Published online Jun 19, 2025. doi: 10.5498/wjp.v15.i6.105889
Revised: March 24, 2025
Accepted: April 27, 2025
Published online: June 19, 2025
Processing time: 92 Days and 2.1 Hours
Sepsis-associated encephalopathy (SAE) is a common complication of sepsis, characterized by cognitive impairment, altered consciousness, and psychiatric symptoms, including anxiety and depression. These psychiatric symptoms often exacerbate the overall prognosis and quality of life of affected patients. However, the underlying metabolic and proteomic features associated with SAE-induced psychiatric symptoms remain poorly understood.
To investigate the clinical manifestations of anxiety and depression in patients with sepsis and SAE and to explore their associated metabolic and proteomic characteristics.
A total of 88 patients were enrolled, comprising 30 healthy controls, 29 patients with sepsis, and 29 with SAE. Anxiety and depression symptoms were evaluated using the Hamilton anxiety rating scale (HAM-A) and Hamilton depression rating scale (HAM-D) in sepsis and SAE. Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA), and quality of life was measured using the 36-Item Short Form Health Survey. Plasma samples were analyzed for metabolomic and proteomic profiling. Metabolic alterations were identified through liquid chromatography-mass spectrometry, while protein expression was assessed using Olink targeted proteomics.
Compared to the sepsis group, patients with SAE exhibited significantly higher levels of anxiety (HAM-A: 15.2 ± 4.0 vs 10.4 ± 3.0, P = 0.012) and depression (HAM-D: 16.0 ± 3.5 vs 9.1 ± 2.3, P = 0.003). Cognitive function, as measured by MoCA, was notably impaired in the SAE group (MoCA: 18.5 ± 4.0 vs 24.5 ± 3.2, P = 0.007). Quality of life scores, particularly in physical functioning, emotional well-being, and mental health, were significantly lower in patients with SAE. Metabolomic and proteomic analyses revealed substantial alterations in oxidative stress and nicotinamide adenine dinucleotide (NAD+) metabolism pathways, with cluster of differentiation (CD) 38 emerging as a potential biomarker associated with psychiatric symptoms in SAE. Further validation in an independent cohort confirmed the diagnostic relevance of CD38.
This study highlights the significant psychological burden of SAE, manifested as anxiety and depression. Multi-omics analysis identified distinct metabolic alterations, particularly in NAD+ metabolism, that may contribute to psychiatric symptom development and progression. Furthermore, CD38 was identified as a promising biomarker for the early detection of SAE, providing potential avenues for early intervention and therapeutic targeting.
Core Tip: Sepsis-associated encephalopathy (SAE) is linked to significant psychiatric symptoms, including anxiety and depression, which worsen patient outcomes. This study integrates multi-omics analysis to identify cluster of differentiation (CD) 38 as a key biomarker associated with nicotinamide adenine dinucleotide (NAD+) metabolism dysregulation in SAE. Elevated CD38 levels correlate with increased psychiatric burden and cognitive decline, suggesting its potential as an early diagnostic marker. A composite biomarker model incorporating CD38 improves the accuracy of predicting SAE. Targeting NAD+ metabolism may offer novel therapeutic strategies for mitigating neuropsychiatric symptoms in patients with SAE. These findings emphasize the need for early psychiatric screening and metabolic interventions in the management of SAE.
- Citation: Wu CR, Zhu HL, Sun YT, Shen SH, Shi PL, Cui YH, Tang JG, Yang CH, Wang SY, Ge XL, Pan SM. Clinical manifestations of anxiety and depression in sepsis-associated encephalopathy and multi-omics identification of cluster of differentiation 38 as an early biomarker. World J Psychiatry 2025; 15(6): 105889
- URL: https://www.wjgnet.com/2220-3206/full/v15/i6/105889.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i6.105889
Sepsis-associated encephalopathy (SAE) is a severe and often overlooked form of brain dysfunction that occurs in patients with sepsis, significantly contributing to increased mortality and long-term complications, including cognitive decline and psychiatric disorders[1]. SAE presents a wide range of neurological impairments, from mild cognitive disturbances to profound coma, making patient management a critical concern in the context of sepsis[2]. In addition to cognitive disturbances, a significant proportion of patients with SAE also experience psychiatric symptoms, including anxiety, depression, and mood swings. These symptoms can significantly worsen their overall prognosis and quality of life, as they often persist long after the acute phase of sepsis has resolved[3]. Recent studies have shown that approximately 30%-50% of patients with SAE experience long-term psychiatric effects, which can complicate rehabilitation and increase the burden on caregivers[4].
Despite advancements in critical care, the early and accurate identification of both sepsis and SAE remains a significant challenge. Current diagnostic methods primarily rely on clinical observations and basic laboratory markers[5], which often lack the necessary sensitivity and specificity to detect these conditions in their early stages. This diagnostic gap emphasizes the urgent need for more reliable biomarkers that can help identify sepsis and SAE early, ultimately improving patient outcomes. Furthermore, psychiatric symptoms, which are often overlooked in the clinical setting, can complicate diagnosis and treatment, further highlighting the need for biomarkers that can identify individuals at high risk of developing SAE. These symptoms can sometimes be subtle or transient, making them difficult to detect without appropriate diagnostic tools, and their early identification is critical for timely intervention.
Although traditional biomarkers such as C-reactive protein, procalcitonin (PCT), and lactate (LA) levels are widely used in the diagnosis of sepsis[6], they do not fully capture the complexity of the underlying pathophysiology and often fail to identify early stages of SAE. Despite advances in understanding the mechanisms of sepsis and SAE[4], early and reliable biomarkers for these conditions are still lacking. This underscores the need for novel biomarkers that can accurately diagnose sepsis and SAE at an early stage, particularly in patients at high risk for developing neurological and psychiatric complications. This research could identify biomarkers that can help screen individuals at high risk of developing SAE. Such early identification is essential for timely intervention, which could significantly improve cognitive and psychiatric symptoms in these patients.
Recent advancements in multi-omics technologies have opened up new opportunities to better understand the molecular alterations underlying sepsis and SAE[7]. Proteomics, in particular, enables the identification of differentially expressed proteins (DEPs) associated with these conditions, providing valuable insights that could serve as biomarkers for early diagnosis[8]. In the context of psychiatric symptoms, proteomic profiling may help identify biomarkers linked to neuroinflammation, stress responses, and neurotransmitter dysregulation, which are thought to contribute to the psychiatric manifestations of SAE. In addition, metabolomics offers complementary perspectives on the metabolic changes occurring in response to sepsis, helping to highlight dysregulated pathways involved in organ dysfunction[9]. These metabolic alterations could be linked to psychiatric symptoms, such as disturbances in the tryptophan-kynurenine pathway, which has been implicated in mood disorders and cognitive dysfunction. The integration of these advanced omics approaches presents a promising strategy to identify novel biomarkers and therapeutic targets, thereby enhancing both diagnostic accuracy and treatment outcomes[10].
In this study, we aimed to investigate the neuropsychiatric burden in patients with SAE and explore the underlying metabolic and proteomic alterations contributing to its pathogenesis. By leveraging multi-omics analysis, we sought to identify key biomarkers associated with anxiety, depression, and cognitive impairment in patients with SAE.
The study recruited all patients with sepsis who were admitted to the intensive care unit (ICU) and Emergency Department of Shanghai Fifth People’s Hospital in 2023, along with healthy controls recruited from the general public. A total of 30 healthy controls (control group), 29 patients with sepsis (sepsis group), and 29 patients with SAE (SAE group) were included. Plasma samples were collected within 24 h of hospital admission from patients with sepsis and SAE.
All participants provided written informed consent, and the study was approved by the Institutional Review Board of Shanghai Fifth People’s Hospital, conducted in accordance with the Declaration of Helsinki. Sepsis was diagnosed according to the Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021[11]. SAE was diagnosed clinically in patients with sepsis who exhibited altered consciousness, such as delirium (assessed by the confusion assessment method for the ICU [CAM-ICU]) or a Glasgow coma scale score < 15, while excluding other potential causes of altered mental status[2]. Exclusion criteria included: (1) Patients who died within 24 hours of admission or were transferred to another hospital; (2) those with a history of traumatic brain injury, brain tumor, stroke, intracranial hemorrhage, brain surgery, intracranial infection, or patients who developed these conditions during hospitalization; (3) those with a history of psychiatric disorders, other neurological diseases, Alzheimer’s disease, or conditions leading to cognitive decline; (4) those with a history of autoimmune or rheumatic diseases; (5) those with a history of malignancies, including intracranial tumors; and (6) those with active substance use disorders (e.g., alcohol dependence, illicit drug use within 30 days) or a history of chronic substance abuse.
Anxiety and depression symptoms were assessed using the Hamilton Anxiety Rating Scale (HAM-A) and Hamilton Depression Rating Scale (HAM-D), respectively. The HAM-A is a clinician-administered scale used to evaluate the severity of anxiety symptoms, including emotional restlessness, tension, and panic attacks. Each item is scored on a 5-point scale, with higher scores indicating more severe anxiety symptoms. The HAM-D is a clinician-administered scale for evaluating the severity of depressive symptoms, assessing mood, loss of interest, insomnia, and self-esteem. Both scales were administered during patient interviews by trained clinicians.
Cognitive function was evaluated using the Montreal Cognitive Assessment (MoCA), a screening tool designed to assess multiple cognitive domains, including memory, attention, language, and visuospatial abilities. Each domain was scored separately, with lower total scores indicating greater cognitive impairment.
Quality of life and functional status were assessed using the 36-Item Short Form Health Survey (SF-36), which evaluates physical functioning, emotional health, social functioning, and mental health. Higher scores on the SF-36 reflect better quality of life and greater functional status.
Psychiatric evaluations (HAM-A, HAM-D, MoCA, SF-36) were conducted within 24 h of admission, before sedation or mechanical ventilation. For patients requiring subsequent sedation, assessments were deferred until sedation cessation and delirium resolution (confirmed via CAM-ICU).
Participants were categorized into three groups: 30 healthy controls, 29 patients with sepsis, and 29 patients with SAE. Quantitative proteomic data were obtained using Olink targeted proteomics technology, and quantitative metabolomic data were collected via liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS).
Olink’s proximity extension assay technology was selected for proteomic profiling owing to its ability to quantify inflammation-related proteins with high sensitivity. For metabolomics, LC-MS/MS was chosen for its broad coverage of polar/nonpolar metabolites and robust reproducibility, enabling comprehensive analysis of SAE-induced metabolic disturbances.
Protein expression levels were analyzed using Olink targeted proteomics technology. Missing values in the data were imputed using the impute.knn function from the impute package. Principal component analysis (PCA) was performed to examine the overall distribution of the samples and assess potential outliers. DEPs were identified by comparing protein expression levels between the sepsis group and the healthy control group, and between the SAE and sepsis groups. Statistical significance was assessed using t-tests, and false discovery rate correction was applied to control for multiple comparisons. Proteins with a log2 fold change (|log2FC|) ≥ 0.5 and adjusted P value < 0.05 were considered significantly different. The top 10 most significant DEPs were visualized using a volcano plot and heatmap. Key proteins were identified by intersecting DEPs with consistent expression trends.
To investigate the biological processes and signaling pathways involved in the key proteins, the R package cluster Profiler was used for Gene Ontology (GO) enrichment analysis, including biological processes, cellular components, and molecular functions, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. Biological pathways were explored with a significance threshold of P < 0.05. Visualization was performed using the R package ggplot2.
Protein-protein interaction networks were constructed using the GeneMANIA platform, which integrates various biological data types, including co-expression, protein interactions, and genetic associations. This network analysis provided insights into potential functional relationships among the DEPs and helped guide subsequent experimental validation.
Metabolomic data were acquired using LC-MS/MS. During the mass spectrometry data analysis, positive and negative ion files were merged, and data entries labeled as “NA” in the compound names were removed. After filtering out exogenous compounds, the data were normalized and log-transformed, with missing values imputed using the impute.knn function. Given the high dimensionality and strong correlations among variables in metabolomic data, multivariate statistical methods such as PCA and partial least squares discriminant analysis were applied to uncover potential insights. PCA, an unsupervised method, was used to reduce dimensionality by generating composite variables through linear combinations of all metabolites, enabling observation of overall distribution trends and group differences using the FactoMineR package. Additionally, partial least squares discriminant analysis, implemented via the ropls package, established a predictive relationship between metabolite expression and sample categories, with variable importance in projection (VIP) scores calculated to evaluate the importance of each metabolite’s expression pattern in sample classification (VIP > 1.0 was used as the selection threshold). Differential analysis between groups was performed using t-tests, and differential metabolites were identified based on VIP > 1.0, |log2FC| ≥ 0.5, and adjusted P value < 0.05. The results were visualized using volcano plots and heatmaps.
Weighted gene co-expression network analysis (WGCNA) is a systems biology approach used to construct weighted co-expression networks among metabolites, clustering metabolites with similar expression patterns into modules, and analyzing the associations between these modules and specific phenotypes. This method is well-suited for handling high-throughput gene expression data, revealing the modular structure of gene networks, and identifying key metabolite sets. First, we calculated the correlation coefficients among metabolites and constructed a weighted co-expression network based on these coefficients. Hierarchical clustering analysis was then used to divide metabolites into distinct modules, with module eigengenes representing each module. Subsequently, we analyzed the correlations between these modules and the phenotypes of interest to identify modules potentially influencing the phenotype. The WGCNA workflow included data normalization, soft-threshold selection, network construction, module identification, module-phenotype correlation analysis, and identification of key metabolites. This approach enabled us to identify biomarkers associated with the studied disease from complex metabolomic data. Using the WGCNA R package, we performed weighted correlation network analysis on metabolomic data from disease samples and all metabolites to identify key modules. An appropriate soft-threshold (β) was selected to ensure that the scale-free topology fit index exceeded 0.9. Pearson correlation analysis was applied to evaluate the relationships between modules and the disease phenotype. The module with the highest trait correlation was defined as the key module, and metabolites within this module were identified as disease-related. The intersection of differentially expressed metabolites and WGCNA module metabolites served as candidate metabolites for subsequent analysis.
The MetaboAnalyst platform was used to perform pathway enrichment analysis on the candidate metabolites, and the results were visualized using the ggplot2 package.
In this study, we applied the least absolute shrinkage and selection operator (LASSO) algorithm for feature selection, a machine learning method commonly used to identify important features in high-dimensional data. By incorporating an L1 regularization term into the loss function, LASSO not only performs variable selection but also improves model sparsity and interpretability by shrinking the coefficients of irrelevant features to zero. In the analysis of feature expression data, the LASSO algorithm effectively identifies key factors associated with disease states from thousands of potential features. Additionally, the regularization property of LASSO helps prevent overfitting and enhances the model’s generalizability. Using this approach, we aimed to identify features that play a critical role in disease onset and progression, providing a scientific basis for subsequent biological validation and clinical application. The glmnet package was used to perform LASSO-based machine learning for feature selection in the groups. The union of the selected features from the comparisons was considered as the set of key metabolites.
In this study, we employed the mixOmics package for the integration of multi-omics data. mixOmics is an R package specifically designed for multivariate analysis of biological datasets, ideal for exploring, reducing dimensionality, and visualizing relationships among heterogeneous omics datasets. The package offers a variety of methods for the simultaneous integration of multiple datasets with a focus on variable selection. It can handle diverse omics data, including transcriptomics, proteomics, and metabolomics, and can process missing values without excluding entire samples with missing data. Using PCA, PLS-DA, and sparse PLS-DA methods from the mixOmics package, we built sparse multivariate models to identify key variables highly associated with biological outcomes. These approaches not only revealed the structural relationships among different datasets but also facilitated the interpretation and understanding of these relationships through graphical representations. The application of mixOmics allowed us to gain deeper insights into the complexity of multi-omics datasets and to identify biologically meaningful patterns and biomarkers. The Pearson algorithm was used to calculate correlations between key proteins and key metabolites, selecting pairs with a correlation coefficient greater than 0.5. The mixOmics package was used to construct a Data Integration Analysis for Biomarker discovery using Latent cOmponents (DIABLO) model. Sparse generalized canonical correlation discriminant analysis was performed to evaluate the ability of biomarkers, and the receiver operating characteristic (ROC) curve was used to assess the discriminatory power of metabolites and proteins for disease classification. The sample plot from the DIABLO model showed that patients with sepsis were significantly distinct from normal control subjects in terms of both proteomics and untargeted metabolomics data. The high correlation between latent components of the two omics datasets indicated good concordance in the DIABLO model. Finally, box plots were used to visualize the expression levels of the biomarkers.
Statistical analysis was performed using descriptive statistics for qualitative data, expressed as n (%), and for quantitative data, normality was assessed using the Shapiro-Wilk test. Data conforming to normal distribution were presented as mean ± SD, while non-normally distributed data were expressed as median (interquartile range). Differences in categorical variables were assessed using Pearson’s χ2 test, while differences in continuous variables were analyzed using t-tests (for normally distributed data) or Mann-Whitney U tests (for non-normally distributed data). First, basic demographic and clinical characteristics (age, weight, height, body mass index, sex, hypertension, diabetes, and hyperlipidemia) were compared between the healthy control group and the sepsis group, with an evaluation of the differential expression of cluster of differentiation (CD) 38 between the two groups. Next, differences in basic characteristics and clinical indicators between the SAE group and the non-encephalopathy group (training set) were analyzed to assess potential factors influencing encephalopathy. Variables with significant differences were further evaluated using ROC curve analysis to assess their diagnostic potential for SAE, with variables categorized based on cut-off values and logistic regression used to create a combined diagnostic model. The diagnostic value of the combined model was assessed with ROC curves. Finally, a separate validation cohort (n = 42; 25 with SAE, 17 without) was used to validate the combined diagnostic model, with differences in basic characteristics and clinical indicators compared between the validation and training sets. The diagnostic performance of the combined model was further validated. P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 24.0 (IBM, Armonk, NY, United States) and R software v4.4.1.
This study included a total of 58 patients with sepsis and 30 control subjects. As shown in Table 1, there were no significant differences in the baseline characteristics between the sepsis group and the control group. The difference in CD38 levels between the two groups was significant (P = 0.002). Among the 58 patients, there were 29 patients with sepsis without encephalopathy (sepsis group) and 29 patients with SAE (SAE group). As shown in Table 2, except for CD38, neuron-specific enolase (NSE), LA, and PCT, there were no significant differences in the baseline characteristics between the SAE group and the sepsis without encephalopathy group.
| Control group (n = 30) | All sepsis group (n = 58) | χ2/t/Z | P value | |
| Age (IQR) | 71.00 (69.00, 79.50) | 78.00 (71.00, 85.00) | 1.897 | 0.058 |
| Weight | 62.19 ± 11.57 | 62.24 ± 11.66 | 0.022 | 0.982 |
| Height (IQR) | 161.00 (155.50, 166.00) | 165.00 (157.25, 170.00) | 1.895 | 0.058 |
| BMI (IQR) | 23.44 (21.41, 25.69) | 22.89 (20.72, 25.20) | 0.527 | 0.598 |
| Gender | 1.161 | 0.281 | ||
| Male | 19 (54.3) | 38 (65.5) | ||
| Female | 16 (45.7) | 20 (34.5) | ||
| Hypertension | 1.887 | 0.170 | ||
| No | 19 (54.3) | 23 (39.7) | ||
| Yes | 16 (45.7) | 35 (60.3) | ||
| Diabetes | 0.573 | 0.449 | ||
| No | 25 (71.4) | 37 (63.8) | ||
| Yes | 10 (28.6) | 21 (36.2) | ||
| Hyperlipidemia | 0.406 | 0.524 | ||
| No | 32 (91.4) | 55 (94.8) | ||
| Yes | 3 (8.6) | 3 (5.2) |
| Sepsis-no encephalopathy(n = 29) | Sepsis-associated encephalopathy | χ2/t/Z | P value | |
| Age (IQR) | 78.00 (73.00, 85.00) | 78.00 (70.00, 85.00) | 0.226 | 0.821 |
| Weight (kg) | 60.28 ± 10.80 | 64.21 ± 12.34 | 1.291 | 0.202 |
| Height (cm) | 162.66 ± 6.73 | 164.55 ± 7.81 | 0.991 | 0.326 |
| BMI (IQR) | 22.49 (19.49, 24.96) | 23.31 (22.03, 25.95) | 1.213 | 0.225 |
| APACHE2 score (IQR) | 16.00 (11.00, 21.00) | 18.00 (13.00, 24.00) | 0.716 | 0.474 |
| Total bilirubin (μmol/L) (IQR) | 10.00 (6.60, 17.40) | 10.10 (8.10, 14.40) | 0.614 | 0.539 |
| Urea nitrogen (mmol/L) (IQR) | 12.60 (8.60, 14.80) | 14.20 (10.40, 24.30) | 1.781 | 0.075 |
| IL-5 (pg/mL) (IQR) | 1.59 (1.59, 1.67) | 1.59 (1.18, 1.69) | 1.339 | 0.180 |
| α-interferon (pg/mL) (IQR) | 0.98 (0.39, 3.07) | 1.47 (0.92, 1.65) | 0.132 | 0.895 |
| IL-2 (pg/mL) (IQR) | 1.27 (0.79, 2.00) | 1.54 (1.22, 2.21) | 1.486 | 0.137 |
| IL-6A (pg/mL) (IQR) | 34.66 (16.65, 76.00) | 35.54 (11.27, 92.62) | 0.148 | 0.883 |
| IL-1β (pg/mL) (IQR) | 3.00 (2.33, 6.76) | 2.33 (1.85, 8.74) | 0.828 | 0.407 |
| IL-10 (pg/mL) (IQR) | 2.23 (1.46, 3.98) | 2.44 (1.42, 3.75) | 0.156 | 0.876 |
| γ-interferon (pg/mL) (IQR) | 2.36 (2.28, 3.52) | 2.36 (2.28, 3.21) | 1.140 | 0.254 |
| IL-8 (pg/mL) (IQR) | 1.54 (1.54, 6.73) | 4.41 (1.54, 10.87) | 1.777 | 0.076 |
| IL-17 (pg/mL) (IQR) | 4.19 (4.19, 5.67) | 4.19 (4.19, 5.24) | 0.665 | 0.506 |
| IL-4 (pg/mL) (IQR) | 0.76 (0.65, 0.82) | 0.67 (0.65, 0.82) | 0.083 | 0.934 |
| IL-12 (pg/mL) (IQR) | 1.62 (1.08, 1.62) | 1.62 (1.12, 1.62) | 0.081 | 0.936 |
| WBC (109/L) (IQR) | 11.18 (8.30, 12.92) | 12.51 (9.56, 14.55) | 1.050 | 0.294 |
| CRP (mg/L) (IQR) | 59.00 (25.00, 108.00) | 94.00 (36.00, 125.00) | 0.996 | 0.319 |
| NSE (ng/mL) (IQR) | 10.67 (8.33, 14.89) | 17.54 (11.23, 22.15) | 2.877 | 0.004 |
| CD38 (ng/mL) (IQR) | 7.37 (6.06, 7.88) | 8.86 (8.30, 10.09) | 4.471 | < 0.001 |
| PCT (ng/mL) (IQR) | 0.22 (0.06, 3.34) | 1.84 (0.58, 6.83) | 2.512 | 0.012 |
| Lactate (mmol/L) (IQR) | 1.80 (1.30, 2.80) | 2.60 (2.10, 4.50) | 2.522 | 0.012 |
| Gender | 1.221 | 0.269 | ||
| Male | 17 (58.62) | 21 (72.41) | ||
| Female | 12 (41.38) | 8 (27.59) | ||
| Diabetes | 0.648 | 0.421 | ||
| No | 19 (65.52) | 16 (55.17) | ||
| Yes | 10 (34.48) | 13 (44.83) | ||
| Hyperlipidemia | 0.358 | 0.550 | ||
| No | 28 (96.55) | 27 (93.10) | ||
| Yes | 1 (3.45) | 2 (6.90) | ||
| Hypertension | 3.658 | 0.056 | ||
| No | 14 (48.28) | 7 (24.14) | ||
| Yes | 15 (51.72) | 22 (75.86) | ||
| Coronary artery disease | 1.105 | 0.293 | ||
| No | 17 (58.62) | 13 (44.83) | ||
| Yes | 12 (41.38) | 16 (55.17) | ||
| CKD/AKI | 2.559 | 0.110 | ||
| No | 15 (51.72) | 9 (31.03) | ||
| Yes | 14 (48.28) | 20 (68.97) | ||
| Liver dysfunction | 2.747 | 0.097 | ||
| No | 22 (75.86) | 16 (55.17) | ||
| Yes | 7 (24.14) | 13 (44.83) | ||
| Fever | 0.672 | 0.412 | ||
| No | 12 (41.38) | 9 (31.03) | ||
| Yes | 17 (58.62) | 20 (68.97) | ||
| Pulmonary infection | 0.345 | 0.557 | ||
| No | 9 (31.03) | 7 (24.14) | ||
| Yes | 20 (68.97) | 22 (75.86) | ||
| Urinary tract infection | 0.892 | 0.345 | ||
| No | 21 (72.41) | 24 (82.76) | ||
| Yes | 8 (27.59) | 5 (17.24) | ||
| Bloodstream infection | 3.219 | 0.073 | ||
| No | 24 (82.76) | 28 (96.55) | ||
| Yes | 5 (17.24) | 1 (3.45) |
Anxiety and depression symptoms were assessed using the HAM-A and HAM-D. In the sepsis group, the mean HAM-A score was 10.4 ± 3.0, while the SAE group had a significantly higher score of 15.2 ± 4.0 (P = 0.012). This difference was further supported by the subdomains: the SAE group exhibited more emotional restlessness (3.4 ± 1.5 vs 2.1 ± 1.3, P = 0.023), higher levels of tension (3.8 ± 1.3 vs 2.3 ± 1.0, P = 0.021), and more panic attacks (2.2 ± 1.1 vs 1.0 ± 0.8, P = 0.045). Additionally, excessive worry was significantly more pronounced in the SAE group (2.1 ± 1.0 vs 1.2 ± 0.7, P = 0.036) (Table 3).
| Sepsis-no encephalopathy (n = 29) | Sepsis-associated encephalopathy (n = 29) | P value | |
| Anxiety symptoms score (HAM-A) | 10.4 ± 3.0 | 15.2 ± 4.0 | 0.012 |
| Emotional restlessness | 2.1 ± 1.3 | 3.4 ± 1.5 | 0.023 |
| Tension | 2.3 ± 1.0 | 3.8 ± 1.3 | 0.021 |
| Panic attacks | 1.0 ± 0.8 | 2.2 ± 1.1 | 0.045 |
| Excessive worry | 1.2 ± 0.7 | 2.1 ± 1.0 | 0.036 |
Regarding depressive symptoms, the mean HAM-D score in the sepsis group was 9.1 ± 2.3, whereas the SAE group scored 16.0 ± 3.5 (P = 0.003). The SAE group exhibited significantly higher scores in several subdomains, including low mood (4.5 ± 1.2 vs 2.1 ± 1.0, P = 0.004), loss of interest (3.9 ± 1.1 vs 1.8 ± 0.9, P = 0.008), insomnia (2.6 ± 0.9 vs 1.2 ± 0.6, P = 0.019), and low self-esteem (2.0 ± 0.8 vs 1.0 ± 0.5, P = 0.028) (Table 4).
| Sepsis-no encephalopathy (n = 29) | Sepsis-associated encephalopathy (n = 29) | P value | |
| Depression symptoms score (HAM-D) | 9.1 ± 2.3 | 16.0 ± 3.5 | 0.003 |
| Low mood | 2.1 ± 1.0 | 4.5 ± 1.2 | 0.004 |
| Loss of interest | 1.8 ± 0.9 | 3.9 ± 1.1 | 0.008 |
| Insomnia | 1.2 ± 0.6 | 2.6 ± 0.9 | 0.019 |
| Low self-esteem | 1.0 ± 0.5 | 2.0 ± 0.8 | 0.028 |
Cognitive function was assessed using the MoCA, and quality of life and functional status were measured using the SF-36. The mean MoCA score in the sepsis group was 24.5 ± 3.2, while the SAE group scored 18.5 ± 4.0 (P = 0.007), indicating significantly poorer cognitive performance in the SAE group. Specifically, the SAE group had lower performance in memory (1.2 ± 0.5 vs 2.0 ± 0.7, P = 0.010), attention (1.5 ± 0.6 vs 2.1 ± 0.8, P = 0.017), language ability (1.8 ± 0.7 vs 2.3 ± 0.6, P = 0.025), and visuospatial ability domains (2.3 ± 0.6 vs 3.0 ± 0.5, P = 0.004) (Table 5).
| Sepsis-no encephalopathy (n = 29) | Sepsis-associated encephalopathy (n = 29) | P value | |
| Cognitive function score (MoCA) | 24.5 ± 3.2 | 18.5 ± 4.0 | 0.007 |
| Memory | 2.0 ± 0.7 | 1.2 ± 0.5 | 0.010 |
| Attention | 2.1 ± 0.8 | 1.5 ± 0.6 | 0.017 |
| Language ability | 2.3 ± 0.6 | 1.8 ± 0.7 | 0.025 |
| Visuospatial ability | 3.0 ± 0.5 | 2.3 ± 0.6 | 0.004 |
In terms of quality of life, the sepsis group had a mean overall score of 72.5 ± 12.5, whereas the SAE group scored 45.0 ± 15.2 (P = 0.015). The SAE group demonstrated significantly lower scores in physical functioning (52.1 ± 11.4 vs 78.3 ± 10.1, P = 0.008), emotional health (42.5 ± 13.8 vs 73.4 ± 14.0, P = 0.003), social functioning (45.6 ± 10.2 vs 70.1 ± 12.0, P = 0.020), and mental health (40.3 ± 13.5 vs 74.0 ± 11.8, P = 0.004) (Table 6).
