Published online Jun 22, 2016. doi: 10.5498/wjp.v6.i2.208
Peer-review started: March 1, 2016
First decision: March 22, 2016
Revised: April 24, 2016
Accepted: May 10, 2016
Article in press: May 11, 2016
Published online: June 22, 2016
Processing time: 110 Days and 19.2 Hours
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder presenting as progressive cognitive decline with dementia that does not, to this day, benefit from any disease-modifying drug. Multiple etiologic pathways have been explored and demonstrate promising solutions. For example, iron ion chelators, such as deferoxamine, are a potential therapeutic solution around which future studies are being directed. Another promising domain is related to thrombin inhibitors. In this minireview, a common pathophysiological pathway is suggested for the pathogenesis of AD to prove that all these mechanisms converge onto the same cascade of neuroinflammatory events. This common pathway is initiated by the presence of vascular risk factors that induce brain tissue hypoxia, which leads to endothelial cell activation. However, the ensuing hypoxia stimulates the production and release of reactive oxygen species and pro-inflammatory proteins. Furthermore, the endothelial activation may become excessive and dysfunctional in predisposed individuals, leading to thrombin activation and iron ion decompartmentalization. The oxidative stress that results from these modifications in the neurovascular unit will eventually lead to neuronal and glial cell death, ultimately leading to the development of AD. Hence, future research in this field should focus on conducting trials with combinations of potentially efficient treatments, such as the combination of intranasal deferoxamine and direct thrombin inhibitors.
Core tip: Patients with Alzheimer’s disease (AD) have not benefited from any disease-modifying drug until now. Multiple etiologic pathways have been explored and suggest promising solutions in the future. The iron chelator deferoxamine is one potential therapeutic solution around which future studies are being directed. Another potential therapeutic solution is related to thrombin inhibitors. In this minireview, a common pathophysiological pathway is suggested for the pathogenesis of AD that is initiated by the presence of vascular risk factors inducing brain tissue hypoxia and endothelial cell activation. In predisposed individuals, this can lead to thrombin activation and iron decompartmentalization. The resulting oxidative stress will eventually lead to neuronal and glial cell death.
- Citation: Bou Khalil R, Khoury E, Koussa S. Linking multiple pathogenic pathways in Alzheimer’s disease. World J Psychiatr 2016; 6(2): 208-214
- URL: https://www.wjgnet.com/2220-3206/full/v6/i2/208.htm
- DOI: https://dx.doi.org/10.5498/wjp.v6.i2.208
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder presenting as progressive cognitive decline with dementia[1-3]. In spite of considerable research, proven disease-modifying drugs are still lacking[1-3]. Currently, all phase 3 drug trials have failed to produce the desired results[1-3]. An increasing amount of literature now supports the vascular-neuronal axis hypothesis in the pathogenesis of AD owing to common risk factors for both AD and cardiovascular conditions[4]. However, it has also become widely established that disturbance in cerebral iron homeostasis also participates to the genesis of AD[5]. In this review, we will attempt to link these multiple physiopathological pathways to prove that these mechanisms converge onto the same cascade of neuroinflammatory events.
Diagnostic criteria categorize dementias into vascular dementias or AD dementias even though mixed forms are frequently encountered[6]. In fact, as presented in a recent review by Hooijmans et al[7], there is an increasing amount of literature supporting the fact that cardiovascular risk factors (i.e., diabetes, hypertension, dyslipidemias) are predisposing factors for vascular and AD dementias, and because the clinical manifestations and pathological findings are also common, the two conditions should not be seen as separate. Accordingly, there seems to be a direct increase in the risk for dementia, including AD, in the presence of these cardiovascular risk factors[8-13]. Hypertension and atherosclerotic disease both bring about vascular changes, causing alterations in the blood brain barrier and cerebral ischemia. Ultimately, this will initiate the pathological process of AD[7]. Indeed, there is in vitro and pathology evidence concerning chronic localized brain ischemia as playing a crucial role in the genesis and progression of AD. The ensuing hypoxia stimulates the production and release of reactive oxygen species and pro-inflammatory proteins[14]. There also appears to be data concerning vascular risk factors and cardiovascular diseases as potentially being able to quicken Aβ 40-42 production, aggregation, and precipitation[6]. On another level, there is also evidence that endothelial dysfunction, due to cerebrovascular risk factors that include diabetes and hypoxia, precedes cognitive decline in AD and might contribute to its pathogenesis through the activation of thrombin[15].
The hypoxia inducible factor 1 (HIF-1) is a sensor for hypoxia, and its levels are increased in the cerebral circulation in both mouse and human models of AD[14]. However, an increasing amount of literature is in favor of an elevation in pro-inflammatory substances in the endothelium of the cerebral microcirculation in oxygen-deficient conditions[14]. Together, those results suggest a relationship between hypoxia and inflammation in the brain. Another phenomenon that occurs in response to hypoxia is the secretion of a strong inducer of angiogenesis known as vascular endothelial growth factor (VEGF). In AD, however, the vascular response to VEGF is deficient, resulting in an increased production of pro-inflammatory and neurotoxic substances[14].
In conclusion, recent studies are in favor of targeting vascular risk factors via lifestyle adjustments (physical exercise, dietary modification and abstinence of smoking) and medications (namely, cholesterol-lowering drugs) to preserve cognitive functions in the aging population and to reduce the progression toward AD. This seems to occur through the reduction of chronic focal ischemia and hypoxia in the brain, which are both harbingers of cerebral inflammation and oxidative stress[7,16].
Thrombin has been demonstrated to be a key regulator of the pro-inflammatory reaction of cerebral endothelial cells in response to ischemic changes[13]. Moreover, prothrombin and thrombin are widely expressed in neurons and particularly in neurofibrillary tangles and senile plaques. This hypothesizes that thrombin could have a role in tau degradation and that a deficiency in this process could cause tau protein accumulation[17,18]. In an animal model on rats, direct intra-cerebral injection of thrombin caused in neuronal death and subsequent cognitive impairment[19]. Likewise, it was shown that thrombin can directly exert neurotoxic effects[20]. Thus, introduction of heparin, an indirect thrombin inhibitor, in the arsenal of AD medications hoped it could enhance the brain’s micro-vasculature via its antithrombotic properties[21]. Accordingly, rats of advanced age displayed a partial but significant improvement of behavioral problems after heparin injection[22]. Moreover, an animal experiment demonstrate protective actions of heparin after injection of amyloid peptide into the amygdala[23]. Furthermore, due to the potential side effects of long-term treatment with heparin, it has been suggested that a more specific treatment that directly inhibits thrombin would be more appropriate in terms of safety and efficacy. Indeed, direct thrombin inhibitors, such as dabigatran, would be a better choice because of their high selectivity in the inhibition of thrombin activity, thus ensuring a better side effect profile than an indirect inhibitor, such as heparin[15,24]. Dabigatran is a competitive reversible non-peptide antagonist of thrombin. Thrombin has many functions: Fibrinogen transformation into fibrin, fibrin strengthening and cross-linking, stimulation of additional thrombin production, platelet activation, and stimulation of protein C, which increases pro-thrombotic activity. Dabigatran inhibits most of these steps[25].
Iron is crucial for the metabolic demands and functions of numerous cells, but when dysregulated, iron can become potentially harmful for these same cells. Iron circulates with the action of its transporter transferrin, which binds iron released in the blood from two sources: Enterocytes (following absorption) and the reticulo-endoplasmic cells. The iron-transferrin complex binds to the transferrin-receptor-1 so that it can be internalized within cells. Iron then enters mitochondria, where it takes part in heme synthesis. Superfluous iron is stowed and detoxified in ferritin[26].
In the central nervous system, iron, a key component for many proteins essential for brain metabolism, is predominantly concentrated in the motor system, and more specific, within glial cells[27,28]. Evidence supports the fact that iron deposition might exert a neurotoxic effect. For example, intracerebral hemorrhage causes extraversion of red blood cells (RBCs) into the parenchyma followed by hemolysis and decompartmentalization of iron, which later promotes long-term neurological deficits and brain atrophy[29-31]. In humans, iron deposition in the endothelium and vascular media is strongly associated with the progression of atherosclerotic lesions[32]. Indeed, atherosclerotic plaques with subsequent neovascularization and intra-plaque hemorrhage may constitute an important source of iron deposition in blood vessels and the brain parenchyma, but in a more localized fashion than what is observed after an intracerebral hemorrhage. Furthermore, in atherosclerotic plaques, cholesterol crystals coincide with glycophorin A (distinctive protein of red cell membranes), implying cholesterol from erythrocyte membranes could participate in the deposition of lipids and expansion of the lipid core following intra-plaque bleeding[33]. Moreover, some data support the fact that hypercholesterolemia might enhance the crossing of iron into the brain parenchyma via an augmentation of endothelial permeability to iron[34]. Hence, the potential efficacy of cholesterol-lowering agents (i.e., statins) in slowing the progression towards AD might be related to their indirect neuroprotective actions of preventing iron-induced neurotoxicity[34-36].
During an intra-plaque hemorrhage, red cells penetrate the oxidative environment of atherosclerotic lesions containing cytotoxic products of lipid peroxidation that can trigger the lysis of RBCs[37]. Hemoglobin released outside RBCs is oxidized and will release high-valence iron compounds with potent oxidative and inflammatory activities[38]. These activities can increase vascular endothelial activation and subsequent thrombin release. However, the known in vitro effect of iron ions on thrombin activity is in favor of the inhibition of its clot-forming effect[39].
In 1991, the first study evaluating the effect of deferoxamine (DFO) in patients suffering from AD was published[40]. DFO was evaluated because of its aluminum chelating properties and because of the evidence linking this metal ion to AD[40]. The study concluded that DFO may slow the progression of AD[40]. However, since then, additional evidence has linked the other metal ion chelated by DFO, iron, with the pathogenesis of AD. In patients with AD, iron accumulation in the cerebral cortex and hippocampus co-localizes with neurofibrillary tangles and senile plaques[41]. In their review, Peters et al[42] hypothesized that amyloid production is actually amplified to compensate for excessive iron levels and “patch” the subsequent vascular damage. High neuronal levels of iron stimulate amyloid protein precursor translation, and along with concomitant abnormal secretase activity, increase extracellular Aβ-42 deposition and tau protein phosphorylation. Peters et al[42] concluded the finding that increased iron deposition increases amyloid production emphasizes the importance of iron management in the treatment of AD.
The ability of iron to interact with oxygen is crucial for cell functioning, but it is also a source of free radicals according to Fenton’s reaction: Fe+n + H2O → Fe +(n-1) + OH- (hydroxyl radicals).
Reactive oxygen species that are formed through this reaction subsequently damage intracellular structures via lipid peroxidation, or induction of DNA mutations[43]. In a 2015 review on vascular dysfunction in AD, Di Marco et al[14] fact found that high levels of lipid peroxidation and DNA oxidation were a frequent observation in AD. Furthermore, they posited that the blood-brain barrier is a central player in oxidative-stress-related tissue injury, in that it is both a source and a target of reactive oxygen species and pro-inflammatory substances. This hypothesis was based on the observation that Aβ plaques contain redox-active metals and that Aβ deposits preferentially locate in perivascular spaces[14].
β-amyloid in itself is a substrate for hydroxyl radicals[44]. Studies utilizing magnetic resonance imaging have found a positive correlation between aging and iron deposition in the brain that renders the brain more vulnerable to iron-mediated oxidative stress[45,46]. Accordingly, iron can attach to phosphorylated tau-proteins and lead to its aggregation, which causes the creation of neurofibrillary tangles[47]. Furthermore, it has been found that intranasal DFO inhibits tau phosphorylation in the brain of transgenic mice with AD[48]. It has also been demonstrated that the potential progression of senile plaques through the affinity of iron ions to β-amyloid and its precursor (i.e., amyloid protein precursor) might be prevented in transgenic mice via administration of intranasal DFO[49].
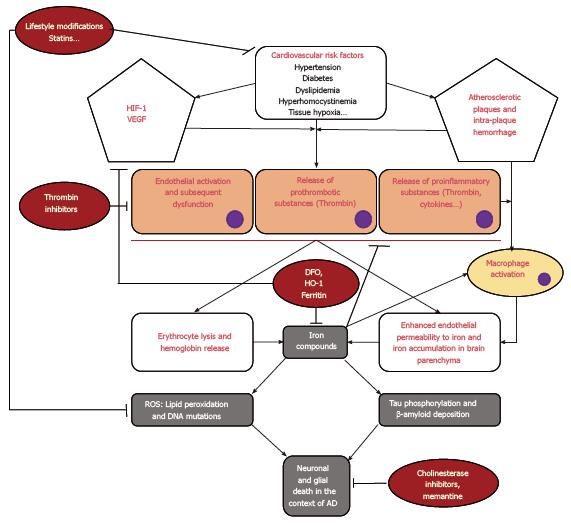
AD has been considered to be caused by a multitude of neuropathogenic pathways that all eventually lead to neuronal death and cognitive impairment. However, in this review, we have demonstrated in a theoretical manner that these multiple pathophysiological pathways (namely the endothelial vascular activation through thrombin activation and the neurotoxic effect of redox species through iron ion decompartmentalization) are actually interlinked (for a schematic representation of these pathophysiological pathways see Figure 1). As a matter of fact, AD might be considered a disease of the neurovascular unit that seems to be triggered by vascular risk factors that affect the endothelium of small vessels and capillaries inside the brain. Vascular risk factors may induce atherosclerotic changes at the bifurcation of vessels, which would lead to tissue hypoxia in the brain parenchyma irrigated by the corresponding vessel. The endothelial cells lining the arterioles and capillaries in the hypoxic brain region are normally highly sensitive to hypoxic changes in the surrounding brain parenchyma and usually secrete substances that include VEGF and HIF-1 to promote neovascularization. When resources for neutralizing the oxidative stress provoked by hypoxia, such as in the elderly brain, are out-weighted by the amount and duration of oxidation, these normal responses become deleterious. A deficient vascular response to tissue hypoxia may promote intra-plaque hemorrhages. Deficient neovascularization and intra-plaque hemorrhages may be the cause of erythrocyte lysis, iron ion deposition in the brain parenchyma due to increased endothelial permeability and thrombin secretion and activation. Erythrocyte lysis liberates more iron ions that will further increase the oxidative stress. Moreover, thrombin activation may increase tissue hypoxia through increased clot formation and vasoconstriction that will also lead to increased oxidative stress. After exposure to oxidative stress compounds, many neuronal and glial cell modifications will ensue, such as lipid peroxidation, DNA mutations, cytoskeleton breakdown, etc. In addition, iron ions may also be responsible for the deposition of β-amyloid plaques and neurofibrillary tangles because it is implicated in enhancing amyloid protein metabolism and tau protein phosphorylation and aggregation. Accordingly, current research in AD therapeutics is focusing on one of the multiple branches of these interlinked pathophysiological pathways. However, a lack of a more global perspective may explain why no disease-modifying treatment has been discovered until now. For example, treatment with DFO may be an interesting solution for iron ion decompartmentalization but this treatment does not take into consideration the positive role that iron may play in the hypoxic tissue by reducing thrombin activation and subsequent clot formation, thus avoiding further hypoxia. Moreover, it becomes more obvious when looking at these interlinked pathways that no one molecule with a single mechanism of action can easily attenuate all the deleterious effects initiated by hypoxia secondary to vascular risk factors. Accordingly, we suggest that future research in this field should focus on testing combinations of potentially efficient treatments, such as the combination of intranasal DFO and direct thrombin inhibitors.
In this minireview, a common physiopathological pathway has been suggested for the pathogenesis of AD. This pathway is initiated by the presence of vascular risk factors that induce brain tissue hypoxia and subsequent endothelial cell activation. The endothelial activation may become dysfunctional in predisposed individuals, leading to thrombin activation and iron ion decompartmentalization. The oxidative stress that results from these modifications in the neurovascular unit will eventually lead to neuronal and glial cell death.
P- Reviewer: Chang NS, Dunbar GL, Das UN, Hortobagyi T, Perry G, Zhang Y S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Castellani RJ, Perry G. Pathogenesis and disease-modifying therapy in Alzheimer’s disease: the flat line of progress. Arch Med Res. 2012;43:694-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Frautschy SA, Cole GM. Why pleiotropic interventions are needed for Alzheimer’s disease. Mol Neurobiol. 2010;41:392-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1109] [Cited by in RCA: 1164] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 4. | Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation. 2011;8:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 316] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 5. | Bandyopadhyay S, Rogers JT. Alzheimer’s disease therapeutics targeted to the control of amyloid precursor protein translation: maintenance of brain iron homeostasis. Biochem Pharmacol. 2014;88:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Honjo K, Black SE, Verhoeff NP. Alzheimer’s disease, cerebrovascular disease, and the β-amyloid cascade. Can J Neurol Sci. 2012;39:712-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Hooijmans CR, Kiliaan AJ. Fatty acids, lipid metabolism and Alzheimer pathology. Eur J Pharmacol. 2008;585:176-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Coker LH, Sidney S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 9. | Nishtala A, Preis SR, Beiser A, Devine S, Hankee L, Seshadri S, Wolf PA, Au R. Midlife cardiovascular risk impacts executive function: Framingham offspring study. Alzheimer Dis Assoc Disord. 2014;28:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | de la Torre JC. Cerebral hemodynamics and vascular risk factors: setting the stage for Alzheimer’s disease. J Alzheimers Dis. 2012;32:553-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 11. | Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4:640-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 340] [Cited by in RCA: 485] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 12. | Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 683] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 13. | Tripathy D, Sanchez A, Yin X, Luo J, Martinez J, Grammas P. Thrombin, a mediator of cerebrovascular inflammation in AD and hypoxia. Front Aging Neurosci. 2013;5:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Di Marco LY, Venneri A, Farkas E, Evans PC, Marzo A, Frangi AF. Vascular dysfunction in the pathogenesis of Alzheimer’s disease--A review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol Dis. 2015;82:593-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 15. | Grammas P, Martinez JM. Targeting thrombin: an inflammatory neurotoxin in Alzheimer’s disease. J Alzheimers Dis. 2014;42 Suppl 4:S537-S544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Sato N, Morishita R. The roles of lipid and glucose metabolism in modulation of β-amyloid, tau, and neurodegeneration in the pathogenesis of Alzheimer disease. Front Aging Neurosci. 2015;7:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | Arai T, Miklossy J, Klegeris A, Guo JP, McGeer PL. Thrombin and prothrombin are expressed by neurons and glial cells and accumulate in neurofibrillary tangles in Alzheimer disease brain. J Neuropathol Exp Neurol. 2006;65:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Reimann-Philipp U, Ovase R, Weigel PH, Grammas P. Mechanisms of cell death in primary cortical neurons and PC12 cells. J Neurosci Res. 2001;64:654-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Mhatre M, Nguyen A, Kashani S, Pham T, Adesina A, Grammas P. Thrombin, a mediator of neurotoxicity and memory impairment. Neurobiol Aging. 2004;25:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Park KW, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons: role of neuronal NADPH oxidase. J Neurosci Res. 2008;86:1053-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Santini V. A general practice trial of Ateroid 200 in 8,776 patients with chronic senile cerebral insufficiency. Mod Probl Pharmacopsychiatry. 1989;23:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Lorens SA, Guschwan M, Hata N, van de Kar LD, Walenga JM, Fareed J. Behavioral, endocrine, and neurochemical effects of sulfomucopolysaccharide treatment in the aged Fischer 344 male rat. Semin Thromb Hemost. 1991;17 Suppl 2:164-173. [PubMed] |
| 23. | Dudas B, Cornelli U, Lee JM, Hejna MJ, Walzer M, Lorens SA, Mervis RF, Fareed J, Hanin I. Oral and subcutaneous administration of the glycosaminoglycan C3 attenuates Abeta(25-35)-induced abnormal tau protein immunoreactivity in rat brain. Neurobiol Aging. 2002;23:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Bou Khalil R. Direct thrombin inhibitors’ potential efficacy in Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2012;27:564-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64:292-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 658] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 26. | Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J. 2011;434:365-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 695] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 27. | Connor JR, Snyder BS, Beard JL, Fine RE, Mufson EJ. Regional distribution of iron and iron-regulatory proteins in the brain in aging and Alzheimer’s disease. J Neurosci Res. 1992;31:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 292] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Morris CM, Candy JM, Oakley AE, Bloxham CA, Edwardson JA. Histochemical distribution of non-haem iron in the human brain. Acta Anat (Basel). 1992;144:235-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 166] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, Xi G. Long-term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg. 2006;104:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 416] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 31. | Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1182] [Cited by in RCA: 1152] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 32. | Ferrari M, Werner GS, Rieber J, Richartz BM, Sigusch HH, Brandstädt A, Mudra H, Figulla HR. No influence of hemochromatosis-related gene mutations on restenosis rate in a retrospective study of 137 patients after coronary stent implantation. Int J Cardiovasc Intervent. 2001;4:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Kolodgie FD, Burke AP, Nakazawa G, Cheng Q, Xu X, Virmani R. Free cholesterol in atherosclerotic plaques: where does it come from? Curr Opin Lipidol. 2007;18:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Ong WY, Halliwell B. Iron, atherosclerosis, and neurodegeneration: a key role for cholesterol in promoting iron-dependent oxidative damage? Ann N Y Acad Sci. 2004;1012:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 36. | Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 970] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 37. | Nagy E, Eaton JW, Jeney V, Soares MP, Varga Z, Galajda Z, Szentmiklósi J, Méhes G, Csonka T, Smith A. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler Thromb Vasc Biol. 2010;30:1347-1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 38. | Jeney V, Balla G, Balla J. Red blood cell, hemoglobin and heme in the progression of atherosclerosis. Front Physiol. 2014;5:379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Azizova OA, Shvachko AG, Aseichev AV. Effect of iron ions on functional activity of thrombin. Bull Exp Biol Med. 2009;148:776-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Crapper McLachlan DR, Dalton AJ, Kruck TP, Bell MY, Smith WL, Kalow W, Andrews DF. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet. 1991;337:1304-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 538] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 41. | Connor JR, Milward EA, Moalem S, Sampietro M, Boyer P, Percy ME, Vergani C, Scott RJ, Chorney M. Is hemochromatosis a risk factor for Alzheimer’s disease? J Alzheimers Dis. 2001;3:471-477. [PubMed] |
| 42. | Peters DG, Connor JR, Meadowcroft MD. The relationship between iron dyshomeostasis and amyloidogenesis in Alzheimer’s disease: Two sides of the same coin. Neurobiol Dis. 2015;81:49-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 43. | Finefrock AE, Bush AI, Doraiswamy PM. Current status of metals as therapeutic targets in Alzheimer’s disease. J Am Geriatr Soc. 2003;51:1143-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Rogers JT, Randall JD, Cahill CM, Eder PS, Huang X, Gunshin H, Leiter L, McPhee J, Sarang SS, Utsuki T. An iron-responsive element type II in the 5’-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J Biol Chem. 2002;277:45518-45528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 399] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 45. | Martin WR, Ye FQ, Allen PS. Increasing striatal iron content associated with normal aging. Mov Disord. 1998;13:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Bartzokis G, Beckson M, Hance DB, Marx P, Foster JA, Marder SR. MR evaluation of age-related increase of brain iron in young adult and older normal males. Magn Reson Imaging. 1997;15:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci USA. 1997;94:9866-9868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 937] [Cited by in RCA: 987] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 48. | Guo C, Wang P, Zhong ML, Wang T, Huang XS, Li JY, Wang ZY. Deferoxamine inhibits iron induced hippocampal tau phosphorylation in the Alzheimer transgenic mouse brain. Neurochem Int. 2013;62:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 49. | Guo C, Wang T, Zheng W, Shan ZY, Teng WP, Wang ZY. Intranasal deferoxamine reverses iron-induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34:562-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |