Published online Jun 22, 2012. doi: 10.5498/wjp.v2.i3.49
Revised: June 19, 2012
Accepted: June 20, 2012
Published online: June 22, 2012
The pathophysiology of depression has been traditionally attributed to a chemical imbalance and critical interactions between genetic and environmental risk factors, and antidepressant drugs suggested to act predominantly amplifying monoaminergic neurotransmission. This conceptualization may be currently considered reductive. The current literature about the pathophysiological mechanisms underlying depression, stress-related disorders and antidepressant treatment was examined. In order to provide a critical overview about neuroplasticity, depression and antidepressant drugs, a detailed Pubmed/Medline, Scopus, PsycLit, and PsycInfo search to identify all papers and book chapters during the period between 1980 and 2011 was performed. Pathological stress and depression determine relevant brain changes such as loss of dendritic spines and synapses, dendritic atrophy as well as reduction of glial cells (both in number and size) in specific areas such as the hippocampus and prefrontal cortex. An increased dendritic arborisation and synaptogenesis may instead be observed in the amygdala as a consequence of depression and stress-related disorders. While hippocampal and prefrontal functioning was impaired, amygdala functioning was abnormally amplified. Most of molecular abnormalities and biological changes of aberrant neuroplasticity may be explained by the action of glutamate. Antidepressant treatment is associated with neurogenesis, gliogenesis, dendritic arborisation, new synapse formation and cell survival both in the hippocampus and prefrontal cortex. Antidepressants (ADs) induce neuroplasticity mechanisms reversing the pathological effects of depression and stress-related disorders. The neuroplasticity hypothesis may explain the therapeutic and prophylactic action of ADs representing a new innovative approach to the pathophysiology of depression and stress-related disorders.
- Citation: Serafini G. Neuroplasticity and major depression, the role of modern antidepressant drugs. World J Psychiatr 2012; 2(3): 49-57
- URL: https://www.wjgnet.com/2220-3206/full/v2/i3/49.htm
- DOI: https://dx.doi.org/10.5498/wjp.v2.i3.49
Major depressive disorder (MDD) is a common and invalidating mental illness affecting approximately 2.5% of the general population. MDD is one of the leading cause of disability and it has been suggested to become the second highest burden of disease (measured in disability-adjusted life years) by 2020[1]. MDD has negative social consequences in terms of reduced employment and psychosocial impairment[2]. The pathophysiology of depression involves both external social stressors and internal genetic vulnerability.
Among all biological theories postulated about MDD, an impairment of neuroplasticity and cellular resilience has been suggested[3]. According to this theory, neural circuits and connections undergo lifelong modifications and reorganizations in response to external or internal environmental stimuli. Adult neurogenesis involves precursors of cell proliferation, migration and differentiation mainly occurring in the dentate gyrus of the hippocampus[4]. Several neurotoxic agents such as chronic stress, excessive concentrations of glutamate, biogenic amines and glucocorticoids may affect the morphology of some neural cells such as hippocampal CA3 pyramidal neurons and pyramidal cells of prefrontal cortex. Neural cells may react to chronic stress debranching apical dendrites or with spine loss and these changes are closely associated to daily periods of resting and activity[5,6]. Interestingly, some antidepressants (ADs) may increase neurotrophin signalling promoting neuronal and synaptic remodelling as well as the formation of new neurons in the hippocampus and prefrontal cortex[3,7-11]. Modern ADs may act enhancing neuroplasticity mechanisms and renewing the impairment in neural circuits contributing to their normalization[3,12,13]. Although precise modifications induced at the synaptic level by ADs are still unclear, it’s well known that ADs may promote neuronal connectivity and strengthen specific synapses or normalize glutamatergic tone which is supposed to be underlying major depression[14]. Pharmacological manipulation of the glutamatergic system in animal models has been shown to reduce stress-induced morphological changes in the hippocampus[11,15,16] and some ADs have been reported to regulate glutamatergic transmission through the inhibition of stress-induced morphological changes in both the hippocampus and amygdala[11].
In order to provide a critical overview about neuroplasticity, depression and ADs, a detailed Pubmed/Medline, Scopus, PsycLit, and PsycInfo search to identify all papers and book chapters during the period between 1980 and 2011 was performed. The search used the following terms: “Major Depression Episode” AND “Affective Disorders” AND “Neuroplasticity” OR “Neurogenesis” OR “Synaptic plasticity” AND “ADs” OR “Antidepressant drugs” OR “Antidepressant medications” OR “Antidepressant agents” AND “Treatment” OR “Intervention” OR “Future implications”. Reference lists of the articles were also manually checked for relevant studies. Included papers were restricted to those in English. Only those articles published in peer-reviewed journals were included. Where a title or abstract seemed to describe a study eligible for inclusion, the full article was obtained and examined to assess its relevance based on the inclusion criteria. Approximately 80 full-text articles met our inclusion criteria and were reviewed. Two independent researchers conducted a two-step literature search. Any discrepancies between the two reviewers who, blind to each other, examined the studies for the possible inclusion were resolved by consultations with a senior author.
Generally, resiliency is the ability to adapt and react to stressful life events and environmental situations. This ability is mediated by the involvement of several brain areas such as the hippocampus, amygdala, and prefrontal cortex playing a key role in either cognitive and affective domains and requiring the involvement of specific neurotransmitter molecules. Neuroplasticity is instead a general term indicating a neural framework in which all the different internal events at either the molecular and systemic levels produce neuronal modifications[14]. In the last decades, the view that the brain is a static structure in which electrical and chemical information are processed within a fixed system has been widely debated. Neural circuits, brain nuclei, neurons and synaptic connections undergo several lifelong modifications and relevant adaptations due to environmental stimuli through the neural plasticity mechanisms. Continuous modifications such as increased axonal growth and collateral sprouting determine the development of new synapses and a retrograde elimination of the pre-existing synapses as well as changes in the dendritic tree and spine density influencing the number of post-synaptic sites[17,18]. Popov and Bochorova[19] found that specific and multifaceted structural changes at synapse level may be induced by mossy fibres and hippocampal pyramidal neurons. Also, hippocampal CA3 pyramidal neurons undergo dendritic shrinkage after chronic stress induced by corticosterone[20-22].
Neurohistological changes associated with pathological stress and antidepressant response are site-specific[23]. Reduced hippocampal volume is one of the most common finding in depressed subjects and longer duration of depressive episodes is known to be closely related to modifications in hippocampal volume[24,25]. It has been suggested that somatodendritic, axonal, synaptic and glial cell number changes are all involved in the inhibition of adult hippocampal neurogenesis. The reduction of hippocampal volume may be observed in post-mortem animal models of either stress and major depression[13,26]. In animal models, stress-induced hippocampal neuropathological changes may be summarized as follows: loss of dendritic spines; decrease in the number and length of dendrites; loss of synapses; loss of glia and impairment of neurogenesis[13,26-30]. The retraction of dendrites and synapses determine a reduction of connectivity, multiple impairments of neurons associated with loss of glia, consequent reduction of neurotransmission, decreased neurogenesis[23]. Recent evidence[31] suggested that both hypercholesterolemia (no post-mortem neuronal loss was found in the brain tissue) and apoptosis (evident only in a small hippocampal area) have not been identified as possible neurotoxic agents. Additionally, Reif et al[32] did not find in a small sample of subjects evidence of reduced neural stem cell proliferation possibly explaining the changes in neurogenesis of depressed patients.
Stress-induced neurohistological changes do not simply interfere with hippocampal functioning but they also affect functioning of other downstream areas. Several structural changes have been shown in the rat prefrontal cortex, a brain area in which a retraction of dendrites and spine loss induced by chronic stress and associated with daily periods resting and activities have been described[5,6]. Post-mortem histopathological studies[33,34] have shown reduced neuronal density, smaller neuronal somata and a relevant reduction in prefrontal cortical thickness. A stress-induced inhibition of cell proliferation and gliogenesis, specifically, a stress-induced dendritic reorganization in pyramidal neurons of the medial prefrontal cortex has been commonly observed. In animal models, stress-induced neurohistological changes in the prefrontal cortex determine loss of dendritic spines, atrophy of the dendritic tree, loss of synapses, decreased number and size of glia[13,26,28,30]. Post-mortem studies in depressed subjects have shown a decrease in neuronal and glial cells (both in number and size), and overall cortical thickness[26]. A glial cells loss was also found in limbic and extralimbic structures, prefrontal, orbitofrontal and cingulated cortices of depressed individuals.
Moreover, structural modifications have been described in the amygdala where an enhanced dendritic arborisation (but not an increase in all classes of amgdaloid neurons) has been shown by Vyas et al[35]. After chronic stress they observed an enhanced dendritic arborisation in the basolateral nucleus of the amygdala and specifically in the pyramidal and excitatory projections of the stellate neurons. Also in animal models, stress-induced neurohistological changes in the amygdala include increased dendritic arborisation and synaptogenesis[13,30]. No alteration was reported in neuronal amygdalar number although a reduced number of glial cells has been demonstrated in depressed patients[36]. Several studies[37,38] using MRI analysis showed an altered amygdalar core. Overall, an increased amygdalar volume determining not only structural but also functional impairments has been described in either stressed animals and depressed subjects. Stress-induced neurohistological modifications in the amygdala were not reversed after some weeks but required longer periods[13].
In addition, chronic stress changes on dendrites and spines influence the expression of several synaptic molecules resulting crucial for the information transfer between neurons. Cooper et al[39] showed that the expression of M6a, particularly the splice variant M6a-Ib, a glycoprotein which appears located in the axonal plasma membrane of glutamatergic neurons may be differently regulated by stress. Chronic stress may differently induce the expression of M6a-Ib in a region-dependent manner down-regulating M6a-Ib in the dentate gyrus granule neurons and CA3 pyramidal neurons and an up-regulating M6a-Ib in the medial prefrontal cortex. This different regulation of targeted glycoproteins induced by chronic stress presumably leads to reduced axonal output in hippocampal neurons also altering the integrity of axons and the information transfer between neurons in different brain regions. Chronic stress may also affect neuron-glia communication inducing a remodelling of hippocampal dendrites and an increased expression of GLT-1 glial glutamate transporter in the dendate gyrus and CA3 hippocampal neurons[16].
Therefore, both pathological stress and major depression result in abnormalities in neuroplasticity response characterized by altered increased activity in the amygdala and impaired hippocampal and prefrontal cortex functioning[40]. The hippocampus is a key structure involved in learning and memory, the prefrontal cortex plays a key role in cognitive functions such as attention, concentration, learning and memory whereas the amygdala plays a fundamental role in social and emotional learning and, particularly, in emotions such as anxiety and fear.
Several lines of evidence demonstrate that some modern ADs may reverse neuroplasticity and neurogenesis modifications induced by chronic stress[13]. In animal models, ADs may reverse and remodel many of the stress-induced neurohistological changes. It is possible to speculate that by reversing the neurohistological effects of stress in animal models, ADs may attenuate depression in human subjects. There is evidence that treatment with modern ADs significantly improves both hippocampal shrinkage[28,41] and functions (e.g., cognitive functions)[42]. Interestingly, Rocher et al[43] suggested that both tianeptine and fluoxetine may reverse the inhibition of long-term potentiation (an interesting prototype of synaptic plasticity) not only in the hippocampus but also in prefrontal cortex. Czeh et al[44] suggested that tianeptine may inhibit the stress-induced reduced number of hippocampal astrocytes reversing morphological modifications observed in the somal volume.
The exact mechanism underlying neuroplasticity dysfunctions is still unclear[45,46]. Evidence suggest a central role of both N-Methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptors activation in inducing morphological changes regulating neuroplasticity such as dendritic length and branching, spine density and volumes of several brain regions, specifically in the hippocampal dentate gyrus[47,48]. Glutamate, in certain concentrations and presumably under the influence of elevated glucocorticoids levels, mediates a structural remodelling of neurons leading to reversible modifications such as reduced neurogenesis, neuronal shrinkage and decreased growth[49]. Several authors[50,51] showed that the inhibition of glutamate release by NMDA receptors prevents this remodelling. Of particular interest is also the evidence suggesting that suicidal ideation in depressed subjects is associated with genes encoding ionotropic glutamate receptors[52].
Some ADs and electroconvulsive shock therapy may reverse reduced neurogenesis, neuronal shrinkage and decreased growth, modifying glutamate impairment in the anterior cingulated of depressed individuals[53]. Drugs having mood stabilization properties with additionally modulating glutamate release may mediate morphological plasticity abnormalities[11] and stress-induced morphological hippocampal/amygdalar changes which are reduced by antidepressant manipulation[11,16]. Specifically, Malberg et al[54] found that tianeptine prevented the retraction of apical dendrites of hippocampal CA3 pyramidal neurons and the increased granule cell proliferation. While tianeptine may prevent glutamate efflux in the basolateral nucleus of the amygdala, this effect seem not to be induced with the administration of fluoxetine. Reznikov et al[55] postulated that the impact of ADs in mediating the stress-induced neuropathological changes was quite specific. Interestingly, Emery et al[56] found that neural stem cells involved in the proliferation and differentiation of adult new neurons extended axons to the CA3 region 2 wk after antidepressant administration explaining at least partially the delayed clinical improvement induced by ADs.
However, what are the molecular mechanisms underlying antidepressant regulation of neuroplasticity and neurogenesis Svenningsson et al[57] suggested that ADs may induce a phosphorilation of AMPA receptors in two main sites of the subunit GluR1: Ser831 which is phosphorilated by protein chinase C or CaMK-II determining elevations in hippocampal currents[58] and Ser845 which appears crucial for protein chinase A amplification of peak current by GluR1 receptors[59]. Imipramine and fluoxetine act increasing the phosphorilation at Ser845 on the subunit GluR1[60,61], while tianeptine may reverse stress-induced changes in glutamate receptors expression[16], impairment of neurogenesis[44] and reduced stress-induced apoptosis in the hippocampus and temporal cortex[62]. Also, other mechanisms of action have been hypothesized. ADs of different classes act enhancing phosphorilation at the c-AMP regulatory element-binding protein (CREB)[63] and CaMK-II[64] as well as electroconvulsive shock therapy has been demonstrated to increase hippocampal CREB phosphorilation[65]. Specific neurotrophic factors such as brain-derived neurotrophic factor (BDNF) binding to tyrosine kinase (TrK) receptors may activate intracellular cascades involving cAMP-dependent protein kinase A (PKA), mitogen-activated protein kinase (MAPK), CaMK-II and also transcription factors such as CREB. CREB is involved in the synthesis of different enzymes and proteins considered crucial in inducing structural changes underlying neuroplasticity. CREB and BDNF are some of the most important effectors of neuroplasticity[13,26]. Additionally, ADs may increase the activity of c-fos, a marker of biochemical activity[66]; some ADs such as tianeptine may reduce c-fos levels reversing its previous stress-induced increase[67]. Tianeptine has been demonstrated to prevent impaired stress-induced amygdalar and prefrontal changes[50,68] also preventing the reduction of length and branching of apical dendrites of the hippocampal CA3 neurons exposed to stress[21,50]. Rocher et al[43] suggested that fluoxetine possesses a similar slower activity blocking the effect of stress in the prefrontal cortex. The final result of all these intracellular signalling cascades is a stimulation of neurogenesis in the dentate gyrus including an increase of glial cells in the complexity of dendritic branching as well as the formation of new synaptic connections[69].
Also, agomelatine has been proposed to promote hippocampal neurogenesis under basal conditions[70,71]. Agomelatine has been found to selectively increase cell proliferation and neurogenesis in the ventral hippocampus and to enhance the survival of newly generated cells throughout the entire hippocampus in rats under either basal and stressful conditions[72,73]. Other evidence[74-76] suggested that agomelatine may stimulate adult neurogenesis in the hippocampal dentate gyrus reducing the increase of glutamate release induced by acute stress in the prefrontal and frontal cortex[77].
Recently, Morley-Fletcher et al[78] found that both a 3- or 6-wk treatment with agomelatine (40-50 mg/kg daily) may reverse the reduced hippocampal levels of phosphorylated CREB in adult prenatal restraint stress rats as well as the reduced hippocampal levels of mGlu2/3 and mGlu5 metabotropic glutamate receptors together with the reduced neurogenesis in the ventral hippocampus (this structucture is specifically involved in encoding memories associated with stress and emotions). In addition, Dagyte et al[79] found that treatment with agomelatine normalized stress-affected neuronal activity and promoted neurogenesis in the hippocampus of rats exposed to chronic footshock stress. They suggested that chronic stress reduced c-Fos expression in the hippocampal dentate gyrus and that chronic agomelatine treatment reversed this effect normalizing neuronal activity to basal levels with enhanced hippocampal cell proliferation and survival in chronically stressed rats. They also reported that chronic mild stress significantly decreased the newborn cell survival and doublecortin expression in the dentate gyrus but these changes can be reversed with agomelatine that completely normalized stress affected cell survival and partly reduced double cortin expression. AlAhmed and Herbert[74] found that agomelatine through an intact diurnal corticosterone rhythm may promote, presumably through its antagonism of the 5HT2C receptor, progenitor cell mitosis in the dentate gyrus. More recently, Dagyte et al[76], found that chronic stress increased total SynI (a regulator of synaptic transmission and plasticity) expression in all layers of the medial prefrontal cortex, whereas agomelatine treatment administered for 3 wk eliminated some of these effects. Chronic agomelatine administration reduced the fraction of phosphorylated SynI in all layers of the medial prefrontal cortex as well as selectively in the outer and middle molecular layers of the hippocampal dentate gyrus.
Chronic agomelatine, but not fluoxetine, increased survival of newly formed cells in the ventral part of the hippocampus without changing their phenotypic differentiation into neurons but promoting cell proliferation and BDNF messenger RNA (mRNA) expression. Molteni et al[80] showed that the expression of BDNF mRNA levels in the prefrontal cortex may be up-regulated preventing the circadian down-regulation of the neurotrophin after acute injection of agomelatine presumably through functional interaction between melatonergic MT1/MT2 and serotonergic 5-HT2C receptors. Additionally, Calabrese et al[81], investigating the effects on mRNA and BDNF protein expression of chronic agomelatine treatment compared to those of venlafaxine, found that only agomelatine produced major transcriptional changes in the hippocampus and increased levels of BDNF in the hippocampus and prefrontal cortex. Considering the different effect on mRNA levels and the similar cumulative effects on BDNF levels in the hippocampus and prefrontal cortex, the authors suggested that different modulatory mechanisms were induced in the two brain regions by agomelatine.
Additionally, recent studies have shown that glucocorticoids are involved in the neurogenic action of ADs[82,83]. The potential role of glucocorticoids in antidepressant-induced neurogenesis is consistent with the evidence that ADs regulate the function of the glucocorticoid receptor (GR)[84-88]. In a recent study[88], it has been identified for the first time that antidepressant-induced changes in neurogenesis are dependent on the GR. The antidepressant sertraline enhances neuronal differentiation and promotes neuronal maturation of human hippocampal progenitor cells through a GR-dependent mechanism associated with GR phosphorylation via protein chinase-A signalling. The authors concluded that this effect is observed only when sertraline is present during the proliferation phase, but suggested a complex regulation of neurogenesis mediated by ADs, with different GR-dependent mechanisms leading to enhanced cell proliferation without changes in neuronal differentiation, or enhanced neuronal differentiation in the presence of decreased cell proliferation.
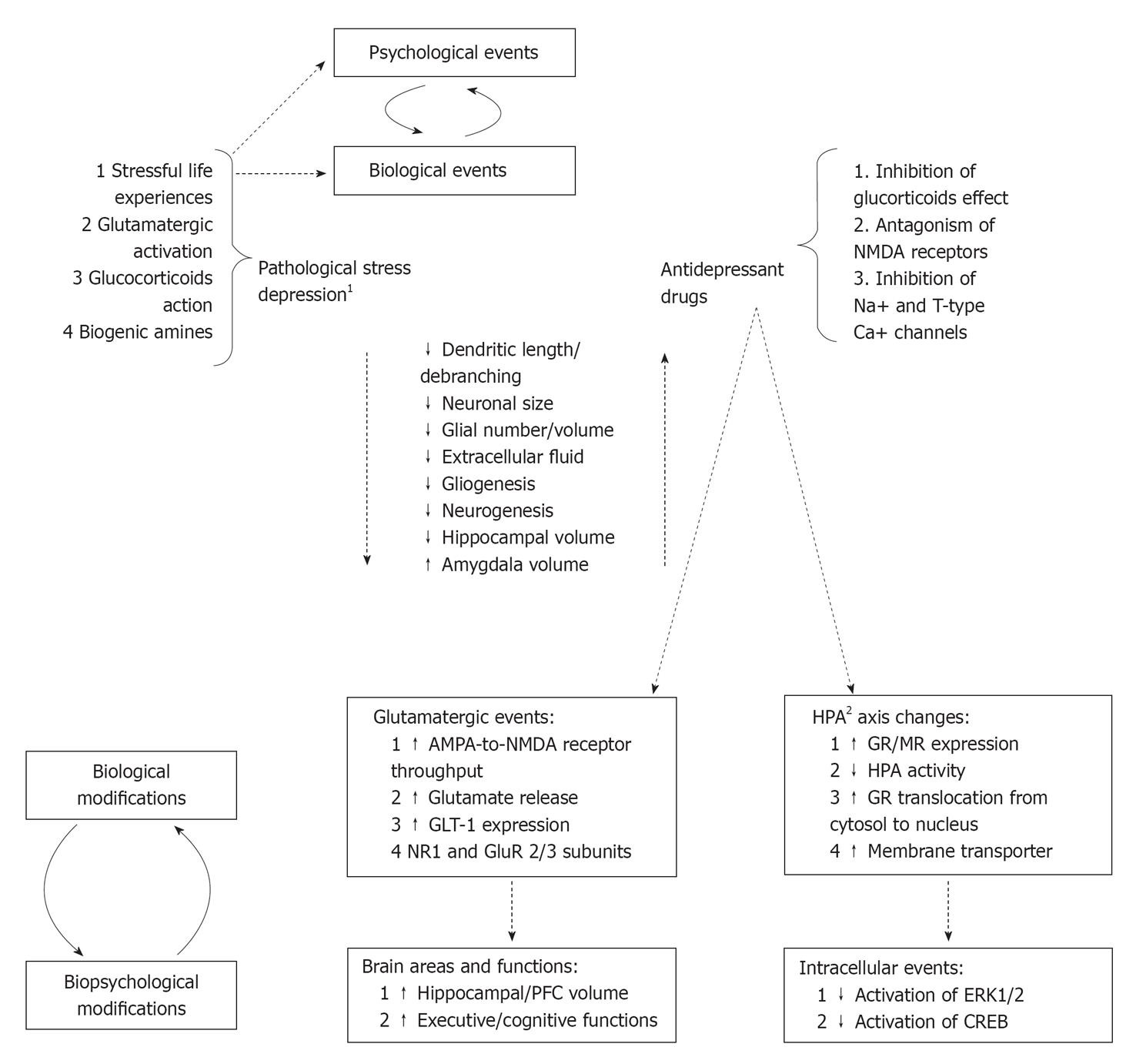
Pathological stress and major depression are associated with loss of dendritic spines, dendritic atrophy and loss of synapses, decrease of glial cells (both in number and size) in the hippocampus and prefrontal cortex. Consequently, the hippocampus, prefrontal cortex, and related downstream structures resulted impaired non only structurally but also in functioning. Pathological stress is also associated with increased dendritic arborisation and formation of new synapses in the amygdala showing an increased volume and an abnormal functioning. The site-specific neurohistological changes explain most of depressive clinical correlates such as anhedonia, loss of motivation, anxiety, fear, and other cognitive dysfunctions. During the last decades, the neurotrophic hypothesis of depression together with the demonstration of ADs to reverse neuroplasticity and neurogenesis modifications induced by chronic stress emerged. Figure 1 summarized the most relevant structural and functional modifications of neural circuits induced by pathological stress and depression as well as modifications/adaptations induced by ADs.
Actually, our knowledge do not allow to conclude whether neuroplasticity and neurogenesis modifications represent the cause or the result of neuropathological processes related to major depression. Another criticism is that the neurotrophic theory is not able alone to explain some experimental findings regarding why ketamine[89,90], scopolamine[91,92] and electroconvulsive shock therapy[93] exert antidepressant properties. Glutamate (NMDA and AMPA receptors activation) is thought to play a crucial role in morphological changes regulating neuroplasticity. Depressive illness and stress-related modifications may affect glutamate receptors and the glutamatergic neurotransmitter system; these alterations, however, may be reversed by the administration of modern ADs. As suggested by Kasper and McEwen[15], ADs may reverse structural and functional modifications underlying depression promoting neuroplasticity mechanisms and presumably with the final result to prevent the illness progression.
Overall, ADs may reverse the stress-induced loss of neuronal cells by reducing the retraction of hippocampal neurons (neuroplasticity) or increasing cell survival and functions (neurogenesis). Also, ADs may reverse the structural and functional consequences of stress in a site-specific manner in both the hippocampus and prefrontal cortex, but not in the amygdala. This presumably explains why, although most of depressive clinical manifestations may be reversed with the administration of ADs, the vulnerability to stress instead remains[94] providing a rationale for the required maintenance of antidepressant therapy after the successful initial treatment of depression. Future longitudinal studies including larger samples of subjects should deeply investigate the potential of ADs as long-term modulators of neuroplasticity and neurogenesis mechanisms, allowing a more detailed understanding of the pathophysiology of major depression.
Peer reviewer: John Fu, MD, PhD, Department of Biostatistics, Saint Louis University School of Public Health, 3545 Lafayette Ave., Suite 300, ST 63104, United States
S- Editor Lu YJ L- Editor A E- Editor Zheng XM
| 1. | World Health Organization. Information on mental disorders management: depression. Geneva: World Health Organization. Available from: http://www.who.int/mental_health/management/depression/definition/en/index.html. |
| 2. | Andersen I, Thielen K, Bech P, Nygaard E, Diderichsen F. Increasing prevalence of depression from 2000 to 2006. Scand J Public Health. 2011;39:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 3. | Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 451] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 4. | Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4410] [Cited by in RCA: 4284] [Article Influence: 158.7] [Reference Citation Analysis (0)] |
| 5. | Perez-Cruz C, Simon M, Czéh B, Flügge G, Fuchs E. Hemispheric differences in basilar dendrites and spines of pyramidal neurons in the rat prelimbic cortex: activity- and stress-induced changes. Eur J Neurosci. 2009;29:738-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Perez-Cruz C, Müller-Keuker JI, Heilbronner U, Fuchs E, Flügge G. Morphology of pyramidal neurons in the rat prefrontal cortex: lateralized dendritic remodeling by chronic stress. Neural Plast. 2007;2007:46276. [PubMed] |
| 7. | Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Castrén E, Võikar V, Rantamäki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 519] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 9. | Sairanen M, O'Leary OF, Knuuttila JE, Castrén E. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience. 2007;144:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764-73, 739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 424] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 11. | McEwen BS, Chattarji S. Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine. Eur Neuropsychopharmacol. 2004;14 Suppl 5:S497-S502. [PubMed] [DOI] [Full Text] |
| 12. | Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull. 2001;35:5-49. [PubMed] |
| 13. | Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88-109. [PubMed] [DOI] [Full Text] |
| 14. | Reznikov LR, Fadel JR, Reagan LP. Glutamate-mediated neuroplasticity deficits in mood disorders. Neuroplasticity: new biochemical mechanisms. London: Springer 2009; 13-26. |
| 15. | Kasper S, McEwen BS. Neurobiological and clinical effects of the antidepressant tianeptine. CNS Drugs. 2008;22:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Reagan LP, Rosell DR, Wood GE, Spedding M, Muñoz C, Rothstein J, McEwen BS. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. Proc Natl Acad Sci USA. 2004;101:2179-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Woolley CS. Effects of estrogen in the CNS. Curr Opin Neurobiol. 1999;9:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Carvalho LA, Garner BA, Dew T, Fazakerley H, Pariante CM. Antidepressants, but not antipsychotics, modulate GR function in human whole blood: an insight into molecular mechanisms. Eur Neuropsychopharmacol. 2010;20:379-387. [PubMed] |
| 19. | Popov VI, Bocharova LS. Hibernation-induced structural changes in synaptic contacts between mossy fibres and hippocampal pyramidal neurons. Neuroscience. 1992;48:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 748] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 21. | Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534-3540. [PubMed] |
| 22. | Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 567] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 23. | Andrade C, Rao NS. How antidepressant drugs act: A primer on neuroplasticity as the eventual mediator of antidepressant efficacy. Indian J Psychiatry. 2010;52:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 387] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 25. | Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 811] [Article Influence: 36.9] [Reference Citation Analysis (1)] |
| 26. | D'Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disord. 2002;4:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 357] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 27. | Sheline YI. Consequences of depression in the hippocampus and other brain regions. Neuroplasticity: A New Approach to the Pathophysiology of Depression. London: Science Press Ltd 2004; 25–37. |
| 28. | Fuchs E. Neuroplasticity: a new approach to the pathophysiology of depression. Neuroplasticity: new biochemical mechanisms. London: Springer 2009; 1-12. |
| 29. | Gorwood P. Clinical consequences of the role of glutamate and neuroplasticity in depressive disorder. Neuroplasticity: New Biochemical Mechanisms. London: Springer 2009; 57–68. |
| 30. | Jay TM. Cellular plasticity and the pathophysiology of depression. Neuroplasticity: New Biochemical Mechanisms. London: Springer 2009; 41–55. |
| 31. | Czéh B, Lucassen PJ. What causes the hippocampal volume decrease in depression Are neurogenesis, glial changes and apoptosis implicated. Eur Arch Psychiatry Clin Neurosci. 2007;257:250-260. [PubMed] |
| 32. | Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 469] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 33. | Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 448] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 34. | Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1041] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 35. | Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810-6818. [PubMed] |
| 36. | Bowley MP, Drevets WC, Ongür D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 354] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 37. | Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 324] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 38. | Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115-118. [PubMed] |
| 39. | Cooper B, Fuchs E, Flügge G. Expression of the axonal membrane glycoprotein M6a is regulated by chronic stress. PLoS One. 2009;4:e3659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 40. | Fuchs E. Animal models of depression. Neuroplasticity: A New Approach to the Pathophysiology of Depression. London: Science Press Ltd 2004; 39–50. |
| 41. | Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136-1143. [PubMed] [DOI] [Full Text] |
| 42. | Yan HC, Cao X, Gao TM, Zhu XH. Promoting adult hippocampal neurogenesis: a novel strategy for antidepressant drug screening. Curr Med Chem. 2011;18:4359-4367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb Cortex. 2004;14:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 241] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 44. | Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98:12796-12801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 792] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 45. | Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 1126] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 46. | Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 321] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 47. | Fifková E, Van Harreveld A. Long-lasting morphological changes in dendritic spines of dentate granular cells following stimulation of the entorhinal area. J Neurocytol. 1977;6:211-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 333] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 48. | Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18:9835-9844. [PubMed] |
| 49. | Virgin CE, Ha TP, Packan DR, Tombaugh GC, Yang SH, Horner HC, Sapolsky RM. Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes: implications for glucocorticoid neurotoxicity. J Neurochem. 1991;57:1422-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 282] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 50. | Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol. 1992;222:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 226] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 51. | Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 684] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 52. | Laje G, Paddock S, Manji H, Rush AJ, Wilson AF, Charney D, McMahon FJ. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry. 2007;164:1530-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 53. | Michael N, Gösling M, Reutemann M, Kersting A, Heindel W, Arolt V, Pfleiderer B. Metabolic changes after repetitive transcranial magnetic stimulation (rTMS) of the left prefrontal cortex: a sham-controlled proton magnetic resonance spectroscopy (1H MRS) study of healthy brain. Eur J Neurosci. 2003;17:2462-2468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 54. | Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104-9110. [PubMed] |
| 55. | Reznikov LR, Grillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur J Neurosci. 2007;25:3109-3114. [PubMed] |
| 56. | Emery DL, Fulp CT, Saatman KE, Schütz C, Neugebauer E, McIntosh TK. Newly born granule cells in the dentate gyrus rapidly extend axons into the hippocampal CA3 region following experimental brain injury. J Neurotrauma. 2005;22:978-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Svenningsson P, Bateup H, Qi H, Takamiya K, Huganir RL, Spedding M, Roth BL, McEwen BS, Greengard P. Involvement of AMPA receptor phosphorylation in antidepressant actions with special reference to tianeptine. Eur J Neurosci. 2007;26:3509-3517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 58. | Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 831] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 59. | Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 643] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 60. | Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, Falke C, Zarate CA, Manji HK. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 61. | Svenningsson P, Tzavara ET, Witkin JM, Fienberg AA, Nomikos GG, Greengard P. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac). Proc Natl Acad Sci USA. 2002;99:3182-3187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 185] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 62. | Lucassen PJ, Fuchs E, Czéh B. Antidepressant treatment with tianeptine reduces apoptosis in the hippocampal dentate gyrus and temporal cortex. Biol Psychiatry. 2004;55:789-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 63. | Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 796] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 64. | Popoli M, Vocaturo C, Perez J, Smeraldi E, Racagni G. Presynaptic Ca2+/calmodulin-dependent protein kinase II: autophosphorylation and activity increase in the hippocampus after long-term blockade of serotonin reuptake. Mol Pharmacol. 1995;48:623-629. [PubMed] |
| 65. | Jeon SH, Seong YS, Juhnn YS, Kang UG, Ha KS, Kim YS, Park JB. Electroconvulsive shock increases the phosphorylation of cyclic AMP response element binding protein at Ser-133 in rat hippocampus but not in cerebellum. Neuropharmacology. 1997;36:411-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Beck CH. Acute treatment with antidepressant drugs selectively increases the expression of c-fos in the rat brain. J Psychiatry Neurosci. 1995;20:25-32. [PubMed] |
| 67. | Duncan GE, Knapp DJ, Johnson KB, Breese GR. Functional classification of antidepressants based on antagonism of swim stress-induced fos-like immunoreactivity. J Pharmacol Exp Ther. 1996;277:1076-1089. [PubMed] |
| 68. | Vouimba RM, Muñoz C, Diamond DM. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress. 2006;9:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Magariños AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol. 1999;371:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 253] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 70. | Hanoun N, Mocaër E, Boyer PA, Hamon M, Lanfumey L. Differential effects of the novel antidepressant agomelatine (S 20098) versus fluoxetine on 5-HT1A receptors in the rat brain. Neuropharmacology. 2004;47:515-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 71. | Païzanis E, Renoir T, Lelievre V, Saurini F, Melfort M, Gabriel C, Barden N, Mocaër E, Hamon M, Lanfumey L. Behavioural and neuroplastic effects of the new-generation antidepressant agomelatine compared to fluoxetine in glucocorticoid receptor-impaired mice. Int J Neuropsychopharmacol. 2010;13:759-774. [PubMed] [DOI] [Full Text] |
| 72. | Banasr M, Soumier A, Hery M, Mocaër E, Daszuta A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry. 2006;59:1087-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 242] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 73. | Daszuta A, Ban M, Soumier A, Hery M, Mocaer E. [Depression and neuroplasticity: implication of serotoninergic systems]. Therapie. 2005;60:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 74. | AlAhmed S, Herbert J. Effect of agomelatine and its interaction with the daily corticosterone rhythm on progenitor cell proliferation in the dentate gyrus of the adult rat. Neuropharmacology. 2010;59:375-379. [PubMed] |
| 75. | Dagyte G, Trentani A, Postema F, Luiten PG, Den Boer JA, Gabriel C, Mocaër E, Meerlo P, Van der Zee EA. The novel antidepressant agomelatine normalizes hippocampal neuronal activity and promotes neurogenesis in chronically stressed rats. CNS Neurosci Ther. 2010;16:195-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Dagyte G, Luiten PG, De Jager T, Gabriel C, Mocaër E, Den Boer JA, Van der Zee EA. Chronic stress and antidepressant agomelatine induce region-specific changes in synapsin I expression in the rat brain. J Neurosci Res. 2011;89:1646-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Tardito D, Milanese M, Bonifacino T, Musazzi L, Grilli M, Mallei A, Mocaer E, Gabriel-Gracia C, Racagni G, Popoli M. Blockade of stress-induced increase of glutamate release in the rat prefrontal/frontal cortex by agomelatine involves synergy between melatonergic and 5-HT2C receptor-dependent pathways. BMC Neurosci. 2010;11:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Morley-Fletcher S, Mairesse J, Soumier A, Banasr M, Fagioli F, Gabriel C, Mocaer E, Daszuta A, McEwen B, Nicoletti F. Chronic agomelatine treatment corrects behavioral, cellular, and biochemical abnormalities induced by prenatal stress in rats. Psychopharmacology (Berl). 2011;217:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 79. | Dagytė G, Crescente I, Postema F, Seguin L, Gabriel C, Mocaër E, Boer JA, Koolhaas JM. Agomelatine reverses the decrease in hippocampal cell survival induced by chronic mild stress. Behav Brain Res. 2011;218:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 80. | Molteni R, Calabrese F, Pisoni S, Gabriel C, Mocaer E, Racagni G, Riva MA. Synergistic mechanisms in the modulation of the neurotrophin BDNF in the rat prefrontal cortex following acute agomelatine administration. World J Biol Psychiatry. 2010;11:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 81. | Calabrese F, Molteni R, Gabriel C, Mocaer E, Racagni G, Riva MA. Modulation of neuroplastic molecules in selected brain regions after chronic administration of the novel antidepressant agomelatine. Psychopharmacology (Berl). 2011;215:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1033] [Cited by in RCA: 987] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 83. | Huang GJ, Herbert J. Stimulation of neurogenesis in the hippocampus of the adult rat by fluoxetine requires rhythmic change in corticosterone. Biol Psychiatry. 2006;59:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 84. | Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 1344] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 85. | Pariante CM, Hye A, Williamson R, Makoff A, Lovestone S, Kerwin RW. The antidepressant clomipramine regulates cortisol intracellular concentrations and glucocorticoid receptor expression in fibroblasts and rat primary neurones. Neuropsychopharmacology. 2003;28:1553-1561. [PubMed] [DOI] [Full Text] |
| 86. | Pariante CM, Kim RB, Makoff A, Kerwin RW. Antidepressant fluoxetine enhances glucocorticoid receptor function in vitro by modulating membrane steroid transporters. Br J Pharmacol. 2003;139:1111-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 87. | Pariante CM, Pearce BD, Pisell TL, Owens MJ, Miller AH. Steroid-independent translocation of the glucocorticoid receptor by the antidepressant desipramine. Mol Pharmacol. 1997;52:571-581. [PubMed] |
| 88. | Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S, Price J, Pariante CM. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry. 2011;16:738-750. [PubMed] |
| 89. | Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2275] [Cited by in RCA: 2577] [Article Influence: 135.6] [Reference Citation Analysis (0)] |
| 90. | aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139-145. [PubMed] |
| 91. | Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 301] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 92. | Drevets WC, Furey ML. Replication of scopolamine's antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 93. | Andrade C, Gangadhar BN, Channabasavanna SM. Further Characterization of Mania as a Side Effect of ECT. Convuls Ther. 1990;6:318-319. [PubMed] |
| 94. | Diamond DM, Campbell A, Park CR, Vouimba RM. Preclinical research on stress, memory, and the brain in the development of pharmacotherapy for depression. Eur Neuropsychopharmacol. 2004;14 Suppl 5:S491-S495. [PubMed] [DOI] [Full Text] |