| Sepsis-no encephalopathy (n = 29) | Sepsis-associated encephalopathy (n = 29) | P value | |
| Quality of life score (SF-36) | 72.5 ± 12.5 | 45.0 ± 15.2 | 0.015 |
| Physical functioning | 78.3 ± 10.1 | 52.1 ± 11.4 | 0.008 |
| Emotional health | 73.4 ± 14.0 | 42.5 ± 13.8 | 0.003 |
| Social functioning | 70.1 ± 12.0 | 45.6 ± 10.2 | 0.020 |
| Mental health | 74.0 ± 11.8 | 40.3 ± 13.5 | 0.004 |
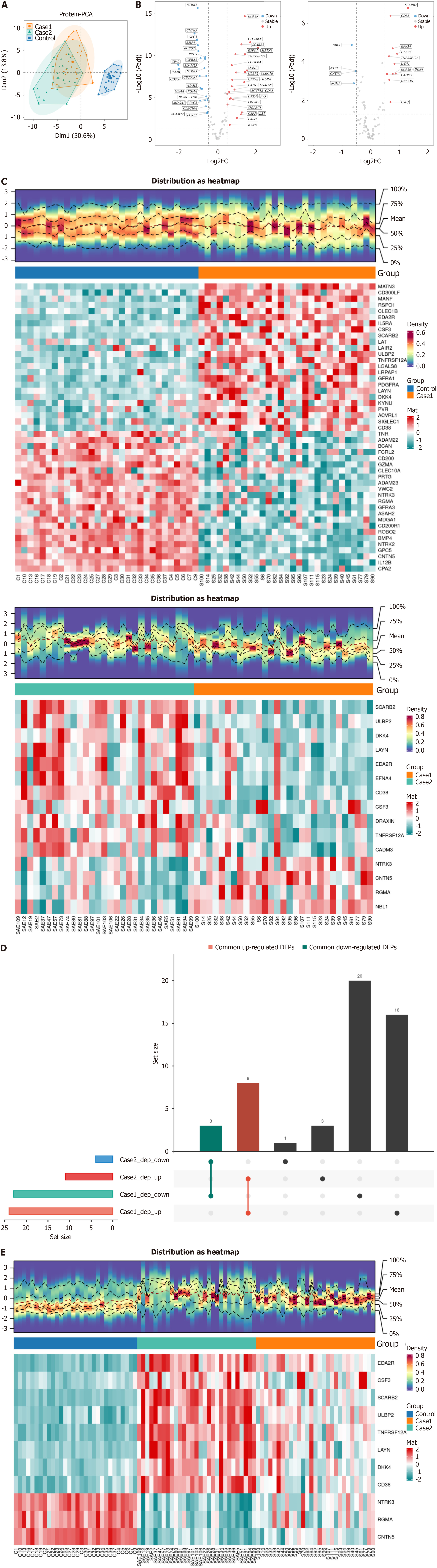
We performed PCA on the proteomic data of 88 proteins to explore the differences between the control group, the sepsis group, and the SAE group. The data were reduced to two dimensions, explaining 30.6% and 13.8% of the variance, respectively. The PCA scatter plot (Figure 1A) showed clear separation between the three groups along these two dimensions, indicating significant differences in protein expression patterns. DEPs were selected using the criteria |log2FC| ≥ 0.5 and adjusted P value < 0.05. In the comparison between the sepsis group and the control group, 47 DEPs were identified, with 24 upregulated and 23 downregulated (Figure 1B and C). A comparison of the SAE and sepsis groups identified 15 DEPs, of which 11 were upregulated and 4 were downregulated (Figure 1B and C).
The intersection of the two comparisons yielded 11 proteins with consistent expression trends, including CSF3, CD38, SCARB2, LAYN, ULBP2, EDA2R, TNFRSF12A, DKK4, CNTN5, NTRK3, and RGMA. Among them, CD38 showed upregulated expression. These 11 proteins were defined as key proteins (Figure 1D and E).
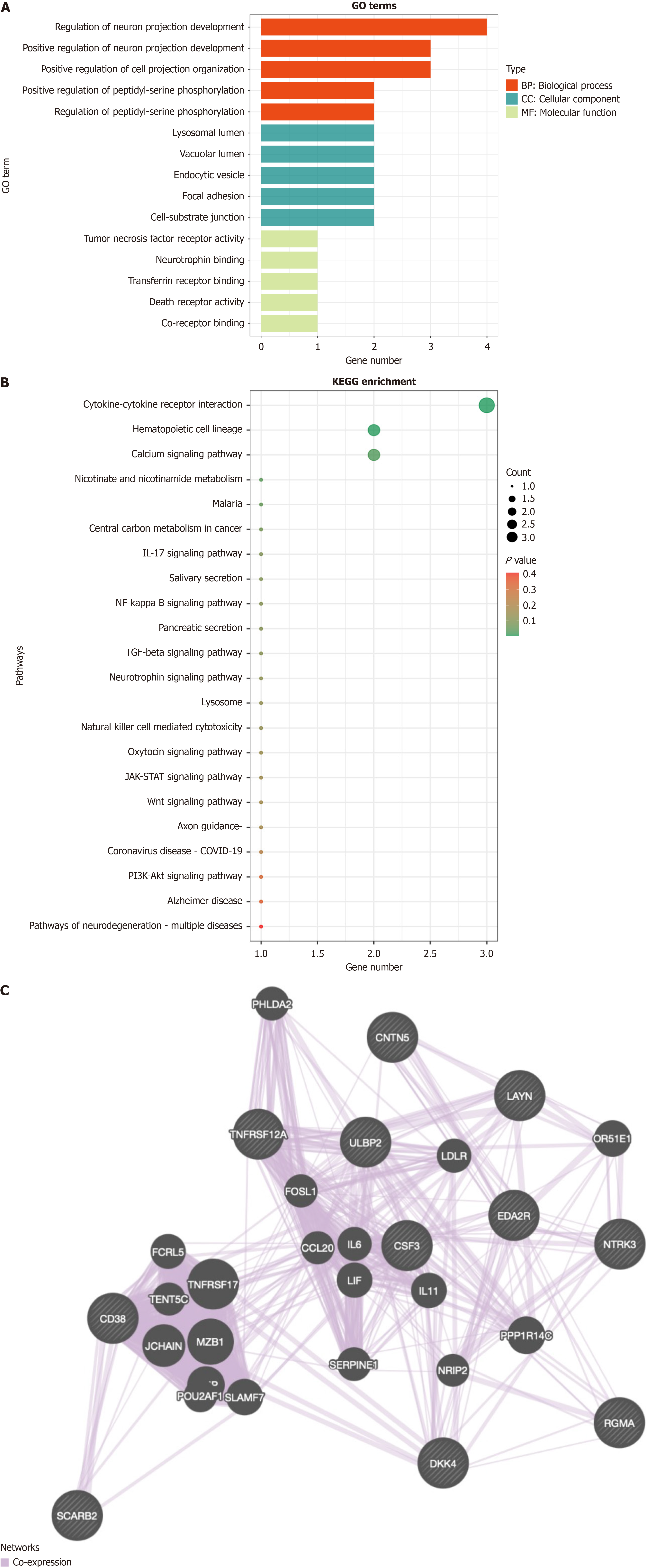
The GO functional enrichment analysis of the 11 key proteins identified 159 terms, comprising 121 biological processes, 9 cellular components, and 29 molecular functions. The following figure presents the top five enriched functions, ranked by the number of enriched terms. Among the biological processes, the proteins were predominantly enriched in the regulation of neuron projection development, positive regulation of neuron projection development, positive regulation of cell projection organization, positive regulation of peptidyl-serine phosphorylation, and regulation of peptidyl-serine phosphorylation. With regard to cellular components, the proteins were enriched in the lysosomal lumen, vacuolar lumen, endocytic vesicle, focal adhesion, and cell-substrate junction. In terms of molecular functions, the proteins were involved in tumor necrosis factor receptor activity, neurotrophin binding, transferrin receptor binding, death receptor activity, and co-receptor binding (Figure 2A).
KEGG pathway enrichment analysis showed that these key proteins were significantly associated with 22 pathways, including the nicotinate and nicotinamide metabolism pathway. While this pathway was not the most significant, it ranked among the top, highlighting the importance of nicotinamide adenine dinucleotide (NAD+) metabolism in the pathogenesis of both sepsis and SAE. Notably, CD38, an upregulated key protein, is known to be an NAD+-degrading enzyme, and its upregulation in sepsis and SAE could directly affect NAD+ levels. Given the association of CD38 with the nicotinate and nicotinamide metabolism pathway, these findings suggest that the upregulation of CD38 may contribute to alterations in NAD+ metabolism, potentially affecting cellular energy metabolism, immune responses, and neuronal damage (Figure 2B).
The results of protein-protein interaction are shown below, where related genes of the proteins are predicted through co-expression, shedding light on their biological roles in sepsis and SAE (Figure 2C).
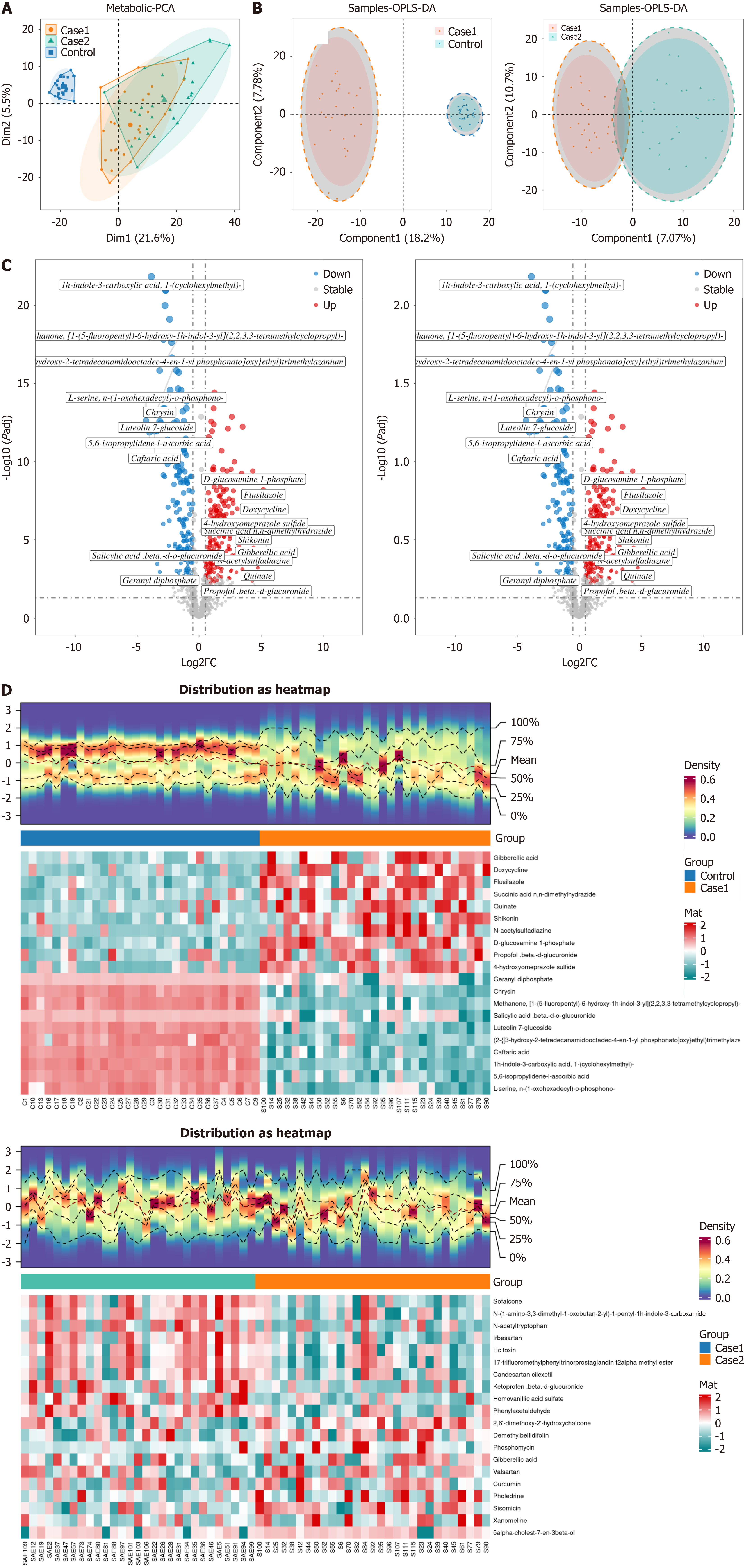
After screening exogenous compounds, a total of 1235 metabolites were identified for subsequent analysis. PCA and partial least squares discriminant analysis were performed on the metabolite data, which were reduced to two principal dimensions explaining 21.6% and 5.5% of the variance. The PCA scatter plot (Figure 3A and B) displayed a clear separation between the three groups along these two dimensions. Differential metabolite analysis using t-tests, with VIP > 1, |log2FC| > 0.5, and adjusted P value < 0.05 as thresholds, identified 401 differential metabolites in the comparison between the sepsis group and the control group, of which 221 were upregulated and 180 were downregulated (Figure 3C and D). A comparison between the SAE and sepsis groups identified 171 differential metabolites, of which 143 were upregulated and 28 were downregulated (Figure 3C and D).
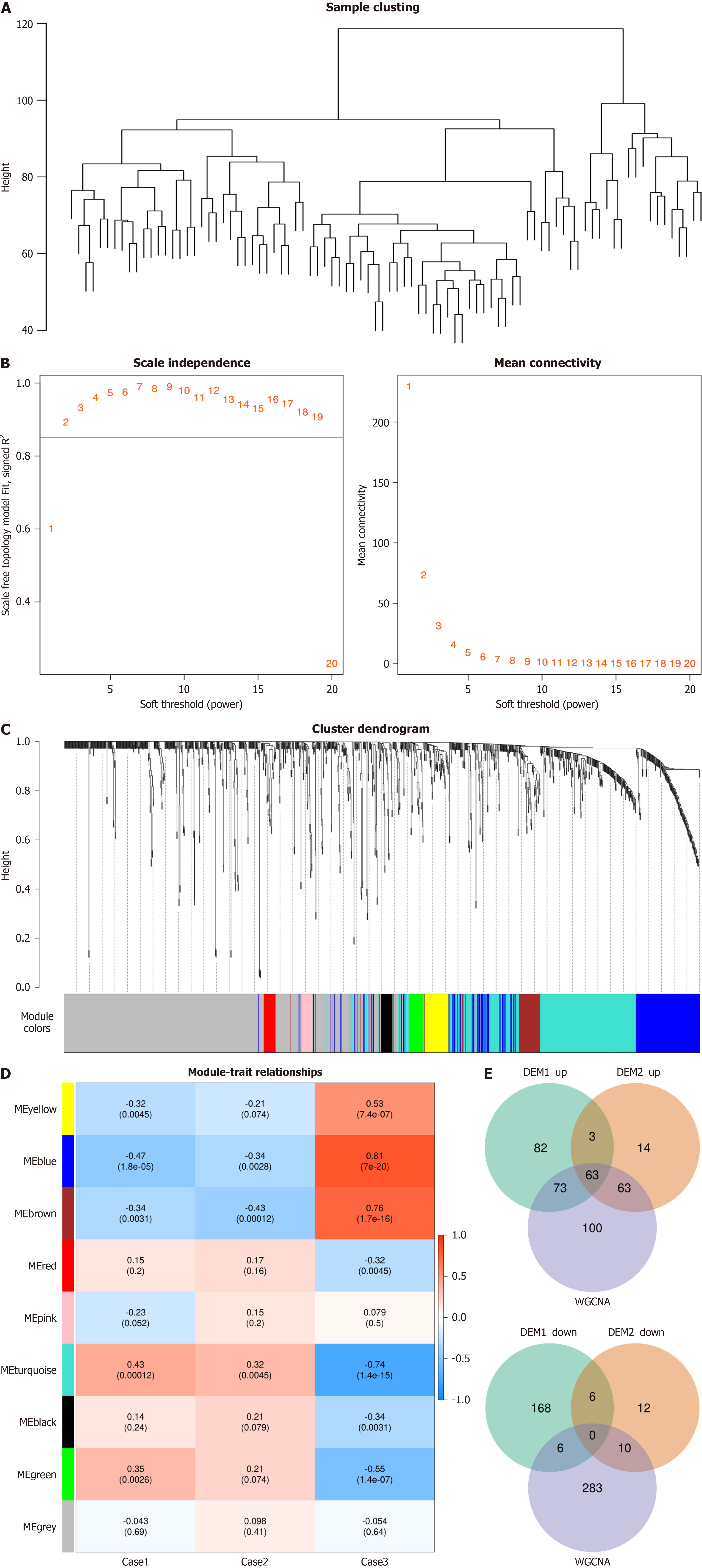
As network module analysis is sensitive to outlier samples, we first constructed a hierarchical clustering tree for all samples (Figure 4A). Next, we selected the most appropriate adjacency function weight parameter according to the scale-free network criterion (Figure 4B). A threshold of 0.85, where the average connectivity is close to 0, was chosen as the cutoff (deep split = 4, min-module size = 20, merge cut height = 0.1) to construct the metabolite modules. As a result, nine metabolite modules were identified, with the gray module being considered non-informative. The disease groups were treated as traits and associated with the metabolite modules, calculating the Pearson correlation coefficient and the corresponding P-values. A heatmap was generated to display the modules (Figure 4C). The turquoise module showed the strongest and most significant positive correlation with the disease; therefore, the 299 metabolites in this module were used for subsequent analysis. Differentially expressed metabolites were overlapped based on the principle of consistent upregulation and downregulation, along with the WGCNA results, resulting in 63 overlapping metabolites (Figure 4D and E).
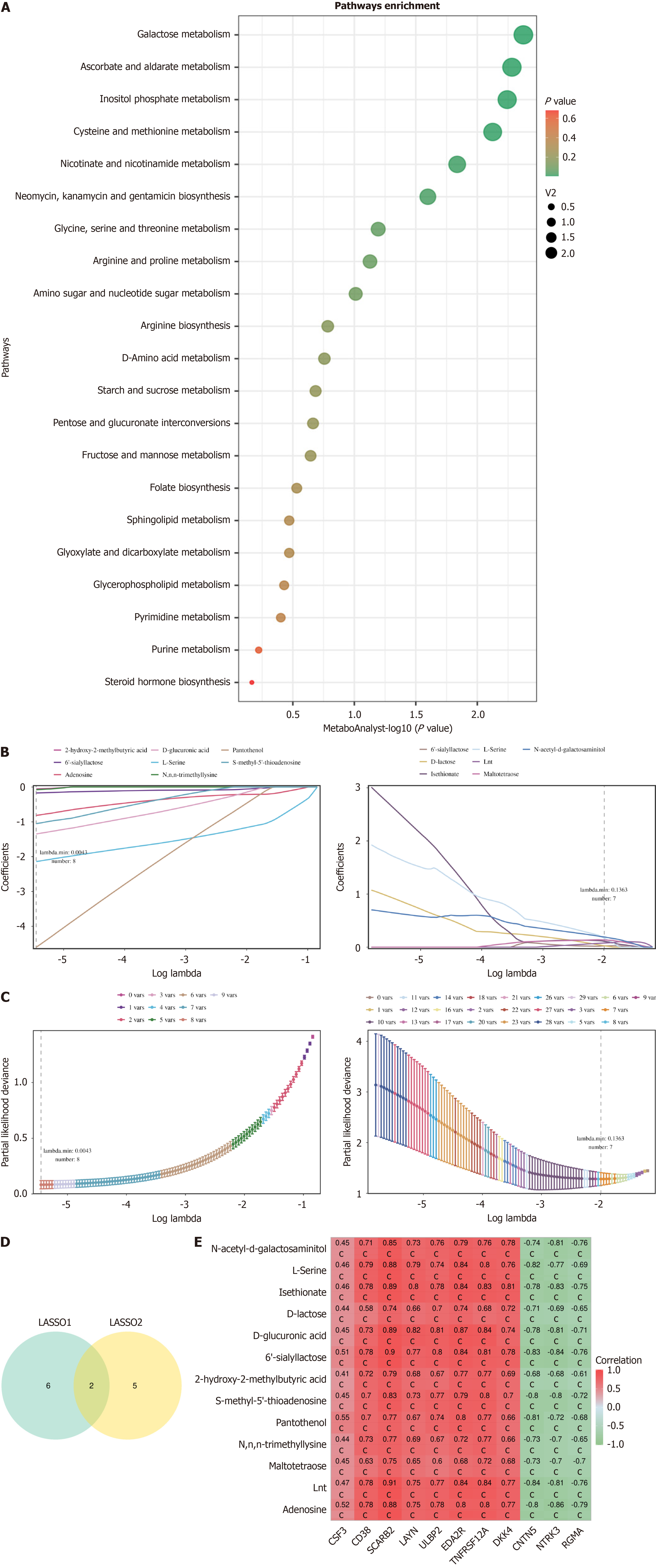
A pathway enrichment analysis of the 63 overlapping differential metabolites was conducted using MetaboAnalyst, revealing their involvement in various metabolic pathways. While the NAD+ metabolism pathway (nicotinate and nicotinamide metabolism) was not the most significant, it ranked among the top pathways, suggesting that NAD+ metabolism may play a critical role in sepsis and SAE. Notably, previous proteomics analysis identified CD38 as a potential biomarker for both sepsis and SAE. CD38 is a well-known NAD+-degrading enzyme, and its interaction with NAD+ metabolism has been extensively documented. Therefore, alterations in CD38 expression in sepsis and SAE could influence NAD+ levels, potentially affecting cellular energy metabolism, immune responses, and neuronal damage. This finding further emphasizes the relevance of the NAD+ metabolism pathway in the pathophysiology of SAE. In addition to NAD+ metabolism, other enriched pathways included galactose metabolism, inositol phosphate metabolism, amino acid metabolism, glucose and fructose metabolism (Figure 5A).
