Published online Dec 22, 2012. doi: 10.5498/wjp.v2.i6.91
Revised: June 14, 2012
Accepted: July 21, 2012
Published online: December 22, 2012
Several parallels exist between platelets and the brain, which make them interesting for studying the neurobiology of psychiatric disorders, such as Alzheimer’s disease, depression, schizophrenia and anxiety disorders. Platelets store, secrete and process the amyloid precursor protein which is cleaved into the β-amyloid (Aβ) peptides. The accumulation of Aβ in brain (plaques) and vessels (Aβ-angiopathy) is a major hallmark in AD. Platelets contain high amounts of serotonin and a dysfunction of the serotoninergic system is involved in the development of several behavior disorders, such as depression, anxiety disorders and self aggressive disturbances. Furthermore, platelets are able to take up dopamine and express various dopamine receptors, which make them to an interesting tool to study the underlying mechanisms of schizophrenia. In summary, platelets are an interesting and easily accessible cell type to study changes related to different psychiatric disorders and platelets proteins may be useful as diagnostic biomarkers for some psychiatric disorders.
- Citation: Ehrlich D, Humpel C. Platelets in psychiatric disorders. World J Psychiatr 2012; 2(6): 91-94
- URL: https://www.wjgnet.com/2220-3206/full/v2/i6/91.htm
- DOI: https://dx.doi.org/10.5498/wjp.v2.i6.91
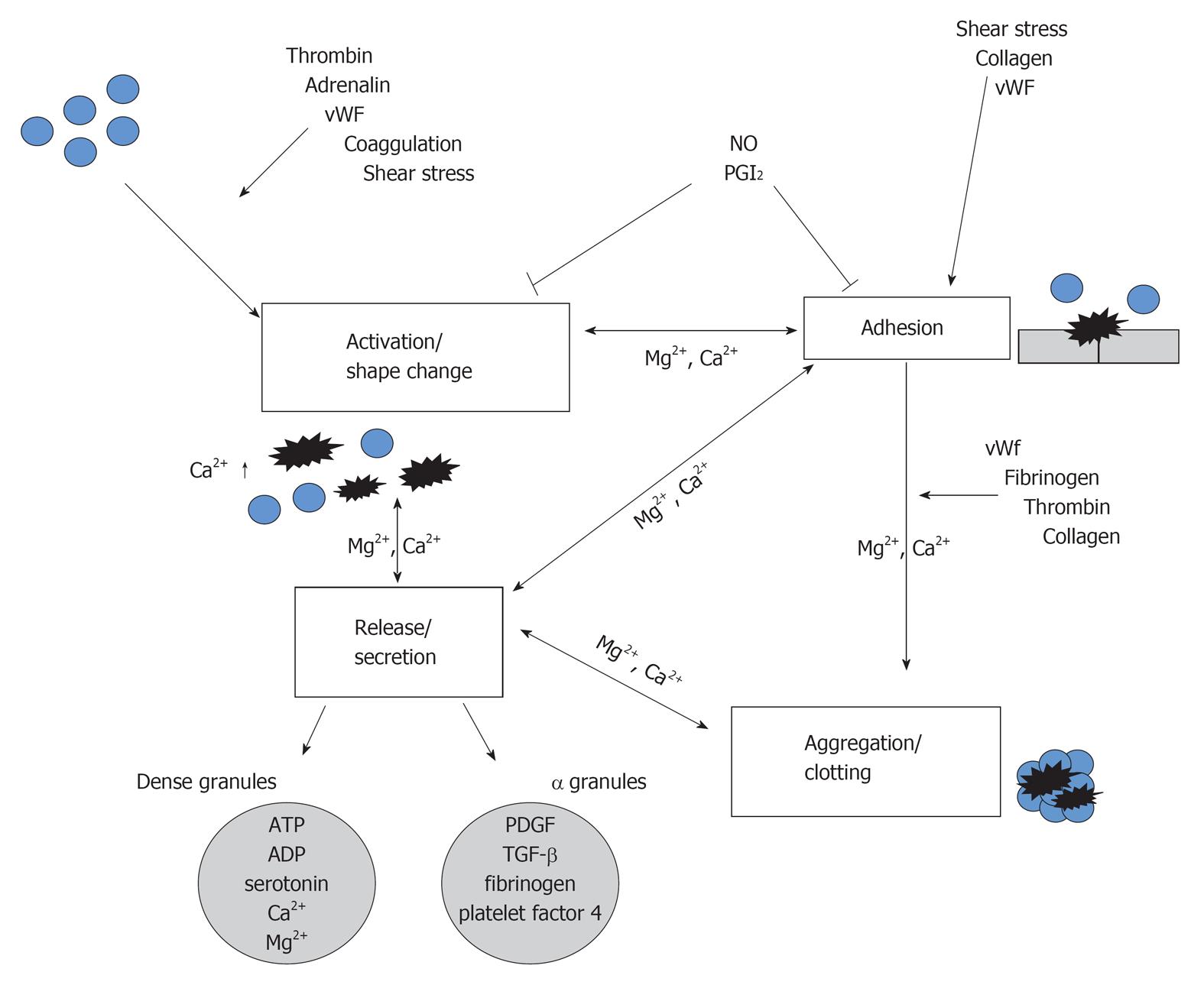
Platelets are small unnucleated blood cells with a size of approximately 3 μm originating from megacaryocytes in the bone marrow, from where they are released into the blood system. Platelets are easily to isolate from the whole blood by different centrifugation step. They possess several important organelles, in which large amounts of proteins are stored and released after platelets activation with different agonists, such as collagen or thrombin. The dense bodies contain serotonin, adenosine-diphosphat, adenosine-triphosphat and calcium, whereas the α granules comprise the highest amount of proteins including von Willebrandt factor, fibronectin, fibrinogen, P-selectin, platelet derived growth factor (PDGF), amyloid-precursor protein, matrix-metalloproteinases, various coagulation and growth factors as well as inflammatory markers. Platelets organelles content is mostly taken up from the plasma, however, platelets are also able to synthesize molecules, such as PDGF, platelet activating factor-4, β-thromboglobulin and thrombospondin. In addition, platelets express various receptors on their surface, including α-2 and β-2 adrenoreceptors, serotonin-, benzodiazepine- and fibrinogen receptors.
In order to characterize isolated platelets the well established platelet markers CD61, CD41 and CD62P were commonly used. CD61 is expressed on resting platelets and serves as a subunit of the receptor for fibrinogen and is also known as the integrin β3 unit which forms together with CD41 the gpIIIa/IIb complex[1]. These glycoproteins are found on platelets and megacaryocytes mediating cell adhesion and binding of fibrinogen to this receptor is required for platelet aggregation and endothelial adherence. CD62P is stored in α-granules of platelets and is a calcium-dependent protein that migrates during platelet activation to the plasma membrane where it mediates platelets interaction with endothelial cells or leukocytes.
Binding of biochemical agonists or shear stress induces changes in the plasma membrane (shape change) and results in platelets activation and secretion of granule contents (Figure 1). Platelets are able to adhere to the subendothelial matrix after vessel wall injury, which induces activation and formation of the hemostatic plug by aggregation (Figure 1). In first line platelets function includes hemostasis[2], but they also participate in inflammatory processes by releasing cytokines[3]. The transport, metabolism, and release of several bioaminergic neurotransmitters (e.g., serotonin) are similar in platelets and neurons and thus platelets serve as a diagnostic tool and an interesting research model in several psychiatric disorders.
Alzheimer’s disease (AD) is a neurodegenerative disorder, which is the most common cause of dementia in the elderly[4,5]. Neuropathology includes β-amyloid (Aβ) plaques in brain and vessels, neurofibrilary tangles with hyperphosphorylated tau protein[6], inflammation[7] and cerebrovascular damage[8]. Platelets are an interesting tool to study the cellular mechanisms of AD, because they contain highest levels of the precursor of β-amyloid (APP) compared to all peripheral tissues[9-12] and all three isoforms of APP (130, 110 and 106 kDa) are detectable in platelets[9,13]. Full length APP is cleaved by a Ca2+ dependent cysteine protease during platelet activation[10] and APP processing is altered in patients suffering from AD[14] resulted in a reduced ratio between the 130 kDa and 106-110 kDa APP isoform[11], suggesting that APP ratios in platelets could be a biomarker for AD. See Review on platelets biomarker in AD[15] and Review on APP as a potential biomarker in AD[16].
The relationship between depression and platelets function has been in focus, because platelets share many similarities with the neuronal monoamine system in the central nervous system. Platelets abnormalities in depressed patients are mainly found in the serotoninergic and noradrenergic system. The uptake, storage and metabolism of serotonin are altered as well as the density of noradrenergic receptors is changed[17]. Basically, serotonin mediates positive affect and mood in the healthy brain, thus the balance of serotonin metabolism may play a central role in the pathogenesis of depression. More than 99% of serotonin in the body is stored in the dense granules of platelets[18] and interestingly, depression is also associated with increased platelet reactivity[19]. Furthermore, antidepressants may protect the vascular system by preventing blood clots[20,21]. See Review Williams (this issue) on platelets in depression[22].
The pathophysiology of schizophrenia is not well understood and there is an increasing body of evidence that several molecular pathways are involved. However, the most common hypothesis promises a link between the disease and excessive levels of dopamine. Platelets may serve as an interesting research tool to investigate underlying mechanism of schizophrenia, because they possess several dopamine receptors and are able to store and release dopamine. Interestingly, platelets of schizophrenic patients show morphological alterations accompanied by the formation of big vacuoles and increased glycogen levels most likely pointing to an altered glycogen metabolism[23]. Oxidative stress may also play a role in development of schizophrenia and accumulation of reactive oxygen species may influence platelets function and reactivity. Indeed, the response of platelets to ADP and collagen is different in schizophrenic patients compared to healthy controls[24]. See review Asor and Ben-Shachar (this issue) on platelets in schizophrenia[25].
The hypothesis of an involvement of the noradrenergic and serotoninergic system in the pathophysiology of anxiety disorders suggests platelets an important role in the study of anxiety disorders. A decreased serotoninergic function with vulnerability towards the development of aggression most likely resulting in the self-aggressive behavior of suicide attempters may be involved in the pathogenesis of suicidality[26,27]. An inverse relationship between the serotonin metabolite 5-hydroxyindoleacetic acid (5HIAA) levels in the cerebrospinal fluid and the past history of suicide attempts may once more link serotoninergic dysfunction and suicide[28]. Beside the serotoninergic dysregulation, platelets of suicidal psychiatric patients showed decreased density of benzodiazepine receptors[29]. See Koudouovoh-Tripp and Sperner-Unterweger (this issue) on platelets in mental stress[30].
Taken together, platelets are an interesting and easily accessible tool to model neuronal function in the periphery and to study neurobiology of several psychiatric disorders which are mostly related to changes in the brain neurotransmitter systems.
Peer reviewer: Gianluca Serafini, MD, Department of Neurosciences, Mental Health and Sensory Organs-Sant’Andrea Hospital, “Sapienza” University of Rome, Via di Grottarossa1035-1039, 00189 Rome, Italy
S- Editor Lu YJ L- Editor A E- Editor Zheng XM
| 1. | Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985;260:11107-11114. [PubMed] |
| 2. | Ware JA, Coller BS. Platelet morphology, biochemistry and function. Williams hematology. New York: McGraw-Hill 1995; 1161-1193. |
| 3. | Horstman LL, Jy W, Ahn YS, Zivadinov R, Maghzi AH, Etemadifar M, Steven Alexander J, Minagar A. Role of platelets in neuroinflammation: a wide-angle perspective. J Neuroinflammation. 2010;7:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 548] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 5. | Dubois MF, Hébert R. The incidence of vascular dementia in Canada: a comparison with Europe and East Asia. Neuroepidemiology. 2001;20:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | McGeer EG, McGeer PL. Inflammatory processes in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 361] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 8. | Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 480] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 9. | Bush AI, Martins RN, Rumble B, Moir R, Fuller S, Milward E, Currie J, Ames D, Weidemann A, Fischer P. The amyloid precursor protein of Alzheimer's disease is released by human platelets. J Biol Chem. 1990;265:15977-15983. [PubMed] |
| 10. | Li QX, Evin G, Small DH, Multhaup G, Beyreuther K, Masters CL. Proteolytic processing of Alzheimer's disease beta A4 amyloid precursor protein in human platelets. J Biol Chem. 1995;270:14140-14147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Borroni B, Agosti C, Marcello E, Di Luca M, Padovani A. Blood cell markers in Alzheimer Disease: Amyloid Precursor Protein form ratio in platelets. Exp Gerontol. 2010;45:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Vignini A, Sartini D, Morganti S, Nanetti L, Luzzi S, Provinciali L, Mazzanti L, Emanuelli M. Platelet amyloid precursor protein isoform expression in Alzheimer's disease: evidence for peripheral marker. Int J Immunopathol Pharmacol. 2011;24:529-534. [PubMed] |
| 13. | Hochstrasser T, Ehrlich D, Marksteiner J, Sperner-Unterweger B, Humpel C. Matrix Metalloproteinase-2 and Epidermal Growth Factor are Decreased in Platelets of Alzheimer Patients. Curr Alzheimer Res. 2011;Epub ahead of print. [PubMed] |
| 14. | Tang K, Hynan LS, Baskin F, Rosenberg RN. Platelet amyloid precursor protein processing: a bio-marker for Alzheimer's disease. J Neurol Sci. 2006;240:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Talib LL, Joaquim HPG, Forlenza OV. Platelet biomarkers in Alzheimer’s disease. World J Psychiatr. 2012;2:95-101. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Evin G, Li QX. Platelets and Alzheimer’s disease: Potential of APP as a biomarker. World J Psychiatr. 2012;2:102-113. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 17. | Ziegelstein RC, Parakh K, Sakhuja A, Bhat U. Platelet function in patients with major depression. Intern Med J. 2009;39:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Skop BP, Brown TM. Potential vascular and bleeding complications of treatment with selective serotonin reuptake inhibitors. Psychosomatics. 1996;37:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 127] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Strike PC, Steptoe A. Psychosocial factors in the development of coronary artery disease. Prog Cardiovasc Dis. 2004;46:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 209] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Ziegelstein RC, Parakh K, Sakhuja A, Bhat U. Depression and coronary artery disease: is there a platelet link. Mayo Clin Proc. 2007;82:1366-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Parakh K, Sakhuja A, Bhat U, Ziegelstein RC. Platelet function in patients with depression. South Med J. 2008;101:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Williams MS. Platelets and depression in cardiovascular disease: A brief review of the current literature. World J Psychiatr. 2012;2:114-123. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Mesa CS. Schizophrenia: cytopathological diagnosis valuation scale. Carib Med J. 1998;60:29-32. |
| 24. | Dietrich-Muszalska A, Olas B. The changes of aggregability of blood platelets in schizophrenia. World J Biol Psychiatry. 2009;10:171-176. [PubMed] |
| 25. | Asor E, Ben-Shachar D. Platelets: A possible glance into brain biological processes in schizophrenia. World J Psychiatr. 2012;2:124-133. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Lauterbach E, Brunner J, Hawellek B, Lewitzka U, Ising M, Bondy B, Rao ML, Frahnert C, Rujescu D, Müller-Oerlinghausen B. Platelet 5-HT2A receptor binding and tryptophan availability in depression are not associated with recent history of suicide attempts but with personality traits characteristic for suicidal behavior. J Affect Disord. 2006;91:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: II. Suicidal behavior. Mol Psychiatry. 2003;8:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 198] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Pandey GN. Altered serotonin function in suicide. Evidence from platelet and neuroendocrine studies. Ann N Y Acad Sci. 1997;836:182-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Marazziti D, Dell'Osso B, Baroni S, Masala I, Di Nasso E, Giannaccini G, Conti L. Decreased density of peripheral benzodiazepine receptors in psychiatric patients after a suicide attempt. Life Sci. 2005;77:3268-3275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Koudouovoh-Tripp P, Sperner-Unterweger B. Influence of mental stress on platelet bioactivity. World J Psychiatr. 2012;2:134-147. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (1)] |