Published online Jun 22, 2016. doi: 10.5498/wjp.v6.i2.221
Peer-review started: January 15, 2016
First decision: March 1, 2016
Revised: March 25, 2016
Accepted: May 10, 2016
Article in press: May 11, 2016
Published online: June 22, 2016
Processing time: 157 Days and 7.6 Hours
AIM: To compare hippocampus and amygdala volumes of patients with vaginismus with those of healthy control subjects.
METHODS: Magnetic resonance imaging was performed on ten patients with vaginismus and ten control subjects matched for age and gender. Volumes of the hippocampus and amygdala were blindly measured.
RESULTS: We found that the mean right amygdala volume of patients with vaginismus were smaller than that of the healthy controls. With regard to hippocampus volumes, the mean left and right hippocampus volumes were smaller than those of the healthy controls.
CONCLUSION: Our present findings suggest that there have been hippocampus and amygdala structural abnormalities in patients with vaginismus. These changes provide the notion that vaginismus may be a fear-related condition.
Core tip: Our present findings suggest that there have been hippocampus and amygdala structural abnormalities in patients with vaginismus. These changes provide the notion that vaginismus may be a fear-related condition.
- Citation: Atmaca M, Baykara S, Ozer O, Korkmaz S, Akaslan U, Yildirim H. Hippocampus and amygdala volumes in patients with vaginismus. World J Psychiatr 2016; 6(2): 221-225
- URL: https://www.wjgnet.com/2220-3206/full/v6/i2/221.htm
- DOI: https://dx.doi.org/10.5498/wjp.v6.i2.221
Vaginismus is described as a condition of no permission of intercourse and vaginal examinations due to spasm of the exterior 1/3 of the vagina. The Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV)[1] describes vaginismus as recurrent or persistent involuntary spasm of the musculature of the outer 1/3 of the vagina that interferes with sexual intercourse[2]. Although many cases of vaginismus are dealed with in sexual function disorders, when we look at its clinical picture, we can notice that vaginismus is also an anxiety related condition in daily practice.
The clinical picture of vaginismus is similar to that observed in phobic states. In usual, somatic components of anxiety accompany with other symptoms of vaginismus. Phobic states, anxiety and related clinical conditions are related to the hypothalamic-pituitary-adrenal axis (HPA). In these conditions, hypothalamic corticotropin-releasing hormone production increases, raising the pituitary release of adrenocorticotropin hormone (ACTH). This increase in ACTH levels leads to increased cortisol release by the adrenal cortex. These hormonal changes can affect many anxiety related symptoms associated with the autonomous nervous system. In this context, in our unpublished study, we performed an investigation to examine QT wave (QTd) and P wave (Pd) dispersions, which are associated with dysfunctioned autonomous nervous system, in the electrocardiogram of patients with vaginismus and healthy control subjects. We found that the mean Pmin value of the patients with vaginismus was statistically significantly lower than that of the healthy controls whereas the mean Pd value of patients with vaginismus was considerably higher than that of healthy control subjects, and that the mean QTmax value of the patients was statistically significantly higher than that of the healthy control subjects, in addition to the finding that the mean QTd value of the patients with vaginismus was considerably higher than that of healthy control subjects, considering that vaginismus patients might be vulnerable to the cardiac problems which are related to cardiac rhythm. This previous ECG study provides another support for the association between anxiety, autonomous system dysfunction and vaginismus.
The hippocampus and amygdala are important limbic and paralimbic brain areas linked to emotional regulation[3,4], with the involement of the hippocampus in learning and verbal memory[5] and amygdala in emotional perception, possibly determining emotional and social behaviors[6,7]. On the other hand, there is an important association between hippocampal region and anxiety. First of all, the hippocampus is a glucocorticoid feedback area. Therefore, it is highly sensitive to endogenous glucocorticoid levels and is an important region affected by stress modulation which is controlled by the HPA[8-10]. In this context, although the hippocampus and amygdala are important regions in anxiety modulation, no study has examined their imaging to date. In this study, we compared hippocampus and amygdala volumes between patients with vaginismus and healthy control subjects.
Ten consecutive female patients meeting DSM-IV criteria for vaginismus and seeking treatment at the Firat University School of Medicine, Department of Psychiatry, Elazig, Turkey were included in the present study. The local ethics committee approved this investigation, according to international guidelines on human research. Written informed consent was obtained from each patient. The mean age of the patients were 27.90 ± 7.25 years. Patients in the present study were those of another unpublished study in which we evaluated P and QT wave dispersions. Diagnoses were obtained by using the Structured Clinical Interview for DSM-IV Disorders-Patient Version[11,12]. In regard to comorbidity, of our patients, one had social anxiety disorder and one had depressive disorder. No other comorbidity was determined. As mentioned in our unpublished study, patients with vaginismus were on a sexual therapy program performed by a senior psychiatry assisstant. All patients and control subjects underwent a detailed physical and neurological examination and clinical assessment to exclude any neurological or comorbid conditions. The healthy control group was composed of also ten females, with a mean age of 27.40 ± 5.38 years. Exclusion criteria included: (1) a history of head trauma; (2) current or lifetime severe medical problems; (3) any current or lifetime neurologic problems; (4) the existence of mental retardation; (5) any problem that prevented them to undergo neuroimaging, particularly existence of cardiac stent; (6) and alcohol/substance abuse within the 6 mo preceding the study. Healthy control subjects were excluded if the following criteria were met: Existence of any DSM-IV axis I disorder, having any first-degree relative with a history of psychiatric disorder, a history of head trauma or seizure, existence of any current or lifetime major medical and neurologic illness, any problem that prevented them to undergo neuroimaging, particularly existence of cardiac stent.
All subjects underwent magnetic resonance imaging (MRI) scans at rest. All in vivo data were collected on a 1.5 T General Electric scanner. In brief, the parameters used for this study were: TR = 2000 ms, TE = 15.6 ms, TI = 700 ms, FOV = 240 mm, echo SPACING = 15.6 ms, 8 echoes, RESOLUTION = 0.9375 mm × 0.9375 mm × 1.328 mm, 128 contiguous slices, 8 min 36 s. The tracing and measurements were done by two neuroradiologists (HY, UA). They were blinded to identity and diagnoses of the subjects. We calculated the intra-class correlation coefficients to be 0.90 for the hippocampus and 0.90 for the amygdala.
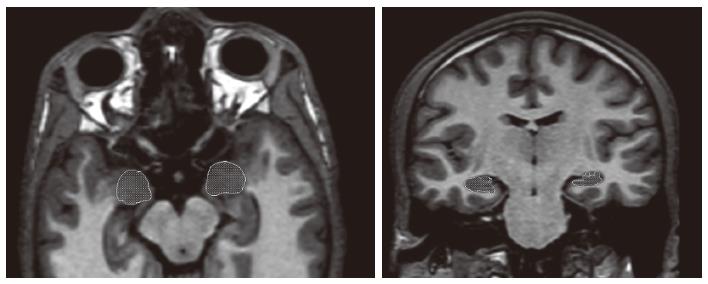
To do manual tracing for anatomical regions, anatomic atlases were used[13-15]. On the other hand, tracings were adapted from Caetano et al[16] and Brambilla et al[17]. When tracing the hippocampus, the process was started on the coronal slice at the line that the superior colliculus was completely connected with the thalamus and was finished one slice before the mamillary bodies appeared. The superior boundary was described as the corona radiata and ambient, and the inferior border was selected as the white matter. The process was finished by tracig of lateral border which was the inferior horn of the lateral ventricle. When tracing the amygdala, the process was started at the point that the mamillary body can be observed. Temporal lobe white matter was accepted as the superior and lateral limits, and the white matter of the parahippocampal gyrus was accepted as the inferior border. The anterior border was described as the limit that the amygdala could not be observed as well. The delimitation of the hippocampus and amygdala is presented in Figure 1.
Statistical analyses were performed using Statistical Package for Social Sciences (SPSS version 16.0, Chicago, Illinois). Independent t test was used to compare volume differences in the left and right hippocampus and amygdala regions in addition to continous variables such as age. For the comparison of categorical variables, χ2 test was performed. In addition, analysis of covariance (ANCOVA) was used, controlling for age. Statistical significance was accepted at P < 0.05.
As given in Table 1, we did not determine any significant differences between patients with vaginismus and healthy control subjects in regard to sex distribution (both groups were composed of female subjects), age, and educational level (P > 0.05).
With regard to amygdala volumes, we found that the mean right amygdala volume was smaller in patients with vaginismus than in the healthy controls (left amygdala: 1799.04 mm3± 289.95 mm3vs 2006.78 mm3± 425.39, P > 0.05; right amygdala: 1816.57 mm3± 271.73 mm3vs 2055.64 mm3± 284.95 mm3, P = 0.07). When controlled for age, ANCOVA demonstrated that the statistically significant difference continued for the right amygdala (P < 0.05).
With regard to hippocampus volumes, we found that the mean left and right hippocampus volumes were smaller in patients with vaginismus than in the healthy controls (left hippocampus: 2505.22 mm3± 223.03 mm3vs 2908.79 mm3± 300.04 mm3, P < 0.05; right hippocampus: 2501.30 mm ± 463.81 mm3vs 2907.61 mm3± 247.34 mm3, P < 0.05). When controlled for age, ANCOVA showed that the statistically significant difference lasted (P < 0.05 for both the left and right hippocampus).
No significant correlation was found between the duration of illness and age, and the volumes of both sides of the hippocampus and amygdala (P > 0.05).
To our knowledge, this is the first study to examine the amygdala and hippocampus volumes in patients with vaginismus, and our results provide the first MRI evidence of reduced amygdala and hippocampus volumes in patients with this disorder. The volumes of both sides of the hippocampus and the right amygdala were significantly smaller in patients with vaginismus than in healthy control subjects. Of particular note is the fact that these findings were obtained in a sample of patients with “pure” vaginismus, with one patient having social anxiety disorder and one having depressive disorder, without any other past or current major psychiatric comorbidity. On the other hand, another important feature of the present investigation was one-to-one matching between the patients and healthy control groups in regard to gender, since all subjects were females, which minimized possible confounding factors such as gender. In addition, by using ANCOVA controlling for age, we eliminated the effects of age. After this control, statistically significant differences continued for both amygdala and hippocampus regions. In fact, since there have been no previous study which evaluated the volumes of any brain region beyond the hippocampus and amygdala, we do not compare our results with those of others. However, our study grıup previously examined the volumes of the hippocampus and amygdala in patients with somatization disorder which is a psychiatric disorder strongly related to both the troubles in the regulation of emotion and copying stress. In that study, we revealed that somatization disordered patients had significantly smaller mean volumes of the left and right amygdala without any differences in regard to whole brain, total gray and white matter or hippocampus volume[18]. In addition, volumes of the hippocampus and amygdala were measured in patients with refractory obsessive-compulsive disorder (OCD)[19]. In that study we found that the mean left and right hippocampus and amygdala volumes of the patients were smaller than those of the healthy controls. Although OCD severity was not correlated with the volume of the left hippocampus, a correlation was noted between the duration of illness and the volumes of both sides of the hippocampus and the left amygdala. In that study, we commented that hippocampus and amygdala abnormalities could be considered in the occurrence of refractoriness to OCD. As can be seen in these investigations, it seems that hippocampus and amygdala structural abnormalities could be related to anxiety itself. However, it is important to perform functional neuroimaging of these regions to show functional relationship between anxiety and these regions. The hippocampus is also a glucocorticoid feedback area. Therefore, it is highly sensitive to endogenous glucocorticoid levels and is an important region affected by stress modulation which is controlled by the HPA[8-10]. In other words, there has been an important relationship between the hippocampus and anxiety. In this context, our findings revealing volumetric changes in the hippocampus and amygdala may suggest an important etiopathogenetic basis for the occurrence of vaginismus which is a fear-related clinical condition. However, these findings should be confirmed by further investigations.
It should be mentioned that our study had several limitations. First of all, although comorbid categorical diagnosis of major psychiatric conditions and substance abuse were part of the exclusion criteria, sub-categorical level of depressive or anxiety symptoms may have influenced our findings. Second, our study group was small. However, it is difficult to find vaginismus patients in such outpatient clinics which is not special for sexual function disorders. Third, we evaluated only hippocampus and amygdala regions which are associated with anxiety and fear. However, we did not examine other brain regions that can be associated with anxiety and fear. Fourth, the cross-sectional design of this study limits the interpretation of our findings. For this reason, clearer conclusions may be made about the role of the hippocampus and amygdala in the pathogenesis of vaginismus through a longitudinal design in which patients are scanned several times. Fifth, we should mention that the technique used in the present study should be compared with other possible alternative methods such as computational morphometry and multivariate approaches.
Consequently, our present findings suggest that there have been hippocampus and amygdala structural abnormalities in patients with vaginismus. These changes provide the notion that vaginismus may be a fear-related condition. Without replication studies, the possibility that findings are due to random chance and idiosyncrasies of small samples cannot be ruled out. Thus, we think that it is important to conceptualize these studies as exploratory, and place an emphasis on replication with larger sample size.
We would like to thank FUBAP for its support.
The hippocampus and amygdala are important limbic and paralimbic brain areas linked to emotional regulation. On the other hand, there is an important association between hippocampal region and anxiety.
The hippocampus is highly sensitive to endogenous glucocorticoid levels and is an important region affected by stress modulation which is controlled by the hypothalamo-pituitary-adrenal axis. In this context, although hippocampus and amygdala are important regions in anxiety modulation, no study has examined their imaging to date. In this study, the authors compared hippocampus and amygdala volumes between patients with vaginismus and healthy control subjects.
The authors for the first time found that the mean right amygdala volumes were smaller in patients with vaginismus than in healthy controls. With regard to hippocampus volumes, they also found that the mean left and right hippocampus volumes were smaller in patients with vaginismus than in healthy controls.
In vaginismus, structural brain alterations compared to healthy subjects might occur.
This is a good cross-sectional study in which the authors examined the volumes of the hippocampus and amygdala of patients with vaginismus.
P- Reviewer: Michetti PM, Tang FR S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Washington, DC: American Psychiatric Association 2000; . |
| 2. | Bystritsky A, Craske M, Maidenberg E, Vapnik T, Shapiro D. Autonomic reactivity of panic patients during a CO2 inhalation procedure. Depress Anxiety. 2000;11:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Soares JC, Mann JJ. The anatomy of mood disorders--review of structural neuroimaging studies. Biol Psychiatry. 1997;41:86-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 535] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 4. | Strakowski SM, Adler CM, DelBello MP. Volumetric MRI studies of mood disorders: do they distinguish unipolar and bipolar disorder? Bipolar Disord. 2002;4:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Reiman EM. The application of positron emission tomography to the study of normal and pathologic emotions. J Clin Psychiatry. 1997;58 Suppl 16:4-12. [PubMed] |
| 6. | Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 809] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 7. | Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 357] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908-3913. [PubMed] |
| 9. | Höschl C, Hajek T. Hippocampal damage mediated by corticosteroids--a neuropsychiatric research challenge. Eur Arch Psychiatry Clin Neurosci. 2001;251 Suppl 2:II81-II88. [PubMed] |
| 10. | Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119:887-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 259] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Çorapçıoğlu A, Aydemir Ö, Yıldız M, Esen A, Köroğlu E. DSM-IV Eksen I Bozuklukları (SCID-I) için yapılandırılmış klinik görüşme, klinik versiyon. Ankara: Hekimler Yayın Birliği 1999; . |
| 12. | First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (February 1996 Final), SCID-I/P: Biometrics Research Department, New York State Psychiatric Institute, 1998. |
| 13. | Duvernoy HM, Bourgouin P. The human brain: surface, three-dimensional sectional anatomy with MRI, and blood supply. New York: Springer 1999; . [DOI] [Full Text] |
| 14. | Bertolino A, Nawroz S, Mattay VS, Barnett AS, Duyn JH, Moonen CT, Frank JA, Tedeschi G, Weinberger DR. Regionally specific pattern of neurochemical pathology in schizophrenia as assessed by multislice proton magnetic resonance spectroscopic imaging. Am J Psychiatry. 1996;153:1554-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 180] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. New York: Thieme Medical Publishers 1996; . |
| 16. | Caetano SC, Hatch JP, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res. 2004;132:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. MRI investigation of temporal lobe structures in bipolar patients. J Psychiatr Res. 2003;37:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Atmaca M, Sirlier B, Yildirim H, Kayali A. Hippocampus and amygdalar volumes in patients with somatization disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1699-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Atmaca M, Yildirim H, Ozdemir H, Ozler S, Kara B, Ozler Z, Kanmaz E, Mermi O, Tezcan E. Hippocampus and amygdalar volumes in patients with refractory obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1283-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |