Published online Mar 22, 2015. doi: 10.5498/wjp.v5.i1.15
Peer-review started: August 27, 2014
First decision: December 17, 2014
Revised: January 13, 2015
Accepted: February 4, 2015
Article in press: February 9, 2015
Published online: March 22, 2015
Processing time: 174 Days and 16.3 Hours
Accumulating translational evidence suggests that the long-chain omega-3 fatty acid docosahexaenoic acid (DHA) plays a role in the maturation and stability of cortical circuits that are impaired in different recurrent psychiatric disorders. Specifically, rodent and cell culture studies find that DHA preferentially accumulates in synaptic and growth cone membranes and promotes neurite outgrowth, dendritic spine stability, and synaptogenesis. Additional evidence suggests that DHA may play a role in microglia-mediated synaptic pruning, as well as myelin development and resilience. In non-human primates n-3 fatty acid insufficiency during perinatal development leads to widespread deficits in functional connectivity in adult frontal cortical networks compared to primates raised on DHA-fortified diet. Preterm delivery in non-human primates and humans is associated with early deficits in cortical DHA accrual. Human preterm birth is associated with long-standing deficits in myelin integrity and cortical circuit connectivity and increased risk for attention deficit/hyperactivity disorder (ADHD), mood, and psychotic disorders. In general, ADHD and mood and psychotic disorders initially emerge during rapid periods of cortical circuit maturation and are characterized by DHA deficits, myelin pathology, and impaired cortical circuit connectivity. Together these associations suggest that early and uncorrected deficits in fetal brain DHA accrual may represent a modifiable risk factor for cortical circuit maturation deficits in psychiatric disorders, and could therefore have significant implications for informing early intervention and prevention strategies.
Core tip: Although the role of perinatal brain omega-3 fatty acid (DHA) accrual on the maturation and long-term stability of cortical circuitry is only beginning to be fully understood, extant translational evidence suggests that DHA plays a role in the initial development and early maturation of cortical circuits. Emerging evidence from human neuroimaging studies further suggests that psychiatric disorders that initially emerge in childhood and adolescence and associated with low blood DHA levels are characterized by frontal circuit deficits compared with healthy developing youth. Based on existing evidence, these associations could have significant implications for informing novel early intervention strategies aimed at reducing the transmission of psychopathology.
- Citation: McNamara RK, Vannest JJ, Valentine CJ. Role of perinatal long-chain omega-3 fatty acids in cortical circuit maturation: Mechanisms and implications for psychopathology. World J Psychiatr 2015; 5(1): 15-34
- URL: https://www.wjgnet.com/2220-3206/full/v5/i1/15.htm
- DOI: https://dx.doi.org/10.5498/wjp.v5.i1.15
Over the past 30 years evidence has emerged from both animal and clinical research implicating long-chain omega-3 (LCn-3) fatty acids in normal brain development and function. The principal LCn-3 fatty acid found in mammalian brain gray matter is docosahexaenoic acid (DHA), which comprises approximately 10%-20% of total fatty acid composition in the adult frontal cortex[1,2]. Although the omega-3 fatty acid precursors of DHA, including α-linolenic acid (ALA, 18:3n-3), eicosapentaenoic acid (EPA, 20:5n-3), and docosapentaenoic acid (22:5n-3), cross the blood-brain barrier, they are rapidly oxidized and consequently comprise < 1% of total brain fatty acid composition[3]. Mammals require a dietary source of n-3 fatty acids to procure and maintain adequate concentrations of DHA in peripheral and central tissues. Although DHA can be biosynthesized from the vegetable short-chain n-3 fatty acid precursor ALA via a series of microsomal desaturase, elongase, and peroxisomal reactions, preformed DHA is significantly more effective than ALA for increasing erythrocyte[4], breast milk[5,6], and cortical gray matter[7,8] DHA concentrations. Primary dietary sources of DHA include cold water fatty fish, milk and eggs fortified with DHA, and fish oil (FO) or algal supplements.
Human infant, childhood, and adolescence are critical developmental periods associated with the formation and establishment of structural and functional connectivity between frontal lobe regions that mediate attention and executive function and limbic structures that mediate emotion and mood[9-11]. During this perinatal period DHA concentrations increase sharply in the frontal cortex[1] and may therefore play an important role in cortical circuit maturation. This is supported in part by recently emerging neuroimaging data that suggests that DHA status is positively correlated with frontal cortex structural and functional integrity in human subjects across the lifespan[12]. Moreover, preterm delivery is associated with early deficits in cortical DHA accrual, long-standing deficits in cortical circuit maturation, and increased risk for developing psychiatric disorders. Lastly, psychiatric disorders which frequently initially emerge during rapid periods of cortical circuit maturation and are characterized by DHA deficits, myelin pathology, and impaired cortical circuit connectivity (see below). These associations support the hypothesis that LCn-3 fatty acids play a role in the early development of cortical circuits and that LCn-3 fatty acid insufficiency may represent a modifiable neurodevelopmental risk factor for psychiatric disorders. This review critically evaluates translational evidence implicating LCn-3 fatty acids in cortical circuit development, highlights plausible molecular and ultrastructural mechanisms, and explores potential relevance to the pathoetiology of recurrent neuropsychiatric disorders.
During perinatal rodent brain development, cortical DHA concentrations increase sharply in conjunction with active periods of neurogenesis, neuroblast migration, and synaptogenesis[13]. For example, there is a 5-fold increase in cortical DHA levels during the late gestation compared with early gestation[13]. DHA preferentially accumulates in neuronal growth cone[14,15] and mature synaptic[16,17] membranes where it modulates membrane signaling dynamics and synaptogenesis[18,19]. DHA also increases neurotrophic factor expression including nerve growth factor (NGF) and brain-derived growth factor (BDNF)[20,21], and promotes neurite outgrowth[22-26]. Dietary-induced deficits in brain DHA accrual during perinatal maturation are associated with reductions in neurogenesis[27,28], delays in neuronal migration and embryonic cortical plate expansion[29,30], and reduced synaptic plasticity and connectivity[24]. Additionally, DHA and its bioactive metabolites are protective against a variety of neuronal insults associated with oxidative stress and lipid peroxidation in the fetal[31-33] and adult rat brain[34-39].
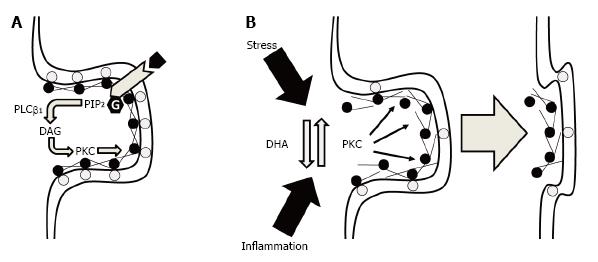
One consequence of changing the fatty acid composition of cellular membranes that is relevant to synaptic maturation and function is the alteration of phospholipid composition. Specifically, perinatal deficits in DHA accrual are associated with selective reductions in neuronal membrane phosphatidylserine concentrations[40,41], whereas perinatal FO supplementation selectively increases neuronal membrane phosphatidylserine concentrations[23,42]. Importantly, phosphatidylserine modulates the activity of signal transduction proteins including protein kinase C (PKC)[43]. PKC plays a pivotal role in filamentous actin (F-actin) cytoskeletal structural plasticity required for neurite outgrowth, growth cone motility, dendritic spine formation and stability, as well as neurotransmitter release dynamics[44]. This response is mediated in part by PKC-mediated phosphorylation of substrate proteins including myristoylated alanine-rich C kinase substrate (MARCKS) which cross-links actin filaments in a phosphorylation-reversible manner[45]. Moreover, MARCKS binds membranes in part by electrostatic interactions between phosphatidylserine and the highly basic phosphorylation site domain in a phosphorylation-reversible manner[46]. Consistent with this mechanism, we demonstrated that perinatal deficits in DHA accrual were associated with a significant reduction in membrane-bound MARCKS, and an associated increase in cytosolic MARCKS, in the rat hippocampus[47]. These findings suggest that lower membrane phosphatidylserine content in response to reduced DHA levels are associated with a dysregulation in the signal transduction processes that regulate F-actin cytoskeletal structural plasticity.
Electrostatic binding of MARCKS with membrane phosphatidylserine also inhibits phospholipase Cβ1-mediated hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) by sequestering PIP2 in lateral membrane domains[48]. Therefore, reductions in MARCKS membrane binding would also be predicted to increase Gqα-linked receptor-initiated hydrolysis of PIP2 into diacylglycerol (DAG) and inositol triphosphate (IP3) which increase PKC activity and intracellular calcium release, respectively. Moreover, free DHA inhibits PKC translocation and activity[49-52], and we previously demonstrated that perinatal deficits in DHA accrual were associated with significant alterations in the subcellular distribution PKC isozymes in the rat hippocampus[47]. Importantly, increases in PKC-mediated phosphorylation of MARCKS leads to a reduction in the tensile strength of the F-actin cytoskeleton and associated deficits in dendritic spine formation and stability[53]. Moreover, higher brain DHA levels are associated with elevated dendritic spine density and resilience[54-56], whereas perinatal deficits in DHA accrual are associated with reductions in synaptic connections[24]. It also relevant that elevations in PKC activity have been implicated in dendritic spine loss in response to chronic stress[57] and chronic inflammation[58,59]. Together, these findings suggest that DHA promotes synapse maturation and stability by decreasing PKC-mediated dismantlement of the F-actin cytoskeleton within synaptic terminals (Figure 1).
In addition to playing a role in the formation of new synapses, PKC, MARCKS, and the F-actin cytoskeleton also play a role in neurotransmitter vesicle trafficking and release efficacy within mature presynaptic terminals[60]. A role of DHA in this dynamic process is supported by findings of alterations in neurotransmitter vesicle distribution within presynaptic terminals[61,62] and abnormalities in the release, i.e., increased basal release and deficits in stimulated release, of multiple neurotransmitter systems including dopamine[63-65], serotonin[66], and acetylcholine[67,68] in the DHA-deficient rat brain. It is relevant therefore that a dyregulation in dopamine[69-73], serotonin[74,75], and acetylcholine[76,77] have been implicated in the pathophysiology and treatment of mood and psychotic disorders as well as neurocognitive impairment. Increased glutamatergic synaptic efficacy is required for the induction of long-term potentiation (LTP) and the formation of new axodendritic synaptic connections subserve the maintenance of LTP[78-80]. Importantly, deficits in DHA accrual during perinatal development are associated with impaired LTP and a significant reduction in glutamate synapses in the rat hippocampus[24]. LTP is also thought to mediate the consolidation and storage of new memories[81,82], and perinatal deficits in DHA accrual are associated with impaired learning on hippocampus-dependent spatial learning tasks[83-85] and olfactory discrimination tasks[86-88]. Together, these findings provide additional support for a role of cortical DHA in activity-dependent synaptic plasticity and synaptogenesis.
Critical to the “fine tuning” of cortical circuits during postnatal development is the pruning of extraneous and aberrant synapses. For example, during the peri-adolescent period there is a substantial (approximately 50%) pruning of glutamatergic connections between the rat frontal cortex and the amygdala[89]. While there is currently little known about the role of DHA in cortical synaptic pruning, in the developing rat visual system DHA deficits were associated with aberrant axonal innervation outside the main terminal zones of the superior colliculus which was transient and consistent with a delay in synaptic pruning[90]. It is also relevant that synaptic pruning is mediated in part by microglial phagocytosis[91,92], and a recent study found that DHA application to cultured microglia stimulated phagocytosis (M2 phenotype) and decreased the production and secretion of pro-inflammatory cytokines including TNF-α (M1 phenotype)[93]. The latter study also demonstrated that DHA application increased microglia BDNF biosynthesis, which was positively correlated with microglia phagocytosis, and BDNF expression is reduced in the frontal cortex of DHA-deficient rats[21]. A second study found that deficits in brain DHA accrual during perinatal development increased microglial pro-inflammatory cytokine production in the neonatal rat hippocampus, consistent with a non-phagocytotic pro-inflammatory phenotype[94]. These preliminary findings suggest that deficits in brain DHA accrual during perinatal development may disrupt synaptic pruning by altering the phenotype of microglia.
An important aspect of cortical circuit maturation is the myelination of axons, and DHA accumulates in myelin during perinatal rat development[17,95]. While there is currently little known about the role of DHA in the maturation of myelin sheaths and axonal conduction, intracerebroventricular administrations of either EPA or DHA in 2-day-old rats increased the expression myelin-related genes in different brain regions[96]. However, maternal FO supplementation, as well as maternal n-3 fatty acid deficiency, during pregnancy and lactation was found to impair auditory brainstem responses in neonates which was interpreted as a slowing of neural signal conduction[97,98]. A subsequent study found that initially lower auditory brainstem responses dissipated by young adulthood[98]. Another study found that maternal DHA supplementation during pregnancy and lactation impaired auditory startle response in neonates[95]. In adult rodents increasing dietary LCn-3 fatty acid status is protective against inflammation-[99] and trauma-induced[100,101] axonal white matter injury, as well as histopathological features in the experimental autoimmune encephalomyelitis model of multiple sclerosis[102]. While these preliminary findings suggest that there are optimal DHA levels required for normal axonal white matter integrity and resilience, additional research is needed to evaluate the role of perinatal brain DHA accrual on myelin integrity and circuit connectivity.
Consistent with rodent studies, DHA concentrations increase sharply in the developing monkey brain during perinatal development[103,104], and baboons born preterm exhibit cortical DHA deficits compared with term births[105,106]. Primate perinatal n-3 fatty acid deficiency is associated with deficits in visual attention[107], polydipsia (excessive thirst)[108], and deficits in visual acuity and electroretinogram abnormalities[103,104]. Electroretinogram abnormalities have also been observed in neonatal baboons born preterm[109]. Consistent with dysregulated dopamine activity, perinatal n-3 fatty acid deficiency is associated increased home cage stereotypy and locomotion bouts[110]. A recent neuroimaging study found that resting-state functional connectivity among prefrontal cortical networks was impaired in young adult monkeys raised on an n-3 fatty acid deficient diet compared with monkeys raised on FO-fortified diet[111]. Specifically, n-3 fatty acid deficient monkeys exhibited reduced connectivity between the dorsal anterior insula (seed region) and ventromedial prefrontal, orbitofrontal, dorsolateral prefrontal cortices as well as with superior temporal sulcus and medial parietal areas. Although not specifically investigated, tracer studies demonstrate monosynaptic connections between monkey orbitofrontal cortex and amygdala[112]. Together these findings suggest that uncorrected deficits in cortical DHA accrual during perinatal brain development leads to reduced connectivity within prefrontal cortical networks in young adult non-human primates.
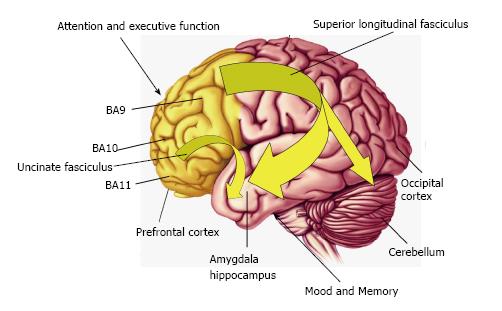
During human neonatal development, DHA accumulates in brain tissue at a rapid rate during the third trimester in association with active periods of neurogenesis, neuroblast migration, differentiation, synaptogenesis, and gray matter expansion[113,114]. Importantly, the third trimester of gestation is also a period associated with the initial formation of connections between brain regions including the uncinate fasciculus and superior longitudinal fasciculus[115]. Postpartum the neonatal brain continues to grow from approximately 350 g at birth to approximately 925 g at 1 year of age[116], during which DHA represents approximately 9% of total cortical fatty acid composition[1,117]. Neonates are wholly reliant on maternal breast milk (or formula) as the sole source of DHA. Term infants fed formulas without DHA consistently exhibit significantly lower erythrocyte and postmortem brain cortex DHA concentrations relative to breastfed infants or infants fed formula containing DHA[118-125]. The recognition that human breast milk DHA represents an important source for postnatal infant brain DHA accrual led to the widespread commercial availability of DHA-fortified infant formula in the United States in 2002.
During early childhood development DHA levels continue to increase in the frontal cortex[1] in association with linear increases in frontal cortex gray matter expansion and myelination[9-11,115], and the maturation of frontal lobe-mediated neurocognitive processes including attention and executive function[10,126]. During adolescent development cortical DHA levels continue to increase to approximately 15% total cortical fatty acids in young adulthood[1], and this increase coincides with frontal cortex synaptic pruning[127-129], white matter expansion and maturation[9-11,130,131]. Human neuroimaging studies indicate that the childhood and adolescent period is associated with the maturation of frontal cortical regions that mediate attention and executive function and the maturation of uncinate fasciculus and superior longitudinal fasciculus functional connectivity between frontal regions and limbic structures that mediate mood including the amygdala[9-11](Figure 2).
As observed in non-human primates[106], human infants born preterm exhibit lower erythrocyte and postmortem cortical DHA concentrations compared with term infants fed the same ALA-fortified formula postpartum[113,114,122,132,133]. Structural imaging studies have found that children and adolescents born preterm exhibit significant reductions in frontal and temporal cortical gray matter volumes, reduced amygdala and hippocampal volumes, reduced corpus callosum and white matter volumes, and enlarged cerebral ventricles[134-142]. Reductions in preterm cortical brain gray matter volume are correlated with functional connectivity deficits[143], and children, adolescents, and adults born preterm exhibit reduced connectivity within prefrontal cortical networks and decreased white matter integrity in different tracts including the uncinate fasciculus and superior fasciculus[144-155]. Importantly, deficits in white matter integrity have been observed in preterm born children with no neonatal ultrasound evidence for intraventricular hemorrhage, periventricular leukomalacia, low-pressure ventriculomegaly, or cytic white matter injury[145]. While these imaging findings suggest that deficits in third trimester DHA accrual may be associated with long-standing deficits in cortical circuit maturation, additional research is needed to determine whether early DHA supplementation can prevent or reverse these deficits.
Studies have also found that decreased white matter integrity in children and adolescents born preterm are associated with cognitive impairment and psychiatric symptoms[145,156-160]. Children and adolescents born preterm exhibit a significantly higher incidence of attention deficits, impulsivity, learning disability, language impairments, hyperactivity, anxiety, motor impairments, and poor social functioning relative to age- and sex-matched term children/adolescents[161,162]. Importantly, preterm birth and/or low birth weight is associated with increased risk for developing attention deficit/hyperactivity disorder (ADHD) in childhood[161,163-165] and mood, anxiety, and psychotic disorders during adolescence and young adulthood independent of multiple confounding variables including maternal history of psychiatric illness[163,166-170]. These findings suggest that deficits in cortical circuit maturation resulting from preterm birth are relevant to the etiology of ADHD in childhood and mood and psychotic disorders during adolescence and young adulthood.
While the contribution of DHA deficits to neurological and cognitive impairments commonly observed in preterm infants and children is poorly understood, fortifying human milk or formula with higher levels of DHA is associated with improvements in visual acuity, sustained attention, and recognition memory compared with infants receiving DHA-free formula[171-174]. However, a systematic review of randomized controlled trials studying the effects of DHA-fortified formula for preterm infants concluded that there was no consistent effect on infant cognition or visual function[175]. It is notable, however, the DHA doses used in the majority of studies (0.2%-0.3% DHA) may have been too low to compensate for intestinal malabsorption, DHA oxidation, and early central DHA deficits in preterm infants. Indeed, a dose-response study found that milk DHA concentrations of 1% were required to increase DHA status in preterm infants to levels similar to term infants[176]. Moreover, deficits in cortical DHA concentrations in preterm baboons were not fully restored to control levels following 4 wk feeding formula fortified with a moderate dose of DHA (0.61%)[106]. It is also relevant that a placebo-controlled structural MRI study found that feeding formula containing lower levels of DHA (0.34%) did not significantly alter white matter volume in premature infants[177], whereas a preliminary intervention study observed improvements in brain white matter volumes in neonates with peroxisomal disorders following supplementation with higher DHA doses (100-600 mg/d)[178]. These and other findings have led to new recommendations for higher dose DHA supplementation for preterm infants to improve neurological and cognitive outcomes[179].
Prospective longitudinal studies have investigated the relationship between fetal cord blood DHA levels and neurodevelopmental outcomes in older children. These studies have found that higher LCn-3 fatty acid intake or cord blood DHA levels are associated with higher movement scores at 7 years of age[180,181], better neurological scores at 5.5 years[182], better visual function at 5 years of age[183], and better recognition memory and associated event-related potentials at approximately 11 years of age[184]. Another study found that higher cord blood DHA or LCn-3 fatty acid levels were associated with lower parent-reported hyperactivity/inattention and emotional symptoms among 416 children at 10 years of age[185]. A longitudinal study also found that higher cord blood DHA status was associated with lower levels of internalizing emotional problems including depression and externalizing conduct problems among 393 children at 7 years of age in subjects fed DHA-free formula but not those fed human milk[186]. The latter finding suggests that increasing postnatal dietary DHA intake can mitigate the emergence of psychiatric problems in youth exposed to low DHA levels in utero. Together, these data suggest that lower cord blood DHA levels are associated with an enduring negative impact on neurocognitive development.
Neonates become wholly reliant on maternal breast milk (or formula) as the sole source of DHA. Human breast milk DHA concentrations are highly correlated with maternal dietary DHA intake[6,187,188] and vary widely across different countries in accordance with habitual dietary fish consumption, e.g., approximately 0.17% of total milk fatty acids in the United States vs approximately 1.1% of total milk fatty acids in Japan[189]. Several studies suggest that longer breastfeeding duration, a putative surrogate for early postnatal DHA intake, is associated with improved white matter microstructure and volume[190,191] and better neurocognitive outcomes in childhood, adolescence, and adulthood[192-194]. Prospective and retrospective studies have also found that shorter breastfeeding duration is associated with increased risk for developing ADHD in childhood[195-198]. However, the latter studies did not determine breast milk DHA concentrations to evaluate contribution to functional outcomes and additional/alternative benefits of longer breastfeeding (i.e., better mother-child attachment) may also play an important role.
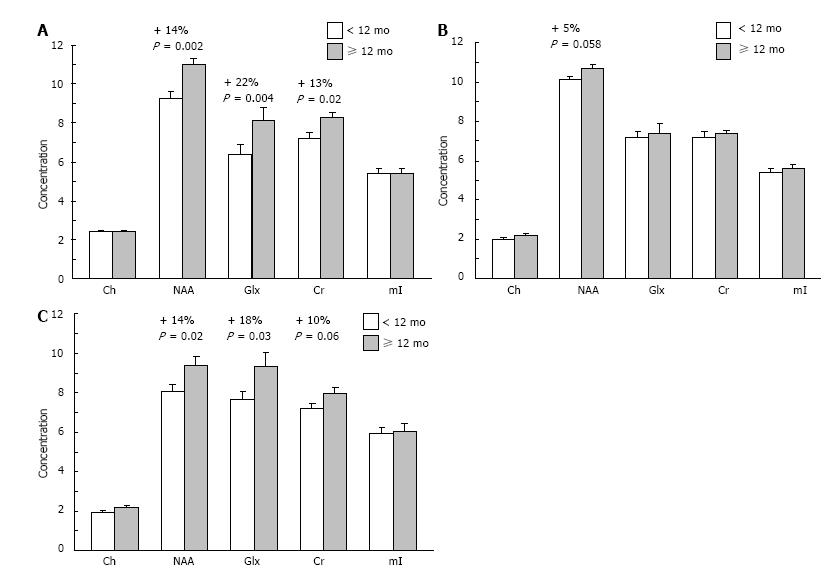
To investigate the relationship between breastfeeding duration and indices of neuronal integrity and function, we conducted a pilot study using proton magnetic resonance spectroscopy (1H MRS) in healthy boys (age 8-10 years, mean 9.1 ± 0.9 years, n = 38). Regions of interest included right and left dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC). Subjects were breastfed for an average of 9.83 ± 1.7 mo (range: 0-42 mo). Following a median split of breastfeeding duration, we compared children who had received ≥ 12 mo (n = 16, mean duration: 20 ± 8.9 mo) of breastfeeding with those who had received < 12 mo (n = 22, mean duration: 2.9 ± 3.2 mo). There were no significant differences in demographic variables between groups. Children receiving ≥ 12 mo of breastfeeding exhibited higher concentrations of N-acetyl aspartate (NAA), a putative marker of neuronal integrity, in the right DLPFC and ACC compared with subjects receiving < 12 mo breastfeeding (Figure 3). Children receiving ≥ 12 mo breastfeeding also exhibited higher levels of glutamate+glutamine (Glx) in the right DLPFC and ACC. These preliminary data suggest that longer durations of breastfeeding may be an important determinant of cortical functional integrity in brain regions mediating attention and executive function in healthy developing children.
In summary, evidence from animal studies suggests that normal brain development requires optimal levels of DHA which has neurotrophic and neuroprotective properties. A potential ultrastructural mechanism mediating the beneficial effects of DHA on synaptic maturation and axo-dendritic connectivity is increased F-actin cytoskeletal stability in pre- and post-synaptic terminals mediated through reductions in PKC activity. Additional evidence from non-human primate and clinical imaging studies suggest that low DHA levels during perinatal development may lead to long-standing impairments in functional connectivity in cortical networks as well as the emergence of cognitive impairment, hyperactivity/inattention and emotional symptoms in children. Taken collectively, these associations support the assertion that cortical DHA accrual during perinatal brain development may play a role in the maturation of human cortical networks mediating cognitive and emotional processes.
Major depressive disorder (MDD), bipolar disorder, schizophrenia, and ADHD are chronic and recurrent neuropsychiatric disorders that are a prominent cause of premature disability. The initial onset of ADHD typically occurs prior to seven years of age, and the initial onset of major mood and psychotic disorders frequently occur during adolescence or early adulthood[199-201]. Functional neuroimaging studies suggest that deficits in the functional maturation of frontal connectivity with limbic structures including the amygdala and/or the striatum are associated with psychopathology[202-206]. For example, event-related fMRI studies have repeatedly observed greater amygdala activation, and associated deficits in orbitofrontal activation, in response to emotional stimuli in youth and adults with bipolar disorder[207-209]. Although the initial onset of major psychiatric disorders commonly coincides with active and dynamic changes in frontal circuit connectivity, and psychopathology is associated with deficits in frontal circuit connectivity, a causal relationship has not been established. Moreover, the etiological mechanisms contributing to frontal circuit connectivity deficits in psychiatric disorders remain poorly understood.
Evidence from cross-national and cross-sectional studies suggests that LCn-3 fatty acid deficiency is relevant to pathophysiology and potentially etiology of different psychiatric disorders. Cross-national epidemiological studies have found that higher per capita intake of fish/seafood, a surrogate for LCn-3 fatty acid intake and status[210-212], is associated with lower lifetime prevalence rates of unipolar and bipolar depression[213-215]. Several population studies have similarly found that lower LCn-3 fatty acid intake is associated with increased risk for developing depressive symptoms[216-221]. It is also relevant that a large percentage of adolescents residing in western countries consume low quantities of LCn-3 fatty acids in their habitual diet[222-225], and lower LCn-3 fatty acid intake by adolescents is associated with a higher prevalence of depressive symptoms[226-228]. Together, these data suggest that higher habitual dietary LCn-3 fatty acid intake may be protective against the development of mood dysregulation.
Habitual dietary LCn-3 fatty acid intake is highly correlated with erythrocyte membrane LCn-3 fatty acid levels[212], and multiple case-control studies have observed significant erythrocyte membrane LCn-3 fatty acid deficits in patients with psychopathology. A meta-analysis of 14 case-control studies found significantly lower erythrocyte EPA and DHA levels in MDD patients[229]. In bipolar patients, three independent studies have observed significant erythrocyte DHA deficits compared with healthy controls[230-232]. Importantly, erythrocyte DHA deficits have also been observed in pediatric and adolescent patients with MDD or bipolar disorder proximal to illness onset[233-235]. Medication-naïve first-episode psychotic patients exhibit erythrocyte DHA deficits compared with healthy controls[236,237], and a recent meta-analysis of 18 case-control studies observed significant DHA deficits in schizophrenic patients[238]. A recent meta-analysis of nine cross-sectional studies observed significantly lower blood DHA levels in ADHD children compared with healthy controls[239]. Together, these case-control studies provide evidence that different psychiatric disorders are characterized by low DHA status which coincide with, and may precede, the initial onset of psychopathology.
Dietary LCn-3 fatty acid supplementation has been found to significantly increase patient erythrocyte LCn-3 fatty acid levels[240-242]. This observation indicates that LCn-3 fatty acid deficits in psychiatric patients are modifiable by increasing dietary LCn-3 fatty acid intake. Importantly, meta-analyses of controlled trials have observed a significant advantage of LCn-3 fatty acid supplementation over placebo for reducing depressive symptoms in patients with MDD[243] or bipolar disorder[244]. Preliminary trials have found that LCn-3 fatty acid supplementation significantly reduces depression and manic symptom severity in pediatric and adolescent patients[235,241,242,245]. Accumulating evidence also suggests that LCn-3 fatty acid supplementation may be efficacious for the treatment of positive and negative symptoms in patients with or at ultra-high risk for developing schizophrenia[240,246,247], and for reducing attention deficits in pediatric and adolescent ADHD patients[239,248]. These data suggest that LCn-3 fatty acid deficits observed in patients with psychiatric disorders are correctable and associated with psychiatric symptom severity.
In general human erythrocyte and frontal cortex DHA levels are positively correlated[1], though non-human primate studies indicate that DHA recuperation occurs more rapidly in erythrocytes than cortical gray matter[2]. A growing number of case-control studies have investigated the fatty acid composition of postmortem frontal gray matter from patients with mood and psychotic disorders. Some studies have observed lower LCn-3 fatty acid levels[249-253] while others have not[254-256]. Our group reported that young adult patients with MDD[249], bipolar disorder[250], and schizophrenia[251] exhibit significant frontal cortex DHA deficits compared with controls. In a preliminary postmortem study, we also found that DHA composition increases sharply in the frontal cortex during normal human adolescent development, and that this increase is significantly blunted in young adult suicide victims[257]. It is also relevant that postmortem brain studies have observed reduced dendritic spine density and synaptic markers in the frontal cortex of patients with mood or psychotic disorders[258-260]. While these findings suggest that psychopathology may be associated with deficits in cortical DHA accrual and reduced synaptic density, limitations associated with the postmortem approach constrain interpretation[261].
Emerging evidence from structural neuroimaging studies provide additional support a beneficial effect of LCn-3 fatty acids on cortical integrity over the lifespan[262-266]. For example, one study found that greater habitual dietary LCn-3 fatty acid intake, which is positively correlated with erythrocyte DHA composition, was associated with larger cortical gray matter volumes in several corticolimbic regions including the anterior cingulate cortex, hippocampus, and amygdala[262]. It is relevant, therefore, that patients with psychiatric disorders commonly exhibit gray matter volume deficits in the anterior cingulate cortex, hippocampus, and amygdala[267-269]. Similar to children and adolescents born preterm, patients with ADHD[270-275], mood disorders[276-283], and psychotic disorders[284-287] also exhibit decreased frontal white matter tract integrity and reduced functional connectivity within cortical networks. Together these findings support the hypothesis that perinatal deficits in DHA accrual may contribute to diminished cortical circuit development observed in major psychiatric disorders.
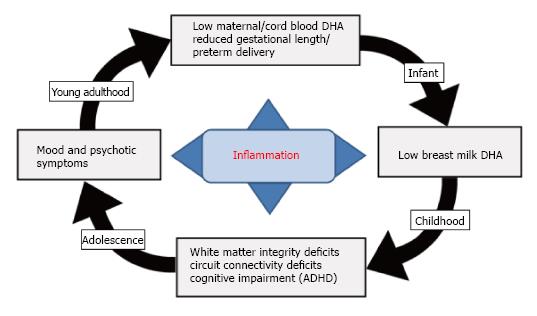
The pathogenic mechanisms underlying major psychiatric disorders are viewed as polygenic and multifactorial, and there is strong evidence for familial transmission and subtotal heritability estimates indicating the important contribution of shared to-be-defined environmental factors[288-293]. Reviewed evidence supports a hypothetical link between dietary LCn-3 fatty acid deficiency and the familial transmission of psychopathology (Figure 4). Specifically, observational and controlled studies suggest that maternal DHA status during pregnancy is an important determinant of gestational length and risk of preterm birth[294-299]. For example, in Japan where maternal DHA status based on breast milk DHA levels (1.1%) is approximately 6-fold higher than breast milk DHA levels in the United Sates (0.17%)[189], the prevalence rate of preterm birth is approximately one-third that observed in United Sates (Japan: 4.3%-5.0% vs United Sates: 11.7%)[300,301]. Importantly, adolescent and young adult females of childbearing potential with mood disorders residing in the United Sates exhibit significant blood DHA deficits compared with healthy women[231,235], and are at increased risk for preterm delivery[302-304]. Risk of preterm delivery is associated with maternal or intrauterine elevations in pro-inflammatory cytokines including interleukin-6 (IL-6)[305-307], and lower LCn-3 fatty acid intake and status is associated with higher serum IL-6 levels[308]. The very low DHA status exhibited by mothers with mood disorders would be anticipated to reduce fetal cortical DHA accrual in utero, increase maternal risk for preterm birth and associated deficits in third trimester fetal cortical DHA accrual, and reduce postnatal fetal DHA accrual secondary to low breast milk DHA levels. Based the reviewed evidence, such perinatal deficits in cortical DHA accrual would be predicted to impair cortical circuit maturation and increase the risk of developing psychopathology during childhood and adolescent development.
Over the past 30 years a body of evidence from animal and clinical studies supports the general assertion that normal brain development requires optimal DHA levels. Rodent studies suggest that cortical DHA has neurotrophic as well as neuroprotective properties in the developing and adult brain, and that dietary-induced reductions in perinatal rat brain DHA accrual are associated with deficits in synaptic maturation and functional plasticity. Deficits in perinatal rat brain DHA accrual also lead to impairments on neurocognitive tasks requiring activity-dependent synaptic plasticity. Decreases in F-actin cytoskeletal stability in pre- and post-synaptic terminals, as well as reduced resilience against neurotoxic, synaptotoxic, and myelinotoxic insults, represent plausible mediating mechanisms. Non-human primate studies further suggest a link between n-3 fatty acid deficiency during perinatal development and long-standing deficits in functional connectivity in cortical networks, hyperactivity, and impairments in visual attention. Evidence from animal studies therefore provide strong evidence for a role of perinatal DHA accrual for the normal maturation of cortical circuits and provide important clues into candidate molecular and ultrastructural mechanisms.
Additional evidence for a role of DHA in normal brain development comes from human studies finding that preterm birth, which results in deficits in third trimester fetal cortical DHA accrual, is associated with enduring prefrontal cortical network connectivity deficits, and a spectrum of neurocognitive impairments which may be mitigated by postnatal high-dose DHA supplementation. Preterm birth is also associated with increased risk for psychiatric disorders including ADHD, psychosis, and mood disorders which are associated with deficits in functional connectivity within cortical networks. More direct evidence is provided by prospective longitudinal studies finding that lower cord blood DHA levels are associated with the emergence of cognitive impairment, hyperactivity/inattention and emotional symptoms in children. Moreover, longer breastfeeding duration (a putative surrogate for early postnatal DHA intake) is associated with improvements in white matter microstructure and volume and better neurocognitive outcomes in childhood, adolescence, and adulthood. Taken in conjunction with non-human primate imaging data, these associations suggest that cortical DHA accrual during perinatal brain development may play a role in the maturation of human cortical networks mediating cognitive and emotional processes that are dysregulated in psychiatric disorders.
It is not currently known whether LCn-3 fatty acid supplementation alone is sufficient to reverse deficits in functional connectivity within cortical networks once established. Indeed, it is possible that cortical circuit maturation deficits secondary to LCn-3 fatty acid deficits may represent a permanent neurodevelopmental “scar” that is potentially irreversible once established. However, LCn-3 fatty acid supplementation has been found to reduce symptom severity in patients with psychiatric disorders and neurological and cognitive symptoms in preterm infants. Moreover, a controlled functional neuroimaging trial found that DHA supplementation increased cortical activity in prefrontal regions, and decreased activity in the temporal cortex and cerebellum, during performance of sustained attention task in healthy developing children[309]. While these findings suggest that increasing DHA status may augment functional connectivity within fronto-limbic networks, additional neuroimaging studies will be required to evaluate this potential therapeutic mechanism.
Although the role of perinatal brain DHA accrual on the maturation and long-term stability of cortical circuitry is only beginning to be fully understood, extant translational evidence suggests that DHA plays a role in the initial development and early maturation of cortical circuits. Emerging evidence from human neuroimaging studies further suggests that psychiatric disorders that initially emerge in childhood and adolescence and associated with low blood DHA levels are characterized by frontal circuit deficits compared with healthy developing youth. Moreover, maternal LCn-3 fatty acid deficiency is associated with increased risk of preterm birth, deficits in functional connectivity with cortical circuits, and ensuing cognitive impairments and mood dysregulation. These associations provide a neurobiological foundation and impetus for additional research to develop a more comprehensive understanding of the requirement for LCn-3 fatty acids during critical periods of neurodevelopment. Based on existing evidence, this research could have significant implications for informing novel early intervention strategies aimed at reducing the transmission of psychopathology.
This work was supported in part by National Institute of Health grants AG034617 and DK097599 to R.K.M., and 3KL2TR000078-0551 to C.J.V. R.K.M. has received research support from NARSAD, Martek Biosciences Inc, The Inflammation Research Foundation (IRF), Ortho-McNeil Janssen, AstraZeneca, Eli Lilly, and was a member of the IRF scientific advisory board. C.J.V. has received research support from the Perinatal Institute at Cincinnati Children’s Hospital.
P- Reviewer: Celikel FC, Yang YK S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull. 2001;56:79-85. |
| 2. | Connor WE, Neuringer M, Lin DS. Dietary effects on brain fatty acid composition: the reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J Lipid Res. 1990;31:237-247. |
| 3. | Chen CT, Liu Z, Ouellet M, Calon F, Bazinet RP. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins Leukot Essent Fatty Acids. 2009;80:157-163. |
| 4. | Barceló-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK. Flaxseed oil and fish-oil capsule consumption alters human red blood cell n-3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n-3 fatty acid. Am J Clin Nutr. 2008;88:801-809. |
| 5. | Francois CA, Connor SL, Bolewicz LC, Connor WE. Supplementing lactating women with flaxseed oil does not increase docosahexaenoic acid in their milk. Am J Clin Nutr. 2003;77:226-233. |
| 6. | Valentine CJ, Morrow G, Pennell M, Morrow AL, Hodge A, Haban-Bartz A, Collins K, Rogers LK. Randomized controlled trial of docosahexaenoic acid supplementation in midwestern U.S. human milk donors. Breastfeed Med. 2013;8:86-91. |
| 7. | Lin YH, Shah S, Salem N. Altered essential fatty acid metabolism and composition in rat liver, plasma, heart and brain after microalgal DHA addition to the diet. J Nutr Biochem. 2011;22:758-765. |
| 8. | Su HM, Bernardo L, Mirmiran M, Ma XH, Corso TN, Nathanielsz PW, Brenna JT. Bioequivalence of dietary alpha-linolenic and docosahexaenoic acids as sources of docosahexaenoate accretion in brain and associated organs of neonatal baboons. Pediatr Res. 1999;45:87-93. |
| 9. | Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:465-470. |
| 10. | Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908-1911. |
| 11. | Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587-597. |
| 12. | McNamara RK. Deciphering the role of docosahexaenoic acid in brain maturation and pathology with magnetic resonance imaging. Prostaglandins Leukot Essent Fatty Acids. 2013;88:33-42. |
| 13. | Green P, Yavin E. Fatty acid composition of late embryonic and early postnatal rat brain. Lipids. 1996;31:859-865. |
| 14. | Auestad N, Innis SM. Dietary n-3 fatty acid restriction during gestation in rats: neuronal cell body and growth-cone fatty acids. Am J Clin Nutr. 2000;71:312S-314S. |
| 15. | Innis SM, de La Presa Owens S. Dietary fatty acid composition in pregnancy alters neurite membrane fatty acids and dopamine in newborn rat brain. J Nutr. 2001;131:118-122. |
| 16. | Suzuki H, Manabe S, Wada O, Crawford MA. Rapid incorporation of docosahexaenoic acid from dietary sources into brain microsomal, synaptosomal and mitochondrial membranes in adult mice. Int J Vitam Nutr Res. 1997;67:272-278. |
| 17. | Yeh YY, Gehman MF, Yeh SM. Maternal dietary fish oil enriches docosahexaenoate levels in brain subcellular fractions of offspring. J Neurosci Res. 1993;35:218-226. |
| 18. | Martin RE. Docosahexaenoic acid decreases phospholipase A2 activity in the neurites/nerve growth cones of PC12 cells. J Neurosci Res. 1998;54:805-813. |
| 19. | Martin RE, Bazan NG. Changing fatty acid content of growth cone lipids prior to synaptogenesis. J Neurochem. 1992;59:318-325. |
| 20. | Ikemoto A, Nitta A, Furukawa S, Ohishi M, Nakamura A, Fujii Y, Okuyama H. Dietary n-3 fatty acid deficiency decreases nerve growth factor content in rat hippocampus. Neurosci Lett. 2000;285:99-102. |
| 21. | Rao JS, Ertley RN, Lee HJ, DeMar JC, Arnold JT, Rapoport SI, Bazinet RP. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36-46. |
| 22. | Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979-988. |
| 23. | Cao D, Xue R, Xu J, Liu Z. Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. J Nutr Biochem. 2005;16:538-546. |
| 24. | Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510-521. |
| 25. | Dagai L, Peri-Naor R, Birk RZ. Docosahexaenoic acid significantly stimulates immediate early response genes and neurite outgrowth. Neurochem Res. 2009;34:867-875. |
| 26. | Ikemoto A, Kobayashi T, Emoto K, Umeda M, Watanabe S, Okuyama H. Effects of docosahexaenoic and arachidonic acids on the synthesis and distribution of aminophospholipids during neuronal differentiation of PC12 cells. Arch Biochem Biophys. 1999;364:67-74. |
| 27. | Beltz BS, Tlusty MF, Benton JL, Sandeman DC. Omega-3 fatty acids upregulate adult neurogenesis. Neurosci Lett. 2007;415:154-158. |
| 28. | Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139:991-997. |
| 29. | Coti Bertrand P, O’Kusky JR, Innis SM. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr. 2006;136:1570-1575. |
| 30. | Yavin E, Himovichi E, Eilam R. Delayed cell migration in the developing rat brain following maternal omega 3 alpha linolenic acid dietary deficiency. Neuroscience. 2009;162:1011-1022. |
| 31. | Green P, Glozman S, Weiner L, Yavin E. Enhanced free radical scavenging and decreased lipid peroxidation in the rat fetal brain after treatment with ethyl docosahexaenoate. Biochim Biophys Acta. 2001;1532:203-212. |
| 32. | Tuzun F, Kumral A, Ozbal S, Dilek M, Tugyan K, Duman N, Ozkan H. Maternal prenatal omega-3 fatty acid supplementation attenuates hyperoxia-induced apoptosis in the developing rat brain. Int J Dev Neurosci. 2012;30:315-323. |
| 33. | Yavin E, Brand A, Green P. Docosahexaenoic acid abundance in the brain: a biodevice to combat oxidative stress. Nutr Neurosci. 2002;5:149-157. |
| 34. | Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke. 2009;40:3121-3126. |
| 35. | Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Polyunsaturated fatty acids induce ischemic and epileptic tolerance. Neuroscience. 2002;109:231-241. |
| 36. | Orr SK, Palumbo S, Bosetti F, Mount HT, Kang JX, Greenwood CE, Ma DW, Serhan CN, Bazinet RP. Unesterified docosahexaenoic acid is protective in neuroinflammation. J Neurochem. 2013;127:378-393. |
| 37. | Ozyurt B, Sarsilmaz M, Akpolat N, Ozyurt H, Akyol O, Herken H, Kus I. The protective effects of omega-3 fatty acids against MK-801-induced neurotoxicity in prefrontal cortex of rat. Neurochem Int. 2007;50:196-202. |
| 38. | Högyes E, Nyakas C, Kiliaan A, Farkas T, Penke B, Luiten PG. Neuroprotective effect of developmental docosahexaenoic acid supplement against excitotoxic brain damage in infant rats. Neuroscience. 2003;119:999-1012. |
| 39. | Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21:1457-1467. |
| 40. | Hamilton L, Greiner R, Salem N, Kim HY. n-3 fatty acid deficiency decreases phosphatidylserine accumulation selectively in neuronal tissues. Lipids. 2000;35:863-869. |
| 41. | Murthy M, Hamilton J, Greiner RS, Moriguchi T, Salem N, Kim HY. Differential effects of n-3 fatty acid deficiency on phospholipid molecular species composition in the rat hippocampus. J Lipid Res. 2002;43:611-617. |
| 42. | Chalon S, Delion-Vancassel S, Belzung C, Guilloteau D, Leguisquet AM, Besnard JC, Durand G. Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. J Nutr. 1998;128:2512-2519. |
| 43. | Huang KP, Huang FL. How is protein kinase C activated in CNS. Neurochem Int. 1993;22:417-433. |
| 44. | Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006;18:276-284. |
| 45. | Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992;356:618-622. |
| 46. | McLaughlin S, Aderem A. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem Sci. 1995;20:272-276. |
| 47. | McNamara RK, Ostrander M, Abplanalp W, Richtand NM, Benoit SC, Clegg DJ. Modulation of phosphoinositide-protein kinase C signal transduction by omega-3 fatty acids: implications for the pathophysiology and treatment of recurrent neuropsychiatric illness. Prostaglandins Leukot Essent Fatty Acids. 2006;75:237-257. |
| 48. | Glaser M, Wanaski S, Buser CA, Boguslavsky V, Rashidzada W, Morris A, Rebecchi M, Scarlata SF, Runnels LW, Prestwich GD. Myristoylated alanine-rich C kinase substrate (MARCKS) produces reversible inhibition of phospholipase C by sequestering phosphatidylinositol 4,5-bisphosphate in lateral domains. J Biol Chem. 1996;271:26187-26193. |
| 49. | Denys A, Hichami A, Maume B, Khan NA. Docosahexaenoic acid modulates phorbol ester-induced activation of extracellular signal-regulated kinases 1 and 2 in NIH/3T3 cells. Lipids. 2001;36:813-818. |
| 50. | Holian O, Nelson R. Action of long-chain fatty acids on protein kinase C activity: comparison of omega-6 and omega-3 fatty acids. Anticancer Res. 1992;12:975-980. |
| 51. | Mirnikjoo B, Brown SE, Kim HF, Marangell LB, Sweatt JD, Weeber EJ. Protein kinase inhibition by omega-3 fatty acids. J Biol Chem. 2001;276:10888-10896. |
| 52. | Seung Kim HF, Weeber EJ, Sweatt JD, Stoll AL, Marangell LB. Inhibitory effects of omega-3 fatty acids on protein kinase C activity in vitro. Mol Psychiatry. 2001;6:246-248. |
| 53. | Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48:77-90. |
| 54. | Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Ashe KH. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron. 2004;43:633-645. |
| 55. | He C, Qu X, Cui L, Wang J, Kang JX. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc Natl Acad Sci USA. 2009;106:11370-11375. |
| 56. | Sakamoto T, Cansev M, Wurtman RJ. Oral supplementation with docosahexaenoic acid and uridine-5’-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res. 2007;1182:50-59. |
| 57. | Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci USA. 2009;106:17957-17962. |
| 58. | Bauer MK, Lieb K, Schulze-Osthoff K, Berger M, Gebicke-Haerter PJ, Bauer J, Fiebich BL. Expression and regulation of cyclooxygenase-2 in rat microglia. Eur J Biochem. 1997;243:726-731. |
| 59. | Kondo S, Kohsaka S, Okabe S. Long-term changes of spine dynamics and microglia after transient peripheral immune response triggered by LPS in vivo. Mol Brain. 2011;4:27. |
| 60. | Vaughan PF, Walker JH, Peers C. The regulation of neurotransmitter secretion by protein kinase C. Mol Neurobiol. 1998;18:125-155. |
| 61. | Yoshida S, Yasuda A, Kawazato H, Sakai K, Shimada T, Takeshita M, Yuasa S, Kobayashi T, Watanabe S, Okuyama H. Synaptic vesicle ultrastructural changes in the rat hippocampus induced by a combination of alpha-linolenate deficiency and a learning task. J Neurochem. 1997;68:1261-1268. |
| 62. | Zimmer L, Delpal S, Guilloteau D, Aïoun J, Durand G, Chalon S. Chronic n-3 polyunsaturated fatty acid deficiency alters dopamine vesicle density in the rat frontal cortex. Neurosci Lett. 2000;284:25-28. |
| 63. | Kodas E, Vancassel S, Lejeune B, Guilloteau D, Chalon S. Reversibility of n-3 fatty acid deficiency-induced changes in dopaminergic neurotransmission in rats: critical role of developmental stage. J Lipid Res. 2002;43:1209-1219. |
| 64. | Zimmer L, Vancassel S, Cantagrel S, Breton P, Delamanche S, Guilloteau D, Durand G, Chalon S. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am J Clin Nutr. 2002;75:662-667. |
| 65. | Zimmer L, Delion-Vancassel S, Durand G, Guilloteau D, Bodard S, Besnard JC, Chalon S. Modification of dopamine neurotransmission in the nucleus accumbens of rats deficient in n-3 polyunsaturated fatty acids. J Lipid Res. 2000;41:32-40. |
| 66. | Kodas E, Galineau L, Bodard S, Vancassel S, Guilloteau D, Besnard JC, Chalon S. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J Neurochem. 2004;89:695-702. |
| 67. | Aïd S, Vancassel S, Linard A, Lavialle M, Guesnet P. Dietary docosahexaenoic acid [22: 6(n-3)] as a phospholipid or a triglyceride enhances the potassium chloride-evoked release of acetylcholine in rat hippocampus. J Nutr. 2005;135:1008-1013. |
| 68. | Aïd S, Vancassel S, Poumès-Ballihaut C, Chalon S, Guesnet P, Lavialle M. Effect of a diet-induced n-3 PUFA depletion on cholinergic parameters in the rat hippocampus. J Lipid Res. 2003;44:1545-1551. |
| 69. | Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci. 1998;18:5901-5907. |
| 70. | Rosa Neto P, Lou H, Cumming P, Pryds O, Gjedde A. Methylphenidate-evoked potentiation of extracellular dopamine in the brain of adolescents with premature birth: correlation with attentional deficit. Ann N Y Acad Sci. 2002;965:434-439. |
| 71. | Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151-1159. |
| 72. | Stein DJ. Depression, anhedonia, and psychomotor symptoms: the role of dopaminergic neurocircuitry. CNS Spectr. 2008;13:561-565. |
| 73. | Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, Logan J, Wong C, Ma Y, Swanson JM. Brain dopamine transporter levels in treatment and drug naïve adults with ADHD. Neuroimage. 2007;34:1182-1190. |
| 74. | Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443-453. |
| 75. | Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85-102. |
| 76. | Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649-684. |
| 77. | Sarter M, Bruno JP. Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: differential afferent regulation of the basal forebrain by telencephalic and brainstem afferents. Neuroscience. 2000;95:933-952. |
| 78. | Bourne JN, Harris KM. Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP. Hippocampus. 2011;21:354-373. |
| 79. | Geinisman Y, Detoledo-Morrell L, Morrell F, Persina IS, Beatty MA. Synapse restructuring associated with the maintenance phase of hippocampal long-term potentiation. J Comp Neurol. 1996;368:413-423. |
| 80. | Muller D, Toni N, Buchs PA. Spine changes associated with long-term potentiation. Hippocampus. 2000;10:596-604. |
| 81. | Jedlicka P, Vlachos A, Schwarzacher SW, Deller T. A role for the spine apparatus in LTP and spatial learning. Behav Brain Res. 2008;192:12-19. |
| 82. | De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog Brain Res. 2008;169:199-207. |
| 83. | Fedorova I, Hussein N, Baumann MH, Di Martino C, Salem N. An n-3 fatty acid deficiency impairs rat spatial learning in the Barnes maze. Behav Neurosci. 2009;123:196-205. |
| 84. | Moriguchi T, Greiner RS, Salem N. Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem. 2000;75:2563-2573. |
| 85. | Moriguchi T, Salem N. Recovery of brain docosahexaenoate leads to recovery of spatial task performance. J Neurochem. 2003;87:297-309. |
| 86. | Greiner RS, Moriguchi T, Hutton A, Slotnick BM, Salem N. Rats with low levels of brain docosahexaenoic acid show impaired performance in olfactory-based and spatial learning tasks. Lipids. 1999;34 Suppl:S239-S243. |
| 87. | Greiner RS, Moriguchi T, Slotnick BM, Hutton A, Salem N. Olfactory discrimination deficits in n-3 fatty acid-deficient rats. Physiol Behav. 2001;72:379-385. |
| 88. | Hichami A, Datiche F, Ullah S, Liénard F, Chardigny JM, Cattarelli M, Khan NA. Olfactory discrimination ability and brain expression of c-fos, Gir and Glut1 mRNA are altered in n-3 fatty acid-depleted rats. Behav Brain Res. 2007;184:1-10. |
| 89. | Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol. 2010;518:2693-2709. |
| 90. | de Velasco PC, Mendonça HR, Borba JM, Andrade da Costa BL, Guedes RC, Navarro DM, Santos GK, Faria-Melibeu Ada C, Campello Costa P, Serfaty CA. Nutritional restriction of omega-3 fatty acids alters topographical fine tuning and leads to a delay in the critical period in the rodent visual system. Exp Neurol. 2012;234:220-229. |
| 91. | Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456-1458. |
| 92. | Schafer DP, Stevens B. Phagocytic glial cells: sculpting synaptic circuits in the developing nervous system. Curr Opin Neurobiol. 2013;23:1034-1040. |
| 93. | Hjorth E, Zhu M, Toro VC, Vedin I, Palmblad J, Cederholm T, Freund-Levi Y, Faxen-Irving G, Wahlund LO, Basun H. Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J Alzheimers Dis. 2013;35:697-713. |
| 94. | Madore C, Nadjar A, Delpech JC, Sere A, Aubert A, Portal C, Joffre C, Layé S. Nutritional n-3 PUFAs deficiency during perinatal periods alters brain innate immune system and neuronal plasticity-associated genes. Brain Behav Immun. 2014;41:22-31. |
| 95. | Haubner L, Sullivan J, Ashmeade T, Saste M, Wiener D, Carver J. The effects of maternal dietary docosahexaenoic acid intake on rat pup myelin and the auditory startle response. Dev Neurosci. 2007;29:460-467. |
| 96. | Salvati S, Natali F, Attorri L, Di Benedetto R, Leonardi F, Di Biase A, Ferri F, Fortuna S, Lorenzini P, Sanchez M. Eicosapentaenoic acid stimulates the expression of myelin proteins in rat brain. J Neurosci Res. 2008;86:776-784. |
| 97. | Church MW, Jen KL, Dowhan LM, Adams BR, Hotra JW. Excess and deficient omega-3 fatty acid during pregnancy and lactation cause impaired neural transmission in rat pups. Neurotoxicol Teratol. 2008;30:107-117. |
| 98. | Church MW, Jen KL, Jackson DA, Adams BR, Hotra JW. Abnormal neurological responses in young adult offspring caused by excess omega-3 fatty acid (fish oil) consumption by the mother during pregnancy and lactation. Neurotoxicol Teratol. 2009;31:26-33. |
| 99. | Tuzun F, Kumral A, Dilek M, Ozbal S, Ergur B, Yesilirmak DC, Duman N, Yilmaz O, Ozkan H. Maternal omega-3 fatty acid supplementation protects against lipopolysaccharide-induced white matter injury in the neonatal rat brain. J Matern Fetal Neonatal Med. 2012;25:849-854. |
| 100. | Bailes JE, Mills JD. Docosahexaenoic acid reduces traumatic axonal injury in a rodent head injury model. J Neurotrauma. 2010;27:1617-1624. |
| 101. | Ward RE, Huang W, Curran OE, Priestley JV, Michael-Titus AT. Docosahexaenoic acid prevents white matter damage after spinal cord injury. J Neurotrauma. 2010;27:1769-1780. |
| 102. | Kong W, Yen JH, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav Immun. 2011;25:872-882. |
| 103. | Anderson GJ, Neuringer M, Lin DS, Connor WE. Can prenatal N-3 fatty acid deficiency be completely reversed after birth? Effects on retinal and brain biochemistry and visual function in rhesus monkeys. Pediatr Res. 2005;58:865-872. |
| 104. | Neuringer M, Connor WE, Lin DS, Barstad L, Luck S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci USA. 1986;83:4021-4025. |
| 105. | Sarkadi-Nagy E, Wijendran V, Diau GY, Chao AC, Hsieh AT, Turpeinen A, Lawrence P, Nathanielsz PW, Brenna JT. Formula feeding potentiates docosahexaenoic and arachidonic acid biosynthesis in term and preterm baboon neonates. J Lipid Res. 2004;45:71-80. |
| 106. | Sarkadi-Nagy E, Wijendran V, Diau GY, Chao AC, Hsieh AT, Turpeinen A, Nathanielsz PW, Brenna JT. The influence of prematurity and long chain polyunsaturate supplementation in 4-week adjusted age baboon neonate brain and related tissues. Pediatr Res. 2003;54:244-252. |
| 107. | Reisbick S, Neuringer M, Gohl E, Wald R, Anderson GJ. Visual attention in infant monkeys: effects of dietary fatty acids and age. Dev Psychol. 1997;33:387-395. |
| 108. | Reisbick S, Neuringer M, Hasnain R, Connor WE. Polydipsia in rhesus monkeys deficient in omega-3 fatty acids. Physiol Behav. 1990;47:315-323. |
| 109. | Diau GY, Loew ER, Wijendran V, Sarkadi-Nagy E, Nathanielsz PW, Brenna JT. Docosahexaenoic and arachidonic acid influence on preterm baboon retinal composition and function. Invest Ophthalmol Vis Sci. 2003;44:4559-4566. |
| 110. | Reisbick S, Neuringer M, Hasnain R, Connor WE. Home cage behavior of rhesus monkeys with long-term deficiency of omega-3 fatty acids. Physiol Behav. 1994;55:231-239. |
| 111. | Grayson DS, Kroenke CD, Neuringer M, Fair DA. Dietary omega-3 fatty acids modulate large-scale systems organization in the rhesus macaque brain. J Neurosci. 2014;34:2065-2074. |
| 112. | Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261-1279. |
| 113. | Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev. 1980;4:121-129. |
| 114. | Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr. 1992;120:S129-S138. |
| 115. | Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48-71. |
| 116. | Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757-767. |
| 117. | Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Extrauterine fatty acid accretion in infant brain: implications for fatty acid requirements. Early Hum Dev. 1980;4:131-138. |
| 118. | Sanders TA, Naismith DJ. A comparison of the influence of breast-feeding and bottle-feeding on the fatty acid composition of the erythrocytes. Br J Nutr. 1979;41:619-623. |
| 119. | Putnam JC, Carlson SE, DeVoe PW, Barness LA. The effect of variations in dietary fatty acids on the fatty acid composition of erythrocyte phosphatidylcholine and phosphatidylethanolamine in human infants. Am J Clin Nutr. 1982;36:106-114. |
| 120. | Auestad N, Montalto MB, Hall RT, Fitzgerald KM, Wheeler RE, Connor WE, Neuringer M, Connor SL, Taylor JA, Hartmann EE. Visual acuity, erythrocyte fatty acid composition, and growth in term infants fed formulas with long chain polyunsaturated fatty acids for one year. Ross Pediatric Lipid Study. Pediatr Res. 1997;41:1-10. |
| 121. | Byard RW, Makrides M, Need M, Neumann MA, Gibson RA. Sudden infant death syndrome: effect of breast and formula feeding on frontal cortex and brainstem lipid composition. J Paediatr Child Health. 1995;31:14-16. |
| 122. | Farquharson J, Jamieson EC, Abbasi KA, Patrick WJ, Logan RW, Cockburn F. Effect of diet on the fatty acid composition of the major phospholipids of infant cerebral cortex. Arch Dis Child. 1995;72:198-203. |
| 123. | Gibson RA, Neumann MA, Makrides M. Effect of dietary docosahexaenoic acid on brain composition and neural function in term infants. Lipids. 1996;31 Suppl:S177-S181. |
| 124. | Jamieson EC, Farquharson J, Logan RW, Howatson AG, Patrick WJ, Weaver LT, Cockburn F. Infant cerebellar gray and white matter fatty acids in relation to age and diet. Lipids. 1999;34:1065-1071. |
| 125. | Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr. 1994;60:189-194. |
| 126. | Conklin HM, Luciana M, Hooper CJ, Yarger RS. Working memory performance in typically developing children and adolescents: behavioral evidence of protracted frontal lobe development. Dev Neuropsychol. 2007;31:103-128. |
| 127. | Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149:582-591. |
| 128. | Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195-205. |
| 129. | Petanjek Z, Judaš M, Šimic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281-13286. |
| 130. | Ducharme S, Hudziak JJ, Botteron KN, Albaugh MD, Nguyen TV, Karama S, Evans AC. Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. J Am Acad Child Adolesc Psychiatry. 2012;51:18-27.e2. |
| 131. | Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex. 2007;17:2760-2768. |
| 132. | Farquharson J, Cockburn F, Patrick WA, Jamieson EC, Logan RW. Infant cerebral cortex phospholipid fatty-acid composition and diet. Lancet. 1992;340:810-813. |
| 133. | Martínez M, Mougan I. Fatty acid composition of human brain phospholipids during normal development. J Neurochem. 1998;71:2528-2533. |
| 134. | Giménez M, Junqué C, Vendrell P, Narberhaus A, Bargalló N, Botet F, Mercader JM. Abnormal orbitofrontal development due to prematurity. Neurology. 2006;67:1818-1822. |
| 135. | Healy E, Reichenberg A, Nam KW, Allin MP, Walshe M, Rifkin L, Murray SR, Nosarti C. Preterm birth and adolescent social functioning-alterations in emotion-processing brain areas. J Pediatr. 2013;163:1596-1604. |
| 136. | Isaacs EB, Lucas A, Chong WK, Wood SJ, Johnson CL, Marshall C, Vargha-Khadem F, Gadian DG. Hippocampal volume and everyday memory in children of very low birth weight. Pediatr Res. 2000;47:713-720. |
| 137. | Kesler SR, Reiss AL, Vohr B, Watson C, Schneider KC, Katz KH, Maller-Kesselman J, Silbereis J, Constable RT, Makuch RW. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008;152:513-520, 520.e1. |
| 138. | Nosarti C, Al-Asady MH, Frangou S, Stewart AL, Rifkin L, Murray RM. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125:1616-1623. |
| 139. | Ment LR, Kesler S, Vohr B, Katz KH, Baumgartner H, Schneider KC, Delancy S, Silbereis J, Duncan CC, Constable RT. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics. 2009;123:503-511. |
| 140. | Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939-1947. |
| 141. | Skranes J, Løhaugen GC, Evensen KA, Indredavik MS, Haraldseth O, Dale AM, Brubakk AM, Martinussen M. Entorhinal cortical thinning affects perceptual and cognitive functions in adolescents born preterm with very low birth weight (VLBW). Early Hum Dev. 2012;88:103-109. |
| 142. | Stewart AL, Rifkin L, Amess PN, Kirkbride V, Townsend JP, Miller DH, Lewis SW, Kingsley DP, Moseley IF, Foster O. Brain structure and neurocognitive and behavioural function in adolescents who were born very preterm. Lancet. 1999;353:1653-1657. |
| 143. | Bäuml JG, Daamen M, Meng C, Neitzel J, Scheef L, Jaekel J, Busch B, Baumann N, Bartmann P, Wolke D. Correspondence Between Aberrant Intrinsic Network Connectivity and Gray-Matter Volume in the Ventral Brain of Preterm Born Adults. Cereb Cortex. 2014;Jun 16; Epub ahead of print. |
| 144. | Allin MP, Kontis D, Walshe M, Wyatt J, Barker GJ, Kanaan RA, McGuire P, Rifkin L, Murray RM, Nosarti C. White matter and cognition in adults who were born preterm. PLoS One. 2011;6:e24525. |
| 145. | Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, Delancy S, Katz KH, Schneider KC, Schafer RJ. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121:306-316. |
| 146. | Giménez M, Junqué C, Narberhaus A, Bargalló N, Botet F, Mercader JM. White matter volume and concentration reductions in adolescents with history of very preterm birth: a voxel-based morphometry study. Neuroimage. 2006;32:1485-1498. |
| 147. | Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz KH, Maller-Kesselman J, Schneider KC, Peterson BS, Rajeevan N, Makuch RW. Alterations in neural connectivity in preterm children at school age. Neuroimage. 2009;48:458-463. |
| 148. | Lubsen J, Vohr B, Myers E, Hampson M, Lacadie C, Schneider KC, Katz KH, Constable RT, Ment LR. Microstructural and functional connectivity in the developing preterm brain. Semin Perinatol. 2011;35:34-43. |
| 149. | Mullen KM, Vohr BR, Katz KH, Schneider KC, Lacadie C, Hampson M, Makuch RW, Reiss AL, Constable RT, Ment LR. Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage. 2011;54:2563-2570. |
| 150. | Nagy Z, Westerberg H, Skare S, Andersson JL, Lilja A, Flodmark O, Fernell E, Holmberg K, Bohm B, Forssberg H. Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatr Res. 2003;54:672-679. |
| 151. | Schafer RJ, Lacadie C, Vohr B, Kesler SR, Katz KH, Schneider KC, Pugh KR, Makuch RW, Reiss AL, Constable RT. Alterations in functional connectivity for language in prematurely born adolescents. Brain. 2009;132:661-670. |
| 152. | Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KA, Martinussen M, Dale AM, Haraldseth O, Brubakk AM. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130:654-666. |
| 153. | Smyser CD, Snyder AZ, Shimony JS, Blazey TM, Inder TE, Neil JJ. Effects of white matter injury on resting state fMRI measures in prematurely born infants. PLoS One. 2013;8:e68098. |
| 154. | Vangberg TR, Skranes J, Dale AM, Martinussen M, Brubakk AM, Haraldseth O. Changes in white matter diffusion anisotropy in adolescents born prematurely. Neuroimage. 2006;32:1538-1548. |
| 155. | White TP, Symington I, Castellanos NP, Brittain PJ, Froudist Walsh S, Nam KW, Sato JR, Allin MP, Shergill SS, Murray RM. Dysconnectivity of neurocognitive networks at rest in very-preterm born adults. Neuroimage Clin. 2014;4:352-365. |
| 156. | Eikenes L, Løhaugen GC, Brubakk AM, Skranes J, Håberg AK. Young adults born preterm with very low birth weight demonstrate widespread white matter alterations on brain DTI. Neuroimage. 2011;54:1774-1785. |
| 157. | Valerdiz Casasola S, Pardo Mindan J. Cystadenofibroma of fallopian tube. Appl Pathol. 1989;7:256-259. |
| 158. | Loe IM, Lee ES, Feldman HM. Attention and internalizing behaviors in relation to white matter in children born preterm. J Dev Behav Pediatr. 2013;34:156-164. |
| 159. | Northam GB, Liégeois F, Chong WK, Wyatt JS, Baldeweg T. Total brain white matter is a major determinant of IQ in adolescents born preterm. Ann Neurol. 2011;69:702-711. |
| 160. | Northam GB, Liégeois F, Chong WK, Baker K, Tournier JD, Wyatt JS, Baldeweg T, Morgan A. Speech and oromotor outcome in adolescents born preterm: relationship to motor tract integrity. J Pediatr. 2012;160:402-408.e1. |
| 161. | Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288:728-737. |
| 162. | Foulder-Hughes LA, Cooke RW. Motor, cognitive, and behavioural disorders in children born very preterm. Dev Med Child Neurol. 2003;45:97-103. |
| 163. | Botting N, Powls A, Cooke RW, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years. J Child Psychol Psychiatry. 1997;38:931-941. |
| 164. | Indredavik MS, Vik T, Heyerdahl S, Kulseng S, Fayers P, Brubakk AM. Psychiatric symptoms and disorders in adolescents with low birth weight. Arch Dis Child Fetal Neonatal Ed. 2004;89:F445-F450. |
| 165. | Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. J Am Acad Child Adolesc Psychiatry. 2010;49:453-463.e1. |
| 166. | Costello EJ, Worthman C, Erkanli A, Angold A. Prediction from low birth weight to female adolescent depression: a test of competing hypotheses. Arch Gen Psychiatry. 2007;64:338-344. |
| 167. | Indredavik MS, Vik T, Evensen KA, Skranes J, Taraldsen G, Brubakk AM. Perinatal risk and psychiatric outcome in adolescents born preterm with very low birth weight or term small for gestational age. J Dev Behav Pediatr. 2010;31:286-294. |
| 168. | Lindström K, Lindblad F, Hjern A. Psychiatric morbidity in adolescents and young adults born preterm: a Swedish national cohort study. Pediatrics. 2009;123:e47-e53. |
| 169. | Nosarti C, Reichenberg A, Murray RM, Cnattingius S, Lambe MP, Yin L, MacCabe J, Rifkin L, Hultman CM. Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry. 2012;69:E1-E8. |
| 170. | Patton GC, Coffey C, Carlin JB, Olsson CA, Morley R. Prematurity at birth and adolescent depressive disorder. Br J Psychiatry. 2004;184:446-447. |
| 171. | Carlson SE, Werkman SH. A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until two months. Lipids. 1996;31:85-90. |
| 172. | Henriksen C, Haugholt K, Lindgren M, Aurvåg AK, Rønnestad A, Grønn M, Solberg R, Moen A, Nakstad B, Berge RK. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics. 2008;121:1137-1145. |
| 173. | Smithers LG, Gibson RA, McPhee A, Makrides M. Higher dose of docosahexaenoic acid in the neonatal period improves visual acuity of preterm infants: results of a randomized controlled trial. Am J Clin Nutr. 2008;88:1049-1056. |
| 174. | Westerberg AC, Schei R, Henriksen C, Smith L, Veierød MB, Drevon CA, Iversen PO. Attention among very low birth weight infants following early supplementation with docosahexaenoic and arachidonic acid. Acta Paediatr. 2011;100:47-52. |
| 175. | Simmer K, Schulzke SM, Patole S. Long-chain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst Rev. 2008;CD000375. |
| 176. | Smithers LG, Gibson RA, McPhee A, Makrides M. Effect of two doses of docosahexaenoic acid (DHA) in the diet of preterm infants on infant fatty acid status: results from the DINO trial. Prostaglandins Leukot Essent Fatty Acids. 2008;79:141-146. |
| 177. | van Wezel-Meijler G, van der Knaap MS, Huisman J, Jonkman EJ, Valk J, Lafeber HN. Dietary supplementation of long-chain polyunsaturated fatty acids in preterm infants: effects on cerebral maturation. Acta Paediatr. 2002;91:942-950. |
| 178. | Martinez M, Vazquez E. MRI evidence that docosahexaenoic acid ethyl ester improves myelination in generalized peroxisomal disorders. Neurology. 1998;51:26-32. |
| 179. | Lapillonne A, Groh-Wargo S, Gonzalez CH, Uauy R. Lipid needs of preterm infants: updated recommendations. J Pediatr. 2013;162:S37-S47. |
| 180. | Bakker EC, Ghys AJ, Kester AD, Vles JS, Dubas JS, Blanco CE, Hornstra G. Long-chain polyunsaturated fatty acids at birth and cognitive function at 7 y of age. Eur J Clin Nutr. 2003;57:89-95. |
| 181. | Bakker EC, Hornstra G, Blanco CE, Vles JS. Relationship between long-chain polyunsaturated fatty acids at birth and motor function at 7 years of age. Eur J Clin Nutr. 2009;63:499-504. |
| 182. | Escolano-Margarit MV, Ramos R, Beyer J, Csábi G, Parrilla-Roure M, Cruz F, Perez-Garcia M, Hadders-Algra M, Gil A, Decsi T. Prenatal DHA status and neurological outcome in children at age 5.5 years are positively associated. J Nutr. 2011;141:1216-1223. |
| 183. | Jacques C, Levy E, Muckle G, Jacobson SW, Bastien C, Dewailly E, Ayotte P, Jacobson JL, Saint-Amour D. Long-term effects of prenatal omega-3 fatty acid intake on visual function in school-age children. J Pediatr. 2011;158:83-90, 90.e1. |
| 184. | Boucher O, Burden MJ, Muckle G, Saint-Amour D, Ayotte P, Dewailly E, Nelson CA, Jacobson SW, Jacobson JL. Neurophysiologic and neurobehavioral evidence of beneficial effects of prenatal omega-3 fatty acid intake on memory function at school age. Am J Clin Nutr. 2011;93:1025-1037. |
| 185. | Kohlboeck G, Glaser C, Tiesler C, Demmelmair H, Standl M, Romanos M, Koletzko B, Lehmann I, Heinrich J. Effect of fatty acid status in cord blood serum on children’s behavioral difficulties at 10 y of age: results from the LISAplus Study. Am J Clin Nutr. 2011;94:1592-1599. |
| 186. | Krabbendam L, Bakker E, Hornstra G, van Os J. Relationship between DHA status at birth and child problem behaviour at 7 years of age. Prostaglandins Leukot Essent Fatty Acids. 2007;76:29-34. |
| 187. | Lauritzen L, Jørgensen MH, Hansen HS, Michaelsen KF. Fluctuations in human milk long-chain PUFA levels in relation to dietary fish intake. Lipids. 2002;37:237-244. |
| 188. | Jensen CL, Maude M, Anderson RE, Heird WC. Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids. Am J Clin Nutr. 2000;71:292S-299S. |
| 189. | Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85:1457-1464. |
| 190. | Deoni SC, Dean DC, Piryatinsky I, O’Muircheartaigh J, Waskiewicz N, Lehman K, Han M, Dirks H. Breastfeeding and early white matter development: A cross-sectional study. Neuroimage. 2013;82:77-86. |
| 191. | Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gadian DG, Lucas A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res. 2010;67:357-362. |
| 192. | Horwood LJ, Darlow BA, Mogridge N. Breast milk feeding and cognitive ability at 7-8 years. Arch Dis Child Fetal Neonatal Ed. 2001;84:F23-F27. |
| 193. | Mortensen EL, Michaelsen KF, Sanders SA, Reinisch JM. The association between duration of breastfeeding and adult intelligence. JAMA. 2002;287:2365-2371. |
| 194. | Oddy WH, Kendall GE, Blair E, De Klerk NH, Stanley FJ, Landau LI, Silburn S, Zubrick S. Breast feeding and cognitive development in childhood: a prospective birth cohort study. Paediatr Perinat Epidemiol. 2003;17:81-90. |
| 195. | Julvez J, Ribas-Fitó N, Forns M, Garcia-Esteban R, Torrent M, Sunyer J. Attention behaviour and hyperactivity at age 4 and duration of breast-feeding. Acta Paediatr. 2007;96:842-847. |
| 196. | Kadziela-Olech H, Piotrowska-Jastrzebska J. The duration of breastfeeding and attention deficit hyperactivity disorder. Rocz Akad Med Bialymst. 2005;50:302-306. |
| 197. | Mimouni-Bloch A, Kachevanskaya A, Mimouni FB, Shuper A, Raveh E, Linder N. Breastfeeding may protect from developing attention-deficit/hyperactivity disorder. Breastfeed Med. 2013;8:363-367. |
| 198. | Shamberger R. Attention-deficit disorder associated with breast-feeding: a brief report. J Am Coll Nutr. 2012;31:239-242. |
| 199. | Burke KC, Burke JD, Rae DS, Regier DA. Comparing age at onset of major depression and other psychiatric disorders by birth cohorts in five US community populations. Arch Gen Psychiatry. 1991;48:789-795. |
| 200. | Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602. |
| 201. | Perlis RH, Dennehy EB, Miklowitz DJ, Delbello MP, Ostacher M, Calabrese JR, Ametrano RM, Wisniewski SR, Bowden CL, Thase ME. Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: results from the STEP-BD study. Bipolar Disord. 2009;11:391-400. |
| 202. | Liston C, Malter Cohen M, Teslovich T, Levenson D, Casey BJ. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point? Biol Psychiatry. 2011;69:1168-1177. |
| 203. | Cocchi L, Bramati IE, Zalesky A, Furukawa E, Fontenelle LF, Moll J, Tripp G, Mattos P. Altered functional brain connectivity in a non-clinical sample of young adults with attention-deficit/hyperactivity disorder. J Neurosci. 2012;32:17753-17761. |
| 204. | Pannekoek JN, van der Werff SJ, Meens PH, van den Bulk BG, Jolles DD, Veer IM, van Lang ND, Rombouts SA, van der Wee NJ, Vermeiren RR. Aberrant resting-state functional connectivity in limbic and salience networks in treatment--naïve clinically depressed adolescents. J Child Psychol Psychiatry. 2014;55:1317-1327. |
| 205. | Benson BE, Willis MW, Ketter TA, Speer A, Kimbrell TA, Herscovitch P, George MS, Post RM. Differential abnormalities of functional connectivity of the amygdala and hippocampus in unipolar and bipolar affective disorders. J Affect Disord. 2014;168:243-253. |
| 206. | Karbasforoushan H, Woodward ND. Resting-state networks in schizophrenia. Curr Top Med Chem. 2012;12:2404-2414. |
| 207. | Passarotti AM, Ellis J, Wegbreit E, Stevens MC, Pavuluri MN. Reduced functional connectivity of prefrontal regions and amygdala within affect and working memory networks in pediatric bipolar disorder. Brain Connect. 2012;2:320-334. |
| 208. | Townsend JD, Torrisi SJ, Lieberman MD, Sugar CA, Bookheimer SY, Altshuler LL. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry. 2013;73:127-135. |
| 209. | Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, DelBello MP, Frangou S, McIntosh A, Phillips ML. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313-325. |
| 210. | Fekete K, Marosvölgyi T, Jakobik V, Decsi T. Methods of assessment of n-3 long-chain polyunsaturated fatty acid status in humans: a systematic review. Am J Clin Nutr. 2009;89:2070S-2084S. |
| 211. | Itomura M, Fujioka S, Hamazaki K, Kobayashi K, Nagasawa T, Sawazaki S, Kirihara Y, Hamazaki T. Factors influencing EPA+DHA levels in red blood cells in Japan. In Vivo. 2008;22:131-135. |
| 212. | Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343-347. |
| 214. | Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222-2227. |
| 215. | Peet M. International variations in the outcome of schizophrenia and the prevalence of depression in relation to national dietary practices: an ecological analysis. Br J Psychiatry. 2004;184:404-408. |
| 216. | Astorg P, Couthouis A, Bertrais S, Arnault N, Meneton P, Guesnet P, Alessandri JM, Galan P, Hercberg S. Association of fish and long-chain n-3 polyunsaturated fatty acid intakes with the occurrence of depressive episodes in middle-aged French men and women. Prostaglandins Leukot Essent Fatty Acids. 2008;78:171-182. |
| 217. | Colangelo LA, He K, Whooley MA, Daviglus ML, Liu K. Higher dietary intake of long-chain omega-3 polyunsaturated fatty acids is inversely associated with depressive symptoms in women. Nutrition. 2009;25:1011-1019. |
| 218. | Murakami K, Miyake Y, Sasaki S, Tanaka K, Arakawa M. Fish and n-3 polyunsaturated fatty acid intake and depressive symptoms: Ryukyus Child Health Study. Pediatrics. 2010;126:e623-e630. |
| 219. | Raeder MB, Steen VM, Vollset SE, Bjelland I. Associations between cod liver oil use and symptoms of depression: the Hordaland Health Study. J Affect Disord. 2007;101:245-249. |
| 220. | Tanskanen A, Hibbeln JR, Tuomilehto J, Uutela A, Haukkala A, Viinamäki H, Lehtonen J, Vartiainen E. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv. 2001;52:529-531. |
| 221. | Timonen M, Horrobin D, Jokelainen J, Laitinen J, Herva A, Räsänen P. Fish consumption and depression: the Northern Finland 1966 birth cohort study. J Affect Disord. 2004;82:447-452. |
| 222. | Clayton EH, Hanstock TL, Watson JF. Estimated intakes of meat and fish by children and adolescents in Australia and comparison with recommendations. Br J Nutr. 2009;101:1731-1735. |
| 223. | Harel Z, Riggs S, Vaz R, White L, Menzies G. Omega-3 polyunsaturated fatty acids in adolescents: knowledge and consumption. J Adolesc Health. 2001;28:10-15. |
| 224. | Lauritzen L, Harsløf LB, Hellgren LI, Pedersen MH, Mølgaard C, Michaelsen KF. Fish intake, erythrocyte n-3 fatty acid status and metabolic health in Danish adolescent girls and boys. Br J Nutr. 2012;107:697-704. |
| 225. | Sichert-Hellert W, Wicher M, Kersting M. Age and time trends in fish consumption pattern of children and adolescents, and consequences for the intake of long-chain n-3 polyunsaturated fatty acids. Eur J Clin Nutr. 2009;63:1071-1075. |
| 226. | Allen KL, Mori TA, Beilin L, Byrne SM, Hickling S, Oddy WH. Dietary intake in population-based adolescents: support for a relationship between eating disorder symptoms, low fatty acid intake and depressive symptoms. J Hum Nutr Diet. 2013;26:459-469. |
| 227. | Oddy WH, Hickling S, Smith MA, O’Sullivan TA, Robinson M, de Klerk NH, Beilin LJ, Mori TA, Syrette J, Zubrick SR. Dietary intake of omega-3 fatty acids and risk of depressive symptoms in adolescents. Depress Anxiety. 2011;28:582-588. |
| 228. | Swenne I, Rosling A, Tengblad S, Vessby B. Omega-3 polyunsaturated essential fatty acids are associated with depression in adolescents with eating disorders and weight loss. Acta Paediatr. 2011;100:1610-1615. |
| 229. | Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140-147. |
| 230. | Chiu CC, Huang SY, Su KP, Lu ML, Huang MC, Chen CC, Shen WW. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol. 2003;13:99-103. |
| 231. | McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126:303-311. |
| 232. | Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, Wagh UV, Debsikdar VB, Mahadik SP. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109-122. |
| 233. | Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids. 2008;43:1031-1038. |
| 234. | Pottala JV, Talley JA, Churchill SW, Lynch DA, von Schacky C, Harris WS. Red blood cell fatty acids are associated with depression in a case-control study of adolescents. Prostaglandins Leukot Essent Fatty Acids. 2012;86:161-165. |
| 235. | McNamara RK, Strimpfel J, Jandacek R, Rider T, Tso P, Welge JA, Strawn JR, DelBello MP. Detection and Treatment of Long-Chain Omega-3 Fatty Acid Deficiency in Adolescents with SSRI-Resistant Major Depressive Disorder. PharmaNutrition. 2014;2:38-46. |
| 236. | Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr Res. 2002;58:1-10. |
| 237. | Reddy RD, Keshavan MS, Yao JK. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophr Bull. 2004;30:901-911. |
| 238. | Hoen WP, Lijmer JG, Duran M, Wanders RJ, van Beveren NJ, de Haan L. Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: a meta-analysis. Psychiatry Res. 2013;207:1-12. |
| 239. | Hawkey E, Nigg JT. Omega-3 fatty acid and ADHD: blood level analysis and meta-analytic extension of supplementation trials. Clin Psychol Rev. 2014;34:496-505. |
| 240. | Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146-154. |
| 241. | Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63:1037-1040. |
| 242. | Wozniak J, Biederman J, Mick E, Waxmonsky J, Hantsoo L, Best C, Cluette-Brown JE, Laposata M. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17:440-447. |
| 243. | Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, Drago F, Caraci F. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One. 2014;9:e96905. |
| 244. | Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73:81-86. |
| 245. | Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163:1098-1100. |
| 246. | Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry. 2002;159:1596-1598. |
| 247. | Mellor JE, Laugharne JD, Peet M. Schizophrenic symptoms and dietary intake of n-3 fatty acids. Schizophr Res. 1995;18:85-86. |
| 248. | Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2011;50:991-1000. |
| 249. | McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford KE, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17-24. |
| 250. | McNamara RK, Jandacek R, Rider T, Tso P, Stanford KE, Hahn CG, Richtand NM. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160:285-299. |
| 251. | McNamara RK, Jandacek R, Rider T, Tso P, Hahn CG, Richtand NM, Stanford KE. Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: gender differences and partial normalization with antipsychotic medications. Schizophr Res. 2007;91:37-50. |
| 252. | Tatebayashi Y, Nihonmatsu-Kikuchi N, Hayashi Y, Yu X, Soma M, Ikeda K. Abnormal fatty acid composition in the frontopolar cortex of patients with affective disorders. Transl Psychiatry. 2012;2:e204. |
| 253. | Conklin SM, Runyan CA, Leonard S, Reddy RD, Muldoon MF, Yao JK. Age-related changes of n-3 and n-6 polyunsaturated fatty acids in the anterior cingulate cortex of individuals with major depressive disorder. Prostaglandins Leukot Essent Fatty Acids. 2010;82:111-119. |
| 254. | Igarashi M, Ma K, Gao F, Kim HW, Greenstein D, Rapoport SI, Rao JS. Brain lipid concentrations in bipolar disorder. J Psychiatr Res. 2010;44:177-182. |
| 255. | Lalovic A, Levy E, Canetti L, Sequeira A, Montoudis A, Turecki G. Fatty acid composition in postmortem brains of people who completed suicide. J Psychiatry Neurosci. 2007;32:363-370. |
| 256. | Yao JK, Leonard S, Reddy RD. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr Res. 2000;42:7-17. |
| 257. | McNamara RK. Developmental long-chain omega-3 fatty acid deficiency and prefrontal cortex pathology in psychiatric disorders. Collins RO, Adams JL, editors. Prefrontal Cortex: Developmental Differences and Role in Neurological Disorders. Hauppauge, NY: Nova Science Publishers 2013; 1-38. |
| 258. | Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65-73. |
| 259. | Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, Lepack A, Majik MS, Jeong LS, Banasr M. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413-1417. |
| 260. | Licznerski P, Duman RS. Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression. Neuroscience. 2013;251:33-50. |
| 261. | McNamara RK, Jandacek R. Investigation of postmortem brain polyunsaturated fatty acid composition in psychiatric disorders: limitations, challenges, and future directions. J Psychiatr Res. 2011;45:44-46. |
| 262. | Conklin SM, Gianaros PJ, Brown SM, Yao JK, Hariri AR, Manuck SB, Muldoon MF. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett. 2007;421:209-212. |
| 263. | Samieri C, Maillard P, Crivello F, Proust-Lima C, Peuchant E, Helmer C, Amieva H, Allard M, Dartigues JF, Cunnane SC. Plasma long-chain omega-3 fatty acids and atrophy of the medial temporal lobe. Neurology. 2012;79:642-650. |
| 264. | Titova OE, Sjögren P, Brooks SJ, Kullberg J, Ax E, Kilander L, Riserus U, Cederholm T, Larsson EM, Johansson L. Dietary intake of eicosapentaenoic and docosahexaenoic acids is linked to gray matter volume and cognitive function in elderly. Age (Dordr). 2013;35:1495-1505. |
| 265. | Virtanen JK, Siscovick DS, Lemaitre RN, Longstreth WT, Spiegelman D, Rimm EB, King IB, Mozaffarian D. Circulating omega-3 polyunsaturated fatty acids and subclinical brain abnormalities on MRI in older adults: the Cardiovascular Health Study. J Am Heart Assoc. 2013;2:e000305. |
| 266. | Walhovd KB, Storsve AB, Westlye LT, Drevon CA, Fjell AM. Blood markers of fatty acids and vitamin D, cardiovascular measures, body mass index, and physical activity relate to longitudinal cortical thinning in normal aging. Neurobiol Aging. 2014;35:1055-1064. |
| 267. | Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740-1748. |
| 268. | Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774-781. |
| 269. | Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, Williams SC. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675-690. |
| 270. | Cao X, Cao Q, Long X, Sun L, Sui M, Zhu C, Zuo X, Zang Y, Wang Y. Abnormal resting-state functional connectivity patterns of the putamen in medication-naïve children with attention deficit hyperactivity disorder. Brain Res. 2009;1303:195-206. |
| 271. | Chuang TC, Wu MT, Huang SP, Weng MJ, Yang P. Diffusion tensor imaging study of white matter fiber tracts in adolescent attention-deficit/hyperactivity disorder. Psychiatry Res. 2013;211:186-187. |
| 272. | Lawrence KE, Levitt JG, Loo SK, Ly R, Yee V, O’Neill J, Alger J, Narr KL. White matter microstructure in subjects with attention-deficit/hyperactivity disorder and their siblings. J Am Acad Child Adolesc Psychiatry. 2013;52:431-440.e4. |
| 273. | Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brain Mapp. 2009;30:2757-2765. |
| 274. | Sun L, Cao Q, Long X, Sui M, Cao X, Zhu C, Zuo X, An L, Song Y, Zang Y. Abnormal functional connectivity between the anterior cingulate and the default mode network in drug-naïve boys with attention deficit hyperactivity disorder. Psychiatry Res. 2012;201:120-127. |
| 275. | Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, Sui M, Cao Q, Hu S, Peng M. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett. 2006;400:39-43. |
| 276. | Adler CM, Adams J, DelBello MP, Holland SK, Schmithorst V, Levine A, Jarvis K, Strakowski SM. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am J Psychiatry. 2006;163:322-324. |
| 277. | Bellani M, Brambilla P. Diffusion imaging studies of white matter integrity in bipolar disorder. Epidemiol Psychiatr Sci. 2011;20:137-140. |
| 278. | Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9-18. |
| 279. | Cullen KR, Klimes-Dougan B, Muetzel R, Mueller BA, Camchong J, Houri A, Kurma S, Lim KO. Altered white matter microstructure in adolescents with major depression: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2010;49:173-183.e1. |
| 280. | Dickstein DP, Gorrostieta C, Ombao H, Goldberg LD, Brazel AC, Gable CJ, Kelly C, Gee DG, Zuo XN, Castellanos FX. Fronto-temporal spontaneous resting state functional connectivity in pediatric bipolar disorder. Biol Psychiatry. 2010;68:839-846. |
| 281. | Guo WB, Liu F, Xue ZM, Gao K, Wu RR, Ma CQ, Liu ZN, Xiao CQ, Chen HF, Zhao JP. Altered white matter integrity in young adults with first-episode, treatment-naive, and treatment-responsive depression. Neurosci Lett. 2012;522:139-144. |
| 282. | Lin F, Weng S, Xie B, Wu G, Lei H. Abnormal frontal cortex white matter connections in bipolar disorder: a DTI tractography study. J Affect Disord. 2011;131:299-306. |
| 283. | Sacher J, Neumann J, Fünfstück T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord. 2012;140:142-148. |
| 284. | Canu E, Agosta F, Filippi M. A selective review of structural connectivity abnormalities of schizophrenic patients at different stages of the disease. Schizophr Res. 2015;161:19-28. |
| 285. | De Peri L, Crescini A, Deste G, Fusar-Poli P, Sacchetti E, Vita A. Brain structural abnormalities at the onset of schizophrenia and bipolar disorder: a meta-analysis of controlled magnetic resonance imaging studies. Curr Pharm Des. 2012;18:486-494. |
| 286. | Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2:e190. |
| 287. | Colombo RR, Schaufelberger MS, Santos LC, Duran FL, Menezes PR, Scazufca M, Busatto GF, Zanetti MV. Voxelwise evaluation of white matter volumes in first-episode psychosis. Psychiatry Res. 2012;202:198-205. |
| 288. | Ehringer MA, Rhee SH, Young S, Corley R, Hewitt JK. Genetic and environmental contributions to common psychopathologies of childhood and adolescence: a study of twins and their siblings. J Abnorm Child Psychol. 2006;34:1-17. |
| 289. | Mortensen PB, Pedersen CB, Melbye M, Mors O, Ewald H. Individual and familial risk factors for bipolar affective disorders in Denmark. Arch Gen Psychiatry. 2003;60:1209-1215. |
| 290. | McNamara RK, Strawn JR. Non-heritable risk factors for bipolar disorder. ), Progression of Bipolar Disorder in Youth: Presentation, Treatment, and Neurobiology. Oxford: Oxford University Press 2014; 109-130. |
| 291. | Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552-1562. |
| 292. | Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet. 2003;123C:48-58. |
| 293. | Tsuang M. Schizophrenia: genes and environment. Biol Psychiatry. 2000;47:210-220. |
| 294. | Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, Georgieff MK, Markley LA, Kerling EH, Shaddy DJ. DHA supplementation and pregnancy outcomes. Am J Clin Nutr. 2013;97:808-815. |
| 295. | Klebanoff MA, Harper M, Lai Y, Thorp J, Sorokin Y, Varner MW, Wapner RJ, Caritis SN, Iams JD, Carpenter MW. Fish consumption, erythrocyte fatty acids, and preterm birth. Obstet Gynecol. 2011;117:1071-1077. |
| 296. | Olsen SF, Sørensen JD, Secher NJ, Hedegaard M, Henriksen TB, Hansen HS, Grant A. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet. 1992;339:1003-1007. |
| 297. | Olsen SF, Østerdal ML, Salvig JD, Weber T, Tabor A, Secher NJ. Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: a randomised clinical trial with fish oil. Eur J Clin Nutr. 2007;61:976-985. |
| 298. | Salvig JD, Lamont RF. Evidence regarding an effect of marine n-3 fatty acids on preterm birth: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2011;90:825-838. |
| 299. | Leventakou V, Roumeliotaki T, Martinez D, Barros H, Brantsaeter AL, Casas M, Charles MA, Cordier S, Eggesbø M, van Eijsden M. Fish intake during pregnancy, fetal growth, and gestational length in 19 European birth cohort studies. Am J Clin Nutr. 2014;99:506-516. |
| 300. | Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Mathews TJ. Births: final data for 2011. Natl Vital Stat Rep. 2013;62:1-69, 72. |
| 301. | Yamada T, Morikawa M, Yamada T, Kishi R, Sengoku K, Endo T, Saito T, Cho K, Minakami H. First-trimester serum folate levels and subsequent risk of abortion and preterm birth among Japanese women with singleton pregnancies. Arch Gynecol Obstet. 2013;287:9-14. |
| 302. | Bodén R, Lundgren M, Brandt L, Reutfors J, Andersen M, Kieler H. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. |
| 303. | Lee HC, Lin HC. Maternal bipolar disorder increased low birthweight and preterm births: a nationwide population-based study. J Affect Disord. 2010;121:100-105. |
| 304. | MacCabe JH, Martinsson L, Lichtenstein P, Nilsson E, Cnattingius S, Murray RM, Hultman CM. Adverse pregnancy outcomes in mothers with affective psychosis. Bipolar Disord. 2007;9:305-309. |
| 305. | Goldenberg RL, Andrews WW, Guerrant RL, Newman M, Mercer B, Iams J, Meis P, Moawad A, Das A, VanDorsten JP. The preterm prediction study: cervical lactoferrin concentration, other markers of lower genital tract infection, and preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 2000;182:631-635. |
| 306. | Murtha AP, Greig PC, Jimmerson CE, Herbert WN. Maternal serum interleukin-6 concentration as a marker for impending preterm delivery. Obstet Gynecol. 1998;91:161-164. |
| 307. | Wakabayashi A, Sawada K, Nakayama M, Toda A, Kimoto A, Mabuchi S, Kinose Y, Nakamura K, Takahashi K, Kurachi H. Targeting interleukin-6 receptor inhibits preterm delivery induced by inflammation. Mol Hum Reprod. 2013;19:718-726. |
| 308. | Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439-446. |
| 309. | McNamara RK, Able J, Jandacek R, Rider T, Tso P, Eliassen JC, Alfieri D, Weber W, Jarvis K, DelBello MP. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am J Clin Nutr. 2010;91:1060-1067. |