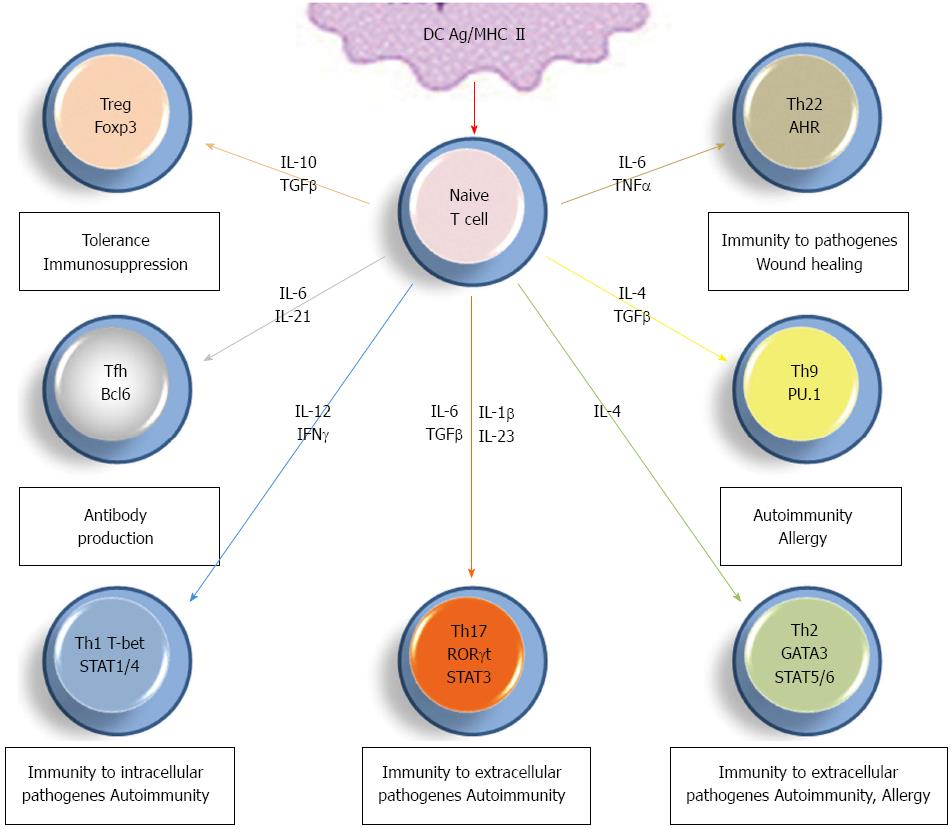
Following antigen recognition on antigen presenting cells, naive Th0 lymphocytes go towards maturation into more specialized subsets depending upon various in situ factors including antigen itself, cellular and cytokinin environment (Figure 1). Differentiation into Th1 cells requires IL-12 induction of signal transducer and activator of transcription (STAT1/4) and subsequent induction of the transcription factor T-bet, with in situ IFN-γ as helper factor. These cells are essential for cell-mediated immune response to intracellular pathogens, cellular immunity and clonal lymphocyte multiplication through their ability to produce IL-2[1,2]. Maturation into Th2 cells seems to be dependent upon weaker T cell receptor signaling and IL-4-dependent or independent GATA-3 transcription factor induction. These Th2 lymphocytes help the development of humoral immunity together with limiting Th1 response[2]. Antibody response is also potentiated by Tfh lymphocytes, characterized by the expression of BCL6 transcription repressor and depends upon the in situ presence of IL-6 and IL-21[14] (Figure 1). Meanwhile, whether Tfh cells directly derive from Th0 cells or from other subsets is still unclear.
Th0-derived Th9 cells produce IL-9 and are polarized by IL-4-induced transcription factors including STAT6, GATA3 together with the presence of TGF-β, required for Smad activation and intracellular expression of PU.1 transcription factor[15]. Other cytokinin environments were shown to induce Th9 cell differentiation but the exact in vivo maturation pathway of these cells remains to be clarified. The presence of Th9 cells is associated with autoimmune and allergic diseases[15], but their definitive role remains unclear. Th22 and Treg cell differentiation is very close to Th17 cells and will be detailed below.
If IL-17 (also known as IL-17A) was identified decades ago, the concept that Th17 cells represent an additional Th cell subset is recent[2,12]. Several cytokines are involved for optimal development of Th17 cells, including IL-6, TGF-β, IL-23, and IL-1β. This cell subset is characterized by the expression of orphan nuclear receptor RORγt together with the production of high levels of IL-17, IL-17F, IL-22, and IFN-γ[7,16-20]. Other factors can regulate differentiation of Th17 cells, such as prostaglandin E2, IL-21 (detailed in[20]). Since their identification, Th17 cells have been largely described for their critical role during the development of inflammation and autoimmunity[11]. Interestingly, Th22 cells were recently described as an additional effector subset during wound healing and tissue reparation[9,21]. These cells develop in response to tumor necrosis factor (TNF)-α and are characterized by the production of IL-22 but not IL-17[21].
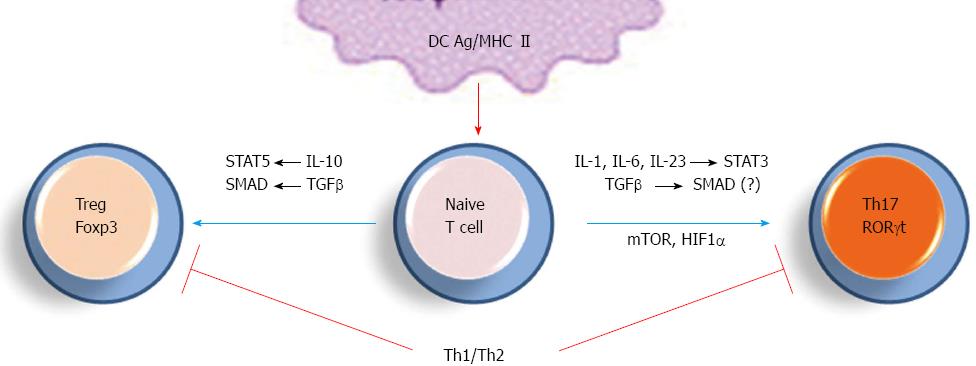
Differentiation of Th0 towards Th17 vs Treg cells is another important checkpoint for immune response and tolerance and many studies addressed factors that may derive this differentiation step (Figure 2)[2,3,22,23]. Cells receiving strong antigenic signaling differentiate into Th17 cells while those receiving lower activation express the transcription factor Foxp3 and polarize into Treg cells[24]. In addition, development toward a Th17 or a Treg phenotype is dependent on the cytokinin environment, such as TGF-β concentration and presence of pro- vs anti-inflammatory cytokines[3,24].
Recently, hypoxia-inducible factor 1 (HIF1)-α and mammalian target of rapamycin (mTOR) were identified as factors positively regulating Th17 differentiation[22,23,25]. In turn, these pathways downregulate Treg cell polarization and constitute potent targets to upregulate Th17 cell development (see below). In addition to IL-17 and IL-17F, Th17 cells are potent producers of IL-22, IL-21, IL-6, TNF-α, CCL20 and IFN-γ, which cooperate together to define the duality of Th17 role: host defense vs inflammation[7,25]. The Th17/Treg ratio is now considered as a critical target for the modulation of inflammatory response and tolerance.
Th17 cells were recently shown to comprise distinct subsets defined by their functions and cytokine secretion profile[24,26]. In addition to initial “regulatory” Th17 cells with important role during immunity to extracellular pathogens[24,25], alternative “inflammatory” Th17 subsets have been identified during autoimmune diseases which require IL-23, IL-1β and IL-6 for their differentiation and were less dependent on TGF-β. Critical distinction between these populations is their cytokine production as regulatory Th17 cells secrete higher levels of IL-10 while inflammatory Th17 cells produce more IL-22, granulocyte macrophage colony-stimulating factor (GM-CSF) and IFN-γ which may explain their proinflammatory property. However, inflammatory Th17 cells seem to comprise various subpopulations that differ by their cytokine release and need further characterization.
The characterization of IL-17 and its production by CD4+ T cells distinct from Th1 and Th2 cells led to subsequent identification of Th17 cells[4,5] and better comprehension of T cell role during chronic inflammatory diseases. This cytokine is also produced by other adaptive immune cells including activated CD8+ T cells under specific cytokinin conditions and are defined as Tc17 cells[27,28] with possible role during inflammatory reaction[29,30]. In addition, IL-17 secreting B lymphocytes were recently reported during immune response to parasite infection[31]. Many experimental inflammatory data enforced IL-17 role during autoimmune diseases[32,33]. Finally, several innate cell subsets also produce IL-17, such as γδT cells, innate lymphoid cells, mast cells and natural killer cells[34].
RA is chronic autoimmune disease affecting 1% of population and characterized by synovial inflammation correlated with leukocyte infiltration and overproduction of multiple inflammatory mediators[35]. Despite the presence of autoantibodies, chemokines, lipidic mediators and/or oxidative burst, decade use of anti-cytokine based therapeutics clearly enforced their role as key pathogenic factors during most steps of RA disease progression. Meanwhile, variations in patient response to available anti-RA therapeutics corroborate the fact that RA is heterogeneous and complex disease due to various immune pathways involved[36]. Although Th1 immunity during RA is established through multiple experimental data and patients’ observations, accumulating evidence points out the contribution of Th17 cells and IL-17 during disease progression. Beside TNF-α and IL-1β, IL-17 seems to be a critical pathogenic factor in RA and is released by both Th17 and mast cells within inflamed joints[37,38]. Recruitment of Th17 in situ during RA appears to be facilitated by CCL20 expression by synoviocytes, the ligand of CCR6, a chemokine receptor known to be expressed by Th17 cells[38].
Compared to healthy individual, arthritic patients present significantly higher serum IL-17 and IL-22 levels, corroborating their clinical scores and cartilage degradation[39,40]. Accordingly, many mouse models of arthritis enforce Th17/IL-17 role during the pathogenesis of RA[41-43]. Interestingly, mice deficient in IL-1 receptor antagonist (IL-1Ra) have increased number of Th17 cells and spontaneous development of arthritis is abrogated in these animals following IL-17 neutralization[44,45]. Nevertheless, after the onset of arthritis, neutralization of IL-17 prevents disease worsening but does not reduce the arthritis score[44]. Beside IL-17, Th17 cells infiltrated the joints produce IL-22, IFN-γ and GM-CSF that are able to activate bone cells, synoviocytes, as well as cells infiltrated the joints such as macrophages. Activation of these cells results in the production of other pro-inflammatory cytokines, lysing enzymes and chemokine, resulting in increasing migration of immune cells in situ[46,47]. Activated macrophages secrete IL-6, IL-1β and IL-23, cytokines that potentiates Th17 cell development, thus enforcing chronic inflammatory response within RA inflammatory joints. Recent data in mice suggest that Th17 may also increase germinal center B cells to produce higher amounts of autoantibodies[48]. While γδT cells also contribute to IL-17 production, these cells seem to have minor role in RA[49,50]. Finally, while TNF-α would be involved during early RA progression, IL-17 would rather contribute to the chronicity and late pathogenic responses[51]. Together, data accumulated over the years suggest the involvement of the IL-17/Th17 pathway during all progression stages of autoimmune arthritis and related inflammation.
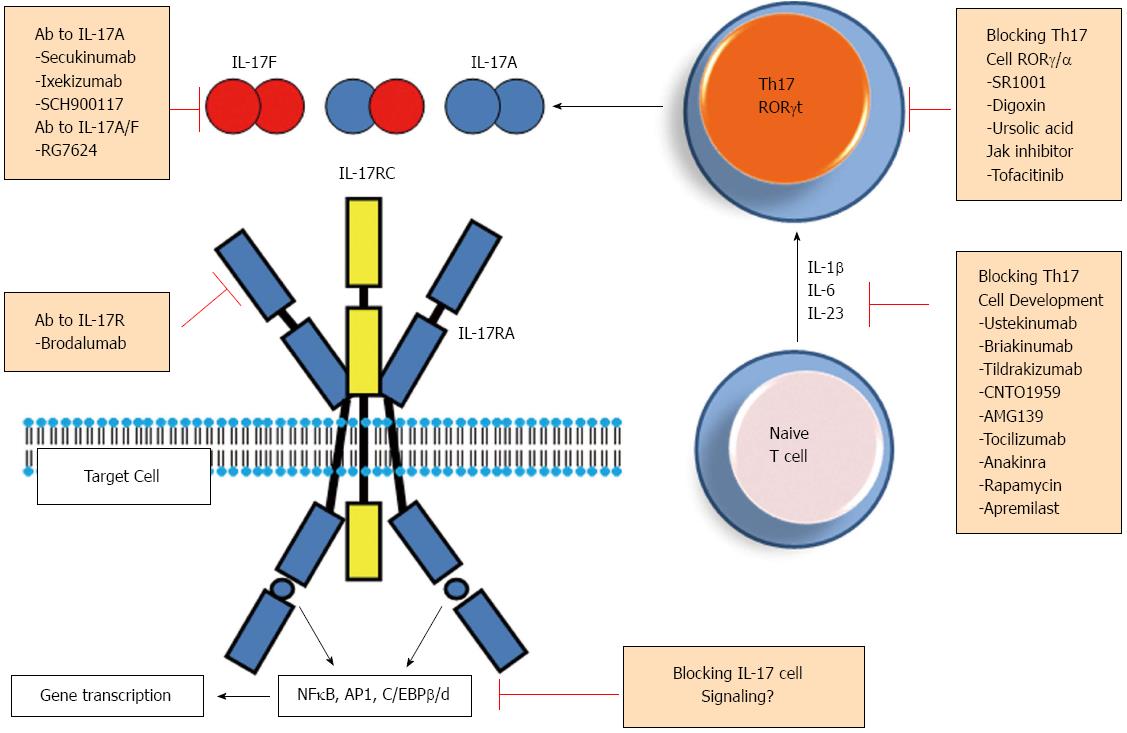
Key role of IL-17/Th17 during the pathogenesis of RA and other major autoimmune inflammatory disorders, such as psoriasis, inflammatory bowel diseases and multiple sclerosis made this pathway a potent target for therapeutic intervention in these affections[52]. Various steps of Th17 cell differentiation or of the secretion of IL-17 and other Th17-related cytokines/factors are now being considered as possible targets to decrease Th17 cell related inflammatory response (Figure 3). Below, we summarize ongoing clinical and experimental attempts addressing this goal.
As IL-6, IL-23 and IL-1β are essential during Th17 polarization and functional maturation, it is believed that beneficial role of inhibitors of IL-1 (e.g., Anakinra)[53] and IL-6 (e.g., tocilizumab)[54] may be in part through their ability to target Th17 cell development (Figure 3). Monoclonal antibodies were developed to block p40 protein, a shared subunit between IL-23 and IL-12 (ustekinumab and briakinumab)[55,56]. Such antibodies inhibit biological activities of both IL-23 and IL-12 and hence prevent the development of Th1 and Th17 cells. Selective inhibition of IL-23 by anti-p19 neutralizing antibodies (such as Tildrakizumab, CNTO1959 and AMG139) is currently under various clinical trials (Figure 3)[56,57]. Involvement of phosphoinositide 3-kinase (PI3K)/AkT activation and subsequent mTOR pathway during optimal differentiation of Th17 cells may also be targeted by rapamycin and results in experimental autoimmune diseases are promising[58,59]. Another factor affecting Th17 cell differentiation from naive T cells is HIF-1α[22,23] but pharmaceutical inhibition of this factor appears to be more difficult, due to its’ wide range of activities in normal cell physiology.
Several studies attempted to inhibit Th17 cell functions by blocking RORγt and/or RORα transcription factors[60-63] essential functional marker for these cells. Small synthetic ligands were identified and block specifically RORγt as shown with a RORγt-dependent galectin 4 -driven reporter system (Digoxin) http://www.nature.com.gate2.inist.fr/nrd/journal/v11/n10/full/nrd3794.html - B151[63], or inhibit both RORγt and RORα activities (SR1001)[61]. Ursolic acid also antagonizes RORγt activity[62] but the use of these molecules requires more in vivo experiments. More recently, tofactinib (Apremilast), a phosphodiesterase-4/JAK pathway inhibitor was shown to decrease IL-17 production from CD4+ T cells[64]. Together, these findings pointed to various available targets to inhibit the differentiation/function of Th17 cells, upstream of IL-17 secretion.
Various antibodies were generated to bind IL-17 and neutralize its activities, most are under clinical trials in RA or/and psoriatic patients including anti-IL-17A (Secukinumab, Ixekinumab, SCH900117, and RG4934)[52,56,65,66] or anti-IL-17A/F (RG7624) antibodies[67]. Another antibody was generated to inhibit IL-17 receptor (brodalumab)[52,67] and was recently shown to block both IL-17RA and IL-17RC subunits (Figure 3). Finally, as IL-17 synergizes with TNF-α to amplify the inflammatory response, inhibition of both cytokines is under clinical trial in RA[68]. Beside IL-17, a specific antibody to IL-22 was generated (Fezakinumab)[69] to inhibit this cytokine, but the trial was discontinued.
Among CD4+ T lymphocytes, Th17 are important functional cells due to their role during infection and autoimmune diseases. Together with Th1 cells, their role during inflammatory pathogenic response in RA patients makes these cells an interesting target for the development of specific biotherapy or co-therapy to decrease both ongoing inflammatory response and disease chronicity.
P- Reviewers: Ozaki K, Ryffel B S- Editor: Zhai HH L- Editor: A E- Editor: Ma S
| 1. | Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145-173. [PubMed] |
| 2. | Nakayamada S, Takahashi H, Kanno Y, O’Shea JJ. Helper T cell diversity and plasticity. Curr Opin Immunol. 2012;24:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 3. | Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414-423. [PubMed] |
| 4. | Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Turner1 H, Murphy TL, Murphy KM, Weaver1 CT. Interleukin 17-producing CD4 effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123-1132. |
| 5. | Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133-1141. [PubMed] |
| 6. | Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. [PubMed] |
| 7. | Boniface K, Blom B, Liu YJ, de Waal Malefyt R. From interleukin-23 to T-helper 17 cells: human T-helper cell differentiation revisited. Immunol Rev. 2008;226:132-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 914] [Cited by in RCA: 875] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 9. | Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857-863. [PubMed] |
| 10. | Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 286] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 11. | Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013;8:477-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 362] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 12. | Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963-970. [PubMed] |
| 13. | Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 378] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 14. | Choi YS, Yang JA, Crotty S. Dynamic regulation of Bcl6 in follicular helper CD4 T (Tfh) cells. Curr Opin Immunol. 2013;25:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Jabeen R, Kaplan MH. The symphony of the ninth: the development and function of Th9 cells. Curr Opin Immunol. 2012;24:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950-957. [PubMed] |
| 17. | Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 754] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 18. | Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121-1133. [PubMed] |
| 19. | Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1380] [Cited by in RCA: 1337] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 20. | Zygmunt B, Veldhoen M. T helper cell differentiation more than just cytokines. Adv Immunol. 2011;109:159-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Akdis M, Palomares O, van de Veen W, van Splunter M, Akdis CA. TH17 and TH22 cells: a confusion of antimicrobial response with tissue inflammation versus protection. J Allergy Clin Immunol. 2012;129:1438-149. [PubMed] |
| 22. | Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1164] [Cited by in RCA: 1423] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 23. | Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 1253] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 24. | Peters A, Lee Y, Kuchroo VK. The many faces of Th17 cells. Curr Opin Immunol. 2011;23:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 25. | Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol Rev. 2013;252:12-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 234] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 26. | Boniface K, Blumenschein WM, Brovont-Porth K, McGeachy MJ, Basham B, Desai B, Pierce R, McClanahan TK, Sadekova S, de Waal Malefyt R. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 27. | Huber M, Heink S, Grothe H, Guralnik A, Reinhard K, Elflein K, Hünig T, Mittrücker HW, Brüstle A, Kamradt T. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol. 2009;39:1716-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh CL, Yeh YC, Chou AH, Chang SR, Hsiao KN, Yu FW. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6. J Leukoc Biol. 2007;82:354-360. [PubMed] |
| 29. | Hijnen D, Knol EF, Gent YY, Giovannone B, Beijn SJ, Kupper TS, Bruijnzeel-Koomen CA, Clark RA. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-γ, IL-13, IL-17, and IL-22. J Invest Dermatol. 2013;133:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 30. | Huber M, Heink S, Pagenstecher A, Reinhard K, Ritter J, Visekruna A, Guralnik A, Bollig N, Jeltsch K, Heinemann C. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest. 2013;123:247-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 31. | Bermejo DA, Jackson SW, Gorosito-Serran M, Acosta-Rodriguez EV, Amezcua-Vesely MC, Sather BD, Singh AK, Khim S, Mucci J, Liggitt D. Trypanosoma cruzi trans-sialidase initiates a program independent of the transcription factors RORγt and Ahr that leads to IL-17 production by activated B cells. Nat Immunol. 2013;14:514-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 32. | Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1240] [Cited by in RCA: 1276] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 33. | Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146-155. [PubMed] |
| 34. | Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1240] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 35. | Schaeverbeke T, Truchetet MÉ, Richez C. When and where does rheumatoid arthritis begin? Joint Bone Spine. 2012;79:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907-916. [PubMed] |
| 37. | Kenna TJ, Brown MA. The role of IL-17-secreting mast cells in inflammatory joint disease. Nat Rev Rheumatol. 2013;9:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803-2812. [PubMed] |
| 39. | Metawi SA, Abbas D, Kamal MM, Ibrahim MK. Serum and synovial fluid levels of interleukin-17 in correlation with disease activity in patients with RA. Clin Rheumatol. 2011;30:1201-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 40. | Zhang L, Li YG, Li YH, Qi L, Liu XG, Yuan CZ, Hu NW, Ma DX, Li ZF, Yang Q. Increased frequencies of Th22 cells as well as Th17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS One. 2012;7:e31000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 41. | Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, van den Berg WB. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650-659. [PubMed] |
| 42. | Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173-6177. [PubMed] |
| 43. | Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41-47. [PubMed] |
| 44. | Koenders MI, Devesa I, Marijnissen RJ, Abdollahi-Roodsaz S, Boots AM, Walgreen B, di Padova FE, Nicklin MJ, Joosten LA, van den Berg WB. Interleukin-1 drives pathogenic Th17 cells during spontaneous arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum. 2008;58:3461-3470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986-5990. [PubMed] |
| 46. | Miossec P. IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect. 2009;11:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 47. | van Hamburg JP, Asmawidjaja PS, Davelaar N, Mus AM, Colin EM, Hazes JM, Dolhain RJ, Lubberts E. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73-83. [PubMed] |
| 48. | Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, Wucherpfennig K, Turley S, Carroll MC, Sobel RA. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35:986-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 379] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 49. | Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35:596-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 831] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 50. | Pöllinger B, Junt T, Metzler B, Walker UA, Tyndall A, Allard C, Bay S, Keller R, Raulf F, Di Padova F. Th17 cells, not IL-17+ γδ T cells, drive arthritic bone destruction in mice and humans. J Immunol. 2011;186:2602-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 51. | Toh ML, Gonzales G, Koenders MI, Tournadre A, Boyle D, Lubberts E, Zhou Y, Firestein GS, van den Berg WB, Miossec P. Role of interleukin 17 in arthritis chronicity through survival of synoviocytes via regulation of synoviolin expression. PLoS One. 2010;5:e13416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1082] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 53. | Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1316] [Cited by in RCA: 1358] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 54. | Ash Z, Emery P. The role of tocilizumab in the management of rheumatoid arthritis. Expert Opin Biol Ther. 2012;12:1277-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, Fretzin S, Kunynetz R, Kavanaugh A. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009;373:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 432] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 56. | Williams SC. New biologic drugs get under the skin of psoriasis. Nat Med. 2012;18:638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Bangert E, Laimer D, Riedl E, Greisenegger E, Horowitz A, Xu D, Kopp T. Anti-IL-23p19 (MK-3222): effects on the hallmarks of inflammation in psoriasis. J Invest Dermatol. 2012;132:S50-S65. |
| 58. | Rommel C, Camps M, Ji H. PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol. 2007;7:191-201. [PubMed] |
| 59. | Camps M, Rückle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Françon B, Martin T, Gretener D, Perrin D, Leroy D, Vitte PA, Hirsch E, Wymann MP, Cirillo R, Schwarz MK, Rommel C. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936-943. [PubMed] |
| 60. | Zhang X, Jin J, Peng X, Ramgolam VS, Markovic-Plese S. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol. 2008;180:6988-6996. [PubMed] |
| 61. | Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidović D. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 441] [Cited by in RCA: 435] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 62. | Xu T, Wang X, Zhong B, Nurieva RI, Ding S, Dong C. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORgamma t protein. J Biol Chem. 2011;286:22707-22710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 63. | Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, Chow J, Manel N, Ciofani M, Kim SV. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature. 2011;472:486-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 485] [Cited by in RCA: 457] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 64. | Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, Gruben D, Wallenstein G, Krishnaswami S, Zwillich SH. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460. [PubMed] |
| 65. | Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, Sloan-Lancaster J. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 371] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 66. | Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis. 2013;72 Suppl 2:ii116-ii123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 245] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 67. | Available from: http://www.roche.com/research_and _development/pipeline/roche_pharma_pipeline.htm. |