Lasso analysis of the overlapping metabolites was performed, yielding an error plot from 10-fold cross-validation and a metabolite coefficient plot (Figure 5B). The metabolites corresponding to the minimum lambda coefficient, where the cross-validation error was at its lowest, were selected by the algorithm (Figure 5C). The results from the LASSO machine learning algorithm identified a total of 13 metabolites: adenosine, Lnt, maltotetraose, N,N,N-trimethyllysine, pantothenol, S-methyl-5’-thioadenosine, 2-hydroxy-2-methylbutyric acid, 6’-sialyllactose, D-glucuronic acid, D-lactose, isethionate, L-serine, and N-acetyl-D-galactosaminitol (Figure 5D). Among these, 6’-sialyllactose and L-serine were identified as overlapping metabolites with potential relevance to both sepsis and SAE. Pearson correlation analysis was used to examine the relationships between key proteins and metabolites. The results revealed significant positive correlations between most key proteins and metabolites (Figure 5E).
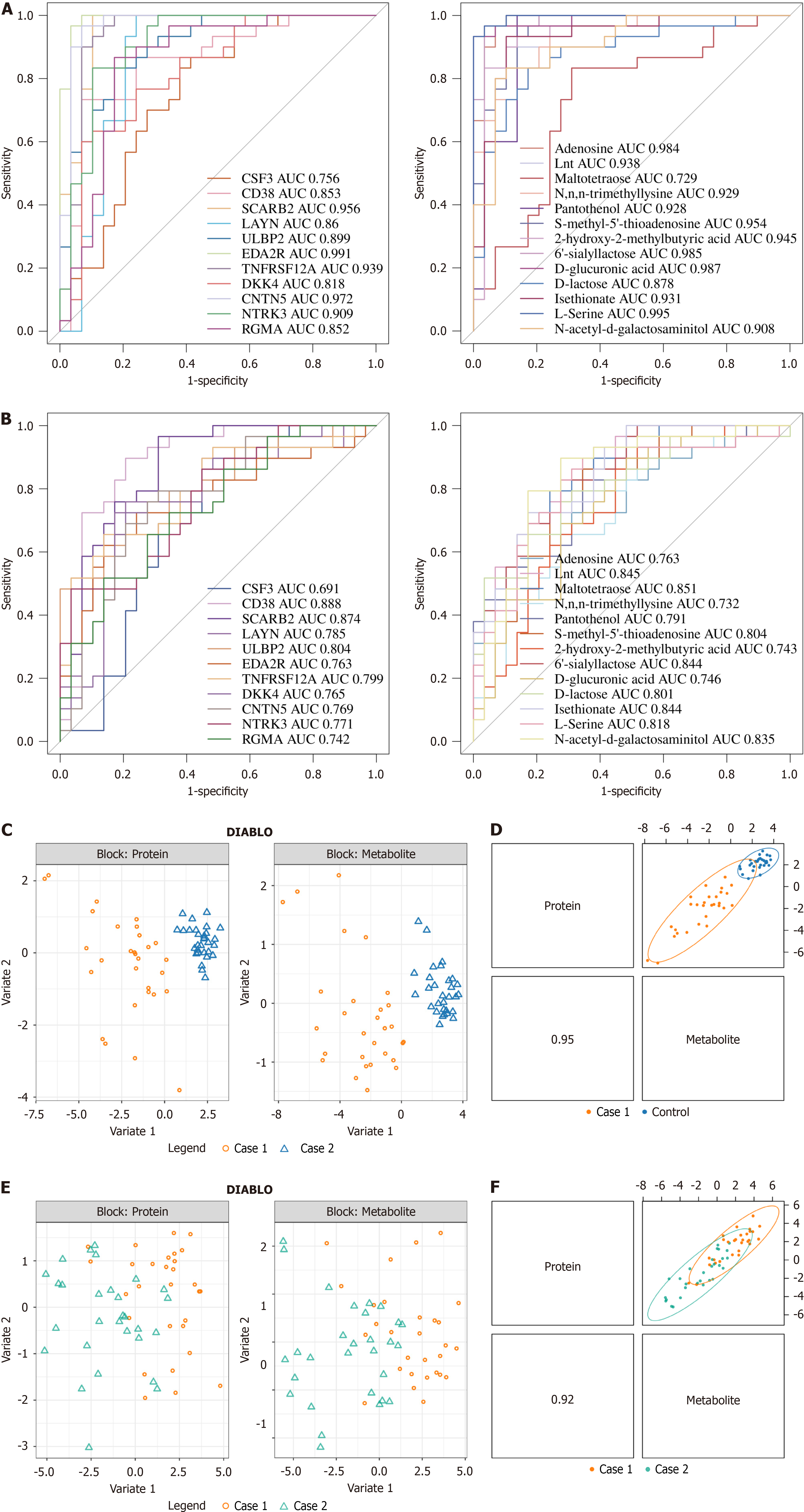
To evaluate the ability of these biomarkers to distinguish between disease and control samples, ROC curves were plotted separately for the protein (Figure 6A and B) and metabolite datasets (Figure 6A and B), and the area under the curve (AUC) was calculated. The AUC values for all biomarkers were greater than 0.7. In both datasets, when comparing sepsis with control samples and SAE with sepsis samples, CD38 consistently showed an AUC greater than 0.8, indicating its strong diagnostic potential.
Additionally, the DIABLO model was constructed to combine proteomics and untargeted metabolomics data for sparse generalized canonical correlation discriminant analysis (Figure 6C-F). The sample plot from the DIABLO model showed that patients with sepsis were significantly different from the control group in terms of both proteomic and non-targeted metabolomic data. The latent components of the two omics datasets were highly correlated, indicating good consistency across the DIABLO model. The Pearson correlation between proteomics and the first component of non-targeted metabolomics data was greater than 0.9. The results plot from the DIABLO model allowed us to visually observe the distribution differences between patients with sepsis and the control group across multiple omics datasets, further confirming the potential value of biomarkers for the diagnosis of sepsis.
To evaluate the diagnostic value of biomarkers for SAE, ROC curve analysis was performed on indicators that showed significant differences between the sepsis groups with and without encephalopathy. As shown in Table 7, CD38 demonstrated the highest diagnostic value for SAE, followed by NSE, while LA and PCT showed lower diagnostic performance. A multivariate logistic regression model was then developed to combine these biomarkers into a composite indicator for predicting the probability of SAE. The model was expressed as follows:
| Cut-off | AUC (95%CI) | P value | Sensitivity | Specificity | |
| CD38 | ≥ 8.18 | 0.842 (0.739-0.945) | < 0.001 | 0.759 | 0.862 |
| NSE | ≥ 17.52 | 0.720 (0.589-0.851) | 0.004 | 0.517 | 0.862 |
| Lactate | ≥ 1.75 | 0.693 (0.555-0.830) | 0.012 | 0.862 | 0.483 |
| PCT | ≥ 0.31 | 0.692 (0.556-0.828) | 0.012 | 0.828 | 0.552 |
| Probabilities | ≥ 0.396 | 0.904 (0.826-0.982) | < 0.001 | 0.862 | 0.862 |
Logit (probabilities) = -3.470 + 2.997 × CD38 + 1.845 × NSE + 1.258 × LA + 1.164 × PCT.
Probabilities = exp (-3.470 + 2.997 × CD38 + 1.845 × NSE + 1.258 × LA + 1.164 × PCT)/[exp (-3.470 + 2.997 × CD38 + 1.845 × NSE + 1.258 × LA + 1.164 × PCT) + 1].
The combined biomarker significantly improved the diagnostic accuracy for SAE. The model was further validated using an independent dataset comprising 42 patients (25 with SAE and 17 without encephalopathy). As shown in Table 8, there were no significant differences in baseline characteristics between the validation and modeling sets. The ROC curve analysis of the validation cohort demonstrated that the composite indicator achieved an AUC (95% confidence interval) of 0.870 (0.773-0.980) with a P value < 0.001. The sensitivity and specificity of the model were 0.920 and 0.647, respectively, indicating that the composite biomarker can be a reliable tool for diagnosing SAE.
| Modeling set (n = 58) | Validation set (n = 42) | χ2/t/Z | P value | |
| Age (IQR) | 78.00 (71.00, 85.00) | 77.50 (70.25, 87.50) | 0.059 | 0.953 |
| Weight (kg) | 62.24 ± 11.66 | 62.24 ± 10.99 | 0.001 | 0.999 |
| Height (cm) | 163.60 ± 7.29 | 164.64 ± 7.91 | 0.679 | 0.499 |
| BMI (IQR) | 22.89 (20.72, 25.20) | 22.90 (20.76, 25.38) | 0.878 | 0.889 |
| APACHE2 score (IQR) | 16.00 (12.00, 22.75) | 18.00 (10.00, 21.00) | 0.276 | 0.782 |
| Total bilirubin (μmol/L) (IQR) | 10.05 (7.00, 15.60) | 11.85 (7.10, 16.03) | 0.475 | 0.635 |
| Urea nitrogen (mmol/L) (IQR) | 12.85 (9.35, 18.25) | 10.20 (6.25, 17.12) | 1.826 | 0.068 |
| IL-5 (pg/mL) (IQR) | 1.59 (1.47, 1.68) | 1.91 (0.72, 2.88) | 0.137 | 0.891 |
| α-interferon (pg/mL) (IQR) | 1.35 (0.72, 1.91) | 1.64 (1.25, 1.89) | 1.823 | 0.068 |
| IL-2 (pg/mL) (IQR) | 1.43 (1.08, 2.16) | 1.54 (0.87, 2.21) | 0.388 | 0.698 |
| IL-6A (pg/mL) (IQR) | 35.10 (11.59, 86.34) | 55.60 (11.86, 425.99) | 1.690 | 0.091 |
| IL-1β (pg/mL) (IQR) | 2.50 (2.33, 6.89) | 2.83 (1.55, 5.16) | 1.513 | 0.130 |
| IL-10 (pg/mL) (IQR) | 2.29 (1.42, 3.92) | 2.75 (1.07, 16.67) | 1.017 | 0.309 |
| γ-interferon (pg/mL) (IQR) | 2.36 (2.28, 3.38) | 2.77 (2.20, 4.72) | 0.744 | 0.457 |
| IL-8 (pg/mL) (IQR) | 2.55 (1.54, 7.40) | 6.06 (1.64, 12.38) | 1.265 | 0.206 |
| IL-17 (pg/mL) (IQR) | 4.19 (4.19, 5.53) | 4.56 (1.89, 11.00) | 0.032 | 0.975 |
| IL-4 (pg/mL) (IQR) | 0.72 (0.65, 0.82) | 0.73 (0.67, 1.03) | 1.761 | 0.078 |
| IL-12 (pg/mL) (IQR) | 1.62 (1.09, 1.62) | 1.48 (0.80, 1.62) | 1.710 | 0.087 |
| WBC (109/L) (IQR) | 11.49 (9.45, 14.01) | 12.04 (6.03, 15.48) | 0.381 | 0.703 |
| CRP (mg/L) (IQR) | 79.50 (27.25, 121.75) | 89.00 (50.25, 151.25) | 1.550 | 0.121 |
| Gender | 0.016 | 0.899 | ||
| Male | 38 (65.52) | 27 (64.29) | ||
| Female | 20 (34.48) | 15 (35.71) | ||
| Diabetes | 0.312 | 0.577 | ||
| No | 35 (60.34) | 23 (54.76) | ||
| Yes | 23 (39.66) | 19 (45.24) | ||
| Hyperlipidemia | 0.166 | 0.684 | ||
| No | 55 (94.83) | 39 (92.86) | ||
| Yes | 3 (5.17) | 3 (7.14) | ||
| Hypertension | 0.300 | 0.584 | ||
| No | 21 (36.21) | 13 (30.95) | ||
| Yes | 37 (63.79) | 29 (69.05) | ||
| Coronary artery disease | 0.090 | 0.764 | ||
| No | 30 (51.72) | 23 (54.76) | ||
| Yes | 28 (48.28) | 19 (45.24) | ||
| CKD/AKI | 0.731 | 0.392 | ||
| o | 24 (41.38) | 21 (50.00) | ||
| Yes | 34 (58.62) | 21 (50.00) | ||
| Liver dysfunction | 0.137 | 0.711 | ||
| No | 38 (65.52) | 29 (69.05) | ||
| Yes | 20 (34.48) | 13 (30.95) | ||
| Fever | 1.750 | 0.186 | ||
| No | 21 (36.21) | 10 (23.81) | ||
| Yes | 37 (63.79) | 32 (76.19) | ||
| Pulmonary infection | 2.532 | 0.112 | ||
| No | 16 (27.59) | 18 (42.86) | ||
| Yes | 42 (72.41) | 24 (57.14) | ||
| Urinary tract infection | 0.027 | 0.870 | ||
| No | 45 (77.59) | 32 (76.19) | ||
| Yes | 13 (22.41) | 10 (23.81) | ||
| Bloodstream infection | 1.532 | 0.216 | ||
| No | 52 (89.66) | 34 (80.95) | ||
| Yes | 6 (10.34) | 8 (19.05) |
This study provides important insights into the neuropsychiatric burden experienced by patients with SAE. Our findings demonstrate that anxiety and depression symptoms are significantly more pronounced in patients with SAE compared to those with sepsis alone, as evidenced by higher HAM-A and HAM-D scores. Additionally, cognitive function was notably impaired in patients with SAE, as reflected by the lower MoCA scores, while their overall quality of life was significantly reduced, particularly in domains related to mental and emotional well-being. These results highlight the substantial psychological burden of SAE and underscore the necessity of integrating mental health assessments into the routine clinical evaluation of patients with SAE. To further investigate the underlying mechanisms of these neuropsychiatric manifestations, we performed plasma metabolomics and proteomics analyses, identifying CD38 as a key biomarker associated with psychiatric symptoms in SAE. Our multi-omics approach revealed significant metabolic alterations, particularly within the NAD+ metabolism pathway. These findings provide new insights into the pathogenesis of SAE and suggest potential targets for early identification of the condition.
SAE is one of the most severe complications of sepsis, contributing to significant morbidity and mortality[12]. The diagnosis and management of SAE remain particularly challenging. SAE presents a broad spectrum of neurological impairments, ranging from subtle cognitive disturbances to profound coma, and is associated with high mortality rates and long-term neurocognitive impairments. A substantial proportion of patients with SAE experience long-term psychiatric sequelae, including anxiety, depression, and mood disorders, which can significantly worsen cognitive dysfunction and prolong recovery times. Our findings underscore the significant impact of psychiatric symptoms on the overall disease burden in SAE. These results are consistent with previous studies indicating that sepsis-related brain dysfunction often manifests with psychiatric symptoms such as anxiety, depression, and cognitive impairment, which can severely hinder long-term recovery and quality of life[4]. From a clinical perspective, this study highlights the urgent need for early screening and management of psychiatric symptoms in patients at risk of developing SAE. Furthermore, our findings suggest that metabolic dysfunction, particularly in oxidative stress and NAD+ metabolism pathways, may play a pivotal role in exacerbating psychiatric symptoms in SAE. The identification of CD38 as a potential biomarker for anxiety and depression in patients with SAE presents new opportunities for targeted interventions, including pharmacological strategies aimed at modulating NAD+ metabolism, which could improve both psychiatric and cognitive outcomes in this patient population.
Currently, the clinical diagnosis of sepsis and SAE mainly relies on physiological and biochemical markers, such as lactate, C-reactive protein, NSE, S100b, and blood routine tests; however, these markers have limited sensitivity and specificity. Although some studies have explored biomarkers related to sepsis, particularly those involved in inflammation and immune responses, these markers have not effectively addressed the early diagnosis of sepsis and its associated encephalopathy[13]. Therefore, the exploration of more sensitive and specific biomarkers has become a major research focus in this field.
One of the significant findings of this study was the marked upregulation of CD38 in both sepsis and SAE groups. CD38, a key enzyme in NAD+ metabolism, is involved in various biological processes, including cellular energy metabolism, immune response, and oxidative stress[14]. Our data indicate that CD38 expression was significantly higher in patients with both sepsis and SAE compared to healthy controls, which is consistent with the immune activation and oxidative stress response associated with sepsis. Existing literature also suggests that the overexpression of CD38 is closely linked to chronic inflammation, immune aging, and the development of various diseases[15-17]. In the context of sepsis, CD38 may regulate NAD+ levels, affecting cellular metabolism and energy production, thus playing a central role in the systemic inflammatory response and brain injury associated with SAE[18]. Therefore, CD38 may not only serve as an early diagnostic biomarker for sepsis but also as a potential tool for early screening of SAE, especially in the early stages when SAE has not yet clearly manifested.
Further analysis revealed a close regulatory relationship between CD38 and the NAD+ metabolism pathway. NAD+ is a critical coenzyme involved in multiple metabolic pathways, including energy metabolism, DNA repair, and cell death regulation[19,20]. Our metabolomics analysis showed that NAD+ and its related metabolites were significantly elevated in patients with sepsis and SAE compared to healthy controls. The increase in NAD+ likely reflects the metabolic imbalance and elevated oxidative stress induced by sepsis, which are closely associated with systemic inflammation and brain dysfunction in sepsis. Previous studies have indicated that abnormal NAD+ metabolism plays a key role in the pathogenesis of various diseases, such as metabolic disorders and neurodegenerative diseases[21], further validating the potential importance of NAD+ metabolism dysregulation in SAE.
Additionally, we identified several differential metabolites through metabolomics analysis, discovering that key metabolites in the NAD+ metabolism pathway, such as adenosine[22], had strong discriminatory power for diagnosing SAE. Changes in the NAD+ metabolic pathway are not only closely related to the onset of sepsis but also tightly connected to the pathological progression of SAE. By jointly analyzing CD38 and NAD+ metabolites[23], we found a significant correlation between the two, suggesting that they may act synergistically in the development of SAE.
The robust diagnostic performance of CD38 (AUC > 0.8) highlights its potential as a bedside biomarker for early SAE detection. Furthermore, the DIABLO model’s integration of proteomic and metabolomic data (Pearson correlation > 0.9) supports the development of multi-omics diagnostic panels. For instance, combining CD38 with dysregulated metabolites could enhance diagnostic specificity. Clinically, this approach may reduce reliance on invasive tests (e.g., lumbar puncture) and enable timely interventions.
While our study highlights the potential diagnostic value of CD38 in SAE, the predictive ability of these biomarkers across different clinical stages remains limited. In the early stages of sepsis, single biomarkers may not fully reflect the complexity of the disease[24]. To enhance diagnostic accuracy, we recommend combining these potential biomarkers with traditional clinical indicators (such as lactate, C-reactive protein, PCT, etc.) to form a multi-layered diagnostic model[25], enabling more precise screening in the early stages of SAE. The integration of CD38 into clinical workflows, alongside established markers like PCT, could enhance early detection of SAE. Targeted interventions may mitigate neuropsychiatric symptoms by restoring metabolic homeostasis. These biomarkers could significantly improve clinical management, enabling early detection and intervention for both cognitive dysfunction and psychiatric disorders, which are critical to improving outcomes and quality of life in patients with SAE. By identifying high-risk individuals early, clinicians can tailor their interventions to address both neurological and psychiatric needs, potentially enhancing recovery and mitigating long-term mental health issues.
CD38-driven NAD+ depletion may contribute to anxiety and depression via multiple pathways. NAD+ deficiency impairs adenosine triphosphate production in hippocampal neurons, disrupting synaptic plasticity and promoting depressive-like behaviors. CD38 activation amplifies microglial release of interleukin-6 and tumor necrosis factor-α, which are implicated in blood-brain barrier disruption and limbic system hyperactivation. Reduced SIRT3 activity (due to NAD+ loss) downregulates BDNF expression, impairing emotional resilience. These mechanisms provide a framework for targeting CD38-NAD+ signaling to alleviate SAE-related neuropsychiatric symptoms.
As the disease progresses, particularly in the middle and late stages of SAE, the expression levels of CD38 significantly increase, making it more valuable for diagnosis during the later stages of the disease[26]. Therefore, the identification of CD38 as a potential biomarker for anxiety and depression in patients with SAE presents new opportunities for targeted interventions, including pharmacological strategies aimed at modulating NAD+ metabolism, which could improve both psychiatric and cognitive outcomes in this patient population. This suggests that interventions targeting NAD+ metabolism may not only help mitigate cognitive deficits commonly associated with SAE but also alleviate anxiety and depression symptoms, which significantly affect patient recovery. Our identification of CD38 as a key biomarker and its link to NAD+ depletion opens new avenues for therapeutic intervention. Restoring NAD+ levels could counteract metabolic stress and improve neuronal resilience in SAE. Conversely, CD38 inhibition might reduce NAD+ degradation and dampen neuroinflammatory pathways. While these approaches have shown promise in preclinical models of neurodegenerative diseases, their efficacy in SAE remains unexplored. Future studies should prioritize translational research to evaluate whether targeting CD38 or NAD+ metabolism improves psychiatric and cognitive outcomes in patients with SAE.
This study has several limitations. First, the sample size, although adequate for preliminary biomarker discovery, was relatively small and derived from a single-center cohort of ICU and emergency department patients. This may limit the generalizability of our findings, necessitating validation in larger, multi-center cohorts with diverse ethnicities, ages, and disease subtypes. Second, while we identified CD38 and NAD+ metabolites as promising biomarkers through multi-omics analysis, their clinical applicability requires further validation across biological fluids (e.g., cerebrospinal fluid, urine) and populations. Third, we did not systematically evaluate all comorbidities (e.g., diabetes) or fully account for the confounding effects of medications (e.g., sedatives) and comorbidities (e.g., chronic kidney disease) on neuropsychiatric outcomes, despite standardized protocols to minimize variability. Fourth, the therapeutic potential of targeting CD38 and NAD+ metabolism remains hypothetical; future studies should prioritize interventional trials (e.g., NAD+ supplementation) and mechanistic explorations in other neuropsychiatric conditions (e.g., post-traumatic brain injury). Finally, the lack of longitudinal follow-up precludes insights into the persistence of neuropsychiatric symptoms after discharge. Future studies should track outcomes at 30- and 90-day intervals to evaluate the burden of chronic SAE and the efficacy of the interventions.
This study highlights the significant neuropsychiatric burden experienced by patients with SAE, particularly in the form of anxiety, depression, and cognitive impairment. Through multi-omics analysis, we identified CD38 as a key biomarker associated with these psychiatric symptoms, with NAD+ metabolism dysregulation emerging as a crucial underlying mechanism. Our findings underscore the need for early screening and management of psychiatric symptoms in these patients, as untreated anxiety and depression may worsen their clinical outcomes and prolong recovery. Furthermore, the identification of CD38 as a potential diagnostic biomarker opens new avenues for targeted interventions, including pharmacological strategies modulating NAD+ metabolism, which could improve both psychiatric and cognitive outcomes.
| 1. | Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 513] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 2. | Sonneville R, Benghanem S, Jeantin L, de Montmollin E, Doman M, Gaudemer A, Thy M, Timsit JF. The spectrum of sepsis-associated encephalopathy: a clinical perspective. Crit Care. 2023;27:386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 3. | Li D, Zhang X, Lu Y, Jing L, Hu H, Song Y, Wu S, Zhu W. Post-sepsis psychiatric disorder: Pathophysiology, prevention, and treatment. Neurol Sci. 2024;45:3093-3105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Hong Y, Chen P, Gao J, Lin Y, Chen L, Shang X. Sepsis-associated encephalopathy: From pathophysiology to clinical management. Int Immunopharmacol. 2023;124:110800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 5. | Barichello T, Generoso JS, Singer M, Dal-Pizzol F. Biomarkers for sepsis: more than just fever and leukocytosis-a narrative review. Crit Care. 2022;26:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 246] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 6. | Zhang L, Zhang X. Serum sTREM-1, PCT, CRP, Lac as Biomarkers for Death Risk Within 28 Days in Patients with Severe Sepsis. Open Life Sci. 2018;13:42-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Babu M, Snyder M. Multi-Omics Profiling for Health. Mol Cell Proteomics. 2023;22:100561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 133] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 8. | Deng YT, You J, He Y, Zhang Y, Li HY, Wu XR, Cheng JY, Guo Y, Long ZW, Chen YL, Li ZY, Yang L, Zhang YR, Chen SD, Ge YJ, Huang YY, Shi LM, Dong Q, Mao Y, Feng JF, Cheng W, Yu JT. Atlas of the plasma proteome in health and disease in 53,026 adults. Cell. 2025;188:253-271.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 9. | Vo DK, Trinh KTL. Emerging Biomarkers in Metabolomics: Advancements in Precision Health and Disease Diagnosis. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 10. | Chen Q, Liang X, Wu T, Jiang J, Jiang Y, Zhang S, Ruan Y, Zhang H, Zhang C, Chen P, Lv Y, Xin J, Shi D, Chen X, Li J, Xu Y. Integrative analysis of metabolomics and proteomics reveals amino acid metabolism disorder in sepsis. J Transl Med. 2022;20:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 11. | Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Hylander Møller M, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. 2021;49:e1063-e1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 1356] [Article Influence: 339.0] [Reference Citation Analysis (2)] |
| 12. | Pan S, Lv Z, Wang R, Shu H, Yuan S, Yu Y, Shang Y. Sepsis-Induced Brain Dysfunction: Pathogenesis, Diagnosis, and Treatment. Oxid Med Cell Longev. 2022;2022:1328729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 13. | Zhu J, Zhang M, Han T, Wu H, Xiao Z, Lin S, Wang C, Xu F. Exploring the Biomarkers of Sepsis-Associated Encephalopathy (SAE): Metabolomics Evidence from Gas Chromatography-Mass Spectrometry. Biomed Res Int. 2019;2019:2612849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Li W, Liang L, Liao Q, Li Y, Zhou Y. CD38: An important regulator of T cell function. Biomed Pharmacother. 2022;153:113395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 15. | Mathur P, Kottilil S, Pallikkuth S, Frasca D, Ghosh A. Persistent CD38 Expression on CD8 + T Lymphocytes Contributes to Altered Mitochondrial Function and Chronic Inflammation in People With HIV, Despite ART. J Acquir Immune Defic Syndr. 2022;91:410-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Chini CCS, Peclat TR, Warner GM, Kashyap S, Espindola-Netto JM, de Oliveira GC, Gomez LS, Hogan KA, Tarragó MG, Puranik AS, Agorrody G, Thompson KL, Dang K, Clarke S, Childs BG, Kanamori KS, Witte MA, Vidal P, Kirkland AL, De Cecco M, Chellappa K, McReynolds MR, Jankowski C, Tchkonia T, Kirkland JL, Sedivy JM, van Deursen JM, Baker DJ, van Schooten W, Rabinowitz JD, Baur JA, Chini EN. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD(+) and NMN levels. Nat Metab. 2020;2:1284-1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 217] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 17. | Hearps AC, Martin GE, Angelovich TA, Cheng WJ, Maisa A, Landay AL, Jaworowski A, Crowe SM. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012;11:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 380] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 18. | Du Y, Zhang H, Guo Y, Song K, Zeng L, Chen Y, Xie Z, Li R. CD38 deficiency up-regulated IL-1β and MCP-1 through TLR4/ERK/NF-κB pathway in sepsis pulmonary injury. Microbes Infect. 2021;23:104845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 19. | Gasperi V, Sibilano M, Savini I, Catani MV. Niacin in the Central Nervous System: An Update of Biological Aspects and Clinical Applications. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 20. | Massudi H, Grant R, Guillemin GJ, Braidy N. NAD+ metabolism and oxidative stress: the golden nucleotide on a crown of thorns. Redox Rep. 2012;17:28-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Verdin E. NAD⁺ in aging, metabolism, and neurodegeneration. Science. 2015;350:1208-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 908] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 22. | Linden J, Koch-Nolte F, Dahl G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu Rev Immunol. 2019;37:325-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 263] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 23. | Fang J, Chen W, Hou P, Liu Z, Zuo M, Liu S, Feng C, Han Y, Li P, Shi Y, Shao C. NAD(+) metabolism-based immunoregulation and therapeutic potential. Cell Biosci. 2023;13:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 24. | Ke L, Lu Y, Gao H, Hu C, Zhang J, Zhao Q, Sun Z, Peng Z. Identification of potential diagnostic and prognostic biomarkers for sepsis based on machine learning. Comput Struct Biotechnol J. 2023;21:2316-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Wu D, Qin H. Diagnostic and prognostic values of immunocyte ratios in patients with sepsis in the intensive care unit. J Infect Dev Ctries. 2023;17:1362-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 26. | Mazeraud A, Righy C, Bouchereau E, Benghanem S, Bozza FA, Sharshar T. Septic-Associated Encephalopathy: a Comprehensive Review. Neurotherapeutics. 2020;17:392-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |