Peer-review started: September 8, 2015
First decision: October 16, 2015
Revised: October 23, 2015
Accepted: December 18, 2015
Article in press: December 21, 2015
Published online: March 22, 2016
Processing time: 196 Days and 14.4 Hours
Brain-derived neurotrophic factor (BDNF), which regulates neuronal survival, growth differentiation, and synapse formation, is known to be associated with depression and post-traumatic stress disorder (PTSD). However, the molecular mechanism for those mental disorders remains unknown. Studies have shown that BDNF is associated with PTSD risk and exaggerated startle reaction (a major arousal manifestation of PTSD) in United States military service members who were deployed during the wars in Iraq and Afghanistan. The frequency of the Met/Met in BDNF gene was greater among those with PTSD than those without PTSD. Among individuals who experienced fewer lifetime stressful events, the Met carriers have significantly higher total and startle scores on the PTSD Checklist than the Val/Val carriers. In addition, subjects with PTSD showed higher levels of BDNF in their peripheral blood plasma than the non-probable-PTSD controls. Increased BDNF levels and startle response were observed in both blood plasma and brain hippocampus by inescapable tail shock in rats. In this paper, we reviewed these data to discuss BDNF as a potential biomarker for PTSD risk and its possible roles in the onset of PTSD.
Core tip: Brain-derived neurotrophic factor (BDNF), which regulates neuronal survival, growth differentiation, and synapse formation, is known to be associated with depression and post-traumatic stress disorder (PTSD). However, the molecular mechanism for those mental disorders remains unknown. In this paper, we reviewed these data to discuss BDNF as a potential biomarker for PTSD risk and its possible roles in the onset of PTSD.
- Citation: Zhang L, Li XX, Hu XZ. Post-traumatic stress disorder risk and brain-derived neurotrophic factor Val66Met. World J Psychiatr 2016; 6(1): 1-6
- URL: https://www.wjgnet.com/2220-3206/full/v6/i1/1.htm
- DOI: https://dx.doi.org/10.5498/wjp.v6.i1.1
Brain-derived neurotrophic factor (BDNF), first discovered in the early 1980s, is considered a member of the nerve growth factor family of neurotrophins[1], which have important roles in the development, physiology, and pathology of mental disorders[2,3]. BDNF is expressed in a number of tissues and cell types, including the brain and blood[4]. In recent years, BDNF has been implicated in a number of psychiatric disorders, such as depression, anxiety, eating disorders, and posttraumatic stress disorder (PTSD) (Table 1). In this review, we will focus on the findings of the association between BDNF and PTSD and expand upon our recent works to provide an argument for the potential role of BDNF in additional psychiatric disorders with their roots in emotional dysregulation, specifically PTSD.
| Mental disorders | Results and references |
| Schizophrenia | Polymorphisms and BDNF[39] |
| The Val66Met allele association[40] | |
| The TrkB receptor decreased in the hippocampus[41] | |
| Up-[41] and down[42] regulated BDNF in the frontal cortices | |
| Major depressive disorder | Antidepressant increases BDNF levels[43] |
| BDNF protein increased in the NAc[44] | |
| The decreased prefrontal cortex is correlated with decreased BDNF and TrkB levels[45,46] | |
| BDNF dose-dependently decreases 5HT uptake[47] | |
| Unclear whether BDNF polymorphisms contribute to expression of MDD symptoms or antidepressant efficacy[48,49] | |
| Bipolar | Both lithium and valproic acid increase BDNF expression in corticolimbic brain[40] |
| BDNF protein levels decreased in post mortem hippocampal tissue[40] | |
| Serum BDNF levels decreased[49] | |
| The V66M BDNF allele strongly correlated to BD[35,50] | |
| PTSD | PTSD risk associated with BDNF Val66Met and BDNF overexpression[15] |
| Blood BDNF levels and PTSD[4,15,18-21] |
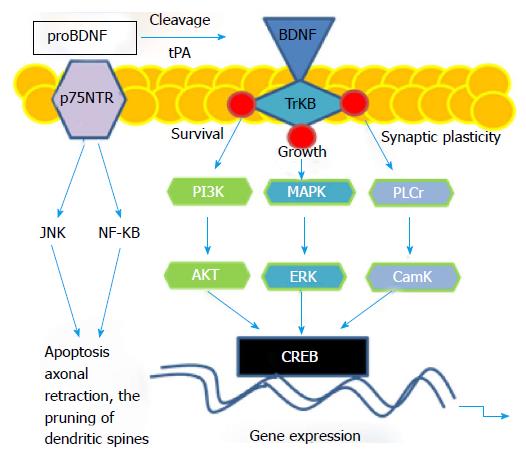
BDNF is a precursor protein (proBDNF) that is proteolytically cleaved to generate mature BDNF[5] via tissue-type plasminogen activator (tPA)/plasminogen[6]. P11 (S100A10), a component of the Annexin II and PTSD associated gene[7,8], greatly enhances the activation of plasmin by tPA[6]. It is suggested that p11 may act through the tPA/plasminogen/BDNF pathway to achieve its antidepressant effect[6]. BDNF binds to either of two functionally different classes of cell surface receptors, the TrkB receptor tyrosine kinase or the p75 neurotrophin receptor (p75NTR), a member of the tumor necrosis factor receptor super family[9]. ProBDNF and mature BDNF differentially interact with the TrkB receptor tyrosine kinase or the p75NTR, respectively[9,10]. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin[11]. ProBDNF induced p75NTR signaling gives rise to an increase in c-Jun N-terminal kinase and nuclear factor κB, regulating apoptosis, axonal retraction, or the pruning of dendritic spines[12]. BDNF-induced TrkB receptor signaling regulates neurotrophic responses via rapid activation of the PI-3 kinase, Ras/MAPK, and Phospholipase C-γ pathways; therefore influencing transcriptional events that affect the cell-cycle, neurite outgrowth, and synaptic plasticity (Figure 1), suggesting that the BDNF plays a key role in stress response and stress-related behaviors[13].
In the animal model, it is found that BDNF protein was over-expressed in the plasma and hippocampus of stressed rats compared with non-stressed controls. These data are in agreement with others showing that stress results in BDNF over-expression in the hippocampus, leading to the hypotheses of a BDNF-related compensatory mechanism[14] and the blood levels of BDNF mirror the hippocampus levels induced by traumatic stress[15]. The rats with up-regulated BDNF in both blood and hippocampus induced by inescapable tail shock demonstrated increases of startle response[15]. Acoustic challenge is known to trigger a range of physiological responses, including startle. The startle reaction (also known as the startle response, the startle reflex, or the alarm reaction) is the psychological and physiological response to a sudden unexpected stimulus, such as a flash of light, a loud noise (acoustic startle reflex), or a quick movement near the face. Abnormality of the startle response, which results from an elevated activation of the autonomic nervous system, is a core symptom of PTSD-hyperarousal[16,17]. These data from the studies in animal model suggests that both BDNF and stress play important roles in startle response, although the mechanism needs to be further analyzed.
Within the last several years, the biological basis of PTSD has been an important focus of research in psychiatry due to the Iraq and Afghanistan wars. There are data showing that the blood BDNF is a potential biomarker for PTSD, the traumatic stress-related disorders, and debilitating psychiatric disorders[4,15,18-21]. A common single nucleotide polymorphism (SNP) in the BDNF gene leading a valine to methionine substitution at position 66 (Val66Met) influences human hippocampal volume[22], memory[23] and susceptibility to PTSD[23]. The BDNF Val66Met polymorphism is associated with sense of coherence, a presumed stress-related protective cognition in a non-clinical community sample[24]. Individuals carrying the Met had decreased activity-dependent BDNF secretion from neurons, leading to impairment of learning[22]. Recently, a study demonstrated that the frequency distribution of Val66Met polymorphism was different between subjects with and without PTSD[15]. The frequencies of Met/Met genotype and Met carriers are significantly higher in individuals with PTSD than those without PTSD. The allelic frequency of Met was two-fold higher (33.3% vs 17.5%) in individuals with PTSD than in non-PTSD controls, supporting the notion that Met carriers have a smaller hippocampal volume relative to Val/Val homozygous[25-27] and decreased volumes in the temporal and occipital lobe grey matter[28].
These data suggest a role of BDNF in the plasticity of the brain, which might be associated with PTSD. It was found that Met carriers performed more poorly than control subjects (Val/Val carrier) on the memory tasks[29]. The interaction of Met-allele and stress can result in depression, anxiety and arousal[30]. There is a significant three-way interaction between Val66Met, serotonin transporter linked promoter region (5-HTTLPR) and maltreatment history in predicting depression[31]. Children with the Met allele and two short alleles of 5-HTTLPR demonstrated the highest depression scores. However, the vulnerability associated with these two genotypes was only evident in the maltreated children[31]. There is a report showing that veterans with psychotic PTSD carried more Met alleles of the BDNF Val66Met than non-psychotic veterans with PTSD or veterans without PTSD[32]. This further supports the linkage between BDNF and PTSD, especially in a military population at war[15]. However, not all studies show the same results. There is a case-control genetic association study showing no relationship between BDNF Val66Met and PTSD diagnosis[23]. It is possible the difference in frequency of trauma exposure, age, and other study conditions among the participants in the studies can explain these divergent results[33]; however, this remains to be determined.
In an association study between BDNF Val66Met and the startle score of PTSD Checklist (PCL), a core symptom of hyperarousal in PTSD is observed to be associated with the polymorphism. The distribution of the Met/Met frequency was significantly different between those with and without exaggerated startle[15]. The frequency of the Met/Met genotype was almost four-fold (12.2% vs 3.3%) higher in subjects with exaggerated startle than in those without exaggerated startle. In addition, the frequency of the Met allele was higher in subjects with exaggerated startle than in those without exaggerated startle (24.4% vs 15.3%), indicating that Met/Met is associated with hyperarousal vulnerability[15]. Since the frequency of Val/Val genotype is higher in the non-startle group than in those endorsing startle reactions, it suggests that Val/Val is related to protection from exaggerated startle reactions and perhaps PTSD. Subjects with fewer stressful life events and carrying the Met/Met homozygote have significantly higher startle scores than those Val carries, indicating that less exposure to stressful life events is associated with higher risk of hyperarousal in Met/Met carriers, but lower risk of hyperarousal in Val carriers[15].
It is found that the BDNF Val66Met does not significantly effect on the PCL total score in the subjects who experienced higher (four or more) stressful life events. However, among those subjects who reported fewer exposures of stressful life events, the Met carriers show higher PCL total scores (i.e., reported greater PTSD symptoms) than Val carriers. Therefore, at a lower exposure of stressful life events, Met carriers had a higher risk of PTSD symptoms, and the presence of Val led to a lower risk of PTSD symptoms[15]. This indicates that there are protective effects at higher levels of stress exposure. A similar phenomenon is observed in a catechol-O-methyltransferase gene association study, which showed that those homozygous for the Met allele demonstrated a high risk for PTSD, independent of the severity of traumatic load[34]. Alternatively, different neuronal mechanisms[35] may be active in minimally exposed and highly exposed individuals who develop PTSD, supporting different underlying trajectories of this disorder and perhaps different treatments[15].
It is also found that at protein levels, subjects with PTSD had significantly higher serum levels of BDNF than the non-PTSD controls[20]. In addition, the BDNF levels in Met carriers are higher than in Val/Val homozygotes[36]. The findings are consistent with other results showing that serum BDNF levels in individuals with PTSD are higher than in age and sex matched controls right after traumatic events[19,37]. However, some studies have shown either significantly lower levels of BDNF among those with PTSD[4] or significant difference of BDNF levels in serum between PTSD and non-PTSD controls[21]. These contradictory results may be due to the different methods used in the various studies. The samples may have been collected from dissimilar population[18,20,21,37], at different time points during the course of the disease[18,20,21,37], or from different animal models[38]. These assumptions need to be further analyzed. Nevertheless, these data suggest that BDNF is associated with PTSD risk at both translational and genomic levels[15]. Therefore, blood levels of BDNF may be of benefit in developing non-invasive diagnostics for PTSD[15].
The association between BDNF and PTSD has been suggested. The frequency of the Met/Met was greater among those with PTSD than non-PTSD controls. In addition, this SNP is associated with exaggerated startle, but not with other items on the PCL. Among individuals who experienced fewer lifetime stressful events, Met carriers have significantly higher total and startle scores on the PCL than Val/Val carries. At protein levels, subjects with PTSD had higher levels of BDNF in their peripheral blood plasma than the non-PTSD controls. In a rodent model, complementing the data from the human subjects, increased BDNF protein levels accompanied by an obvious elevation of the startle response were obtained in both blood plasma and brain hippocampus by inescapable tail shock. Therefore, protein BNDF in the blood and startle test, aside from genotype, and neuroimaging could also serve as biomarkers to direct more personalized PTSD treatment. Future studies on patient cohorts will elucidate whether these biomarkers, particular BDNF for PTSD prove to be useful in a clinical setting.
P- Reviewer: Malan L, Pivac N S- Editor: Qiu S L- Editor: A E- Editor: Jiao XK
| 1. | Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123-131. [PubMed] |
| 2. | Chuu JY, Taylor JL, Tinklenberg J, Noda A, Yesavage J, Murphy GM. The brain-derived neurotrophic factor Val66Met polymorphism and rate of decline in Alzheimer’s disease. J Alzheimers Dis. 2006;9:43-49. [PubMed] |
| 3. | Jiang X, Xu K, Hoberman J, Tian F, Marko AJ, Waheed JF, Harris CR, Marini AM, Enoch MA, Lipsky RH. BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 2005;30:1353-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Dell’Osso L, Carmassi C, Del Debbio A, Catena Dell’Osso M, Bianchi C, da Pozzo E, Origlia N, Domenici L, Massimetti G, Marazziti D. Brain-derived neurotrophic factor plasma levels in patients suffering from post-traumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:899-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Chen ZY, Bath K, McEwen B, Hempstead B, Lee F. Impact of genetic variant BDNF (Val66Met) on brain structure and function. Novartis Found Symp. 2008;289:180-188; discussion 188-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Tsai SJ. The P11, tPA/plasminogen system and brain-derived neurotrophic factor: Implications for the pathogenesis of major depression and the therapeutic mechanism of antidepressants. Med Hypotheses. 2007;68:180-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Su TP, Zhang L, Chung MY, Chen YS, Bi YM, Chou YH, Barker JL, Barrett JE, Maric D, Li XX. Levels of the potential biomarker p11 in peripheral blood cells distinguish patients with PTSD from those with other major psychiatric disorders. J Psychiatr Res. 2009;43:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Zhang L, Li H, Su TP, Barker JL, Maric D, Fullerton CS, Webster MJ, Hough CJ, Li XX, Ursano R. p11 is up-regulated in the forebrain of stressed rats by glucocorticoid acting via two specific glucocorticoid response elements in the p11 promoter. Neuroscience. 2008;153:1126-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1625] [Cited by in RCA: 1737] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 10. | Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1244] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 11. | Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455-5463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 781] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 12. | Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203-233. [PubMed] |
| 13. | Liu QR, Walther D, Drgon T, Polesskaya O, Lesnick TG, Strain KJ, de Andrade M, Bower JH, Maraganore DM, Uhl GR. Human brain derived neurotrophic factor (BDNF) genes, splicing patterns, and assessments of associations with substance abuse and Parkinson’s Disease. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 169] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Faure J, Uys JD, Marais L, Stein DJ, Daniels WM. Early maternal separation alters the response to traumatization: resulting in increased levels of hippocampal neurotrophic factors. Metab Brain Dis. 2007;22:183-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Zhang L, Benedek DM, Fullerton CS, Forsten RD, Naifeh JA, Li XX, Hu XZ, Li H, Jia M, Xing GQ. PTSD risk is associated with BDNF Val66Met and BDNF overexpression. Mol Psychiatry. 2014;19:8-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Zhang L, Hu XZ, Li H, Li X, Smerin S, Benedek DM, Ursano R. Startle response related genes. Med Hypotheses. 2011;77:685-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Jovanovic T, Norrholm SD, Sakoman AJ, Esterajher S, Kozarić-Kovacić D. Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. Int J Psychophysiol. 2009;71:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Angelucci F, Ricci V, Gelfo F, Martinotti G, Brunetti M, Sepede G, Signorelli M, Aguglia E, Pettorruso M, Vellante F. BDNF serum levels in subjects developing or not post-traumatic stress disorder after trauma exposure. Brain Cogn. 2014;84:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Grassi-Oliveira R, Stein LM, Lopes RP, Teixeira AL, Bauer ME. Low plasma brain-derived neurotrophic factor and childhood physical neglect are associated with verbal memory impairment in major depression--a preliminary report. Biol Psychiatry. 2008;64:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Hauck S, Kapczinski F, Roesler R, de Moura Silveira E, Magalhães PV, Kruel LR, Schestatsky SS, Ceitlin LH. Serum brain-derived neurotrophic factor in patients with trauma psychopathology. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:459-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Matsuoka Y, Nishi D, Noguchi H, Kim Y, Hashimoto K. Longitudinal changes in serum brain-derived neurotrophic factor in accident survivors with posttraumatic stress disorder. Neuropsychobiology. 2013;68:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Brooks SJ, Nilsson EK, Jacobsson JA, Stein DJ, Fredriksson R, Lind L, Schiöth HB. BDNF polymorphisms are linked to poorer working memory performance, reduced cerebellar and hippocampal volumes and differences in prefrontal cortex in a Swedish elderly population. PLoS One. 2014;9:e82707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Zhang H, Ozbay F, Lappalainen J, Kranzler HR, van Dyck CH, Charney DS, Price LH, Southwick S, Yang BZ, Rasmussen A. Brain derived neurotrophic factor (BDNF) gene variants and Alzheimer’s disease, affective disorders, posttraumatic stress disorder, schizophrenia, and substance dependence. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:387-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Surtees PG, Wainwright NW, Willis-Owen SA, Sandhu MS, Luben R, Day NE, Flint J. The brain-derived neurotrophic factor Val66Met polymorphism is associated with sense of coherence in a non-clinical community sample of 7335 adults. J Psychiatr Res. 2007;41:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59:812-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 353] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 26. | Harrisberger F, Spalek K, Smieskova R, Schmidt A, Coynel D, Milnik A, Fastenrath M, Freytag V, Gschwind L, Walter A. The association of the BDNF Val66Met polymorphism and the hippocampal volumes in healthy humans: a joint meta-analysis of published and new data. Neurosci Biobehav Rev. 2014;42:267-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, Ashtari M, Napolitano B, Bilder RM, Kane JM. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 283] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 28. | Ho BC, Milev P, O’Leary DS, Librant A, Andreasen NC, Wassink TH. Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch Gen Psychiatry. 2006;63:731-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690-6694. [PubMed] |
| 30. | Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams LM. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14:681-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 385] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 31. | Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 437] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 32. | Pivac N, Kozaric-Kovacic D, Grubisic-Ilic M, Nedic G, Rakos I, Nikolac M, Blazev M, Muck-Seler D. The association between brain-derived neurotrophic factor Val66Met variants and psychotic symptoms in posttraumatic stress disorder. World J Biol Psychiatry. 2012;13:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Rakofsky JJ, Ressler KJ, Dunlop BW. BDNF function as a potential mediator of bipolar disorder and post-traumatic stress disorder comorbidity. Mol Psychiatry. 2012;17:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Kolassa IT, Kolassa S, Ertl V, Papassotiropoulos A, De Quervain DJ. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val(158)Met polymorphism. Biol Psychiatry. 2010;67:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 35. | Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 627] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 36. | Harris SE, Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ. The brain-derived neurotrophic factor Val66Met polymorphism is associated with age-related change in reasoning skills. Mol Psychiatry. 2006;11:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Hauck S, Gomes F, Silveira Júnior Ede M, Almeida E, Possa M, Ceitlin LH. Serum levels of brain-derived neurotrophic factor in acute and posttraumatic stress disorder: a case report study. Rev Bras Psiquiatr. 2009;31:48-51. [PubMed] |
| 38. | Bazak N, Kozlovsky N, Kaplan Z, Matar M, Golan H, Zohar J, Richter-Levin G, Cohen H. Pre-pubertal stress exposure affects adult behavioral response in association with changes in circulating corticosterone and brain-derived neurotrophic factor. Psychoneuroendocrinology. 2009;34:844-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | van Haren NE, Bakker SC, Kahn RS. Genes and structural brain imaging in schizophrenia. Curr Opin Psychiatry. 2008;21:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Hashimoto R, Takei N, Shimazu K, Christ L, Lu B, Chuang DM. Lithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: an essential step for neuroprotection against glutamate excitotoxicity. Neuropharmacology. 2002;43:1173-1179. [PubMed] |
| 41. | Takahashi M, Shirakawa O, Toyooka K, Kitamura N, Hashimoto T, Maeda K, Koizumi S, Wakabayashi K, Takahashi H, Someya T. Abnormal expression of brain-derived neurotrophic factor and its receptor in the corticolimbic system of schizophrenic patients. Mol Psychiatry. 2000;5:293-300. [PubMed] |
| 42. | Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8:592-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 418] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 43. | Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 643] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 44. | Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1425] [Cited by in RCA: 1756] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 45. | Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 625] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 46. | Pandey GN, Ren X, Rizavi HS, Conley RR, Roberts RC, Dwivedi Y. Brain-derived neurotrophic factor and tyrosine kinase B receptor signalling in post-mortem brain of teenage suicide victims. Int J Neuropsychopharmacol. 2008;11:1047-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Mössner R, Daniel S, Albert D, Heils A, Okladnova O, Schmitt A, Lesch KP. Serotonin transporter function is modulated by brain-derived neurotrophic factor (BDNF) but not nerve growth factor (NGF). Neurochem Int. 2000;36:197-202. [PubMed] |
| 48. | Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1030] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 49. | Sanacora G. New understanding of mechanisms of action of bipolar medications. J Clin Psychiatry. 2008;69 Suppl 5:22-27. [PubMed] |
| 50. | Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim Y-, Tsan G, Schaffner S, Kirov G, Jones I, Owen M. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry. 2002;7:579-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 420] [Article Influence: 19.1] [Reference Citation Analysis (0)] |