Published online Mar 22, 2018. doi: 10.5498/wjp.v8.i1.20
Peer-review started: October 2, 2017
First decision: November 27, 2017
Revised: December 17, 2017
Accepted: January 7, 2018
Article in press: January 7, 2018
Published online: March 22, 2018
Processing time: 177 Days and 19.4 Hours
To investigate the repeatability of proton magnetic resonance spectroscopy in the in vivo measurement of human cerebral levels of choline-containing compounds (Cho).
Two consecutive scans were carried out in six healthy resting subjects at a magnetic field strength of 1.5 T. On each occasion, neurospectroscopy data were collected from 64 voxels using the same 2D chemical shift imaging (CSI) sequence. The data were analyzed in the same way, using the same software, to obtain the values for each voxel of the ratio of Cho to creatine. The Wilcoxon related-samples signed-rank test, coefficient of variation (CV), repeatability coefficient (RC), and intraclass correlation coefficient (ICC) were used to assess the repeatability.
The CV ranged from 2.75% to 33.99%, while the minimum RC was 5.68%. There was excellent reproducibility, as judged by significant ICC values, in 26 voxels. Just three voxels showed significant differences according to the Wilcoxon related-samples signed-rank test.
It is therefore concluded that when CSI multivoxel proton neurospectroscopy is used to measure cerebral choline-containing compounds at 1.5 T, the reproducibility is highly acceptable.
Core tip: Proton neurospectroscopy is a powerful tool allowing the assessment of cerebral metabolites. As such, it is increasingly being introduced into the practice of psychiatry for the investigation of cerebral choline-containing compounds in patients, as well as being used as a research tool. However, it is important to establish the reproducibility of this sensitive technique. In the present study, we show that this technique (using 2D chemical shift imaging) gives a level of reproducibility that is highly acceptable. These results should further encourage the use of this technique, which, in principle, is available on all standard MRI scanners, in psychiatric practice.
- Citation: Puri BK, Egan M, Wallis F, Jakeman P. Repeatability of two-dimensional chemical shift imaging multivoxel proton magnetic resonance spectroscopy for measuring human cerebral choline-containing compounds. World J Psychiatr 2018; 8(1): 20-26
- URL: https://www.wjgnet.com/2220-3206/full/v8/i1/20.htm
- DOI: https://dx.doi.org/10.5498/wjp.v8.i1.20
In vivo magnetic resonance proton spectroscopy studies of the human brain pose a technical challenge given that the water signal is four orders of magnitude greater than signals from metabolites of interest, and also because of the narrow range of the chemical shift, spin-spin coupling complicating the spectral pattern, and the higher scalp lipid signal compared with cerebral metabolite signals; nevertheless, choline-containing compounds (Cho) such as phosphoryl- and glycerophosphoryl-choline can be measured using this technique[1].
In contrast to the commonly used method of single-voxel spectroscopy (SVS), chemical shift imaging (CSI) is a multi-voxel technique. Thus, in neuroimaging, 2D-CSI has the distinct advantage over SVS of allowing larger areas of the brain to be studied during scanning, so that areas showing abnormal signals and also those appearing normal in structural magnetic resonance images can be included[2]. CSI can also be carried out in three dimensions, which should improve spatial resolution and the signal-to-noise ratio; however, 2D-CSI is more resistant to motion artefact, which can be a problem when scanning the brain, than 3D-CSI[3]. Furthermore, image quality is better with 2D-CSI compared with 3D-CSI at a usual magnetic field strength of 1.5 T or 3 T[4-6].
Choline is an alcohol which, in the human brain, is particularly abundant in phosphatidylcholine (in which it is attached, as a polar head group, via a phosphate group, to the Sn3 position of the glycerol backbone) membrane phospholipid molecules; Cho take part in membrane biosynthesis and breakdown[1]. Thus, measurement of Cho has clinical and research value. One example is in relation to chronic fatigue syndrome (also known as myalgic encephalomyelitis or systemic exertion intolerance disease), which is currently of unknown etiology. The first systematic proton neurospectroscopy study of this condition showed a significantly higher level of Cho in the occipital cortex in patients compared with matched healthy controls, and also loss of the spatial variation of Cho that is normally expected[7]. Given that such increased levels are associated with abnormal membrane phospholipid metabolism[8], this finding, which was essentially confirmed later by another group in respect of the basal ganglia[9], suggests that chronic fatigue syndrome/myalgic encephalomyelitis is associated with abnormal phospholipid metabolism in neuroglial membranes[1,7]. It has been suggested that this, in turn, might result from chronic viral infection[10]. Based on this Cho finding, a potential therapeutic approach to this difficult-to-treat disorder, involving long-chain polyunsaturated fatty acids, has been suggested[11,12]. A second example relates to dyslexia, which is another important neuropsychiatric disorder of unknown etiology, in which the first systematic proton neurospectroscopy study revealed decreased Cho in the left temporo-parietal lobe[13]. This finding could have resulted from reduced left temporo-parietal phospholipid metabolism[14], which would be consistent with the findings from the first systematic 31-phosphorus neurospectroscopy study of this disorder[15]. In turn, this has led to suggestions of potential therapeutic interventions[16].
2D-CSI may also be useful clinically in evaluating patients with acute onset of neuropsychiatric systemic lupus erythematosus[2]. Another important clinical use of 2D-CSI is in relation to grading gliomas when used in combination with diffusion kurtosis imaging and dynamic susceptibility-weighted contrast-enhanced MRI[17]. Indeed, in a brain histopathological study, it has been shown that 2D-CSI combined with perfusion MRI are associated with high sensibility and high specificity in differentiating between glioblastoma multiforme and cerebral metastases and also in distinguishing between grade III and grade IV gliomas[18]. It is therefore important to ascertain the reproducibility of 2D-CSI.
We present the results of the first study to investigate the repeatability of proton magnetic resonance spectroscopy 2D-CSI in the in vivo measurement of human cerebral levels of Cho at a magnetic field strength of 1.5 T.
This study was a repeated-measures pilot study in six individuals. The study was approved by the Research Ethics Committee. All participants gave written informed consent. Immediately after undergoing MRI scanning (including 2D-CSI), each participant remained lying in the scanner and the scanning protocol, including the 2D-CSI, was repeated.
The cohort consisted of six healthy volunteers, three males and three females. Their mean age was 44.1 years (range 26 to 58 years).
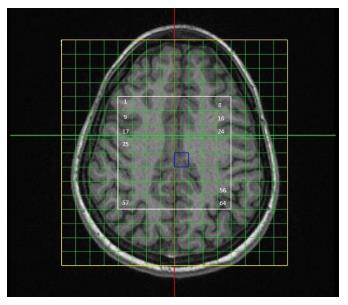
All measurements were carried out using a 1.5-T Siemens Symphony TIM (Total Imaging Matrix) scanner (Siemens Medical Systems, Erlangen, Germany) using a standard head matrix coil. Proton spectra were acquired using a 64-voxel 2D-CSI spin-echo spectroscopy sequence with TE = 30 ms, TR = 1500 ms, number of averages = 4, field of view = 160 mm × 160 mm, and thickness = 15 mm. Figure 1 shows the location of the voxels. Spectral analysis was carried out using the Siemens spectroscopy task card (Siemens Medical Systems, Erlangen, Germany). This automated software analysis was objective and clearly obviated the need for inter-observer analysis.
The main endpoint of this study was the ratio of Cho to creatine (Cr) for each voxel. The coefficient of variation (CV), repeatability coefficient (RC), and intraclass correlation coefficient (ICC) were used to assess the repeatability. The repeatability coefficient was calculated as 1.96 × (standard deviation of the mean difference between two measurements), after the method proposed by Bland and Altman as being more appropriate than the correlation coefficient when assessing the level of agreement between two methods of clinical measurement[19]. The CV was calculated as (the standard deviation of the mean difference between two measurements)/(the mean of all measurements) and was assessed in order to allow comparison of the results of the present study with those of previous studies of the reproducibility of proton magnetic resonance (albeit without CSI). Differences between the results of the two scans were analyzed using the Wilcoxon related-samples signed-rank test (a repeated-measures nonparametric test). A P-value of less than 0.05 was taken to be statistically significant. Statistical tests were carried out using the software package IBM SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, NY, United States).
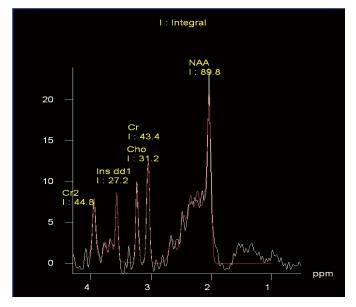
There were no technical difficulties in carrying out this study and all 2D-CSI proton neurospectroscopy data were included in the analyses. Figure 2 illustrates an example of a fitted spectrum from this study using the Siemens software.
The Wilcoxon related-samples signed-rank test results for all 64 voxels are shown in Table 1, using the voxel nomenclature given in Figure 1. Three voxels showed a significant difference between successive scans, namely voxels 3, 10 and 21.
| Voxel | Median Cho/Cr at first scan | Median Cho/Cr at second scan | Wilcoxon related-samples signed-rank test (P value) |
| 1 | 0.681 | 0.624 | 0.345 |
| 2 | 0.825 | 0.836 | 0.917 |
| 3 | 0.897 | 0.924 | 0.046 |
| 4 | 0.819 | 0.785 | 0.917 |
| 5 | 0.822 | 0.812 | 0.753 |
| 6 | 0.998 | 1.060 | 0.917 |
| 7 | 0.868 | 0.902 | 0.600 |
| 8 | 0.661 | 0.708 | 0.463 |
| 9 | 0.751 | 0.579 | 0.075 |
| 10 | 0.878 | 0.799 | 0.028 |
| 11 | 0.970 | 1.043 | 0.173 |
| 12 | 0.793 | 0.860 | 0.173 |
| 13 | 0.822 | 0.765 | 0.600 |
| 14 | 0.993 | 0.989 | 0.753 |
| 15 | 0.923 | 0.894 | 0.600 |
| 16 | 0.714 | 0.682 | 0.463 |
| 17 | 0.760 | 0.644 | 0.249 |
| 18 | 0.947 | 0.855 | 0.116 |
| 19 | 1.027 | 1.045 | 0.345 |
| 20 | 0.751 | 0.828 | 0.173 |
| 21 | 0.898 | 0.816 | 0.046 |
| 22 | 1.063 | 1.056 | 0.173 |
| 23 | 0.942 | 1.022 | 0.917 |
| 24 | 0.728 | 0.747 | 0.116 |
| 25 | 0.713 | 0.705 | 0.249 |
| 26 | 0.941 | 0.984 | 0.917 |
| 27 | 0.940 | 0.961 | 0.345 |
| 28 | 0.808 | 0.831 | 0.753 |
| 29 | 0.853 | 0.851 | 0.600 |
| 30 | 1.030 | 1.083 | 0.600 |
| 31 | 0.991 | 0.929 | 0.249 |
| 32 | 0.709 | 0.696 | 0.173 |
| 33 | 0.660 | 0.660 | 0.600 |
| 34 | 0.892 | 0.922 | 0.345 |
| 35 | 0.948 | 0.905 | 0.917 |
| 36 | 0.732 | 0.718 | 0.600 |
| 37 | 0.750 | 0.724 | 0.345 |
| 38 | 1.032 | 0.904 | 0.173 |
| 39 | 0.982 | 0.946 | 0.345 |
| 40 | 0.700 | 0.744 | 0.116 |
| 41 | 0.627 | 0.597 | 0.917 |
| 42 | 0.866 | 0.854 | 0.249 |
| 43 | 0.871 | 0.793 | 0.600 |
| 44 | 0.591 | 0.595 | 0.600 |
| 45 | 0.573 | 0.584 | 0.753 |
| 46 | 0.793 | 0.875 | 0.600 |
| 47 | 0.903 | 0.940 | 0.249 |
| 48 | 0.563 | 0.686 | 0.075 |
| 49 | 0.571 | 0.575 | 0.463 |
| 50 | 0.779 | 0.817 | 0.345 |
| 51 | 0.740 | 0.777 | 0.116 |
| 52 | 0.539 | 0.533 | 0.463 |
| 53 | 0.546 | 0.500 | 0.463 |
| 54 | 0.767 | 0.752 | 0.463 |
| 55 | 0.812 | 0.838 | 0.046 |
| 56 | 0.530 | 0.583 | 0.345 |
| 57 | 0.498 | 0.507 | 0.917 |
| 58 | 0.692 | 0.715 | 0.463 |
| 59 | 0.607 | 0.683 | 0.463 |
| 60 | 0.466 | 0.472 | 0.173 |
| 61 | 0.569 | 0.451 | 0.173 |
| 62 | 0.825 | 0.677 | 0.753 |
| 63 | 0.638 | 0.639 | 0.753 |
| 64 | 0.530 | 0.625 | 0.600 |
The values of the mean CV, RC and ICC (together with corresponding P values) are given in Table 2. The CV ranged from 2.75% (voxel 3) to 33.99% (voxel 58). The minimum RC was 5.68% (voxel 3). Many of the ICC values were statistically significant, particularly for central and more caudal voxels, but also for some rostral voxels.
| Voxel | Mean coefficient of variation | Repeatability coefficient | Intraclass correlation coefficient (P value) |
| 1 | 0.080 | 0.159 | 0.492 (0.236) |
| 2 | 0.057 | 0.194 | 0.866 (0.032) |
| 3 | 0.028 | 0.057 | 0.982 (< 0.0001) |
| 4 | 0.115 | 0.386 | -1.877 (0.822) |
| 5 | 0.101 | 0.260 | -0.066 (0.523) |
| 6 | 0.117 | 0.446 | 0.372 (0.335) |
| 7 | 0.053 | 0.171 | 0.829 (0.046) |
| 8 | 0.076 | 0.206 | 0.158 (0.429) |
| 9 | 0.191 | 0.607 | -0.233 (0.612) |
| 10 | 0.049 | 0.113 | 0.901 (0.003) |
| 11 | 0.071 | 0.191 | 0.702 (0.081) |
| 12 | 0.091 | 0.225 | 0.509 (0.197) |
| 13 | 0.076 | 0.213 | 0.724 (0.100) |
| 14 | 0.078 | 0.233 | 0.696 (0.124) |
| 15 | 0.089 | 0.277 | 0.735 (0.105) |
| 16 | 0.091 | 0.236 | -0.024 (0.509) |
| 17 | 0.290 | 1.287 | -3.314 (0.949) |
| 18 | 0.095 | 0.226 | 0.811 (0.029) |
| 19 | 0.058 | 0.226 | 0.816 (0.052) |
| 20 | 0.058 | 0.137 | 0.370 (0.273) |
| 21 | 0.058 | 0.106 | 0.569 (0.071) |
| 22 | 0.064 | 0.214 | 0.780 (0.048) |
| 23 | 0.055 | 0.238 | 0.941 (0.005) |
| 24 | 0.258 | 6.467 | 0.152 (0.43) |
| 25 | 0.192 | 0.305 | 0.866 (0.028) |
| 26 | 0.044 | 0.129 | 0.974 (0.001) |
| 27 | 0.062 | 0.205 | 0.833 (0.033) |
| 28 | 0.087 | 0.242 | 0.652 (0.159) |
| 29 | 0.059 | 0.170 | 0.749 (0.088) |
| 30 | 0.061 | 0.205 | 0.851 (0.034) |
| 31 | 0.046 | 0.114 | 0.983 (< 0.001) |
| 32 | 0.093 | 0.174 | 0.907 (0.007) |
| 33 | 0.126 | 0.244 | 0.872 (0.024) |
| 34 | 0.057 | 0.146 | 0.829 (0.033) |
| 35 | 0.066 | 0.230 | 0.793 (0.071) |
| 36 | 0.063 | 0.151 | 0.883 (0.024) |
| 37 | 0.036 | 0.079 | 0.960 (0.002) |
| 38 | 0.096 | 0.314 | 0.397 (0.284) |
| 39 | 0.087 | 0.269 | -0.162 (0.584) |
| 40 | 0.122 | 0.222 | 0.643 (0.116) |
| 41 | 0.185 | 0.225 | 0.917 (0.011) |
| 42 | 0.055 | 0.138 | 0.950 (0.002) |
| 43 | 0.083 | 0.235 | 0.691 (0.132) |
| 44 | 0.106 | 0.217 | 0.553 (0.216) |
| 45 | 0.106 | 0.369 | 0.229 (0.397) |
| 46 | 0.182 | 0.547 | -0.256 (0.588) |
| 47 | 0.054 | 0.136 | 0.942 (0.003) |
| 48 | 0.175 | 0.274 | -0.074 (0.555) |
| 49 | 0.102 | 0.134 | 0.961 (0.002) |
| 50 | 0.053 | 0.121 | 0.970 (0.001) |
| 51 | 0.039 | 0.081 | 0.946 (0.001) |
| 52 | 0.056 | 0.116 | 0.870 (0.03) |
| 53 | 0.068 | 0.134 | 0.795 (0.064) |
| 54 | 0.071 | 0.171 | 0.928 (0.007) |
| 55 | 0.083 | 0.117 | 0.945 (0.001) |
| 56 | 0.148 | 0.277 | 0.108 (0.448) |
| 57 | 0.195 | 0.296 | 0.887 (0.022) |
| 58 | 0.340 | 0.547 | 0.582 (0.188) |
| 59 | 0.177 | 0.384 | 0.143 (0.43) |
| 60 | 0.135 | 0.177 | 0.883 (0.011) |
| 61 | 0.143 | 0.230 | 0.620 (0.135) |
| 62 | 0.200 | 0.691 | 0.512 (0.253) |
| 63 | 0.180 | 0.432 | 0.833 (0.046) |
| 64 | 0.273 | 0.714 | -0.957 (0.73) |
There have been no previous studies of the repeatability of proton neurospectroscopy 2D-CSI in the in vivo measurement of human cerebral levels of Cho at a magnetic field strength of 1.5 T. Previous in vivo studies of the reproducibility of proton magnetic resonance spectroscopy measurements have used single voxel techniques and have reported “within day” CV values for human hepatic fat of between 0.3% and 8.5%[20-25]. Thus the results of the present study compare favorably with these reports, which is all the more impressive given that cerebral tissue is more heterogeneous than hepatic tissue. There have been few cerebral single-voxel proton reproducibility studies. Schirmer and Auer reported CVs for absolute human brain concentrations of the main metabolites Cho, Cr and N-acetylaspartate, ranging from 3.8% to 6.4%[26]; the present results compare very well with these.
Van Werven and colleagues reported a “within day” RC value for hepatic fat (using a single voxel technique at 3 T) of 0.4%. Again, the present result of a minimum voxel RC of over 5% compares very well this result. Twenty-six of the voxels in the present study had an ICC which was statistically significant, indicating a high level of agreement for these voxels.
Just three voxels had median Cho to Cr ratios which were different between scans. From Figure 1 it can be seen that these voxels (numbers 3, 10 and 21) have locations in sulcal regions of the brain. It is therefore possible that the poor reproducibility in these three voxels might be a function of “bleeding” in the neurospectroscopy data acquisition. Voxel “bleeding” refers to contamination with signals derived from any of the six adjacent voxels, and is an analogue of artifactual Gibbs ringing in structural MRI[27]. In the present case, the contaminating signals could have arisen from the low-signal sulcal spaces.
In conclusion, in this first study of its type, the reproducibility of proton magnetic resonance spectroscopy in the in vivo measurement of human cerebral levels of Cho at a field strength of 1.5 T using 2D-CSI has been found to be very acceptable. These findings should further encourage the use of this technique in psychiatric clinical practice as well as in research studies of neuropsychiatric disorders. Already, neurospectroscopy is proving helpful in studies of schizophrenia, major depressive disorder, forensic psychiatry (e.g., posttraumatic stress disorder), chronic fatigue syndrome (myalgic encephalomyelitis or systemic exertion intolerance disease), and neuropsychiatric presentations in organic disorders, in which it has an important role to play in aiding diagnosis[16,28,29]. Given the present finding of a highly acceptable level of reproducibility of 2D-CSI, it would be appropriate in future to apply this technique to the follow-up of such patients, including monitoring their response to treatment.
In vivo magnetic resonance proton spectroscopy studies of the brain can be used to measure Cho. In contrast to the commonly used method of SVS, CSI is a multi-voxel technique. Thus, compared with SVS, 2D-CSI allows larger areas of the brain to be studied, so that areas showing abnormal signals and also those appearing normal in structural MRI can be included. Compared with 3D-CSI, 2D-CSI is more resistant to motion artefact, which can be a problem when scanning the brain, and image quality is better at a usual clinical magnetic field strength of 1.5 T or 3 T.
Brain choline is particularly abundant in phosphatidylcholine membrane phospholipid molecules; Cho take part in membrane biosynthesis and breakdown. Thus, measurement of Cho has clinical and research value. For example, in chronic fatigue syndrome (also known as myalgic encephalomyelitis or systemic exertion intolerance disease), which is of unknown etiology, the first systematic proton neurospectroscopy study showed a significantly higher level of Cho in the occipital cortex in patients compared with matched healthy controls, and also loss of the spatial variation of Cho that is normally expected. This finding, which was essentially confirmed later by another group in respect of the basal ganglia, suggests that this disorder is associated with abnormal phospholipid metabolism in neuroglial membranes and has led to the suggestion of a potential therapeutic approach. A second example is dyslexia, also of unknown etiology, in which the first systematic proton neurospectroscopy study revealed decreased Cho in the left temporo-parietal lobe. This finding could have resulted from reduced left temporo-parietal phospholipid metabolism, which would be consistent with the findings from the first systematic 31-phosphorus neurospectroscopy study of dyslexia. In turn, this has led to suggestions of potential therapeutic interventions. 2D-CSI may also be useful clinically in evaluating patients with acute onset of neuropsychiatric symptoms. Another important clinical use of 2D-CSI is in relation to grading gliomas. It is therefore important to ascertain the reproducibility of 2D-CSI.
The aim of this study was to investigate the repeatability of proton magnetic resonance spectroscopy 2D-CSI in the in vivo measurement of human cerebral levels of Cho.
A repeated-measures study in six individuals was carried out using a 1.5-T Siemens Symphony TIM scanner and a standard head matrix coil. Proton spectra were acquired using a 64-voxel 2D-CSI spin-echo spectroscopy sequence. Spectral analysis was carried out using the Siemens spectroscopy task card. The main endpoint was the ratio of Cho to Cr for each voxel. The CV, RC, and ICC were used to assess the repeatability. There have been no previous studies of the repeatability of proton neurospectroscopy 2D-CSI in the in vivo measurement of human cerebral levels of Cho at a magnetic field strength of 1.5 T.
There was a minimum voxel RC of over 5%, which compared favorably with previous studies of the liver; the present results were all the more impressive given the much more heterogeneous nature of the brain compared with hepatic tissue. Twenty-six voxels had an ICC which was statistically significant, indicating a high level of agreement for these voxels. Just three voxels had median Cho to Cr ratios which were significantly different between scans. These three voxels were located in sulcal brain regions. Thus the poor reproducibility in these three voxels might be a function of “bleeding” in the neurospectroscopy data acquisition.
In this first study of its type, the reproducibility of proton magnetic resonance spectroscopy in the in vivo measurement of human cerebral levels of Cho at a field strength of 1.5 T using 2D-CSI has been found to be very acceptable. Overall, the present findings should further encourage the use of this technique in psychiatric clinical practice as well as in research studies of neuropsychiatric disorders.
Overall, the results of this study are highly encouraging for the use of this technique in neuropsychiatric research and clinical practice. Further studies should be carried out to determine whether sulcal voxels should routinely be omitted from longitudinal comparison studies.
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hosak L, Razek AAKA S- Editor: Kong JX L- Editor: A E- Editor: Wang CH
| 1. | Cox IJ, Puri BK. In vivo MR spectroscopy in diagnosis and research of neuropsychiatric disorders. Prostaglandins Leukot Essent Fatty Acids. 2004;70:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Sundgren PC, Jennings J, Attwood JT, Nan B, Gebarski S, McCune WJ, Pang Y, Maly P. MRI and 2D-CSI MR spectroscopy of the brain in the evaluation of patients with acute onset of neuropsychiatric systemic lupus erythematosus. Neuroradiology. 2005;47:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Ramamurthy NK, Moosavi B, McInnes MD, Flood TA, Schieda N. Multiparametric MRI of solid renal masses: pearls and pitfalls. Clin Radiol. 2015;70:304-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Fischer MA, Donati OF, Chuck N, Blume IN, Hunziker R, Alkadhi H, Nanz D. Two- versus three-dimensional dual gradient-echo MRI of the liver: a technical comparison. Eur Radiol. 2013;23:408-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Ramalho M, Herédia V, de Campos RO, Dale BM, Azevedo RM, Semelka RC. In-phase and out-of-phase gradient-echo imaging in abdominal studies: intra-individual comparison of three different techniques. Acta Radiol. 2012;53:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Marin D, Soher BJ, Dale BM, Boll DT, Youngblood RS, Merkle EM. Characterization of adrenal lesions: comparison of 2D and 3D dual gradient-echo MR imaging at 3 T--preliminary results. Radiology. 2010;254:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Puri BK, Counsell SJ, Zaman R, Main J, Collins AG, Hajnal JV, Davey NJ. Relative increase in choline in the occipital cortex in chronic fatigue syndrome. Acta Psychiatr Scand. 2002;106:224-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Ruiz-Cabello J, Cohen JS. Phospholipid metabolites as indicators of cancer cell function. NMR in Biomedicine. 1992;5:226-233. [DOI] [Full Text] |
| 9. | Chaudhuri A, Condon BR, Gow JW, Brennan D, Hadley DM. Proton magnetic resonance spectroscopy of basal ganglia in chronic fatigue syndrome. Neuroreport. 2003;14:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Puri BK. Long-chain polyunsaturated fatty acids and the pathophysiology of myalgic encephalomyelitis (chronic fatigue syndrome). J Clin Pathol. 2007;60:122-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Puri BK, Holmes J, Hamilton G. Eicosapentaenoic acid-rich essential fatty acid supplementation in chronic fatigue syndrome associated with symptom remission and structural brain changes. Int J Clin Pract. 2004;58:297-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Puri BK. The use of eicosapentaenoic acid in the treatment of chronic fatigue syndrome. Prostaglandins Leukot Essent Fatty Acids. 2004;70:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Rae C, Lee MA, Dixon RM, Blamire AM, Thompson CH, Styles P, Talcott J, Richardson AJ, Stein JF. Metabolic abnormalities in developmental dyslexia detected by 1H magnetic resonance spectroscopy. Lancet. 1998;351:1849-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Puri BK, Richardson AJ. Brain phospholipid metabolism in dyslexia assessed by magnetic resonance spectroscopy. Phospholipid Spectrum Disorders in Psychiatry and Neurology. Carnforth Lancashire: Marius Press 2003; 501-508. |
| 15. | Richardson AJ, Cox IJ, Sargentoni J, Puri BK. Abnormal cerebral phospholipid metabolism in dyslexia indicated by phosphorus-31 magnetic resonance spectroscopy. NMR Biomed. 1997;10:309-314. [PubMed] [DOI] [Full Text] |
| 16. | Puri BK. Proton and 31-phosphorus neurospectroscopy in the study of membrane phospholipids and fatty acid intervention in schizophrenia, depression, chronic fatigue syndrome (myalgic encephalomyelitis) and dyslexia. Int Rev Psychiatry. 2006;18:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Van Cauter S, De Keyzer F, Sima DM, Sava AC, D’Arco F, Veraart J, Peeters RR, Leemans A, Van Gool S, Wilms G. Integrating diffusion kurtosis imaging, dynamic susceptibility-weighted contrast-enhanced MRI, and short echo time chemical shift imaging for grading gliomas. Neuro Oncol. 2014;16:1010-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Bendini M, Marton E, Feletti A, Rossi S, Curtolo S, Inches I, Ronzon M, Longatti P, Di Paola F. Primary and metastatic intraaxial brain tumors: prospective comparison of multivoxel 2D chemical-shift imaging (CSI) proton MR spectroscopy, perfusion MRI, and histopathological findings in a group of 159 patients. Acta Neurochir (Wien). 2011;153:403-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307-310. [PubMed] |
| 20. | van Werven JR, Hoogduin JM, Nederveen AJ, van Vliet AA, Wajs E, Vandenberk P, Stroes ES, Stoker J. Reproducibility of 3.0 Tesla magnetic resonance spectroscopy for measuring hepatic fat content. J Magn Reson Imaging. 2009;30:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Machann J, Stefan N, Schick F. (1)H MR spectroscopy of skeletal muscle, liver and bone marrow. Eur J Radiol. 2008;67:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Johnson NA, Walton DW, Sachinwalla T, Thompson CH, Smith K, Ruell PA, Stannard SR, George J. Noninvasive assessment of hepatic lipid composition: Advancing understanding and management of fatty liver disorders. Hepatology. 2008;47:1513-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Machann J, Thamer C, Schnoedt B, Stefan N, Haring HU, Claussen CD, Fritsche A, Schick F. Hepatic lipid accumulation in healthy subjects: a comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn Reson Med. 2006;55:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Thomas EL, Hamilton G, Patel N, O’Dwyer R, Doré CJ, Goldin RD, Bell JD, Taylor-Robinson SD. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;54:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462-E468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 1184] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 26. | Schirmer T, Auer DP. On the reliability of quantitative clinical magnetic resonance spectroscopy of the human brain. NMR Biomed. 2000;13:28-36. [PubMed] |
| 27. | Bertholdo D, Watcharakorn A, Castillo M. Brain proton magnetic resonance spectroscopy: introduction and overview. Neuroimaging Clin N Am. 2013;23:359-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 28. | Port JD, Puri BK. Magnetic resonance spectroscopy in psychiatry. Clinical MR Neuroimaging: Diffusion, Perfusion and Spectroscopy. Cambridge: Cambridge University Press 2010; 566-592. |
| 29. | Puri BK. Neurospectroscopy. Forensic Psychiatry: Fundamentals and Clinical Practice. Boca Raton, Florida, United States: CRC Press 2017; 37-38. |