Published online Jun 28, 2018. doi: 10.5498/wjp.v8.i2.51
Peer-review started: March 19, 2018
First decision: May 8, 2018
Revised: May 15, 2018
Accepted: June 8, 2018
Article in press: June 9, 2018
Published online: June 28, 2018
Processing time: 101 Days and 4.9 Hours
Glutamate is the predominant excitatory neurotransmitter in the human brain and it has been shown that prolonged activation of the glutamatergic system leads to nerve damage and cell death. Following release from the pre-synaptic neuron and synaptic transmission, glutamate is either taken up into the pre-synaptic neuron or neighbouring glia by transmembrane glutamate transporters. Excitatory amino acid transporter (EAAT) 1 and EAAT2 are Na+-dependant glutamate transporters expressed predominantly in glia cells of the central nervous system. As the most abundant glutamate transporters, their primary role is to modulate levels of glutamatergic excitability and prevent spill over of glutamate beyond the synapse. This role is facilitated through the binding and transportation of glutamate into astrocytes and microglia. The function of EAAT1 and EAAT2 is heavily regulated at the levels of gene expression, post-transcriptional splicing, glycosylation states and cell-surface trafficking of the protein. Both glutamatergic dysfunction and glial dysfunction have been proposed to be involved in psychiatric disorder. This review will present an overview of the roles that EAAT1 and EAAT2 play in modulating glutamatergic activity in the human brain, and mount an argument that these two transporters could be involved in the aetiologies of schizophrenia and affective disorders as well as represent potential drug targets for novel therapies for those disorders.
Core tip: Following release from the presynaptic neuron, the majority of glutamate within the human cortex is taken up into glia cells where it is converted into glutamine for recycling back into glutamate. Glutamate transporters excitatory amino acid transporter (EAAT) 1 and EAAT2 are predominantly localized in the glial plasma membrane, and are responsible for the majority of glutamate uptake within the human brain. Here we provide a comprehensive review of the unique regulation of EAAT1 and EAAT2 mRNA and protein in health and psychiatric disorder, and in response to medication use.
- Citation: Parkin GM, Udawela M, Gibbons A, Dean B. Glutamate transporters, EAAT1 and EAAT2, are potentially important in the pathophysiology and treatment of schizophrenia and affective disorders. World J Psychiatr 2018; 8(2): 51-63
- URL: https://www.wjgnet.com/2220-3206/full/v8/i2/51.htm
- DOI: https://dx.doi.org/10.5498/wjp.v8.i2.51
Glutamate has long been recognized as the principal excitatory neurotransmitter of the mammalian brain[1]. It has been shown that glutamate concentration in the CNS extracellular space (approximately 0.6-2 μmol/L)[2-4] is comparatively lower than levels in cell cytoplasm (approximately 1-10 mmol/L)[5-7]. It is necessary for the level of extracellular glutamate to be kept low to allow for a high signal-to-noise ratio following the release of glutamate into the synapse and to prevent glutamate-mediated neuronal degeneration[8-10] as high levels of extracellular glutamate causes excitotoxicity and nerve damage[10]. The extracellular/intracellular glutamate gradient is at least partly maintained through the activity of Na+-dependent excitatory amino acid transporters (EAATs) which are in the membrane of pre-synaptic neurons and glia. Whilst glutamate uptake into astrocytes is also mediated by Na+-independent, chloride-dependent antiporters, this family of transporters appears to be responsible for less than 5% overall glutamate uptake[11]. Thus, this review will focus solely on the Na+-dependent EAAT family.
The EAAT family of transporters consists of five Na+-dependent high-affinity glutamate transporters termed EAAT1 [also known as solute carrier family 1 member 3 (SLC1A3)][12,13], EAAT2/SLC1A2[13,14], EAAT3/SLC1A1[13,15], EAAT4/SLC1A6[16] and EAAT5/SLC1A7[17]. In this review, the accepted nomenclature of “EAAT” will be used in discussing data from both humans and other mammalians. These subtypes are quite differentiated - EAAT1, EAAT2 and EAAT3 only share 51%-55% amino acid sequence homology[18].
The functionality of the glutamate transporters reflects their coupling to the electrochemical potential gradients of Na+, K+ and H+/OH-. Specifically, glutamate is co-transported across the plasma membrane 1:2-3 with Na+ and 1:1 H+ (or counter-transport of OH-) and counter-transported 1:1 with K+[4,19,20]. This ionic association provides a net positive charge to glutamate transport[20] however a relatively slow turnover rate of approximately 70 ms makes it unlikely that this electrogenic attribute contributes significantly to the electrochemical gradient of the cell[21,22]. Furthermore, this slow turnover rate suggests that the transporters act first to buffer glutamate away from the synapse, and transport glutamate into glia at a slower rate. The quantity of charge transferred per molecule of glutamate is highly voltage dependant due to the existence of a thermodynamically uncoupled, transporter substrate-specific movement of chloride ions through the transporter[16,23]. The ion- and voltage- dependant uptake of glutamate makes this process highly susceptible to changes in the immediate cellular environment and plasma membrane potential.
Some of the functional properties of the EAATs can be attributed to their differential localisation. EAAT3 and EAAT5 are exclusively neuron-specific[17,24], with EAAT5 expression restricted to neurons and Müller cells of the retina[17]. By contrast, EAAT1 and EAAT2 are predominantly localised on astrocytes and are highly expressed in the cerebellum and hippocampus, respectively[25-27]. EAAT1, EAAT2 and EAAT3 have been reported to make up approximately 20%, 80% and 1% of all cell-surface glutamate transporters in the adult rat hippocampus, respectively[24]. EAAT4 is found in Purkinje neurons of the cerebellum[28].
Glial metabolism of glutamate is now recognised as a major factor in the control of glutamatergic neurotransmission[9], as, following the release of glutamate from the pre-synaptic neuron, the majority of the neurotransmitter diffuses out of the synaptic cleft where it is taken up into glial cells[29-31]. This effectively means that the astrocytic EAATs play a significant role in controlling the extent of glutamatergic activation by preventing neurotransmitter spill-over into neighbouring synapses[20,22,32].
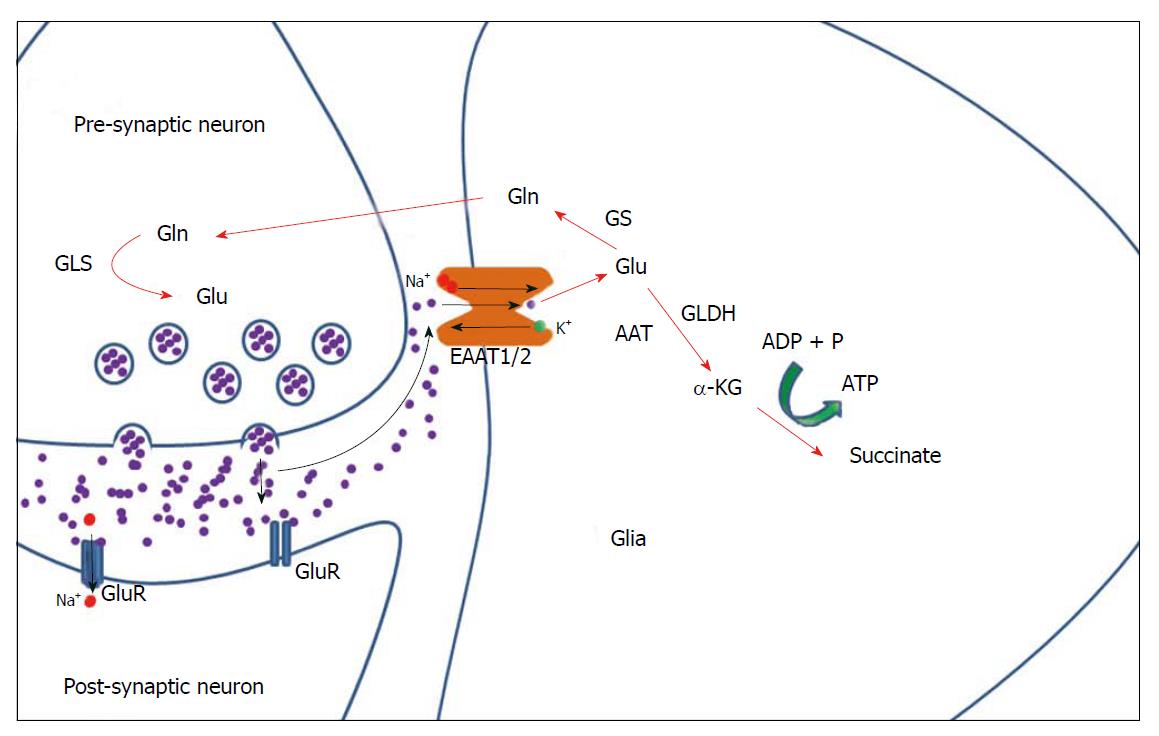
Once taken up by astrocytes, glutamate is either converted to glutamine by the glia-specific glutamine synthetase (GS)[33], or converted to α-ketoglutarate (α-KG) (also known as 2-oxoglutarate) through oxidation by glutamate dehydrogenase (GLDH) or transamination by aspartate aminotransferase[34,35]. α-KG may then be converted to succinate with a by-production of ATP (Figure 1) as part of the tricarboxylic acid cycle[34,35]. The formation of glutamine - the predominant metabolic pathway for glutamate - is followed by the transport of glutamine to neurons where it undergoes synthesis back into glutamate via the enzyme glutaminase[36] (Figure 1).
It is now acknowledged that glutamate excitotoxicity can potentially lead to problems in CNS functioning[8]. Significantly, it is now being speculated that glutamatergic excitotoxicity may in part be due to a reduction in glutamate uptake by astrocytic glutamate transporters and subsequent conversion of glutamate to glutamine by GS. Such an hypothesis is supported by research showing that inhibition or antisense oligonucleotide-knockdown of astrocytic glutamate transporters EAAT1 and EAAT2 results in excitotoxic oligodendrocyte death and nerve damage[37]. Moreover, whilst glutamate is usually taken into astrocytes under physiological conditions, it has been suggested that Ca2+-dependent activation of select signalling molecules, intracellular increase in Na+ and glutamate, ATP depletion or cell swelling can lead to glutamate release through EAAT transporter reversal/reverse uptake[11,38]. Such an outcome represents another mechanism that can lead to glutamate excitotoxicity.
The focus of this review is the potential involvement of glutamate transporters in the aetiology of psychiatric disorders. At present, most evidence implicating glutamate transporters in the aetiologies of psychiatric disorders is from the study of EAAT1 and EAAT2. Hence the remainder of this review will focus on those two forms of glutamate transporters.
The EAAT1 gene has been localised to human chromosome 5p11-12[39] and 5p13[40,41]. EAAT1 is highly expressed in the glial cells of the cerebellar Purkinje cell layer[12,13,42] and generally is expressed at higher levels in astrocytes and oligodendrocytes compared to microglia[43].
The EAAT1 gene encodes a 59 kDa protein that undergoes glycosylation to produce native 64 kDa and 70 kDa glycoproteins[44-46]. Post-translational modification of EAAT1 is developmentally regulated, with glycosylated EAAT1 increasing and non-glycosylated EAAT1 decreasing after birth[47]. Glycosylation does not affect EAAT1 transport activity, however research by Conradt et al[46] suggests that the addition of carbohydrates does impact EAAT1 homodimerisation. This is significant because it has been suggested that activation of astrocytes increases homodimerisation of EAAT1 and trafficking of the transporter to the plasma membrane[48]. In addition to homodimers, EAAT1 protein has shown potential to form homotrimers[49]. Moreover, it has been reported that cell-surface protein expression of EAAT1 is upregulated by exogenous glutamate[50-52], a process suggested to be mediated through transporter activity[51] and/or ionotropic glutamate receptor signalling[50,52]. EAAT1 protein levels have also been reported as being downregulated by the group I metabotropic glutamate receptor (mGluR) agonist, DHPG, which selectively activates mGluR1 and 5, and upregulated by the group II mGluR agonist, DCG-IV, which activates mGluR 2 and 3[53]. Further work is therefore required to fully elucidate which glutamate receptors are critical in controlling levels of EAAT1 at the cell surface.
At the level of protein localisation, high levels of EAAT1 protein can be observed on rat astrocytic membranes opposed to synaptically dense regions of the CNS and at higher levels on astrocytes facing capillaries, pia mater or stem dendrites[27]. Although predominantly a glial transporter, EAAT1 protein has also been detected in deep cerebellar rat neurons and transient protein expression of EAAT1 has been observed in cultured rat embryonic hippocampal neurons[26,54]. Rat neuronal EAAT1 protein expression appears to be restricted to perinuclear localizations, in particular the Golgi apparatus and associated vesicles[26]. Soluble factors secreted from astrocytes appear to induce the repression of neuronal protein expression of EAAT1[55], which supports the aforementioned lack of EAAT1 protein at the neuronal cell surface. It is possible that, at least in the adult brain, neuronal EAAT1 is redundant, and may be upregulated where there is an inadequate presence of EAAT1-expressing astroglia.
The EAAT1 gene is expressed in its full length coding sequence of 10 exons[39] and as three splice variants which encode shorter protein isoforms known as EAAT1a, EAAT1b and EAAT1c.
EAAT1a lacks exon 3 - which ordinarily encodes the first and second transmembrane domain and the first extracellular loop[45] - and has been detected in rat brain and retina[45,56]. Significantly, it has been proposed that the splicing of exon 3 may reverse the orientation of EAAT1a within the membrane, and therefore the direction of glutamate transport[45]. EAAT1a has been shown to be predominantly expressed within internal vesicles, rather than on the cell membrane, in an osteocyte cell line[57]. Interestingly, an unglycosylated variant of EAAT1a protein has also been detected in rat brain[45]; as glycosylation has previously been associated with trafficking of EAAT1 to the cell surface, this may explain the localization of EAAT1a to internal vesicles.
mRNA for EAAT1b, which lacks exon 9[58], has been detected in both human grey matter and axonal tracts at levels that are 10%-20% that of the full length EAAT1[58]. EAAT1b localization has been associated with the endoplasmic reticulum[58], intracellular inclusions and the plasma membrane[59], where it appears to have no functional glutamate uptake activity[58]. Rather, it has been suggested that EAAT1b negatively regulates levels of full-length EAAT1 at the cell surface by preventing the insertion of the latter into the plasma membrane[58], possibly by interacting directly with full length EAAT1. EAAT1b appears to be particularly localised to cortical neurons[59] where it is suggested to play a role in preventing ectopic neuronal expression of EAAT1. Immunoperoxidase-labelling of EAAT1b was associated with the plasma membrane for morphologically healthy neurons and in punctate intracellular inclusions of neurons that appeared degenerate[59]. Furthermore, EAAT1b expression was increased in neuronal populations - and to a lesser extent astrocytes - that were subject to hypoxia, in histologically degenerate brain regions which also displayed a downregulation of glial EAAT1[60].
EAAT1c mRNA, which lacks exon 5 and 6 of the full coding sequence, has been detected in astrocytes and oligodendrocytes of the human cortex, retina and optic nerve, as well as pig forebrain, midbrain, hindbrain and cerebellum[61]. The approximately 47 kDa glia-specific EAAT1c protein has been shown to have an intracellular perinuclear localization, with rapid redistribution to the cell surface following astrocytic stimulation[61]. Furthermore, EAAT1c does not undergo glycosylation[61]. Unfortunately the only study to examine EAAT1c was not able to determine its function, with the two most likely (default) candidates remaining as a regulator of full length EAAT1 cell surface expression, or as a bona fide transporter trafficked to the cell surface under appropriate conditions.
The EAAT2 gene is localized within human cytogenetic bands 11p12-13[62], and is responsible for the majority of glutamate uptake within the EAAT family (see[63] for review). EAAT2 expression appears to be restricted to the brain and placenta in humans[13] and has also been detected in cultured hippocampal neurons from rat embryo[64]. The mRNA expression of EAAT2 was found to be greater in cultured rat oligodendrocytes than astrocytes or microglia[43].
While EAAT2 protein expression has an overall homogenous brain distribution, it predominates across the forebrain with a particular focus in the hippocampus[26,65,66]. EAAT2 appears to be a predominantly glial glutamate transporter, with up to 80% of EAAT2 protein detected in glia plasma membrane, 6% localized to plasma membrane of pre-synaptic neurons and 8% to the axonal plasma membrane in the stratum radiatum of the rat hippocampal subregion CA1[67]. In line with the detection of EAAT2 mRNA[64], EAAT2 protein expression has similarly been recorded in cultured rat embryonic hippocampal neurons[54,64]. Like EAAT1, EAAT2 protein undergoes glycosylation, which produces a 5-15 kDa shift in molecular weight[48,68] and mediates its cell surface expression[68]. EAAT2 also exists in the plasma membrane as a multimer, with the potential to form dimers and trimers[49,69].
EAAT2 would seem to have a complex role in development as it is transiently detected in neurons throughout ovine forebrain and cerebellum at 71 d gestation, and lost by 136 d gestation[70]. In addition, EAAT2 and glial fibrillary acidic protein (GFAP) have been reported to not be expressed by the same cells at 71 d ovine gestation but showed region-specific colocalization by 136 d gestation[70]. A similar transition from prenatal axonal pathways to astrocytic EAAT2 protein expression was also seen in rat[47]. Interestingly, it appears as if neuronal soluble factors may be required for proper expression of EAAT2 in neighbouring astrocytes[53,71] with EAAT2 protein levels quantitatively higher in the synaptically dense regions of glial cells[27].
The human EAAT2 gene consists of 11 exons[72]. Two functional splice variants of EAAT2, termed EAAT2b and EAAT2c, contain unique C-terminal domains and have been detected in rat brain (EAAT2b) and retina (EAAT2c)[73,74]. Additional splice variants which share the C-terminals of EAAT2 and EAAT2b, but have a unique N-terminal domain, have also been detected in mouse and rat liver and referred to as mGLT-1A/rGLT-1A and mGLT-1B, respectively[75] (see[74] for a comparison of amino acid terminal sequences). While the unique N-terminals has been proposed to regulate tissue-specific expression, the function of the differing C-terminal of mGLT-1A and mGLT-1B is less clear - the authors propose that it may be related to the retention time of the transporter in the plasma membrane[75]. As mGLT-1A/rGLT-1A and mGLT-1B are localized to the liver[75], they will not be discussed further in this review. Rather, further evidence for the role of the C-terminus can be presented using data on rat EAAT2b, which will be discussed in the following paragraph.
Full length EAAT2 (commonly referred to as EAAT2a) expression is about 25-fold and 10-fold higher than EAAT2b in human and rat brain, respectively[76]. EAAT2b protein is similarly found in glia, localized close to or within the plasma membrane[77-79], however transcript and protein have also been detected in neurons[78]. While EAAT2 is constitutively trafficked to the cell surface membrane, localization of EAAT2b is mediated through its C-terminal, which is predicted to interact with the postsynaptic density-95/Discs large/zona occludens-1 (PDZ) domain-containing protein disks large homolog-1 (DLG1)[80]. The interaction between EAAT2b and DLG1 is itself regulated through AMPA-associated intracellular calcium levels, with exogenous glutamate resulting in dissociation of EAAT2b and DLG1 and subsequent internalization of EAAT2b[80]. EAAT2b also coimmunoprecipitates with the excitatory postsynaptic density scaffolding protein, PSD-95, as well as the ionotropic N-methyl-D-aspartate receptor (NMDAR), both found within the postsynaptic neuron[81]. Significantly, EAAT2 has also been detected in these protein complexes, through the indirect formation of a hetero-oligomer with EAAT2b[81]. This suggests that EAAT2b may assist in conditional neuronal cell-sur-face expression of EAAT2. EAAT2b represented 6% of total rat hippocampal EAAT2 variants at 8 wk of age, compared to EAAT2 at 90%, whereas the equally functional EAAT2c sits at just 1%[77].
EAAT2c is made up of exons 1-10 from the EAAT2 transcript plus a unique eleventh exon and C-terminus spliced from intron 10 - thereby losing the original eleventh exon from EAAT2 - similarly contains a PDZ-binding domain and is pre-synaptically expressed in the rat and human retina[74].
Aberrant EAAT2 splice variants which skip exons have also been discovered: In particular, EAAT2 exon7skipping and EAAT2 exon9skipping lack glutamate transport functionality and must form multimers with functional EAAT2 or EAAT2b (see[82] for review). These splice variants add another layer of complexity to what has been considered a predominantly astrocytic glutamate transporter.
Despite belonging to the same family of transporters, sharing 52% amino acid identity[18] and being localized within the same astrocytic plasma membrane[49], EAAT1 and EAAT2 display many differences in their functionality. EAAT1 protein levels have been shown to function approximately 6 times slower than EAAT2, and be expressed at a level approximately 6 times higher in the adult rat cerebellar molecular layer[21,65]. On the other hand, EAAT2 protein levels are upwards of 4 times higher than EAAT1 in the adult murine hippocampus[65]. Furthermore, unlike EAAT1, cell-surface protein expression of EAAT2 appears unaffected by exogenous glutamate levels but rather, is regulated by neuronal soluble factors[50-52]. Finally, within the EAAT family, only EAAT2 can be competitively inhibited by kainic acid (KA) and dihydrokainic acid (DHK)[13]. Conversely, KA has been shown to increase EAAT1 protein levels[50]. It is possible that this increase in EAAT1 protein in response to KA is a homeostatic mechanism, counterbalancing for the inhibition of EAAT2.
The fact that EAAT1 and EAAT2, and their functionally distinct splice variants, may be differentially regulated by internal and external factors presents us with two distinct transporters that are part of a system that is highly responsive to cellular physiology. In the following paragraphs, we will present the current knowledge surrounding EAAT1 and EAAT2 in psychiatric illness, as well as their responsiveness to medication and potential as drug targets (refer to Table 1 for a summary).
| EAAT1 | Genetic studies | |
| BD | SNP rs2731880 T/T genotype associated with worse working memory and selective attention during a depressive episode[102] | |
| SNP rs2731880 T/T genotype increased negative fMRI BOLD coupling between the amygdala and AnCg[103] | ||
| Scz | SNP rs2731880 T/T genotype associated with worse executive function, verbal fluency and verbal memory[104] | |
| No association between EAAT1 SNPs rs1428973, rs2033267, rs426040, rs4869684, rs1544795, rs3776585, rs962686, rs2303716, rs3776586, rs1049524, rs1529461 and Scz[112] | ||
| mRNA studies | ||
| MDD | ↓Lower levels in the DLPFC[83], AnCg[83], locus coeruleus[105] and hippocampus[106] | |
| ↑Higher cortical levels in suicide completers with a MDD diagnosis compared to those without a diagnosis[118] | ||
| Scz | ↑Higher mRNA in the cerebellar vermis[113], AnCg[114], thalamus[115] and prefrontal cortex[116] | |
| →No change in the DLPFC or primary visual cortex[76,114] | ||
| ↓Lower levels in the prefrontal cortex of subjects who completed suicide compared to those who did not[117] | ||
| Medication use | ↑Haloperidol has been associated with an increase in EAAT1 mRNA in the thalamic medial dorsal nucleus[121] | |
| ↑Chronic sodium valproate resulted in an upregulation of EAAT1 mRNA in chick cerebellar BGC culture[110] | ||
| Protein studies | ||
| Scz | ↓Decreased in the prefrontal cortex[114] | |
| ↓N-glycosylation of EAAT1 monomer was decreased in the AnCg[114,119] | ||
| PTSD | ↓Hippocampal EAAT1 protein was lower in a single prolonged stress (SPS) rat model of PTSD[108] | |
| Medication use | →Clozapine did not affect EAAT1 protein levels in rat[113,122] | |
| ↑Chronic sodium valproate resulted in an upregulation of EAAT1 protein in rat hippocampus and chick cerebellar BGC culture[109,110] | ||
| EAAT2 | Genetic studies | |
| Scz | SNP rs4354668 G/G associated with poorer working memory performance[104,138] and a reduction in frontal grey matter[139] | |
| mRNA studies | ||
| MDD | ↓Lower levels in DLPFC and AnCg[83] | |
| ↑Higher levels in subjects who had completed suicide without a diagnosis of MDD compared to those with a diagnosis[118,127] | ||
| ↓Lower levels in the hippocampus, cerebral cortex and striatum of a rat model of depression[128,129] | ||
| Scz | ↓Lower levels in the parahippocampal gyrus[140] and prefrontal cortex[141] | |
| ↑Higher levels in the thalamus[115] and prefrontal cortex[142] | ||
| →No change in EAAT2 or EAAT2b mRNA in the DLPFC or primary visual cortex[76] | ||
| Medication use | ↓Clozapine decreased levels in hippocampal CA1, parietal temporal, frontal and cingulate cortical[144], and striatal[145] brain regions of male Sprague-Dawley rats | |
| ↓Haloperidol decreased frontal and cingulate cortical[144], as well as striatal[145], EAAT2 expression in rat | ||
| ↓Levels were higher in untreated subjects with Scz than in those prescribed typical or atypical antipsychotics[142] | ||
| ↓Increased levels caused by chronic stress were normalised by tianeptine treatment in rat[130] | ||
| ↓Increased hippocampal levels caused by stress were normalised by lithium administration in rat[137] | ||
| ↑Fluoxetine increased rat hippocampal and cortical levels[136] | ||
| ↑Tranylcypromine increased levels in rat amygdala[136] | ||
| Protein studies | ||
| Scz | ↓ N-glycosylation of EAAT2 multimer was lower in the DLPFC[119] | |
| ↑ EAAT2b increased in extrasynaptic membrane/cytosol fractions from the DLPFC[143] | ||
| PTSD | ↓Hippocampal EAAT2 protein was lower in the single prolonged stress (SPS) rat model of PTSD[108] | |
| Medication use | ↓Clozapine decreased protein levels in astrocyte culture[147] | |
| ↓Clozapine reduced protein levels in the cerebral cortex of adult rats[146] | ||
| ↓Increased levels caused by chronic stress were normalised by tianeptine treatment in rat[130] | ||
| →Increases in EAAT2b protein caused by chronic stress were unaffected by tianeptine treatment in rat[130] | ||
| ↑Chronic sodium valproate increased hippocampal EAAT2 protein in rat[109] | ||
Glial dysfunction has been implicated in a range of psychiatric illnesses, including major depressive disorders (MDD)[83,84], schizophrenia[85], bipolar disorders (BD)[86] and post-traumatic stress disorder (PTSD)[87]. Glia dysfunction has also been associated with suicide completion[88,89]. The association between the glutamatergic neurotransmitter system and psychiatric illness is not new[90,91]; originally based on the observation that phenylcyclidine (PCP), and later ketamine - both NMDA receptor antagonists - could induce schizophrenia-like positive and negative symptoms, as well as cognitive impairment[92-94]. While glutamatergic dysfunction is also hypothesized to be involved in other neurodevelopmental disorders such as autism[95] and attention deficit hyperactivity disorder[96,97], neurodegenerative disorders such as dementia[98,99], substance abuse/addiction[100] and chronic pain[101] (the latter two referenced reviews are written with a focus on EAATs), these topics are outside the scope of the current review.
The rs2731880 (C/T) single nucleotide polymorphism (SNP) of EAAT1 has been associated with deficits in working memory and selective attention in patients with Type 1 bipolar disorder during a depressive episode, with T/T homozygotes displaying significantly worse performance[102]. Furthermore, bipolar disorder patients with the rs2731880 T/T genotype have displayed an overall negative correlation between amygdala and subgenual anterior cingulate cortex (AnCg) functional magnetic resonance imaging (fMRI) blood-oxygen-level dependent (BOLD) contrast imaging during a task which involved the processing of emotional or neutral faces, whereas in carriers of the C allele the coupling was absent[103]. SNP rs2731880 is a putative functional polymorphism within the promoter region of EAAT1, with the T/T genotype proposed to be associated with lower expression[104]. In support of the hypothesis that lower EAAT1 expression is associated with affective disorders, lower levels of EAAT1 mRNA have been reported in the human dorsolateral prefrontal cortex (DLPFC)[83], AnCg[83], locus coeruleus[105] and hippocampus[106] from subjects with MDD. Interestingly, Group II mGluR receptors - the agonists of which have been shown to upregulate EAAT1 protein levels[53] - are also decreased in MDD[107].
Furthermore, Feng et al[108] detected an increase in CSF glutamate levels and decrease in hippocampal EAAT1 protein levels in the single prolonged stress (SPS) rat model for PTSD. Interestingly, administration of fibroblast growth factor 2 (FGF2) alleviated the SPS-induced PTSD-like behaviour, promoted glutamate uptake and increased EAAT1 protein expression, thereby suggesting that astrocyte activation (and EAAT1 upregulation) may be advantageous in the treatment of PTSD[108].
Short-term sodium valproate treatment augmented EAAT1 translocation to the cell membrane, whereas prolonged or chronic sodium valproate treatment resulted in an upregulation of EAAT1 mRNA and protein levels, as well as glutamate transport and production of glutamine[109,110]. The ability of sodium valproate treatment to increase EAAT1 mRNA and protein levels, which are downregulated in affective disorders, contributes EAAT1 dynamics to an understanding of the medication’s effectiveness[111]. This is not surprising, given that sodium valproate is used to treat both epilepsy - a disorder of excitotoxicity - and bipolar disorders.
Carriers of the rs2731880 SNP T/T genotype with a diagnosis of schizophrenia performed worse in tests of executive function, verbal fluency and verbal memory than the C carrier group[104]. This association has overlap with cognitive performance of subjects with bipolar disorder I[102]. Furthermore, Deng et al[112] analysed 11 EAAT1 SNPS - exclusive of rs2731880 - in a Japanese population and found no association between EAAT1 genotype and schizophrenia. These genotypic association studies suggest that while there may exist a relationship between EAAT1 genotype and cognition, particularly within the context of psychiatric disorder, it is not a susceptibility locus specific to either schizophrenia or bipolar disorders.
Levels of EAAT1 mRNA have been reported as higher in the cerebellar vermis[113], AnCg[114], thalamus[115] and prefrontal cortex[116] of subjects with schizophrenia. In comparison, other studies have found no changes in EAAT1 expression in the DLPFC[76,114] or primary visual cortex[76] of subjects with schizophrenia. However, lower levels of EAAT1 mRNA were found in the prefrontal cortices of subjects with schizophrenia who completed suicide relative to those who did not[117], a confounding factor that many studies have not taken into consideration. Conversely, it has also been reported that EAAT1 mRNA levels were higher in the cortex of suicide completers without a prior diagnosis of MDD but not those with the diagnosis[118]. These data suggest complex expression x diagnoses x suicide factors that need to be considered when contemplating the role of EAAT1 in psychiatric disorders. Finally, monomeric EAAT1 protein expression was decreased in the DLPFC of elderly subjects with schizophrenia[114], while N-glycosylation of EAAT1 protein monomer was decreased in the AnCg[119]. Animal models have shown that EAAT1 knock-out mice displayed locomotor hyperactivity in response to a novel environment which was exacerbated by NMDAR antagonists – two phenotypes considered to be relevant models for the positive symptoms of schizophrenia[120].
Interestingly, the locomotor hyperactivity in EAAT1 knock-out mice could be normalised by treatment with haloperidol or the mGluR 2/3 agonist LY379268[120]. As EAAT1 protein levels have previously been shown to increase with administration of the mGluR 2/3 agonist DCG-IV[53], the data in EAAT1 knockout mice suggests that activation of mGluR 2/3 may impact on a function downstream of glial glutamate uptake. Haloperidol has similarly been associated with an increase in EAAT1 RNA in the thalamic medial dorsal nucleus in subjects with schizophrenia[121]. On the other hand, administration of clozapine did not appear to affect EAAT1 protein levels in treated Sprague-Dawley rats[113,122], possibly due to the fact that clozapine, but not haloperidol, increases NMDAR-mediated neurotransmission through synaptobrevin-associated glial release of glutamate and
In summary, current data suggest an overall decrease in EAAT1 mRNA in affective disorders, while an increase in EAAT1 mRNA and decrease in EAAT1 protein is associated with schizophrenia; results which are further complicated by suicide completion and medication use. These factors must be taken into consideration when studying EAAT1, and the glutamatergic system as a whole, in terms of treatment for psychiatric illness.
EAAT2 translation may be regulated by a large range of molecules, including the stress-related glucocorticoids[124-126], creating a putative link between EAAT2 protein levels and stress-induced biological responses. To date, one study has recorded a lower levels of EAAT2 mRNA in the DLPFC and AnCg of subjects with MDD[83]. Interestingly, as with EAAT1, levels of EAAT2 mRNA were higher in the cortex of subjects who had completed suicide without a history of MDD, but not in those with a prior diagnosis[118,127]. EAAT2 mRNA was also lower in the hippocampus and cerebral cortex of learned helplessness rats – an established animal model of depression[128] and in the hippocampus, striatum, and frontal cortex of prenatally, restraint- stressed juvenile rats displaying increased behavioural despair[129].
Hippocampal EAAT2 protein levels were also observed to be lower in a SPS rat model of PTSD, which, like EAAT1 protein levels, could be alleviated by treatment with FGF2[108]. Interestingly, a rat model of chronic stress produced upregulated EAAT2 mRNA and protein levels in the hippocampus[130], suggesting that EAAT2 regulation may respond differently to the type, duration and severity of stress stimuli. Finally, amygdala specific DHK-inhibition of EAAT2 activity in rat resulted in reduced social interaction – a behavioural phenotype that could be blocked by the NMDA receptor antagonist, AP5[131].
Cerebrospinal fluid glutamate levels have been reported as higher in patients with obsessive compulsive disorder (OCD)[132,133]. To date however, an association between OCD and the glutamate transporters has only been proposed for the neuronal EAAT3, which is significantly less involved in glutamate uptake when compared to EAAT1 and EAAT2[134]. That said, astrocyte-specific inducible knockout of EAAT2 in adolescent - but not prenatal or adult - mice has been shown to result in glutamatergic hyperexcitability-related pathological repetitive self-grooming and tic-like head shakes[135]. Interestingly, these mice did not present with increased anxiety or social impairments[135].
It seems that the lower EAAT2 expression associated with depression and learned helplessness can be rescued by mood stabilizers. Chronic sodium valproate treatment increased EAAT2 protein levels in the rat hippocampus, but not other brain regions[109]. The antidepressant fluoxetine (class: SSRI) also produced rat hippocampal and cortical increases in EAAT2 expression, while tranylcypromine (class: monoamine oxidase inhibitor) resulted in an amygdala-specific increase[136]. In contrast to a depressive state, increases in EAAT2 mRNA and protein caused by chronic stress could be normalised by the antidepressant tianeptine in rat[130]. Interestingly, EAAT2b protein, but not mRNA, was also increased by chronic stress, however remained unaffected by tianeptine treatment[130]. A similar stress-induced increase in hippocampal EAAT2 mRNA expression was countered by food-based administration of lithium in rat[137]. This increase in EAAT2 expression in response to chronic stress lies in stark contrast to the previously mentioned decreases in EAAT2 expression associated with depression and learned helplessness, and suggests that EAAT2 is highly responsive to, or correlated with, different mood states.
EAAT2 SNP rs4354668 (T/G), located in the gene promoter region and associated with lower transport activity, has been correlated to cognitive dysfunction in schizophrenia, with the lower activity G allele linked to poorer working memory performance[104,138] and a reduction in frontal grey matter[139]. EAAT2 mRNA levels have been reported as lower in the parahippocampal gyrus -but not other hippocampal regions[140] and prefrontal cortex[141], and higher in the thalamus of subjects with schizophrenia[115]. In contrast, Matute et al[142] found an increase in EAAT2 expression in the prefrontal cortex. Finally, Lauriat et al[76] found no change in EAAT2 or EAAT2b mRNA in the DLPFC or primary visual cortex of subjects with schizophrenia, however the authors acknowledge the potential masking effect of antipsychotics on their results. N-glycosylation of the EAAT2 multimer was reduced in the DLPFC from subjects with schizophrenia[119], which may be associated with ER retention and reduced trafficking of EAAT2 to the plasma membrane[68]. The splice variant EAAT2b was increased in extra-synaptic membrane/cytosol post-mortem fractions from the DLPFC of subjects with schizophrenia[143]. As EAAT2b cell-surface expression is internalised in response to increases in intracellular calcium[80], it is possible that the elevated cytosolic localization of EAAT2b is a countermeasure to excitotoxicity.
Clozapine treatment has been reported to decrease EAAT2 expression in hippocampal CA1, parietal temporal, frontal and cingulate cortical[144], and striatal[145] brain regions of male Sprague-Dawley rats. EAAT2 protein levels and glutamate uptake were similarly reduced in the cerebral cortex of clozapine-treated adult rats with an accompanying increase in extracellular glutamate[146]. Clozapine also induced a decrease in EAAT2 protein in astrocyte culture, which was accompanied by a reduction in glutamate uptake[147]. This response to clozapine, which contrasts with the lack of effect that clozapine had on EAAT1 expression (discussed earlier in this review), suggests once again that the two EAAT subtypes are intrinsically different. Haloperidol similarly decreased frontal and cingulate cortical[144], as well as striatal[145], EAAT2 expression in rat. Matute and colleagues have provided support to the argument that antipsychotic drug treatment can affect EAAT2 expression by showing the higher levels of EAAT2 mRNA in the prefrontal cortex of untreated subjects with schizophrenia were not detectable in those with the disorder who had received typical or atypical antipsychotics[142].
Given the differential expression of EAAT1 and EAAT2 throughout brain development[47] and their importance in normal brain development[148], it is not surprisingly that abnormal levels of these glutamate transporters have been found in the pathophysiology of psychiatric illness. It is imperative, however, that the subtype splice variants and glycosylation states be taken into consideration when researching the EAATs, as their unique attributes make them just as susceptible to disorder. The ability to analysis EAAT dynamics in a pre-mortem setting will assist in understanding the cause for their dysregulation and through that, the glutamatergic role in psychiatric disorder. Such information will allow for the prescription of medication with an understanding of how it may, or may not, affect the glutamatergic system. This review concludes with the contention that the EAAT family is dynamically regulated by a range of internal and external factors and offer a viable means to region-specific, subtype-specific therapeutic targets with the potential to respond to the immediate environment. However, a better understanding of the dynamic regulation of EAATs within the convoluted context of psychiatric disorder will be advantageous in advancing drug discovery.
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chakrabarti S, Hosak L, Pasquini M, Tcheremissine OV S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130:1007S-1015S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1069] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 2. | Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984;43:1369-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2330] [Cited by in RCA: 2167] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 3. | Lehmann A, Isacsson H, Hamberger A. Effects of in vivo administration of kainic acid on the extracellular amino acid pool in the rabbit hippocampus. J Neurochem. 1983;40:1314-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 184] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Bouvier M, Szatkowski M, Amato A, Attwell D. The glial cell glutamate uptake carrier countertransports pH-changing anions. Nature. 1992;360:471-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 270] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Erecińska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35:245-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 489] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Perry TL, Hansen S, Berry K, Mok C, Lesk D. Free amino acids and related compounds in biopsies of human brain. J Neurochem. 1971;18:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 150] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Perry TL, Berry K, Hansen S, Diamond S, Mok C. Regional distribution of amino acids in human brain obtained at autopsy. J Neurochem. 1971;18:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 222] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3529] [Cited by in RCA: 3578] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 9. | Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3344] [Cited by in RCA: 3522] [Article Influence: 146.8] [Reference Citation Analysis (0)] |
| 10. | Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1631] [Cited by in RCA: 1688] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 11. | Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci USA. 1992;89:10955-10959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 880] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 13. | Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559-5569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 756] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 14. | Pines G, Danbolt NC, Bjørås M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 963] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 15. | Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1010] [Cited by in RCA: 1021] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 16. | Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 875] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 17. | Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci USA. 1997;94:4155-4160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 720] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 18. | Kanai Y, Smith CP, Hediger MA. A new family of neurotransmitter transporters: the high-affinity glutamate transporters. FASEB J. 1993;7:1450-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Stallcup WB, Bulloch K, Baetge EE. Coupled transport of glutamate and sodium in a cerebellar nerve cell line. J Neurochem. 1979;32:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 424] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 21. | Grewer C, Rauen T. Electrogenic glutamate transporters in the CNS: molecular mechanism, pre-steady-state kinetics, and their impact on synaptic signaling. J Membr Biol. 2005;203:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP. Kinetics of a human glutamate transporter. Neuron. 1995;14:1019-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 325] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 449] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 24. | Holmseth S, Dehnes Y, Huang YH, Follin-Arbelet VV, Grutle NJ, Mylonakou MN, Plachez C, Zhou Y, Furness DN, Bergles DE. The density of EAAC1 (EAAT3) glutamate transporters expressed by neurons in the mammalian CNS. J Neurosci. 2012;32:6000-6013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 25. | Schmitt A, Asan E, Püschel B, Kugler P. Cellular and regional distribution of the glutamate transporter GLAST in the CNS of rats: nonradioactive in situ hybridization and comparative immunocytochemistry. J Neurosci. 1997;17:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1208] [Cited by in RCA: 1326] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 27. | Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 642] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 28. | Yamada K, Watanabe M, Shibata T, Tanaka K, Wada K, Inoue Y. EAAT4 is a post-synaptic glutamate transporter at Purkinje cell synapses. Neuroreport. 1996;7:2013-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Schousboe A, Svenneby G, Hertz L. Uptake and metabolism of glutamate in astrocytes cultured from dissociated mouse brain hemispheres. J Neurochem. 1977;29:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 279] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | McLennan H. The autoradiographic localization of L-[3h]glutamate in rat brain tissue. Brain Res. 1976;115:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 127] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Gundersen V, Shupliakov O, Brodin L, Ottersen OP, Storm-Mathisen J. Quantification of excitatory amino acid uptake at intact glutamatergic synapses by immunocytochemistry of exogenous D-aspartate. J Neurosci. 1995;15:4417-4428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Bergles DE, Dzubay JA, Jahr CE. Glutamate transporter currents in bergmann glial cells follow the time course of extrasynaptic glutamate. Proc Natl Acad Sci USA. 1997;94:14821-14825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 199] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 905] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 34. | Farinelli SE, Nicklas WJ. Glutamate metabolism in rat cortical astrocyte cultures. J Neurochem. 1992;58:1905-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Yu AC, Schousboe A, Hertz L. Metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. J Neurochem. 1982;39:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 247] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Hassel B, Bachelard H, Jones P, Fonnum F, Sonnewald U. Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. J Cereb Blood Flow Metab. 1997;17:1230-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 131] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Domercq M, Etxebarria E, Pérez-Samartín A, Matute C. Excitotoxic oligodendrocyte death and axonal damage induced by glutamate transporter inhibition. Glia. 2005;52:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 557] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 39. | Stoffel W, Sasse J, Düker M, Müller R, Hofmann K, Fink T, Lichter P. Human high affinity, Na(+)-dependent L-glutamate/L-aspartate transporter GLAST-1 (EAAT-1): gene structure and localization to chromosome 5p11-p12. FEBS Lett. 1996;386:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Kirschner MA, Arriza JL, Copeland NG, Gilbert DJ, Jenkins NA, Magenis E, Amara SG. The mouse and human excitatory amino acid transporter gene (EAAT1) maps to mouse chromosome 15 and a region of syntenic homology on human chromosome 5. Genomics. 1994;22:631-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Takai S, Yamada K, Kawakami H, Tanaka K, Nakamura S. Localization of the gene (SLC1A3) encoding human glutamate transporter (GluT-1) to 5p13 by fluorescence in situ hybridization. Cytogenet Cell Genet. 1995;69:209-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Torp R, Danbolt NC, Babaie E, Bjørås M, Seeberg E, Storm-Mathisen J, Ottersen OP. Differential expression of two glial glutamate transporters in the rat brain: an in situ hybridization study. Eur J Neurosci. 1994;6:936-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 142] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 43. | Kondo K, Hashimoto H, Kitanaka J, Sawada M, Suzumura A, Marunouchi T, Baba A. Expression of glutamate transporters in cultured glial cells. Neurosci Lett. 1995;188:140-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Schulte S, Stoffel W. UDP galactose:ceramide galactosyltransferase and glutamate/aspartate transporter. Copurification, separation and characterization of the two glycoproteins. Eur J Biochem. 1995;233:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Huggett J, Vaughan-Thomas A, Mason D. The open reading frame of the Na(+)-dependent glutamate transporter GLAST-1 is expressed in bone and a splice variant of this molecule is expressed in bone and brain. FEBS Lett. 2000;485:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Conradt M, Storck T, Stoffel W. Localization of N-glycosylation sites and functional role of the carbohydrate units of GLAST-1, a cloned rat brain L-glutamate/L-aspartate transporter. Eur J Biochem. 1995;229:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 47. | Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci. 1997;17:8363-8375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 414] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 48. | Escartin C, Brouillet E, Gubellini P, Trioulier Y, Jacquard C, Smadja C, Knott GW, Kerkerian-Le Goff L, Déglon N, Hantraye P. Ciliary neurotrophic factor activates astrocytes, redistributes their glutamate transporters GLAST and GLT-1 to raft microdomains, and improves glutamate handling in vivo. J Neurosci. 2006;26:5978-5989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Haugeto O, Ullensvang K, Levy LM, Chaudhry FA, Honoré T, Nielsen M, Lehre KP, Danbolt NC. Brain glutamate transporter proteins form homomultimers. J Biol Chem. 1996;271:27715-27722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 389] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 50. | Gegelashvili G, Civenni G, Racagni G, Danbolt NC, Schousboe I, Schousboe A. Glutamate receptor agonists up-regulate glutamate transporter GLAST in astrocytes. Neuroreport. 1996;8:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Duan S, Anderson CM, Stein BA, Swanson RA. Glutamate induces rapid upregulation of astrocyte glutamate transport and cell-surface expression of GLAST. J Neurosci. 1999;19:10193-10200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 230] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 52. | Gegelashvili G, Danbolt NC, Schousboe A. Neuronal soluble factors differentially regulate the expression of the GLT1 and GLAST glutamate transporters in cultured astroglia. J Neurochem. 1997;69:2612-2615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 191] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 53. | Gegelashvili G, Dehnes Y, Danbolt NC, Schousboe A. The high-affinity glutamate transporters GLT1, GLAST, and EAAT4 are regulated via different signalling mechanisms. Neurochem Int. 2000;37:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 169] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 54. | Plachez C, Danbolt NC, Récasens M. Transient expression of the glial glutamate transporters GLAST and GLT in hippocampal neurons in primary culture. J Neurosci Res. 2000;59:587-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Plachez C, Martin A, Guiramand J, Récasens M. Astrocytes repress the neuronal expression of GLAST and GLT glutamate transporters in cultured hippocampal neurons from embryonic rats. Neurochem Int. 2004;45:1113-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Macnab LT, Williams SM, Pow DV. Expression of the exon 3 skipping form of GLAST, GLAST1a, in brain and retina. Neuroreport. 2006;17:1867-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Huggett JF, Mustafa A, O’neal L, Mason DJ. The glutamate transporter GLAST-1 (EAAT-1) is expressed in the plasma membrane of osteocytes and is responsive to extracellular glutamate concentration. Biochem Soc Trans. 2002;30:890-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Vallejo-Illarramendi A, Domercq M, Matute C. A novel alternative splicing form of excitatory amino acid transporter 1 is a negative regulator of glutamate uptake. J Neurochem. 2005;95:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Macnab LT, Pow DV. Central nervous system expression of the exon 9 skipping form of the glutamate transporter GLAST. Neuroreport. 2007;18:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Sullivan SM, Macnab LT, Björkman ST, Colditz PB, Pow DV. GLAST1b, the exon-9 skipping form of the glutamate-aspartate transporter EAAT1 is a sensitive marker of neuronal dysfunction in the hypoxic brain. Neuroscience. 2007;149:434-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Lee A, Anderson AR, Beasley SJ, Barnett NL, Poronnik P, Pow DV. A new splice variant of the glutamate-aspartate transporter: cloning and immunolocalization of GLAST1c in rat, pig and human brains. J Chem Neuroanat. 2012;43:52-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Li X, Francke U. Assignment of the gene SLC1A2 coding for the human glutamate transporter EAAT2 to human chromosome 11 bands p13-p12. Cytogenet Cell Genet. 1995;71:212-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Robinson MB. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem Int. 1998;33:479-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 189] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 64. | Brooks-Kayal AR, Munir M, Jin H, Robinson MB. The glutamate transporter, GLT-1, is expressed in cultured hippocampal neurons. Neurochem Int. 1998;33:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751-8757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 481] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 66. | Sutherland ML, Delaney TA, Noebels JL. Glutamate transporter mRNA expression in proliferative zones of the developing and adult murine CNS. J Neurosci. 1996;16:2191-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 67. | Furness DN, Dehnes Y, Akhtar AQ, Rossi DJ, Hamann M, Grutle NJ, Gundersen V, Holmseth S, Lehre KP, Ullensvang K. A quantitative assessment of glutamate uptake into hippocampal synaptic terminals and astrocytes: new insights into a neuronal role for excitatory amino acid transporter 2 (EAAT2). Neuroscience. 2008;157:80-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 68. | Kalandadze A, Wu Y, Fournier K, Robinson MB. Identification of motifs involved in endoplasmic reticulum retention-forward trafficking of the GLT-1 subtype of glutamate transporter. J Neurosci. 2004;24:5183-5192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Gendreau S, Voswinkel S, Torres-Salazar D, Lang N, Heidtmann H, Detro-Dassen S, Schmalzing G, Hidalgo P, Fahlke C. A trimeric quaternary structure is conserved in bacterial and human glutamate transporters. J Biol Chem. 2004;279:39505-39512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 70. | Northington FJ, Traystman RJ, Koehler RC, Martin LJ. GLT1, glial glutamate transporter, is transiently expressed in neurons and develops astrocyte specificity only after midgestation in the ovine fetal brain. J Neurobiol. 1999;39:515-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 71. | Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, Longuemare MC. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J Neurosci. 1997;17:932-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 395] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 72. | Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, Fisher PB. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2). Proc Natl Acad Sci USA. 2003;100:1955-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 73. | Lauriat TL, McInnes LA. EAAT2 regulation and splicing: relevance to psychiatric and neurological disorders. Mol Psychiatry. 2007;12:1065-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 74. | Rauen T, Wiessner M, Sullivan R, Lee A, Pow DV. A new GLT1 splice variant: cloning and immunolocalization of GLT1c in the mammalian retina and brain. Neurochem Int. 2004;45:1095-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Utsunomiya-Tate N, Endou H, Kanai Y. Tissue specific variants of glutamate transporter GLT-1. FEBS Lett. 1997;416:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 76. | Lauriat TL, Dracheva S, Chin B, Schmeidler J, McInnes LA, Haroutunian V. Quantitative analysis of glutamate transporter mRNA expression in prefrontal and primary visual cortex in normal and schizophrenic brain. Neuroscience. 2006;137:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Holmseth S, Scott HA, Real K, Lehre KP, Leergaard TB, Bjaalie JG, Danbolt NC. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience. 2009;162:1055-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 78. | Schmitt A, Asan E, Lesch KP, Kugler P. A splice variant of glutamate transporter GLT1/EAAT2 expressed in neurons: cloning and localization in rat nervous system. Neuroscience. 2002;109:45-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 79. | Reye P, Sullivan R, Fletcher EL, Pow DV. Distribution of two splice variants of the glutamate transporter GLT1 in the retinas of humans, monkeys, rabbits, rats, cats, and chickens. J Comp Neurol. 2002;445:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Underhill SM, Wheeler DS, Amara SG. Differential regulation of two isoforms of the glial glutamate transporter EAAT2 by DLG1 and CaMKII. J Neurosci. 2015;35:5260-5270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 81. | González-González IM, García-Tardón N, Giménez C, Zafra F. Splice variants of the glutamate transporter GLT1 form hetero-oligomers that interact with PSD-95 and NMDA receptors. J Neurochem. 2009;110:264-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 82. | O’Donovan SM, Sullivan CR, McCullumsmith RE. The role of glutamate transporters in the pathophysiology of neuropsychiatric disorders. NPJ Schizophr. 2017;3:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 83. | Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE Jr, Akil H, Watson SJ, Jones EG. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci USA. 2005;102:15653-15658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 498] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 84. | Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets. 2013;14:1225-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 449] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 85. | Bernstein HG, Steiner J, Bogerts B. Glial cells in schizophrenia: pathophysiological significance and possible consequences for therapy. Expert Rev Neurother. 2009;9:1059-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 86. | Peng L, Li B, Verkhratsky A. Targeting astrocytes in bipolar disorder. Expert Rev Neurother. 2016;16:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 87. | Xia L, Zhai M, Wang L, Miao D, Zhu X, Wang W. FGF2 blocks PTSD symptoms via an astrocyte-based mechanism. Behav Brain Res. 2013;256:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 88. | Ernst C, Nagy C, Kim S, Yang JP, Deng X, Hellstrom IC, Choi KH, Gershenfeld H, Meaney MJ, Turecki G. Dysfunction of astrocyte connexins 30 and 43 in dorsal lateral prefrontal cortex of suicide completers. Biol Psychiatry. 2011;70:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 89. | Torres-Platas SG, Hercher C, Davoli MA, Maussion G, Labonté B, Turecki G, Mechawar N. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology. 2011;36:2650-2658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 90. | Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 741] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 91. | de Sousa RT, Loch AA, Carvalho AF, Brunoni AR, Haddad MR, Henter ID, Zarate CA, Machado-Vieira R. Genetic Studies on the Tripartite Glutamate Synapse in the Pathophysiology and Therapeutics of Mood Disorders. Neuropsychopharmacology. 2017;42:787-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 92. | Allen RM, Young SJ. Phencyclidine-induced psychosis. Am J Psychiatry. 1978;135:1081-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 332] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 93. | Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2312] [Cited by in RCA: 2384] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 94. | Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 468] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 95. | Fung LK, Hardan AY. Developing Medications Targeting Glutamatergic Dysfunction in Autism: Progress to Date. CNS Drugs. 2015;29:453-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 96. | Akutagava-Martins GC, Salatino-Oliveira A, Genro JP, Contini V, Polanczyk G, Zeni C, Chazan R, Kieling C, Anselmi L, Menezes AM. Glutamatergic copy number variants and their role in attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2014;165B:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 97. | Bauer J, Werner A, Kohl W, Kugel H, Shushakova A, Pedersen A, Ohrmann P. Hyperactivity and impulsivity in adult attention-deficit/hyperactivity disorder is related to glutamatergic dysfunction in the anterior cingulate cortex. World J Biol Psychiatry. 2016;1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 98. | Danysz W, Parsons CG, Mobius HJ, Stoffler A, Quack G. Neuroprotective and symptomatological action of memantine relevant for Alzheimer’s disease--a unified glutamatergic hypothesis on the mechanism of action. Neurotox Res. 2000;2:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 99. | Francis PT. Glutamatergic systems in Alzheimer’s disease. Int J Geriatr Psychiatry. 2003;18:S15-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 100. | Spencer S, Kalivas PW. Glutamate Transport: A New Bench to Bedside Mechanism for Treating Drug Abuse. Int J Neuropsychopharmacol. 2017;20:797-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 101. | Gegelashvili G, Bjerrum OJ. Glutamate Transport System as a Novel Therapeutic Target in Chronic Pain: Molecular Mechanisms and Pharmacology. Adv Neurobiol. 2017;16:225-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Poletti S, Locatelli C, Pirovano A, Colombo C, Benedetti F. Glutamate EAAT1 transporter genetic variants influence cognitive deficits in bipolar disorder. Psychiatry Res. 2015;226:407-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 103. | Riberto M, Poletti S, Lorenzi C, Vai B, Brioschi S, Benedetti F. Excitatory amino acid transporters 1 affects corticolimbic circuitry during implicit processing of negative emotional stimuli in bipolar disorder. Eur Neuropsychopharmacol. 2017;27:S712. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 104. | Spangaro M, Bosia M, Zanoletti A, Bechi M, Mariachiara B, Pirovano A, Lorenzi C, Bramanti P, Smeraldi E, Cavallaro R. Exploring effects of EAAT polymorphisms on cognitive functions in schizophrenia. Pharmacogenomics. 2014;15:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 105. | Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry. 2011;16:634-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 268] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 106. | Medina A, Burke S, Thompson RC, Bunney W Jr, Myers RM, Schatzberg A, Akil H, Watson SJ. Glutamate transporters: a key piece in the glutamate puzzle of major depressive disorder. J Psychiatr Res. 2013;47:1150-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 107. | McOmish CE, Pavey G, Gibbons A, Hopper S, Udawela M, Scarr E, Dean B. Lower [3H]LY341495 binding to mGlu2/3 receptors in the anterior cingulate of subjects with major depressive disorder but not bipolar disorder or schizophrenia. J Affect Disord. 2016;190:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 108. | Feng D, Guo B, Liu G, Wang B, Wang W, Gao G, Qin H, Wu S. FGF2 alleviates PTSD symptoms in rats by restoring GLAST function in astrocytes via the JAK/STAT pathway. Eur Neuropsychopharmacol. 2015;25:1287-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 109. | Hassel B, Iversen EG, Gjerstad L, Taubøll E. Up-regulation of hippocampal glutamate transport during chronic treatment with sodium valproate. J Neurochem. 2001;77:1285-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 110. | Aguirre G, Rosas S, López-Bayghen E, Ortega A. Valproate-dependent transcriptional regulation of GLAST/EAAT1 expression: involvement of Ying-Yang 1. Neurochem Int. 2008;52:1322-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 111. | Ghodke-Puranik Y, Thorn CF, Lamba JK, Leeder JS, Song W, Birnbaum AK, Altman RB, Klein TE. Valproic acid pathway: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2013;23:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 270] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 112. | Deng X, Shibata H, Takeuchi N, Rachi S, Sakai M, Ninomiya H, Iwata N, Ozaki N, Fukumaki Y. Association study of polymorphisms in the glutamate transporter genes SLC1A1, SLC1A3, and SLC1A6 with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 113. | Wilmsdorff MV, Blaich C, Zink M, Treutlein J, Bauer M, Schulze T, Schneider-Axmann T, Gruber O, Rietschel M, Schmitt A. Gene expression of glutamate transporters SLC1A1, SLC1A3 and SLC1A6 in the cerebellar subregions of elderly schizophrenia patients and effects of antipsychotic treatment. World J Biol Psychiatry. 2013;14:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 114. | Bauer D, Gupta D, Harotunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr Res. 2008;104:108-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 115. | Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2001;158:1393-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 116. | Scarr E, Udawela M, Thomas EA, Dean B. Changed gene expression in subjects with schizophrenia and low cortical muscarinic M1 receptors predicts disrupted upstream pathways interacting with that receptor. Mol Psychiatry. 2018;23:295-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 117. | Kim S, Choi KH, Baykiz AF, Gershenfeld HK. Suicide candidate genes associated with bipolar disorder and schizophrenia: an exploratory gene expression profiling analysis of post-mortem prefrontal cortex. BMC Genomics. 2007;8:413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 118. | Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, Rehal S, Klempan T, Gratton A, Benkelfat C. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. 2009;4:e6585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 285] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 119. | Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr Res. 2010;117:92-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 120. | Karlsson RM, Tanaka K, Heilig M, Holmes A. Loss of glial glutamate and aspartate transporter (excitatory amino acid transporter 1) causes locomotor hyperactivity and exaggerated responses to psychotomimetics: rescue by haloperidol and metabotropic glutamate 2/3 agonist. Biol Psychiatry. 2008;64:810-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 121. | McCullumsmith RE, O’Donovan SM, Drummond JB, Benesh FS, Simmons M, Roberts R, Lauriat T, Haroutunian V, Meador-Woodruff JH. Cell-specific abnormalities of glutamate transporters in schizophrenia: sick astrocytes and compensating relay neurons? Mol Psychiatry. 2016;21:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 122. | Melone M, Bragina L, Conti F. Clozapine-induced reduction of glutamate transport in the frontal cortex is not mediated by GLAST and EAAC1. Mol Psychiatry. 2003;8:12-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 123. | Tanahashi S, Yamamura S, Nakagawa M, Motomura E, Okada M. Clozapine, but not haloperidol, enhances glial D-serine and L-glutamate release in rat frontal cortex and primary cultured astrocytes. Br J Pharmacol. 2012;165:1543-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 124. | Tian G, Lai L, Guo H, Lin Y, Butchbach ME, Chang Y, Lin CL. Translational control of glial glutamate transporter EAAT2 expression. J Biol Chem. 2007;282:1727-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 125. | Zschocke J, Bayatti N, Clement AM, Witan H, Figiel M, Engele J, Behl C. Differential promotion of glutamate transporter expression and function by glucocorticoids in astrocytes from various brain regions. J Biol Chem. 2005;280:34924-34932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 126. | Autry AE, Grillo CA, Piroli GG, Rothstein JD, McEwen BS, Reagan LP. Glucocorticoid regulation of GLT-1 glutamate transporter isoform expression in the rat hippocampus. Neuroendocrinology. 2006;83:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 127. | Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, Turecki G. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry. 2009;14:175-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 128. | Zink M, Vollmayr B, Gebicke-Haerter PJ, Henn FA. Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression. Neuropharmacology. 2010;58:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 129. | Zhang XH, Jia N, Zhao XY, Tang GK, Guan LX, Wang D, Sun HL, Li H, Zhu ZL. Involvement of pGluR1, EAAT2 and EAAT3 in offspring depression induced by prenatal stress. Neuroscience. 2013;250:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 130. | Reagan LP, Rosell DR, Wood GE, Spedding M, Muñoz C, Rothstein J, McEwen BS. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. Proc Natl Acad Sci USA. 2004;101:2179-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 131. | Lee Y, Gaskins D, Anand A, Shekhar A. Glia mechanisms in mood regulation: a novel model of mood disorders. Psychopharmacology (Berl). 2007;191:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 132. | Chakrabarty K, Bhattacharyya S, Christopher R, Khanna S. Glutamatergic dysfunction in OCD. Neuropsychopharmacology. 2005;30:1735-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 133. | Bhattacharyya S, Khanna S, Chakrabarty K, Mahadevan A, Christopher R, Shankar SK. Anti-brain autoantibodies and altered excitatory neurotransmitters in obsessive-compulsive disorder. Neuropsychopharmacology. 2009;34:2489-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 134. | Pittenger C. Glutamatergic agents for OCD and related disorders. Curr Treat Options Psychiatry. 2015;2:271-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 135. | Aida T, Yoshida J, Nomura M, Tanimura A, Iino Y, Soma M, Bai N, Ito Y, Cui W, Aizawa H. Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice. Neuropsychopharmacology. 2015;40:1569-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 136. | Zink M, Rapp S, Donev R, Gebicke-Haerter PJ, Thome J. Fluoxetine treatment induces EAAT2 expression in rat brain. J Neural Transm (Vienna). 2011;118:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 137. | Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc Natl Acad Sci USA. 2004;101:3973-3978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 157] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 138. | Spangaro M, Bosia M, Zanoletti A, Bechi M, Cocchi F, Pirovano A, Lorenzi C, Bramanti P, Benedetti F, Smeraldi E. Cognitive dysfunction and glutamate reuptake: effect of EAAT2 polymorphism in schizophrenia. Neurosci Lett. 2012;522:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 139. | Poletti S, Radaelli D, Bosia M, Buonocore M, Pirovano A, Lorenzi C, Cavallaro R, Smeraldi E, Benedetti F. Effect of glutamate transporter EAAT2 gene variants and gray matter deficits on working memory in schizophrenia. Eur Psychiatry. 2014;29:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 140. | Ohnuma T, Tessler S, Arai H, Faull RL, McKenna PJ, Emson PC. Gene expression of metabotropic glutamate receptor 5 and excitatory amino acid transporter 2 in the schizophrenic hippocampus. Brain Res Mol Brain Res. 2000;85:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 141. | Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Expression of the human excitatory amino acid transporter 2 and metabotropic glutamate receptors 3 and 5 in the prefrontal cortex from normal individuals and patients with schizophrenia. Brain Res Mol Brain Res. 1998;56:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 154] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 142. | Matute C, Melone M, Vallejo-Illarramendi A, Conti F. Increased expression of the astrocytic glutamate transporter GLT-1 in the prefrontal cortex of schizophrenics. Glia. 2005;49:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 143. | Shan D, Mount D, Moore S, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal partitioning of hexokinase 1 suggests disruption of a glutamate transport protein complex in schizophrenia. Schizophr Res. 2014;154:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 144. | Schmitt A, Zink M, Petroianu G, May B, Braus DF, Henn FA. Decreased gene expression of glial and neuronal glutamate transporters after chronic antipsychotic treatment in rat brain. Neurosci Lett. 2003;347:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 145. | Schneider JS, Wade T, Lidsky TI. Chronic neuroleptic treatment alters expression of glial glutamate transporter GLT-1 mRNA in the striatum. Neuroreport. 1998;9:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 146. | Melone M, Vitellaro-Zuccarello L, Vallejo-Illarramendi A, Pérez-Samartin A, Matute C, Cozzi A, Pellegrini-Giampietro DE, Rothstein JD, Conti F. The expression of glutamate transporter GLT-1 in the rat cerebral cortex is down-regulated by the antipsychotic drug clozapine. Mol Psychiatry. 2001;6:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 147. | Vallejo-Illarramendi A, Torres-Ramos M, Melone M, Conti F, Matute C. Clozapine reduces GLT-1 expression and glutamate uptake in astrocyte cultures. Glia. 2005;50:276-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 148. | Matsugami TR, Tanemura K, Mieda M, Nakatomi R, Yamada K, Kondo T, Ogawa M, Obata K, Watanabe M, Hashikawa T. From the Cover: Indispensability of the glutamate transporters GLAST and GLT1 to brain development. Proc Natl Acad Sci USA. 2006;103:12161-12166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |