Revised: April 18, 2012
Accepted: July 4, 2012
Published online: August 3, 2012
Rheumatoid arthritis (RA) is the most common inflammatory disease of the musculoskeletal system primarily affecting the joints. It is characterized by massive synovial hyperplasia and subsequent destruction of articular cartilage and bone. Although various aspects in the pathogenesis of RA remain unclear, genetic, environmental and of course immunological factors have been involved. Defects in apoptosis seem to play a role in both initiation and perpetuation of RA. Apo2 ligand/ tumor necrosis factor (TNF) related apoptosis-inducing ligand (Apo2L/TRAIL) is a cytokine that belongs to the TNF superfamily capable of inducing apoptosis on tumor cells through activation of the extrinsic pathway. Besides this function, like other members of the TNF superfamily, Apo2L/TRAIL has been shown to exert important functions in the regulation of the immune system. Concerning pathological conditions, the Apo2L/TRAIL signaling pathway plays an important role in the response to infections, in immune surveillance against tumors and in autoimmune diseases such as RA. Furthermore, its implication in suppression of autoimmunity suggests that Apo2L/TRAIL has potential as therapeutic agent not only in cancer but also in autoimmune diseases. In fact, Apo2L/TRAIL-based therapies have been shown effective in various animal models of RA. This review summarizes the current knowledge on the biology of Apo2L/TRAIL and its role in RA.
- Citation: Martinez-Lostao L, Anel A. Role of Apo2L/TRAIL in immunity: Applications to rheumatoid arthritis. World J Rheumatol 2012; 2(1): 1-11
- URL: https://www.wjgnet.com/2220-3214/full/v2/i1/1.htm
- DOI: https://dx.doi.org/10.5499/wjr.v2.i1.1
Multicellular organisms use apoptosis, the major mechanism of programmed cell death, to eliminate cells that are superfluous or that are irreparably damaged[1,2]. Apoptosis plays a pivotal role during development and controls homeostasis of tissues throughout adult live[3]. A wide variety of stimuli can trigger apoptosis such as severe damage caused by heat shock, cytotoxic drugs, radiation infection, tumor transformation, and cellular stress. Moreover, an aberrant regulation of apoptosis is implicated in the pathogenesis of a variety of major human diseases. Excessive apoptosis is implicated in neurodegenerative diseases, such as Alzheimer’s and Huntington’s diseases[4], acquired immune deficiency syndrome[5], ischemic heart disease[6], stroke[7], and infertility[8]. In contrast, deficiency in apoptosis plays a key role in the pathogenesis of cancer[9] and is also involved in certain autoimmune disorders[10].
There are two major apoptotic pathways: the intrinsic or mitochondrial and the extrinsic or death receptor-mediated. The intrinsic pathway is activated by intracellular events and depends on proteins of the Bcl-2 family that control the release of apoptogenic factors from the mitochondria[11]. In contrast, the extrinsic pathway is triggered by signals received through extracellular protein ligands that bind to proapoptotic death receptors (DR) thereby initiating an intracellular signaling cascade leading to apoptosis[12].
Mitochondrial outer-membrane permeabilization is involved in the intrinsic pathway allowing the release of proapoptotic factors such as cytochrome c, Second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI (Smac/DIABLO) and apoptosis inducing factor from the mitochondria to the cytosol[13-15], a process controlled by the Bcl-2 protein family[16]. Once released from mitochondria to the cytosol, cytochrome c induces the formation of a multimeric complex called the apoptosome, containing the adaptor protein Apaf-1 and the initiator caspase-9. Caspase-9 is activated into the apoptosome and in turn cleaves effector caspases ultimately leading to apoptosis[17].
The extrinsic pathway transmits apoptotic signals from extracellular ligands through DRs to the intracellular apoptotic machinery. Six different DRs are known: Fas, tumor necrosis factor (TNF)R1, DR3, TNF-related apoptosis-inducing ligand (TRAIL)-R1 or DR4, and TRAIL-R2 or DR5, and DR6[18]. These DRs interact with their corresponding ligands, which belong to TNF superfamily (FasL, TNF, TL1A and TRAIL, respectively). This interaction induces receptor oligomerization and activation of caspase cascade, ultimately leading to apoptosis[12].
Apo2L/TRAIL, a member of the TNF protein superfamily, was initially described as a death ligand capable of inducing apoptosis in transformed cells while sparing normal cells[19,20]. Subsequently, a variety of studies, including those with knockout mice for TRAIL and TRAIL-R have been conducted to unravel the physiological role of this cytokine[21-23]. These studies revealed that Apo2L/TRAIL-TRAIL-R signaling is implicated in the regulation of the homeostasis of the immune system. Thus Apo2L/TRAIL can be considered as an additional mechanism necessary to prevent the development of autoimmunity[24,25].
Rheumatoid arthritis (RA) is the most common inflammatory disease of the musculoskeletal system[26,27]. Although RA frequently shows systemic involvement, it primarily affects the joints, where chronic synovial inflammation and subsequent destruction of the articular cartilage and bone are the hallmarks of the disease. This synovial hyperplasia is caused by a massive invasion of inflammatory cells and by extensive increase of resident synovial cells also called fibroblast-like synoviocytes (FLS), which generates a heterogeneous tissue known as pannus. RA-FLS play a pivotal role in both initiation and perpetuation of RA[28,29]. A body of evidence has demonstrated that FLS undergo substantial changes referred to as tumor-like transformation, being active drivers of joint destruction in RA[30,31]. Among the cellular characteristics that distinguish FLS are production of cytokines, chemokines and growth factors as well as alterations in growth and apoptosis. The later is of particular interest, because the resistance of RA-FLS to apoptotic signals provides one explanation to the development of pannus and joint destruction. Concerning inflammatory cells, T lymphocytes, mainly CD4+ T cells with a memory phenotype, but also CD8+ T cells, macrophages and B cells are present in the sublining tissue. It has also been described alterations in apoptosis in infiltrating T lymphocytes that together with alterations in RA-FLS may lead to the creation of a proinflammatory microenvironment into the joint that contributes to chronic disease maintenance.
The major aim of this review is to provide a summary of the current data on the role of the death ligand Apo2L/TRAIL in the pathogenesis as well as its use as therapeutic agent in RA.
Apo2L/TRAIL was independently identified by two different groups as the third member of the TNF superfamily that induces apoptosis[19,20]. Apo2L/TRAIL is capable of binding to a complex system of receptors with different affinities and possibly distinct signaling outcomes. Five receptors for Apo2L/TRAIL are known in humans called TRAIL-R1/DR4, TRAIL-R2/DR5, TRAIL-R3/DcR1 and TRAIL-R4/DcR2[12]. Apo2L/TRAIL can bind a soluble receptor termed osteoprotegerin (OPG). Only DR4 and DR5 possess a death domain (DD) in their intracellular portion and are capable of transmitting the proapoptotic signal[32,33] by inducing the formation of the death-inducing signaling complex (DISC)[34,35]. DcR1 and DcR2 are two non-apoptotic cell-bound receptors for Apo2L/TRAIL[36,37]. Apo2L/TRAIL can also bind, with rather low affinity, to a soluble receptor called OPG[38]. OPG binds with high affinity and inhibits the action of another TNF superfamily member termed receptor activator of nuclear factor kappa B (NFκB) ligand (RANKL) involved in bone metabolism. Nevertheless, it is rather unlikely that Apo2L/TRAIL-OPG interaction may play a physiological role, at least in vivo, since Apo2L/TRAIL and DR5 knockout mice do not display a phenotype with alteration in bone metabolism[39,40].
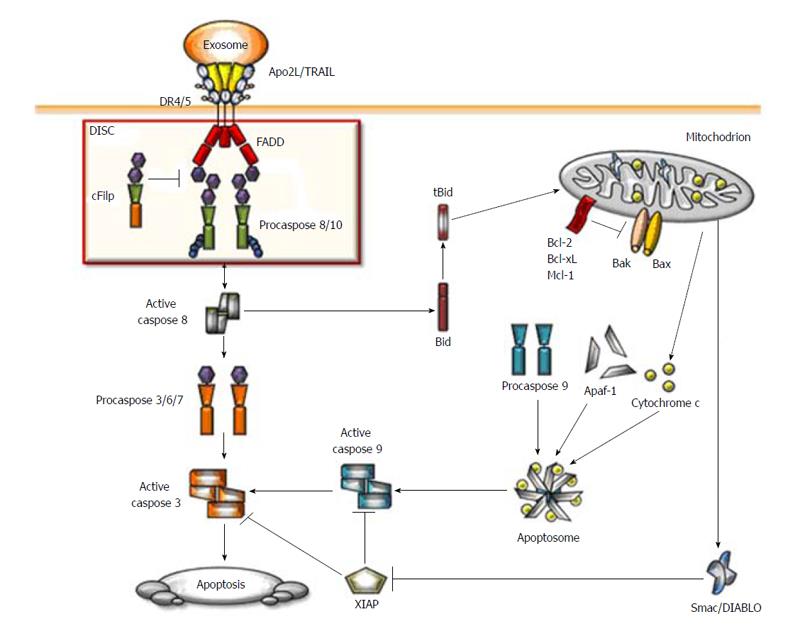
The initial step of the Apo2L/TRAIL-induced apoptosis is the binding of the trimeric ligand to DR4 or DR5. This interaction induces clustering of the receptors that recruits the adaptor protein Fas-associated DD (FADD) which in turn promotes the assembly of the DISC[34,41]. DD of FADD binds to the homologue domain of the DRs thereby exposing the death-effector domain of procaspase-8 or -10. Recruitment of procaspase-8 to the DISC induces its activation by autocleavage, release into the cytosol, where active caspase 8 activates the effector caspases-3, -6 and -7, the ultimate executors of the apoptotic program of cell death (Figure 1).
Apo2L/TRAIL apoptotic signaling pathway is regulated at different levels to prevent unwanted caspase activation. In fact, not all proteins present in the DISC are proapoptotic. Cellular FLICE inhibitory protein (cFLIP), which shares high sequence homology with caspase-8 and -10, inhibits caspase activation at the DISC by competing for binding to FADD. There are two splice variants of cFLIP, a longer (cFLIPL) and a shorter version (cFLIPS)[42]. However, the role of cFLIPL is controversial. Some studies have reported that cFLIPL is an antiapoptotic protein that works in a similar manner to cFLIPS[43]. In contrast, other studies describe cFLIPL as a proapoptotic molecule[44].
Other mechanisms of distinct nature have been described that may modulate Apo2L/TRAIL signaling. Post-translational modifications of DRs by O-glycosylation seem to be important to promote clustering of DR4 and DR5, after ligand binding which mediate recruitment and activation of the apical caspase-8[45]. Recently, ubiquitylation of caspase-8 after death receptor ligation by Apo2L/TRAIL has been showed as a crucial mechanism which promotes the full activation of caspase-8[46].
Finally, it has been described that Apo2L/TRAIL has more diverse effects than apoptosis. Among the non-apoptotic effects of Apo2L/TRAIL, it has been reported induction of proliferation, migration and survival signals, in distinct cell types. Receptor interacting protein (RIP), which is able to activate the inhibitor of NFκB kinase-complex (IKK complex) in TNF signaling, has been described to be present in the Apo2L/TRAIL DISC[47]. RIP promotes phosphorylation of IKK, which phosphorylates IκB leading to its degradation. Degradation of inhibitor of NFκB (IκB) promotes phosphorylation of NFκB thereby activating this transcription factor[48]. Furthermore, Apo2L/TRAIL can activate other proinflammatory intracellular signaling pathways such as mitogen-activated kinase (MAPK), phosphatydilinositol 3-kinase (PI3K) and c-Jun N-terminal kinase[49]. This pro-survival effects induced by Apo2L/TRAIL are crucial in the resistance of distinct tumor cells to Apo2L/TRAIL-induced apoptosis[50] and also seem to be important in the pathogenesis of some autoimmune diseases such as RA[51].
Both Apo2L/TRAIL and TRAIL-R deficient mice do not display any overt developmental defects[39,52,53] revealing that, Apo2L/TRAIL signaling is not essential for normal embryonic development.
The major roles of Apo2L/TRAIL have been found in the immune system playing a role in shaping and regulating the immune response. This is not surprising as it was already suggested by the inducible expression of Apo2L/TRAIL in immune cells such as monocytes, dendritic cells (DCs) and natural killer (NK) cells[54-56].
In case of T cells, Apo2L/TRAIL expression is absent in naive T cells, whereas expression of Apo2L/TRAIL protein was increased on both CD4+ and CD8+ after T-cell receptor or phytohaemagglutinin stimulation[57,58]. Surface Apo2L/TRAIL in activated T cells seems to be stabilized by type I interferons[57].
In human T cell blasts Apo2L/TRAIL and FasL are stored into intracytoplasmic pre-lysosomal compartments with the structure of multivesicular bodies[59]. Apo2L/TRAIL, and FasL, are rapidly released to the supernatant of activated human T cells associated with microvesicles/exosomes of 100 nm of diameter with the death ligands on their surface[25,60].
Although non-activated CD4+ and CD8+ T cells express DR4 and DR5, they are resistant to Apo2L/TRAIL-mediated apoptosis[61,62]. However, activation of T cells with interleukin (IL)-2 resulted in Apo2L/TRAIL susceptibility. In fact, Apo2L/TRAIL is implicated in the homeostasis of the immune response by induction of activation-induced cell death (AICD) of human T cells[63]. This process is dependent on the action of death ligands, especially on FasL[64,65], but Apo2L/TRAIL also plays a role in AICD[66]. The effect of Apo2L/TRAIL was more pronounced on the CD8+ T cell population[67]. Inhibition of IL-2-dependent T cell blast growth, mainly in the CD8+ T cell population, by Apo2L/TRAIL does not require re-stimulation and would suggest an additional immune-regulatory role of this death ligand[25,66,67].
There is a CD8+ T cell population which is primed in the absence of CD4 T cells, the so-called “helpless” CD8+ T cells. These cells are unable to undergo the second round of clonal expansion[68]. The memory CD8+ T cells generated in this manner die by Apo2L/TRAIL-mediated AICD upon re-stimulation[69].
Apo2L/TRAIL also seems to be involved in the regulation of T helper 1 and T helper 2 responses[70] and has been recently implicated in the induction of cell proliferation of the CD4+ CD25+ regulatory T cell population[71,72].
Apo2L/TRAIL signaling has been implicated in intrathyimic negative selection[23,73]. However, it is still a controversial subject. These studies suggested that negative selection was at least partially impaired in TRAIL knockout mice or in the presence of soluble blocking DR5. In contrast other studies using TRAIL knockout mice and a neutralizing anti-mouse TRAIL mAb showed that Apo2L/TRAIL signaling does not play a role in this process[74]. Supporting this finding normal negative selection has been described in DR5 knockout mice suggesting that Apo2L/TRAIL receptor signaling in not required for negative selection[53].
Immune effector cells involved in the fight against infections, such as NK cells and cytotoxic T cells, express Apo2L/TRAIL when they are activated and exert their cytotoxic function, at least in part, via Apo2L/TRAIL signaling[62,75-79].
Finally, Apo2L/TRAIL has been implicated in immunosurveillance against cancer[35,52,80]. Although Apo2L/TRAIL has a suppression role in the growth of grafted tumor and experimental metastasis, the importance of endogenous ligand in the immunosurveillance against primary tumors is still a matter of debate. Mice deficient in Apo2L/TRAIL and TRAIL-R do not spontaneously develop tumors in early age[39,53]. However, TRAIL-deficient mice develop more lymphomas than wild-type mice when they are aged[81]. Neutralization of Apo2L/TRAIL signaling enhanced fibrosarcoma development in methylcholanthrene-induced tumors in mice[39]. In the absence of Apo2L/TRAIL or TRAIL-R, mice develop more lymphomas, carcinogen–induced tumors, skin carcinoma and lymphoma metastasis[21,22,81]. In any case, a definitive role of endogenous Apo2L/TRAIL in tumor suppression of primary tumors has not been yet well established and further studies in autochthonous tumor development models will be needed[82].
The resistance of RA-FLS to apoptotic signals has been associated with the phenotype of these cells and it may provide an explanation to the development of pannus and consequently the joint destruction[83]. Resistance of FLS to apoptosis occurs at different levels. For example, Bcl-2 expression is induced by TNFα and IL-1β in cultured FLS. Moreover, there is a direct correlation between Bcl-2-expression and the extent of the synovial lining thickening and inflammation[84]. IL-15, a cytokine with pleiotropic effects, increases Bcl-2 and Bcl-xL levels in FLS[85]. Mcl-1 is also induced after cytokine stimulation and is found in RA synovium correlating with synovial inflammation[86]. The apoptotic protein Bcl-2/adenovirus E1B 19-kd protein-interacting protein 3 is induced in RA synovium in response to hypoxia but is pro-apoptotic action is inhibited by TNFα and IL-1β providing a link between inflammation and resistance to apoptosis in RA[87].
Although FLS express a variety of death receptors such as Fas/CD95, TRAILR1 and TRAILR2 and also TNFR1[88-90], various data indicate that FLS are relatively resistant to receptor-mediated apoptosis. TNFα is able to induce soluble Fas thus decreasing the susceptibility of FLS to Fas/CD95-induced apoptosis[89]. DcR3 is expressed in FLS in a TNFα-dependent manner and is able to prevent Fas/CD95-induced apoptosis[91]. LIGHT, another member of the TNF superfamily, is found in RA and also prevents FLS from Fas/CD95-induced apoptosis[92]. The expression of FLIP is high in RA mainly at sites of cartilage destruction[93]. It has been suggested that the expression of FLIP depends on the stage of disease[94]. While in RA patients with short duration of the disease showed decreased levels of apoptosis accompanied by high expression of FLIP, in patients with a long-term RA, increased levels of apoptosis were associated with low levels of FLIP. Again a connection between inflammation and resistance to apoptosis is achieved because TNFα can induce the expression of FLIP[95]. Post-translational modifications also play a role in FLS apopotosis. Small ubiquitin-like modifier 1 (SUMO-1) is highly expressed in FLS and SUMO-1-mediated modification protects cells from Fas- and TNFR1-induced apoptosis[96].
On the other hand, FLS also contribute to the accumulation of infiltrating cells by regulating their response to apoptosis through cellular interaction and soluble factors. FLS produce large amounts of stromal cell-derived factor 1α which is able to inhibit T cell apoptosis through activation of PI3-kinase and MAPK pathways[97]. B cells co-cultured with FLS are protected from apoptosis through a Vascular cell adhesion protein 1 and α4β1 integrin-dependent mechanism[98]. Reduced apoptosis has been associated with increased expression of Bcl-xL[99]. The B cell-activating factor of the TNF family, that is involved in prosurvival B cell signaling, is also produced by FLS after engagement of α5β1 integrins of the cell surface[100].
In summary, FLS resistance to apoptosis contributes significantly to the pathogenesis of RA. The tumor-like transformation of FLS not only leads to profound changes in the responsiveness of these cells to apoptotic stimuli. In addition, it increases the persistence of inflammatory cells by modulating its resistance to cell death.
Autoimmune diseases result from the inappropriate recognition of self-antigens due to defects in the regulation of the immune system. Apo2L/TRAIL signaling seems to be able to modulate the autoimmune disease and to be implicated in a variety of autoimmune diseases. An increased number of studies have consistently shown that Apo2L/TRAIL is capable of inhibiting autoimmune diseases in a variety of animal models. In these studies, Apo2L/TRAIL seems to play distinct roles ranging from inhibiting inflammation, to inhibiting cell cycle progression, proliferation of auto-reactive T cells as well as cytokine and antibody production.
Although TRAIL- and TRAIL-deficient mice do no display spontaneous autoimmune diseases, many studies have identified profound effects when autoimmunity is induced in these mice or in the presence of Apo2L/TRAIL signaling-blocking agents. In these studies, it has been shown that mice were more susceptible to induced-autoimmune diabetes[23,101-103]. It is noteworthy that double FasL mutant (gld) and TRAIL knockout mice developed an extreme and fatal lymphoproliferative disease which was more severe than that due to mutation in FasL alone[104]. Apo2L/TRAIL is also implicated in experimental autoimmune thyroiditis[71,105,106]. The most widely used mouse model which mimics multiple sclerosis is experimental autoimmune encephalomyelitis (EAE). Blockade of Apo2L/TRAIL signaling led to a high degree of inflammation in the central nervous system[107,108]. However, a reduction of the clinical severity of EAE is observed when TRAIL-R2-Fc, an Apo2L/TRAIL signaling blocking agent, was injected into central nervous system in mice in which EAE was previously induced[109].
A variety of studies have implicated Apo2L/TRAIL the pathogenesis of RA. In a mice model of RA [collagen-induced arthritis (CIA)], the chronic blockade of Apo2L/TRAIL exacerbated autoimmune arthritis, leading to profound hyperproliferation of synovial cells and arthritogenic lymphocytes and increasing the production of autoantibodies and proinflammatory cytokines[110]. In this study, Apo2L/TRAIL inhibited autoimmune inflammation by blocking cell cycle progression rather than by inducing apoptosis of inflammatory cells. TRAIL-deficient mice were also more susceptible to CIA. In line with this, TRAIL-deficient C57BL/6 mice developed the typical symptoms when immunized with collagen whereas C56BL/6 wild-type mice were not susceptible to CIA[23].
Although numerous studies have examined the role of Apo2L/TRAIL in autoimmune diseases in experimental animal models, less is known of the role of Apo2L/TRAIL in human autoimmune diseases. Most of the studies have shown expression of DR4 and/or DR5 in FLS from RA patients[51,90,111,112]. However, in one study neither Apo2L/TRAIL nor its receptors were detectable on lymphocytes or synovial fibroblasts obtained from synovial fluid (SF) from RA patients[113]. Nevertheless, RA SF macrophages expressed the decoy receptor DcR1. On the other hand, it has been demonstrated that T lymphocytes from RA synovial fluids were activated and expressed a similar pattern of Apo2L/TRAIL than human T cell blasts or T cells in the SF of traumatic patients[114]. RA T cells were insensitive to Fas-mediated regulation, as previously reported[115] but remarkably, they were more sensitive than in vitro activated T cells to regulation by Apo2L/TRAIL. Nevertheless, it was detected very low amounts of bioactive FasL and Apo2L/TRAIL associated with exosomes in SF from RA patients as compared with SF from traumatic arthritis patients[114].
Conversely, a dual role of Apo2L/TRAIL has been suggested in RA which is characterized by expansion of FLS. It has been reported that Apo2L/TRAIL induced RA FLS proliferation in a dose-dependent manner through a mechanism involving MAPK and PI3K/Akt signaling[51]. Previous studies have demonstrated a relative in vitro sensitivity of RA FLS to Apo2L/TRAIL[90] which is increased upon treatment with actinomycin D[116]. However, more recent studies indicated that only a fraction of FLS are sensitive to Apo2L/TRAIL-induced apoptosis[117], depending on their proliferative state[118], while proliferation is induced in another fraction after rApo2L/TRAIL treatment[51,117]. More recently, it has been reported that Apo2L/TRAIL-induced apoptosis varied in FLS from different RA patients and that susceptibility of FLS to apoptosis induced by Apo2L/TRAIL inversely correlated with disease severity of RA patients[119].
Although Apo2L/TRAIL-based therapies have been mostly used in cancer, its therapeutic value in autoimmune diseases has been also proposed. In this line, a number of therapeutic strategies involving Apo2L/TRAIL have been currently used to treat various experimental autoimmune diseases such as experimental autoimmune thyroiditis[71,105] and experimental autoimmune encephalomyelitis[10,72,120,121].
Concerning RA, distinct Apo2L/TRAIL-based therapeutic approaches have been used for treatment of arthritic joints. CIA was induced in DBA/1 mice and then animals received an intra-articular injection of an adenovirus carrying the mouse TRAIL gene[110]. This local treatment reduced disease score. Interestingly, in this study TRAIL had no effect on apoptosis of inflammatory cells either in vivo or in vitro but inhibited DNA synthesis and prevented cell cycle progression of lymphocytes in vitro. A similar therapeutic strategy had been used in a rabbit model of RA. In IL-1β-induced arthritis in rabbits, intra-articular gene transfer using an adenoviral vector carrying human Apo2L/TRAIL gene ameliorated disease in treated arthritic joints. Apo2L/TRAIL gene transfer was able to induce apoptosis in cells within the synovial cell lining, to reduce leukocyte infiltration and to stimulate matrix synthesis[122]. Gene transfer-based therapeutic strategy which modulates Apo2L/TRAIL receptor expression may sensitize RA synoviocytes to Apo2L/TRAIL. Primary cultures established from RA synovial cells showed an increase of DcR2 correlating with Apo2L/TRAIL resistance of these cells. A combined treatment with a DcR2 silencing RNA approach and gene transfer using an adenoviral vector carrying human Apo2L/TRAIL eliminated apoptosis-resistant RA synovial fibroblasts[123].
Other therapy strategy for treatment of RA has been the use of rApo2L/TRAIL. Using the previously described rabbit model of IL-1β-induced arthritis, intra-articular injection of human rApo2L/TRAIL into arthritic joints induced apoptosis of the synovial cells and reduced leukocyte infiltration. Furthermore, treatment with rApo2L/TRAIL had not adverse effects neither locally on cartilage metabolism nor systemic on hepatic function[124]. Treatment with human rApo2L/TRAIL was also reported in a CIA mouse model. Soluble rApo2L/TRAIL was capable of significantly reducing the severity and incidence of CIA, joint swelling, erythema, and edema. Inflammatory cell infiltration, cartilage destruction, and bone erosion were also significantly reduced in joints of TRAIL-treated mice in a dose-dependent manner. Treatment with rApo2L/TRAIL was also effective systemically decreasing the levels of proinflammatory cytokines and anti-collagen-specific antibodies in the sera of CIA mice[125].
Other Apo2L/TRAIL-based therapeutic strategy has been the use of genetically modified DCs in mouse models. In a CIA model on DBA/1j mice, in vivo administration of genetically modified DC infected with an adenovirus expressing inducible TRAIL and pulsed with collagen II significantly decreased the incidence of arthritis and infiltration of T cells in joints[126]. Interestingly, adenoviral vector carrying Apo2L/TRAIL was not toxic to DCs or mice but could induce activated T cells to undergo apoptosis in the spleen. Anti-human DR5 mAb (TRA-8) has been also uses as treatment in adjuvant arthritis in rats, a rat model of RA[127]. Hind paw inflammation was ameliorated after treatment with TRA-8 decreasing synovial hyperplasia due to induction of apoptosis in synovial cells and infiltration of inflammatory cells.
Novel Apo2L/TRAIL formulations have been developed to improve its biological half-life, stability and/or bioactivity and have been used as treatment for RA in distinct animal models. Nano-sized complexes (nanocomplexes) based on hyaluronic acid and polyethylene glycol (PEG)-derivatized human TRAIL (PEG-TRAIL) formed by N-terminal specific PEGylation has been used in a CIA mouse model[128]. The therapeutic effect of this formulation injected intra-peritoneally was higher than soluble TRAIL, concerning clinical scores and histology. Additionally, sustained delivery of PEG-TRAIL resulted in significant reduction of serum inflammatory cytokines and collagen-specific antibodies that are responsible for the pathogenesis of RA. As previously discussed, infiltrating T lymphocytes in synovial fluid (SF) from RA patients, although resistant to Fas, were unexpectedly more susceptible to human rApo2L/TRAIL than were in vitro activated T cells. However, the amount of bioactive Apo2L/TRAIL associated with exosomes in SF from RA patients was extremely low compared with SF from control patients with traumatic arthritis[114]. Consequently, administration of Apo2L/TRAIL associated to the surface of liposomes resembling the natural exosomes may be a reasonable therapeutic strategy in RA. Treatment of the arthritic knee joints by intra-articular injection with human rApo2L/TRAIL associated with liposomes (LUV-Apo2L/TRAIL) in an antigen-induced arthritis rabbit model showed a higher effectiveness than soluble rApo2L/TRAIL reducing joint swelling. Histological parameters such as synovial hyperplasia, inflammatory infiltrate vascularity and formation of villi were also significantly reduced when arthritic joint were treated with LUV-Apo2L/TRAIL[129]. In consequence, the association of Apo2L/TRAIL to liposome surface improves its bioactivity. Interestingly, treatment with this Apo2L/TRAIL novel formulation did not have adverse effects previously described for soluble form of Apo2L/TRAIL such as hepatotoxicity.
Since the first description of Apo2L/TRAIL, more than ten years ago, and the identification of its two cognate pro-apoptotic receptors, Apo2L/TRAIL signaling has provided a unique novel model system for studying the extrinsic apoptotic pathway. During the last decade, a body of evidence has accumulated illustrating that Apo2L/TRAIL is clearly implicated not only in cancer but also in immunity. Immunosuppressive and immunoregulatory functions important for immune homeostasis, immunosurveillance and autoimmunity have been demonstrated for Apo2L/TRAIL.
Biological therapies such as anti-TNF and anti-IL1 agents have been successfully used in RA. However, these therapies targeting immune system do not have a response over 60%. Therefore, other therapeutic approaches have been set up. In line with this, apart from the use of Apo2L/TRAIL as anti-tumor therapy, an increasing number of studies have shown that this molecule is a promising therapeutic agent to treat autoimmune diseases including RA. Distinct studies using in vivo animal models of RA have provided evidences that Apo2L/TRAIL is capable of diminishing the incidence and the severity of the autoimmune disease. A variety of experimental approaches, including gene transfer, soluble molecule, pro-apoptotic agonistic receptor antibodies and lately, novel Apo2L/TRAIL formulations based on association of the death ligand with different kind of nanoparticles have been used as treatment for arthritis in several animal models. In summary, Apo2L/TRAIL signaling is a promising molecular target for autoimmune disease immunotherapeutics.
In spite of these promising data obtained in RA, further studies are required to optimally exploit the Apo2L/TRAIL-TRAIL pathway in this disease. In this line, Apo2L/TRAIL-based nanoparticles have been shown to improve its biological half-life, stability and bioactivity compared with the soluble form and could open new perspectives in the use of Apo2L/TRAIL as therapeutic agent in RA. With regard to the route of possible administration of Apo2L/TRAIL-based therapy, in most of studies carried out in animal models of RA, administration of Apo2L/TRAIL has been performed intra-articularly. Further studies should be performed in order to establish the viability of a systemic administration, more feasible in humans given the large number of involved joints.
Pending the outcomes of clinical trials targeting the Apo2L/TRAIL pathway in patients with cancer, clinical trials could be considered to determine the therapeutic efficacy of targeting the Apo2L/TRAIL in patients with RA. Regardless, Apo2L/TRAIL has appeared as a significant molecule in immune system regulation, with a promising future as treatment in RA.
The authors thank Dr. Julian Pardo for their critical comments through preparation of this manuscript. The authors also acknowledge Dr. Avi Ashkenazi for their support through the years.
Peer reviewers: Chang-Hee Suh, Professor, Department of Rheumatology, Ajou University School of Medicine, San 5, Wonchon-dong, Yeongtong-gu, Suwon 443-721, South Korea; Hazem M Youssef, MD, FRCP, Rheumatology Unit, Aberdeen Royal Infirmary, Aberdeen, AB25 2ZN, United Kingdom
S- Editor Wu X L- Editor Stewart G E- Editor Wu X
| 1. | Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3451] [Cited by in RCA: 3569] [Article Influence: 170.0] [Reference Citation Analysis (0)] |
| 2. | Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1729] [Cited by in RCA: 1928] [Article Influence: 113.4] [Reference Citation Analysis (0)] |
| 3. | Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9960] [Cited by in RCA: 9992] [Article Influence: 188.5] [Reference Citation Analysis (0)] |
| 4. | Vila M, Przedborski S. Targeting programmed cell death in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 377] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 5. | Roshal M, Zhu Y, Planelles V. Apoptosis in AIDS. Apoptosis. 2001;6:103-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Geng YJ. Molecular mechanisms for cardiovascular stem cell apoptosis and growth in the hearts with atherosclerotic coronary disease and ischemic heart failure. Ann N Y Acad Sci. 2003;1010:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Holcík M. Targeting endogenous inhibitors of apoptosis for treatment of cancer, stroke and multiple sclerosis. Expert Opin Ther Targets. 2004;8:241-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Said TM, Paasch U, Glander HJ, Agarwal A. Role of caspases in male infertility. Hum Reprod Update. 2004;10:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7:1001-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 327] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 10. | Cretney E, Shanker A, Yagita H, Smyth MJ, Sayers TJ. TNF-related apoptosis-inducing ligand as a therapeutic agent in autoimmunity and cancer. Immunol Cell Biol. 2006;84:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2769] [Cited by in RCA: 2758] [Article Influence: 106.1] [Reference Citation Analysis (0)] |
| 12. | Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4271] [Cited by in RCA: 4190] [Article Influence: 155.2] [Reference Citation Analysis (0)] |
| 13. | van Gurp M, Festjens N, van Loo G, Saelens X, Vandenabeele P. Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun. 2003;304:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 279] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 14. | Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3067] [Cited by in RCA: 2968] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 16. | Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 489] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 17. | Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 787] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 18. | LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 653] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 19. | Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687-12690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1354] [Cited by in RCA: 1381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 20. | Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2187] [Cited by in RCA: 2204] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 21. | Finnberg N, Klein-Szanto AJ, El-Deiry WS. TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J Clin Invest. 2008;118:111-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Grosse-Wilde A, Voloshanenko O, Bailey SL, Longton GM, Schaefer U, Csernok AI, Schütz G, Greiner EF, Kemp CJ, Walczak H. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J Clin Invest. 2008;118:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 23. | Lamhamedi-Cherradi SE, Zheng SJ, Maguschak KA, Peschon J, Chen YH. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL-/- mice. Nat Immunol. 2003;4:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 263] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Falschlehner C, Schaefer U, Walczak H. Following TRAIL's path in the immune system. Immunology. 2009;127:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 235] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Anel A, Bosque A, Naval J, Pineiro A, Larrad L, Alava MA, Martinez-Lorenzo MJ. Apo2L/TRAIL and immune regulation. Front Biosci. 2007;12:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | McInnes IB, O'Dell JR. State-of-the-art: rheumatoid arthritis. Ann Rheum Dis. 2010;69:1898-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 27. | Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2254] [Cited by in RCA: 2432] [Article Influence: 162.1] [Reference Citation Analysis (0)] |
| 28. | Neumann E, Lefèvre S, Zimmermann B, Gay S, Müller-Ladner U. Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol Med. 2010;16:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 296] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 29. | Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1503] [Cited by in RCA: 1457] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 30. | Müller-Ladner U, Kriegsmann J, Franklin BN, Matsumoto S, Geiler T, Gay RE, Gay S. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1996;149:1607-1615. [PubMed] |
| 31. | Fassbender HG. Histomorphological basis of articular cartilage destruction in rheumatoid arthritis. Coll Relat Res. 1983;3:141-155. [PubMed] |
| 32. | Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1352] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 33. | Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity. 1997;7:821-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 550] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 34. | Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 753] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 35. | Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1924] [Cited by in RCA: 1927] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 36. | Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 490] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 37. | Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1165] [Article Influence: 41.6] [Reference Citation Analysis (1)] |
| 38. | Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363-14367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 879] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 39. | Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356-1361. [PubMed] |
| 40. | Labrinidis A, Liapis V, Thai le M, Atkins GJ, Vincent C, Hay S, Sims NA, Zannettino AC, Findlay DM, Evdokiou A. Does Apo2L/TRAIL play any physiologic role in osteoclastogenesis. Blood. 2008;111:5411-542; autor reply 5413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Sprick MR, Weigand MA, Rieser E, Rauch CT, Juo P, Blenis J, Krammer PH, Walczak H. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 615] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 42. | Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem. 2001;276:20633-20640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 428] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 43. | Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schröter M, Burns K, Mattmann C. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 1920] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 44. | Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, Briand C, Grütter MG. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277:45162-45171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 360] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 45. | Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 468] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 46. | Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR, Ashkenazi A. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 501] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 47. | Harper N, Farrow SN, Kaptein A, Cohen GM, MacFarlane M. Modulation of tumor necrosis factor apoptosis-inducing ligand- induced NF-kappa B activation by inhibition of apical caspases. J Biol Chem. 2001;276:34743-34752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | Ehrhardt H, Fulda S, Schmid I, Hiscott J, Debatin KM, Jeremias I. TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-kappaB. Oncogene. 2003;22:3842-3852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 214] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 49. | Varfolomeev E, Maecker H, Sharp D, Lawrence D, Renz M, Vucic D, Ashkenazi A. Molecular determinants of kinase pathway activation by Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem. 2005;280:40599-40608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 211] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 50. | Falschlehner C, Emmerich CH, Gerlach B, Walczak H. TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol. 2007;39:1462-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 344] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 51. | Morel J, Audo R, Hahne M, Combe B. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces rheumatoid arthritis synovial fibroblast proliferation through mitogen-activated protein kinases and phosphatidylinositol 3-kinase/Akt. J Biol Chem. 2005;280:15709-15718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 52. | Sedger LM, Glaccum MB, Schuh JC, Kanaly ST, Williamson E, Kayagaki N, Yun T, Smolak P, Le T, Goodwin R. Characterization of the in vivo function of TNF-alpha-related apoptosis-inducing ligand, TRAIL/Apo2L, using TRAIL/Apo2L gene-deficient mice. Eur J Immunol. 2002;32:2246-2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, Lenz LL, Cado D, Riley LW, Winoto A. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 54. | Kayagaki N, Yamaguchi N, Nakayama M, Takeda K, Akiba H, Tsutsui H, Okamura H, Nakanishi K, Okumura K, Yagita H. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J Immunol. 1999;163:1906-1913. [PubMed] |
| 55. | Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343-1354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 372] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 56. | Fanger NA, Maliszewski CR, Schooley K, Griffith TS. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J Exp Med. 1999;190:1155-1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 313] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 57. | Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: A novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 1999;189:1451-1460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 369] [Cited by in RCA: 360] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 58. | Ehrlich S, Infante-Duarte C, Seeger B, Zipp F. Regulation of soluble and surface-bound TRAIL in human T cells, B cells, and monocytes. Cytokine. 2003;24:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Monleón I, Martínez-Lorenzo MJ, Monteagudo L, Lasierra P, Taulés M, Iturralde M, Piñeiro A, Larrad L, Alava MA, Naval J. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol. 2001;167:6736-6744. [PubMed] |
| 60. | Martínez-Lorenzo MJ, Anel A, Gamen S, Monle n I, Lasierra P, Larrad L, Piñeiro A, Alava MA, Naval J. Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. J Immunol. 1999;163:1274-1281. [PubMed] |
| 61. | Wendling U, Walczak H, Dörr J, Jaboci C, Weller M, Krammer PH, Zipp F. Expression of TRAIL receptors in human autoreactive and foreign antigen-specific T cells. Cell Death Differ. 2000;7:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Mirandola P, Ponti C, Gobbi G, Sponzilli I, Vaccarezza M, Cocco L, Zauli G, Secchiero P, Manzoli FA, Vitale M. Activated human NK and CD8+ T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity. Blood. 2004;104:2418-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 63. | Martínez-Lorenzo MJ, Alava MA, Gamen S, Kim KJ, Chuntharapai A, Piñeiro A, Naval J, Anel A. Involvement of APO2 ligand/TRAIL in activation-induced death of Jurkat and human peripheral blood T cells. Eur J Immunol. 1998;28:2714-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Dhein J, Walczak H, Bäumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature. 1995;373:438-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1240] [Cited by in RCA: 1278] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 65. | Muppidi JR, Siegel RM. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat Immunol. 2004;5:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 66. | Bosque A, Pardo J, Martínez-Lorenzo MJ, Iturralde M, Marzo I, Piñeiro A, Alava MA, Naval J, Anel A. Down-regulation of normal human T cell blast activation: roles of APO2L/TRAIL, FasL, and c- FLIP, Bim, or Bcl-x isoform expression. J Leukoc Biol. 2005;77:568-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Bosque A, Pardo J, Martínez-Lorenzo MJ, Lasierra P, Larrad L, Marzo I, Naval J, Anel A. Human CD8+ T cell blasts are more sensitive than CD4+ T cell blasts to regulation by APO2L/TRAIL. Eur J Immunol. 2005;35:1812-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Shedlock DJ, Whitmire JK, Tan J, MacDonald AS, Ahmed R, Shen H. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J Immunol. 2003;170:2053-2063. [PubMed] |
| 69. | Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 487] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 70. | Zhang XR, Zhang LY, Devadas S, Li L, Keegan AD, Shi YF. Reciprocal expression of TRAIL and CD95L in Th1 and Th2 cells: role of apoptosis in T helper subset differentiation. Cell Death Differ. 2003;10:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | Wang SH, Chen GH, Fan Y, Van Antwerp M, Baker JR. Tumor necrosis factor-related apoptosis-inducing ligand inhibits experimental autoimmune thyroiditis by the expansion of CD4+CD25+ regulatory T cells. Endocrinology. 2009;150:2000-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Hirata S, Matsuyoshi H, Fukuma D, Kurisaki A, Uemura Y, Nishimura Y, Senju S. Involvement of regulatory T cells in the experimental autoimmune encephalomyelitis-preventive effect of dendritic cells expressing myelin oligodendrocyte glycoprotein plus TRAIL. J Immunol. 2007;178:918-925. [PubMed] |
| 73. | Corazza N, Brumatti G, Jakob S, Villunger A, Brunner T. TRAIL and thymocyte apoptosis: not so deadly. Cell Death Differ. 2004;11 Suppl 2:S213-S215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Cretney E, Uldrich AP, Berzins SP, Strasser A, Godfrey DI, Smyth MJ. Normal thymocyte negative selection in TRAIL-deficient mice. J Exp Med. 2003;198:491-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 75. | Sato K, Hida S, Takayanagi H, Yokochi T, Kayagaki N, Takeda K, Yagita H, Okumura K, Tanaka N, Taniguchi T. Antiviral response by natural killer cells through TRAIL gene induction by IFN-alpha/beta. Eur J Immunol. 2001;31:3138-3146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 76. | Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J Immunol. 2008;181:4918-4925. [PubMed] |
| 77. | Saitou Y, Shiraki K, Fuke H, Inoue T, Miyashita K, Yamanaka Y, Yamaguchi Y, Yamamoto N, Ito K, Sugimoto K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand and tumor necrosis factor-related apoptosis-inducing ligand receptors in viral hepatic diseases. Hum Pathol. 2005;36:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Mundt B, Kühnel F, Zender L, Paul Y, Tillmann H, Trautwein C, Manns MP, Kubicka S. Involvement of TRAIL and its receptors in viral hepatitis. FASEB J. 2003;17:94-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 79. | Warke RV, Martin KJ, Giaya K, Shaw SK, Rothman AL, Bosch I. TRAIL is a novel antiviral protein against dengue virus. J Virol. 2008;82:555-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 80. | Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1721] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 81. | Zerafa N, Westwood JA, Cretney E, Mitchell S, Waring P, Iezzi M, Smyth MJ. Cutting edge: TRAIL deficiency accelerates hematological malignancies. J Immunol. 2005;175:5586-5590. [PubMed] |
| 82. | Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29:4752-4765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 286] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 83. | Korb A, Pavenstädt H, Pap T. Cell death in rheumatoid arthritis. Apoptosis. 2009;14:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 84. | Perlman H, Georganas C, Pagliari LJ, Koch AE, Haines K, Pope RM. Bcl-2 expression in synovial fibroblasts is essential for maintaining mitochondrial homeostasis and cell viability. J Immunol. 2000;164:5227-5235. [PubMed] |
| 85. | Kurowska M, Rudnicka W, Kontny E, Janicka I, Chorazy M, Kowalczewski J, Ziółkowska M, Ferrari-Lacraz S, Strom TB, Maśliński W. Fibroblast-like synoviocytes from rheumatoid arthritis patients express functional IL-15 receptor complex: endogenous IL-15 in autocrine fashion enhances cell proliferation and expression of Bcl-x(L) and Bcl-2. J Immunol. 2002;169:1760-1767. [PubMed] |
| 86. | Liu H, Eksarko P, Temkin V, Haines GK, Perlman H, Koch AE, Thimmapaya B, Pope RM. Mcl-1 is essential for the survival of synovial fibroblasts in rheumatoid arthritis. J Immunol. 2005;175:8337-8345. [PubMed] |
| 87. | Kammouni W, Wong K, Ma G, Firestein GS, Gibson SB, El-Gabalawy HS. Regulation of apoptosis in fibroblast-like synoviocytes by the hypoxia-induced Bcl-2 family member Bcl-2/adenovirus E1B 19-kd protein-interacting protein 3. Arthritis Rheum. 2007;56:2854-2863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Wang J, Li C, Liu Y, Mei W, Yu S, Liu C, Zhang L, Cao X, Kimberly RP, Grizzle W. JAB1 determines the response of rheumatoid arthritis synovial fibroblasts to tumor necrosis factor-alpha. Am J Pathol. 2006;169:889-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 89. | Drynda A, Quax PH, Neumann M, van der Laan WH, Pap G, Drynda S, Meinecke I, Kekow J, Neumann W, Huizinga TW. Gene transfer of tissue inhibitor of metalloproteinases-3 reverses the inhibitory effects of TNF-alpha on Fas-induced apoptosis in rheumatoid arthritis synovial fibroblasts. J Immunol. 2005;174:6524-6531. [PubMed] |
| 90. | Miranda-Carús ME, Balsa A, Benito-Miguel M, De Ayala CP, Martín-Mola E. Rheumatoid arthritis synovial fluid fibroblasts express TRAIL-R2 (DR5) that is functionally active. Arthritis Rheum. 2004;50:2786-2793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Hayashi S, Miura Y, Nishiyama T, Mitani M, Tateishi K, Sakai Y, Hashiramoto A, Kurosaka M, Shiozawa S, Doita M. Decoy receptor 3 expressed in rheumatoid synovial fibroblasts protects the cells against Fas-induced apoptosis. Arthritis Rheum. 2007;56:1067-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 92. | Pierer M, Brentano F, Rethage J, Wagner U, Hantzschel H, Gay RE, Gay S, Kyburz D. The TNF superfamily member LIGHT contributes to survival and activation of synovial fibroblasts in rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1063-1070. [PubMed] |
| 93. | Schedel J, Gay RE, Kuenzler P, Seemayer C, Simmen B, Michel BA, Gay S. FLICE-inhibitory protein expression in synovial fibroblasts and at sites of cartilage and bone erosion in rheumatoid arthritis. Arthritis Rheum. 2002;46:1512-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Catrina AI, Ulfgren AK, Lindblad S, Grondal L, Klareskog L. Low levels of apoptosis and high FLIP expression in early rheumatoid arthritis synovium. Ann Rheum Dis. 2002;61:934-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Bai S, Liu H, Chen KH, Eksarko P, Perlman H, Moore TL, Pope RM. NF-kappaB-regulated expression of cellular FLIP protects rheumatoid arthritis synovial fibroblasts from tumor necrosis factor alpha-mediated apoptosis. Arthritis Rheum. 2004;50:3844-3855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 96. | Meinecke I, Cinski A, Baier A, Peters MA, Dankbar B, Wille A, Drynda A, Mendoza H, Gay RE, Hay RT. Modification of nuclear PML protein by SUMO-1 regulates Fas-induced apoptosis in rheumatoid arthritis synovial fibroblasts. Proc Natl Acad Sci U S A. 2007;104:5073-5078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 97. | Nanki T, Hayashida K, El-Gabalawy HS, Suson S, Shi K, Girschick HJ, Yavuz S, Lipsky PE. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J Immunol. 2000;165:6590-6598. [PubMed] |
| 98. | Reparon-Schuijt CC, van Esch WJ, van Kooten C, Rozier BC, Levarht EW, Breedveld FC, Verweij CL. Regulation of synovial B cell survival in rheumatoid arthritis by vascular cell adhesion molecule 1 (CD106) expressed on fibroblast-like synoviocytes. Arthritis Rheum. 2000;43:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 99. | Hayashida K, Shimaoka Y, Ochi T, Lipsky PE. Rheumatoid arthritis synovial stromal cells inhibit apoptosis and up-regulate Bcl-xL expression by B cells in a CD49/CD29-CD106-dependent mechanism. J Immunol. 2000;164:1110-1116. [PubMed] |
| 100. | Alsaleh G, Messer L, Semaan N, Boulanger N, Gottenberg JE, Sibilia J, Wachsmann D. BAFF synthesis by rheumatoid synoviocytes is positively controlled by alpha5beta1 integrin stimulation and is negatively regulated by tumor necrosis factor alpha and Toll-like receptor ligands. Arthritis Rheum. 2007;56:3202-3214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 101. | Lamhamedi-Cherradi SE, Zheng S, Tisch RM, Chen YH. Critical roles of tumor necrosis factor-related apoptosis-inducing ligand in type 1 diabetes. Diabetes. 2003;52:2274-2278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 102. | Mi QS, Ly D, Lamhamedi-Cherradi SE, Salojin KV, Zhou L, Grattan M, Meagher C, Zucker P, Chen YH, Nagle J. Blockade of tumor necrosis factor-related apoptosis-inducing ligand exacerbates type 1 diabetes in NOD mice. Diabetes. 2003;52:1967-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 103. | Kang S, Park EJ, Joe Y, Seo E, Park MK, Seo SY, Chung HY, Yoo YH, Kim DK, Lee HJ. Systemic delivery of TNF-related apoptosis-inducing ligand (TRAIL) elevates levels of tissue inhibitor of metalloproteinase-1 (TIMP-1) and prevents type 1 diabetes in nonobese diabetic mice. Endocrinology. 2010;151:5638-5646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 104. | Sedger LM, Katewa A, Pettersen AK, Osvath SR, Farrell GC, Stewart GJ, Bendall LJ, Alexander SI. Extreme lymphoproliferative disease and fatal autoimmune thrombocytopenia in FasL and TRAIL double-deficient mice. Blood. 2010;115:3258-3268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 105. | Wang SH, Cao Z, Wolf JM, Van Antwerp M, Baker JR. Death ligand tumor necrosis factor-related apoptosis-inducing ligand inhibits experimental autoimmune thyroiditis. Endocrinology. 2005;146:4721-4726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 106. | Fang Y, Sharp GC, Yagita H, Braley-Mullen H. A critical role for TRAIL in resolution of granulomatous experimental autoimmune thyroiditis. J Pathol. 2008;216:505-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 107. | Hilliard B, Wilmen A, Seidel C, Liu TS, Göke R, Chen Y. Roles of TNF-related apoptosis-inducing ligand in experimental autoimmune encephalomyelitis. J Immunol. 2001;166:1314-1319. [PubMed] |
| 108. | Cretney E, McQualter JL, Kayagaki N, Yagita H, Bernard CC, Grewal IS, Ashkenazi A, Smyth MJ. TNF-related apoptosis-inducing ligand (TRAIL)/Apo2L suppresses experimental autoimmune encephalomyelitis in mice. Immunol Cell Biol. 2005;83:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 109. | Aktas O, Smorodchenko A, Brocke S, Infante-Duarte C, Schulze Topphoff U, Vogt J, Prozorovski T, Meier S, Osmanova V, Pohl E. Neuronal damage in autoimmune neuroinflammation mediated by the death ligand TRAIL. Neuron. 2005;46:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 110. | Song K, Chen Y, Göke R, Wilmen A, Seidel C, Göke A, Hilliard B, Chen Y. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J Exp Med. 2000;191:1095-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 280] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 111. | Miyashita T, Kawakami A, Nakashima T, Yamasaki S, Tamai M, Tanaka F, Kamachi M, Ida H, Migita K, Origuchi T. Osteoprotegerin (OPG) acts as an endogenous decoy receptor in tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis of fibroblast-like synovial cells. Clin Exp Immunol. 2004;137:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 112. | Ichikawa K, Liu W, Fleck M, Zhang H, Zhao L, Ohtsuka T, Wang Z, Liu D, Mountz JD, Ohtsuki M. TRAIL-R2 (DR5) mediates apoptosis of synovial fibroblasts in rheumatoid arthritis. J Immunol. 2003;171:1061-1069. [PubMed] |
| 113. | Perlman H, Nguyen N, Liu H, Eslick J, Esser S, Walsh K, Moore TL, Pope RM. Rheumatoid arthritis synovial fluid macrophages express decreased tumor necrosis factor-related apoptosis-inducing ligand R2 and increased decoy receptor tumor necrosis factor-related apoptosis-inducing ligand R3. Arthritis Rheum. 2003;48:3096-3101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 114. | Martínez-Lorenzo MJ, Anel A, Saez-Gutierrez B, Royo-Cañas M, Bosque A, Alava MA, Piñeiro A, Lasierra P, Asín-Ungría J, Larrad L. Rheumatoid synovial fluid T cells are sensitive to APO2L/TRAIL. Clin Immunol. 2007;122:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 115. | Zhang J, Bárdos T, Mikecz K, Finnegan A, Glant TT. Impaired Fas signaling pathway is involved in defective T cell apoptosis in autoimmune murine arthritis. J Immunol. 2001;166:4981-4986. [PubMed] |
| 116. | Park YW, Ji JD, Lee JS, Ryang DW, Yoo DH. Actinomycin D renders cultured synovial fibroblasts susceptible to tumour necrosis factor related apoptosis-inducing ligand (TRAIL)-induced apoptosis. Scand J Rheumatol. 2003;32:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 117. | Audo R, Combe B, Coulet B, Morel J, Hahne M. The pleiotropic effect of TRAIL on tumor-like synovial fibroblasts from rheumatoid arthritis patients is mediated by caspases. Cell Death Differ. 2009;16:1227-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 118. | Pundt N, Peters MA, Wunrau C, Strietholt S, Fehrmann C, Neugebauer K, Seyfert C, van Valen F, Pap T, Meinecke I. Susceptibility of rheumatoid arthritis synovial fibroblasts to FasL- and TRAIL-induced apoptosis is cell cycle-dependent. Arthritis Res Ther. 2009;11:R16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 119. | Audo R, Calmon-Hamaty F, Baeten D, Bruyer A, Combe B, Hahne M, Morel J. Mechanisms and clinical relevance of TRAIL-triggered responses in the synovial fibroblasts of patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:904-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 120. | Hirata S, Senju S, Matsuyoshi H, Fukuma D, Uemura Y, Nishimura Y. Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand. J Immunol. 2005;174:1888-1897. [PubMed] |
| 121. | Razmara M, Hilliard B, Ziarani AK, Murali R, Yellayi S, Ghazanfar M, Chen YH, Tykocinski ML. Fn14-TRAIL, a chimeric intercellular signal exchanger, attenuates experimental autoimmune encephalomyelitis. Am J Pathol. 2009;174:460-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 122. | Yao Q, Wang S, Gambotto A, Glorioso JC, Evans CH, Robbins PD, Ghivizzani SC, Oligino TJ. Intra-articular adenoviral-mediated gene transfer of trail induces apoptosis of arthritic rabbit synovium. Gene Ther. 2003;10:1055-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 123. | Terzioglu E, Bisgin A, Sanlioglu AD, Ulker M, Yazisiz V, Tuzuner S, Sanlioglu S. Concurrent gene therapy strategies effectively destroy synoviocytes of patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:783-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Yao Q, Seol DW, Mi Z, Robbins PD. Intra-articular injection of recombinant TRAIL induces synovial apoptosis and reduces inflammation in a rabbit knee model of arthritis. Arthritis Res Ther. 2006;8:R16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 125. | Jin CH, Chae SY, Kim TH, Yang HK, Lee EY, Song YW, Jo DG, Lee KC. Effect of tumor necrosis factor-related apoptosis-inducing ligand on the reduction of joint inflammation in experimental rheumatoid arthritis. J Pharmacol Exp Ther. 2010;332:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 126. | Liu Z, Xu X, Hsu HC, Tousson A, Yang PA, Wu Q, Liu C, Yu S, Zhang HG, Mountz JD. CII-DC-AdTRAIL cell gene therapy inhibits infiltration of CII-reactive T cells and CII-induced arthritis. J Clin Invest. 2003;112:1332-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 127. | Li W, Liu Z, Zhuang G, Yin P, Tao H, Qiu J, Hu Q, Zhang J. Anti-DR5 mAb ameliorate adjuvant arthritis rats through inducing synovial cells apoptosis. Exp Biol Med (Maywood). 2009;234:1468-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 128. | Kim YJ, Chae SY, Jin CH, Sivasubramanian M, Son S, Choi KY, Jo DG, Kim K, Chan Kwon I, Lee KC. Ionic complex systems based on hyaluronic acid and PEGylated TNF-related apoptosis-inducing ligand for treatment of rheumatoid arthritis. Biomaterials. 2010;31:9057-9064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 129. | Martinez-Lostao L, García-Alvarez F, Basáñez G, Alegre-Aguarón E, Desportes P, Larrad L, Naval J, Martínez-Lorenzo MJ, Anel A. Liposome-bound APO2L/TRAIL is an effective treatment in a rabbit model of rheumatoid arthritis. Arthritis Rheum. 2010;62:2272-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |