Published online Jun 19, 2025. doi: 10.5498/wjp.v15.i6.104809
Revised: March 11, 2025
Accepted: April 21, 2025
Published online: June 19, 2025
Processing time: 147 Days and 7.2 Hours
Hypobaric hypoxia exposure (HHE) often causes neuropsychiatric disorders. Due to its complex mechanism, efficient strategies for alleviating HHE-induced anxiety- and depression-like behaviors remain limited.
To characterize alterations in the oral and gut microbiota following HHE and to explore a potential microbiota-based intervention to mitigate associated psychia
C57BL/6J mice were exposed to simulated high-altitude hypoxia (5000 m) for 1, 3, 5, or 7 days. Behavioral assessments, including the open field test, elevated plus maze, and forced swim test, were conducted to evaluate anxiety- and depression-like behaviors. Oral and fecal microbiota were analyzed using 16S rRNA sequen
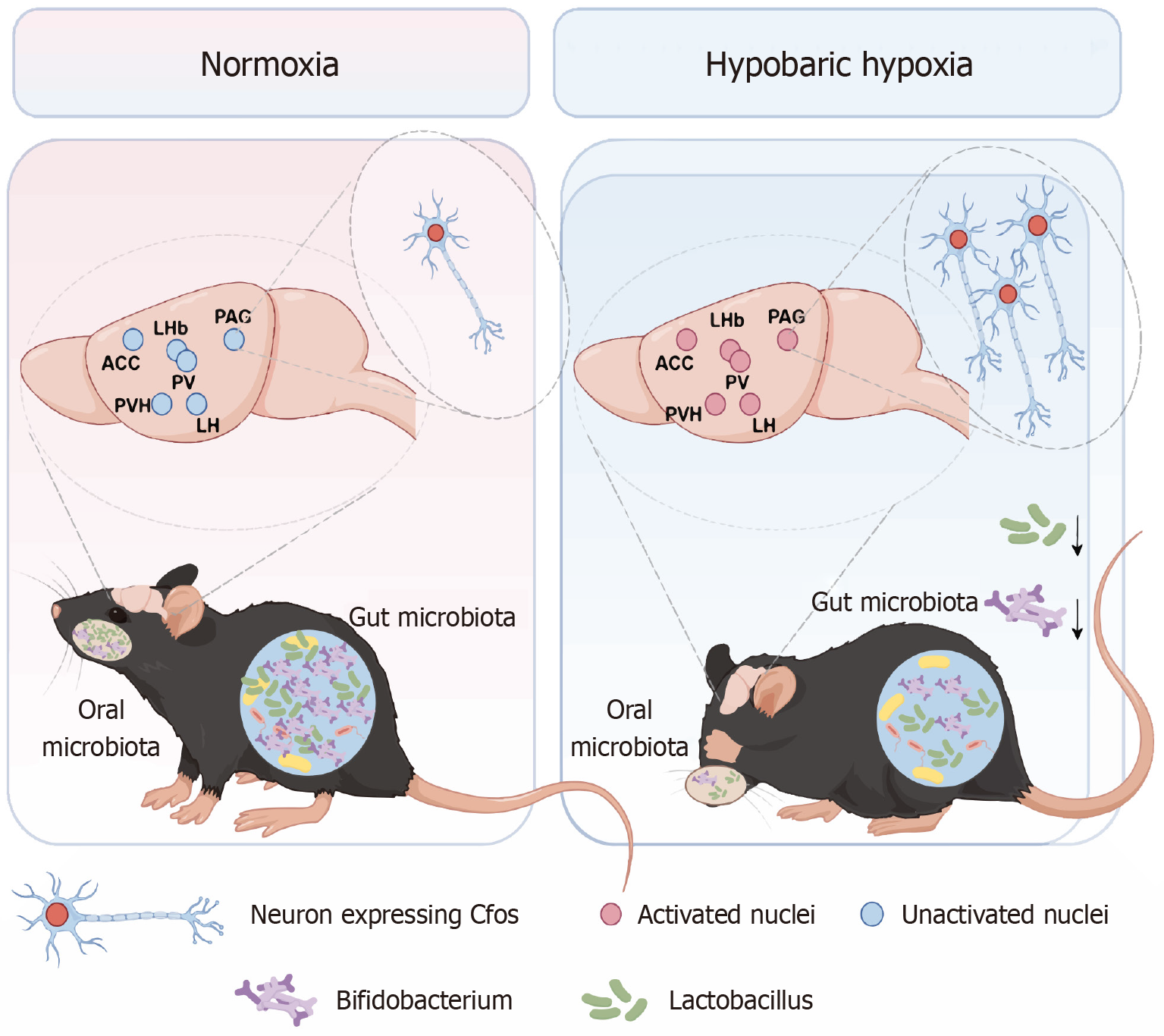
Behavioral tests revealed that HHE caused anxiety- and depression-like behaviors, which were most pronounced after 1 day of exposure. The IF data revealed significantly increased expression of c-Fos in various brain nuclei after HHE, including the anterior cingulate cortex, paraventricular thalamic nucleus, lateral habenula nucleus, paraventricular hypothalamic nucleus, lateral hypothalamus, and periaqueductal gray. The 16S rRNA sequencing results demonstrated a sharp decline in the abundance of Lactobacillus in the oral microbiota of mice exposed to HH1 and a marked decrease in the abundance of Lactobacillus and Bifidobacterium in the fecal microbiota of mice exposed to three days of HHE. Finally, oral administration and gavage of Lactobacillus significantly alleviated anxiety- and depression-like behaviors in HH1 mice.
HHE caused significant variations in the oral and fecal microbiota of mice. Lactobacillus supplementation alleviated anxiety- and depression-like behaviors in mice. Improving oral flora may relieve HHE-induced psychiatric disorders.
Core Tip: The effects of hypobaric hypoxia (HH) exposure (HHE) on anxiety- and depression-like behaviors in mice were analyzed. HHE for one day activated several brain nuclei, including the anterior cingulate cortex, paraventricular thalamic nucleus, lateral habenula, paraventricular hypothalamic nucleus, lateral hypothalamus, and anterior periaqueductal gray. A significant decrease in Lactobacillus abundance was found in the oral microbiota following one day of HH. Both oral application and gavage administration of a Lactobacillus complex effectively alleviated anxiety- and depression-like behaviors in mice subjected to one day of HH.
- Citation: Chang WY, Qin QZ, Li XT, Wang JJ, Chen Y, Ruan HQ, Qu YN, Jiang XX, He HX. Modulating oral microbiota ameliorates hypobaric hypoxia-induced anxiety- and depression-like behaviors in mice. World J Psychiatry 2025; 15(6): 104809
- URL: https://www.wjgnet.com/2220-3206/full/v15/i6/104809.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i6.104809
Plateau landforms, characterized by high altitudes, low atmospheric pressure, and reduced oxygen levels, are found globally and present unique environmental and climatic challenges compared with plains. Due to the imperative of balanced regional development and the demands of various occupations, a remarkable number of individuals are required to reside at high altitudes for extended or intermittent periods. Studies have demonstrated a link between high-altitude conditions and psychiatric disorders, indicating that hypobaric hypoxia (HH) exposure (HHE) may negatively affect emotional well-being and increase the risk of anxiety and depression[1-7]. Consequently, understanding the physiological and psychological effects of high-altitude environments is essential for developing preventive strategies against neuropsychiatric disorders.
Recent research has increasingly concentrated on the mechanisms underlying anxiety- and depression-like behaviors triggered by HHE. The gut microbiota has emerged as a critical factor in maintaining normal brain function, and its diversity is notably affected by HHE[8-10]. Mounting evidence highlights a close association between changes in the gut microbiota and the onset of anxiety and depression. Studies indicated that the gut microbiota could influence brain neurotransmitter activity by producing short-chain fatty acids (SCFAs) and modulating key neurotransmitters, such as serotonin and dopamine, which can contribute to negative emotional states[11-13]. Additionally, metabolites from gut microbiota, such as SCFAs, are capable of crossing the blood-brain barrier (BBB) and influencing emotional regulation[14]. Inflammatory markers are increasingly recognized as significant indicators of anxiety and depression, and several studies have reported that alterations in specific gut bacterial communities are correlated with shifts in inflammatory cytokines and immune responses[15,16]. For instance, previous research has demonstrated that gut microbiota, including symbiotic, probiotic, and pathogenic bacteria, can coactivate neural pathways and central nervous system signaling, potentially fostering the progression of anxiety and depression[17]. These findings suggest that HHE modifies the composition and functionality of gut microbiota, ultimately influencing the brain's ability to regulate emotions and increasing the likelihood of anxiety and depressive disorders.
Given the close relationship between the oral and gut microbiota, the oral microbiota, the second largest microbial community in humans and directly exposed to environmental factors, merits further investigation to determine its susceptibility to HHE and its potential role in anxiety and depression. Research on the correlation between oral microbiota and emotional disorders has revealed that patients with depression exhibit significantly lower oral microbial diversity compared with healthy individuals, identifying microbial diversity as a potential indicator of mental health[18,19]. Moreover, recent studies have revealed specific associations between oral microbiota and mental health conditions, including anxiety and depression. For instance, a particular saliva-resident bacterium, Eggerthia, has been linked to these disorders in datasets, such as the United Kingdom Biobank and FinnGen, presenting a promising diagnostic target and facilitating early intervention strategies for managing anxiety and depression[20]. Researchers have also explored the mechanisms by which oral microbiota can influence anxiety and depression. Metabolites produced by oral microbiota, including SCFAs, amino acids, phenolic compounds, and bioactive molecules, can affect brain activity and emotional states, potentially contributing to these mental health conditions. Such metabolites may aggravate emotional disorders through diverse pathways. For instance, chronic neuroinflammation is closely associated with depression and anxiety, and certain metabolites of the oral microbiota may weaken the BBB, enabling these compounds to enter the brain and intensify neuroinflammation. These metabolites can also alter the brain’s inflammatory profile by impacting the synthesis and release of neurotransmitters. Alternatively, metabolites from oral microbiota may enter the gut via saliva, subsequently influencing gut microbial composition and functionality. This, in turn, affects neurotransmitter levels through the gut-brain axis, ultimately shaping emotional states and behavioral patterns[18]. These findings highlight the critical role of oral microbiota and their metabolites in emotional regulation, particularly in the progression of anxiety and depression.
Considering the potential function of the microbiota in HHE-induced psychiatric disorders, this study investigated the effects of HHE on the behaviors of mice and the changes in their oral and gut microbiota. The results revealed that improving oral flora can alleviate HHE-induced anxiety- and depression-like behaviors in mice.
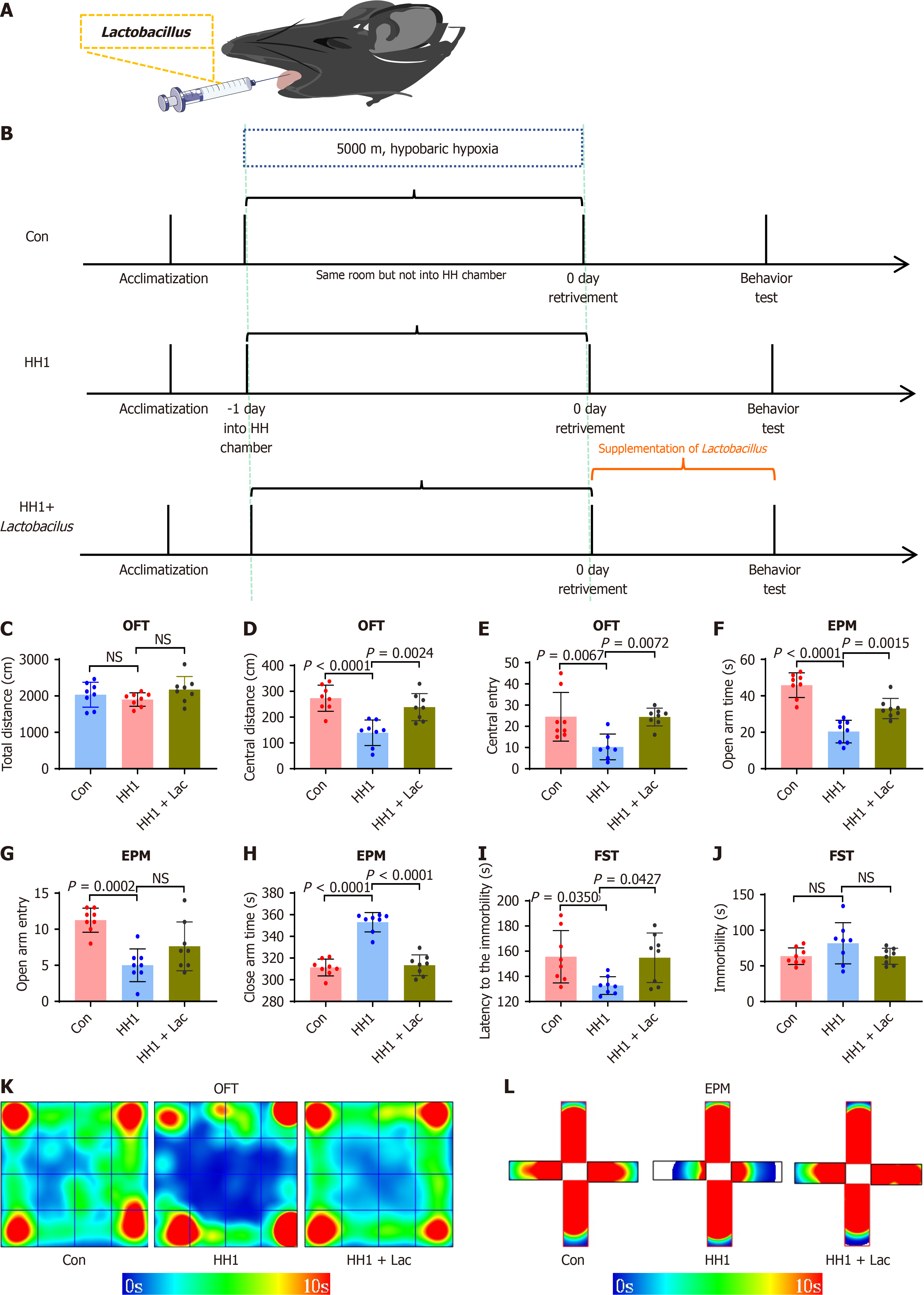
In this study, 7-week-old C57BL/6J mice (SPFbiotech, Haidian, Beijing, China) were used, and housed under standard conditions (12/12 hours light/dark cycle) with ad libitum access to food and water. After a 1-week acclimation period, the experiments began when mice reached 8 weeks of age.
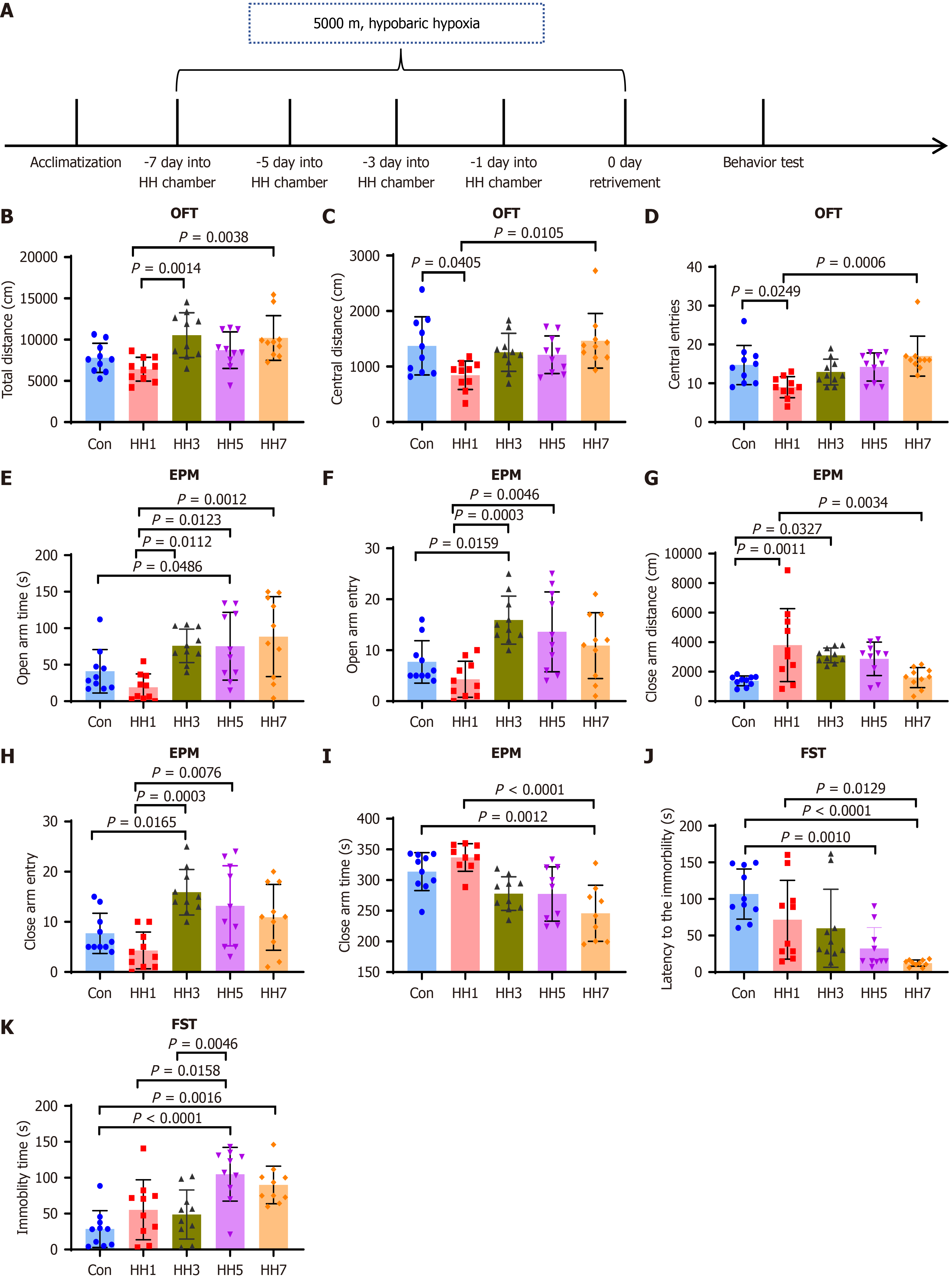
To simulate high-altitude hypoxic conditions, an HH chamber (Fenglei Aeronautics Ordnance Co., Ltd., Guizhou, China) was utilized to replicate an altitude of 5000 m. On the day of the experiment, mice were deprived of food and water for 1 hour before exposure. All fifty mice were first acclimated for 30 minutes in a high-altitude hypoxia simulation room. forty mice in the experimental group were then placed inside the HH chamber, and the chamber was sealed to gradually increase the simulated altitude to 5000 m. After stabilization, the condition of mice was monitored, and the experimenter exited the chamber. Ten mice in the control group (Con) remained outside the chamber, while were otherwise exposed to identical conditions.
After one day of HHE (HH1), the chamber altitude was gradually lowered to normal levels. Once the chamber was safely opened, mice in the experimental group were retrieved and returned to the animal laboratory along with those in the Con. All subsequent experimental procedures were initiated 30 minutes later.
Oral microbiota collection: Under sterile conditions, mice were anesthetized with isoflurane (30 μL/g; Meilun, Dalian, Liaoning, China) and placed in a laminar flow cabinet. To collect oral microbiota, 10 μL of sterile phosphate-buffered saline (PBS) was applied to the periodontal mucosa to facilitate microbial transfer. A sterilized dental brush was thereafter used to gently scrub the mucosa until the PBS solution was absorbed. The liquid-soaked portion of the brush was immediately frozen in liquid nitrogen and stored at -80 °C for subsequent 16S rRNA sequencing.
Fecal microbiota collection: Fecal samples were collected by gently stimulating the rectal and anal regions of mice to induce defecation. The freshly excreted feces were immediately transferred into sterile 1.5 mL centrifuge tubes, flash-frozen in liquid nitrogen, and stored at -80 °C for subsequent 16S rRNA gene sequencing.
Before testing, all equipment was thoroughly cleaned to eliminate residual odors and to minimize stress. Mice were acclimated to the behavioral testing room for at least 1 hour before testing.
Each mouse was placed in the center of an open field (40 cm × 40 cm × 40 cm) and allowed to explore freely for 5 minutes. Movement trajectories were recorded using Visual Track software (Shanghaixinruan, Minhang, Shanghai, China), which automatically calculated total movement distance, time spent in the central area, and the number of entries into the central area. These metrics assessed spontaneous activity and anxiety levels. After each test, the field was cleaned with alcohol to remove odors.
Mice were placed in the central area of the elevated maze, facing one of the open arms, and allowed to explore for 5 minutes. Movement trajectories were recorded using Visual Track software, which calculated time spent and distance traveled in the open and closed arms, along with the number of entries into each arm. These metrics served as indicators of anxiety severity.
The forced swim test (FST) was conducted in a water-filled chamber (23 °C-25 °C). Mice were placed in water for 6 minutes, and behavioral data from the final 4 minutes were recorded for analysis. Immobility time and latency were measured to evaluate depression-like behaviors. Testing equipment for open field test (OFT), elevated plus maze (EPM), and FST was obtained from Zhongshikeji Co., Ltd. (Chaoyang, Beijing, China).
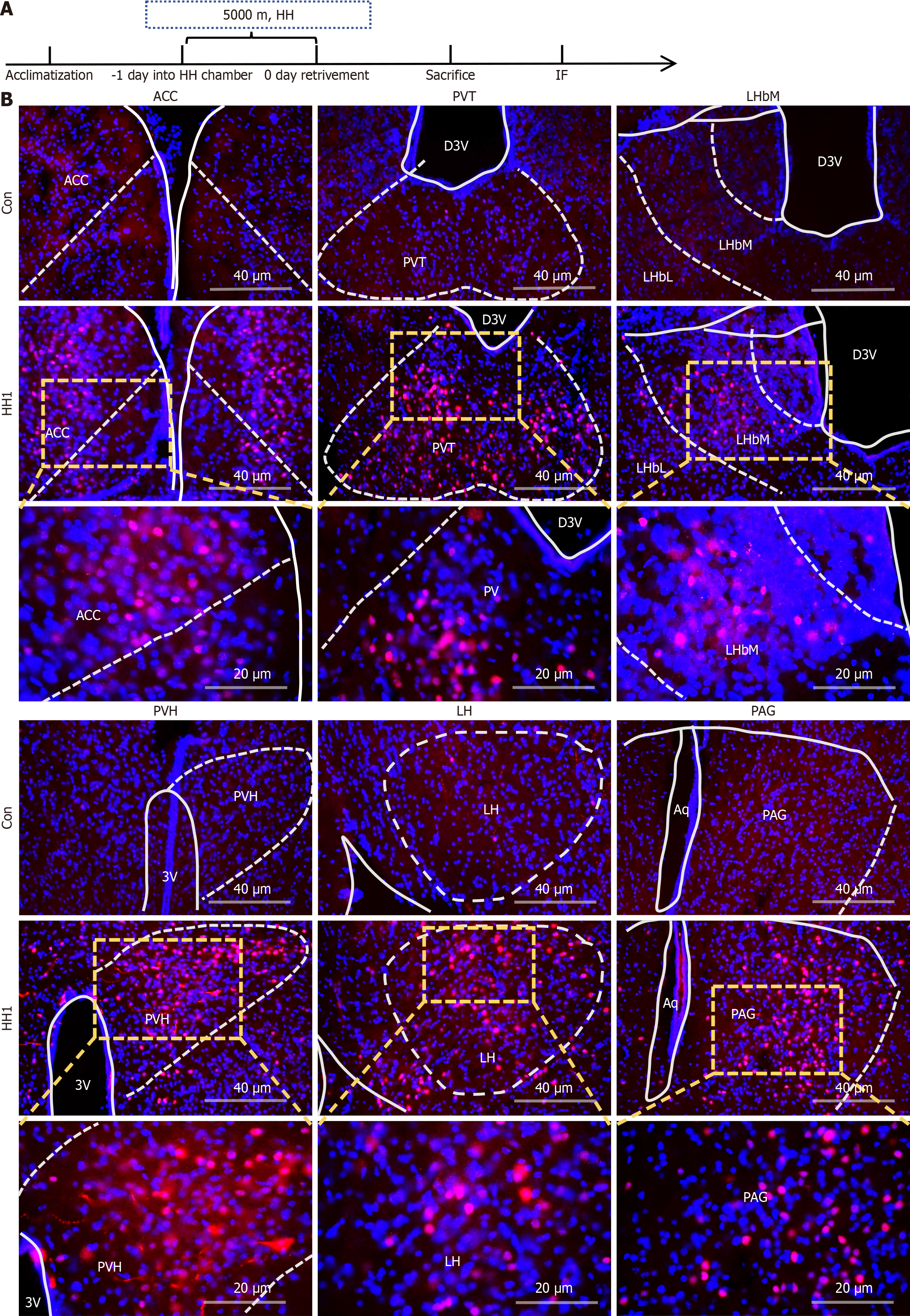
Thirty minutes after HHE, mice were anesthetized with isoflurane and perfused with 40 g/L paraformaldehyde. Brain tissues were dehydrated using sucrose gradients (0.15 g/mL and 0.3 g/mL), embedded into optimal cutting temperature compound, and sectioned into 25-30 μm slices. After blocking with PBS containing Triton and BSA, brain slices were incubated overnight with a 1:500 dilution of anti-C-Fos antibody (Abcam, Cambridge, United Kingdom), followed by a 1:200 dilution of Cy3 secondary antibody. DAPI was used for nuclear staining. Fluorescence and confocal microscopy were employed to visualize c-Fos protein expression in brain regions, providing insights into the activation of specific brain nuclei under HH.
Oral and fecal microbiota samples were subjected to 16S rRNA sequencing (Majorbio, Pudong, Shanghai, China). The analysis included DNA extraction, PCR amplification, library construction, and sequencing. Changes in microbial composition, alpha and beta diversities, and the abundance of specific taxa were analyzed to evaluate the impact of HHE on oral and gut microbiota.
A probiotic formulation containing Lactobacillus rhamnosus (L. rhamnosus) DSM17648, Lactobacillus acidophilus (L. acidophilus) DDS-1, and L. reuteri (L. reuteri) UALR-06 (Yikejin, Changsha, Hunan, China) was suspended in sterile distilled water. Eight mice in the HH1 + Lac group received oral administration of the probiotic solution 30 minutes after removal of the HH chamber. Supplementation was conducted once daily for 7 consecutive days. To enhance interaction with the oral microbiota, the solution was applied directly to the periodontal mucosa and retained for 5 minutes. Sixteen mice collectively in control and HH1 groups received equivalent volumes of sterile distilled water following the same administration protocol.
After 1 week, all groups underwent behavioral tests (OFT, EPM, and FST) as described. This supplementation protocol aimed to evaluate the potential of probiotics in mitigating anxiety- and depression-like behaviors induced by HH. The schematic diagram of this study is illustrated in Figure 1.
Data were analyzed using GraphPad Prism 8.3.0 software (GraphPad Software Inc., San Diego, CA, United States) and were presented as mean ± SE of the mean. Comparisons between the two groups were performed using an unpaired t-test. One-way and two-way analysis of variance were employed to compare multiple groups. P value < 0.05 was considered statistically significant.
Mice were randomly divided into five groups that were exposed to HH for 0, 1, 3, 5, or 7 days (Figure 2A), designated as the Con, HH1, three days of HHE (HH3), five days of HHE (HH5), and seven days of HHE (HH7) groups, respectively. Behavioral tests were conducted in each group of mice after modeling (Figure 2). The results of the behavioral tests revealed that mice in the HH1 group exhibited remarkable anxiety- and depression-like behaviors. The results of OFT indicated no significant difference in the total distance traveled among the five groups (Figure 2B), suggesting that high-altitude HH did not negatively impact the locomotor activity of mice. However, the HH1 group exhibited a significant decrease in the number of entries into the center area and the distance traveled in the center compared with the Con, HH3, HH5, and HH7 groups (Figure 2C and D). In the EPM, the HH1 group showed a reduced number of entries into the open arms and a shorter distance traveled in the open arms compared with the Con, HH3, HH5, and HH7 groups (Figure 2E and F). However, the distance traveled in the closed arms by mice in the HH1 group was significantly higher compared with that in other groups (Figure 2G-I), indicating that mice in the HH1 group preferred to spend more time in the dark and more enclosed closed arms. This finding suggests the occurrence of an anxiety-like behavior in mice in the HH1 group. In the subsequent FST, the HH1 group similarly displayed a trend of shorter immobility latency and extended immobility time (Figure 2J and K).
Cardiac perfusion was performed to extract the whole brains of mice exposed to HH1. After fixation, dehydration, embedding, and immunofluorescence staining (IF), samples were photographed via fluorescence and confocal microscopy (Figure 3). The results revealed a significant upregulation of c-Fos protein expression in neuronal nuclei in specific brain regions of the HH1 group compared with the Con group. This suggests the activation of distinct neuronal populations potentially mediating the anxiety- and depression-like behaviors observed post-HHE. The activated regions included the anterior cingulate cortex (ACC), paraventricular nuclei of the thalamus (PV), lateral habenula nuclei (LHb), paraventricular nuclei of the hypothalamus (PVH), lateral hypothalamus (LH), and the anterior periaqueductal gray (PAG; Figure 3B). Additionally, increased expression of Iba-1, a microglial marker, was found in brain regions, such as the ACC, PV, PVH, and PAG following HHE. This indicates not only neuronal activation, but also microglial activation in these areas, suggesting a potential inflammatory response contributing to the observed behavioral alterations (Figure 4).
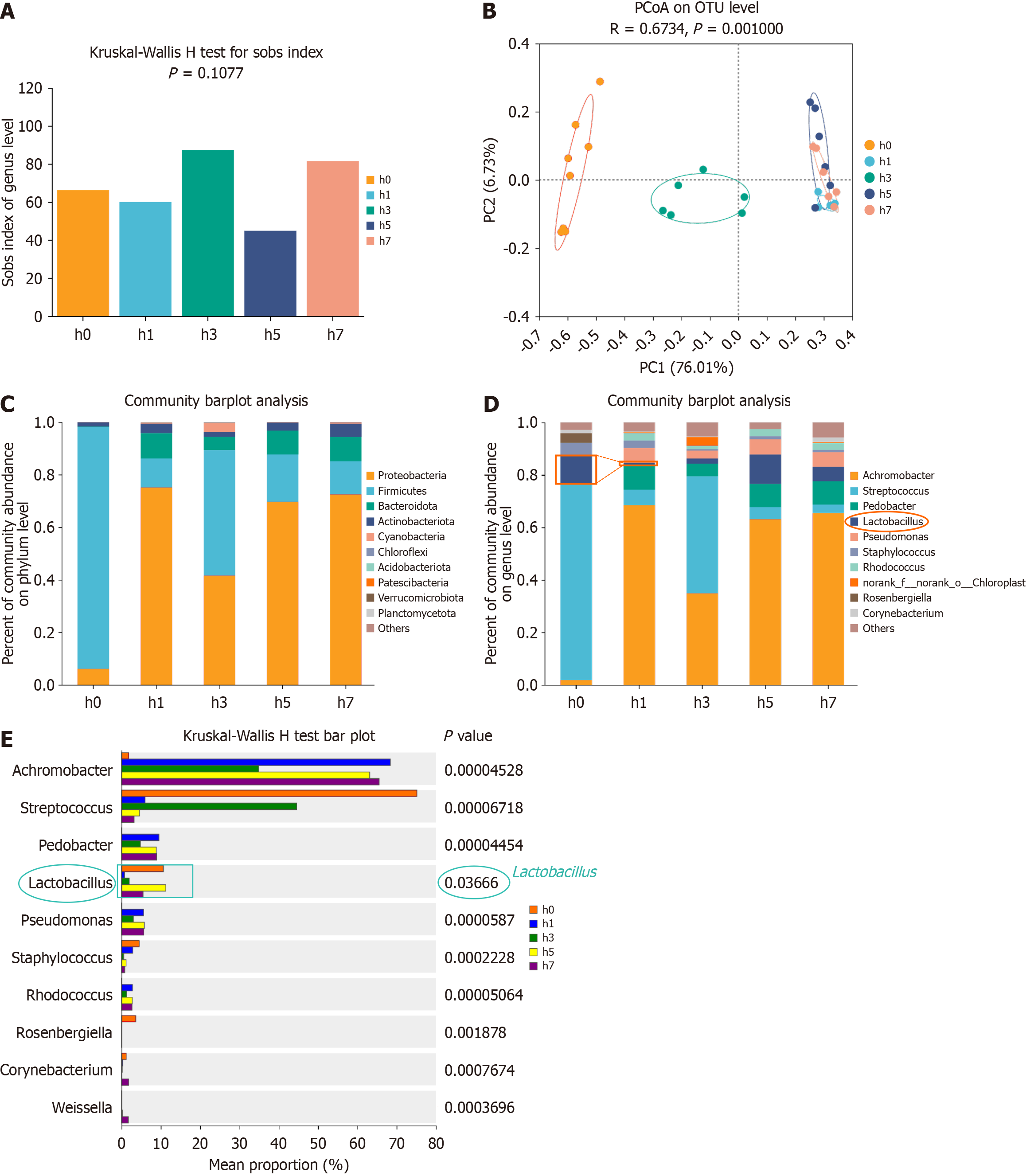
To investigate the impact of HH on oral microbiota, 16S rRNA gene sequencing was performed on oral and fecal samples from mice exposed to HH1 for varying durations. The sequencing yielded 3965559 optimized sequences, totaling 1676232749 bases, with an average read length of 422 bp. Taxonomic annotation identified 1 domain, 1 kingdom, 32 phyla, 85 classes, 212 orders, 348 families, 669 genera, 1003 species, and 1971 operational taxonomic units. Alpha diversity analysis using the Sobs index indicated that HH exposure influenced the oral microbiota's diversity across the HH1, HH3, HH5, and HH7 groups. However, these changes were not statistically significant compared with the Con group (Figure 5A; P = 0.1077), indicating no significant difference in species richness between the Con and HHE groups (1, 3, 5, and 7 days). Principal coordinate analysis (PCoA) based on Bray-Curtis distances demonstrated distinct clustering between the Con and HH-exposed groups, indicating alterations in microbial community structure due to HHE. Notably, no complete separation was found among the HH1, HH5, and HH7 groups (Figure 5B; R = 0.6734, P = 0.001000). The predominant phyla in the oral microbiota included Bacteroidetes, Firmicutes, and Actinobacteria (Figure 5C). The primary characteristic of the oral microbiota in the HHE mice was a reduction in Lactobacillus abundance, which significantly decreased in the HH1 and HH3 groups, reaching very low levels, and began increasing on day 5 and slightly decreasing again on day 7. The abundance of Corynebacterium also significantly decreased in the HH1, HH3, and HH5 groups (Figure 5D). Differential testing in the species composition analysis revealed a significant difference in Lactobacillus abundance between the HH1 and HH3 groups compared with that in the Con group (Figure 5E; P = 0.03666). These results suggest a significant decrease in Lactobacillus abundance in the oral microbiota of mice after 1 and 3 days of HHE.
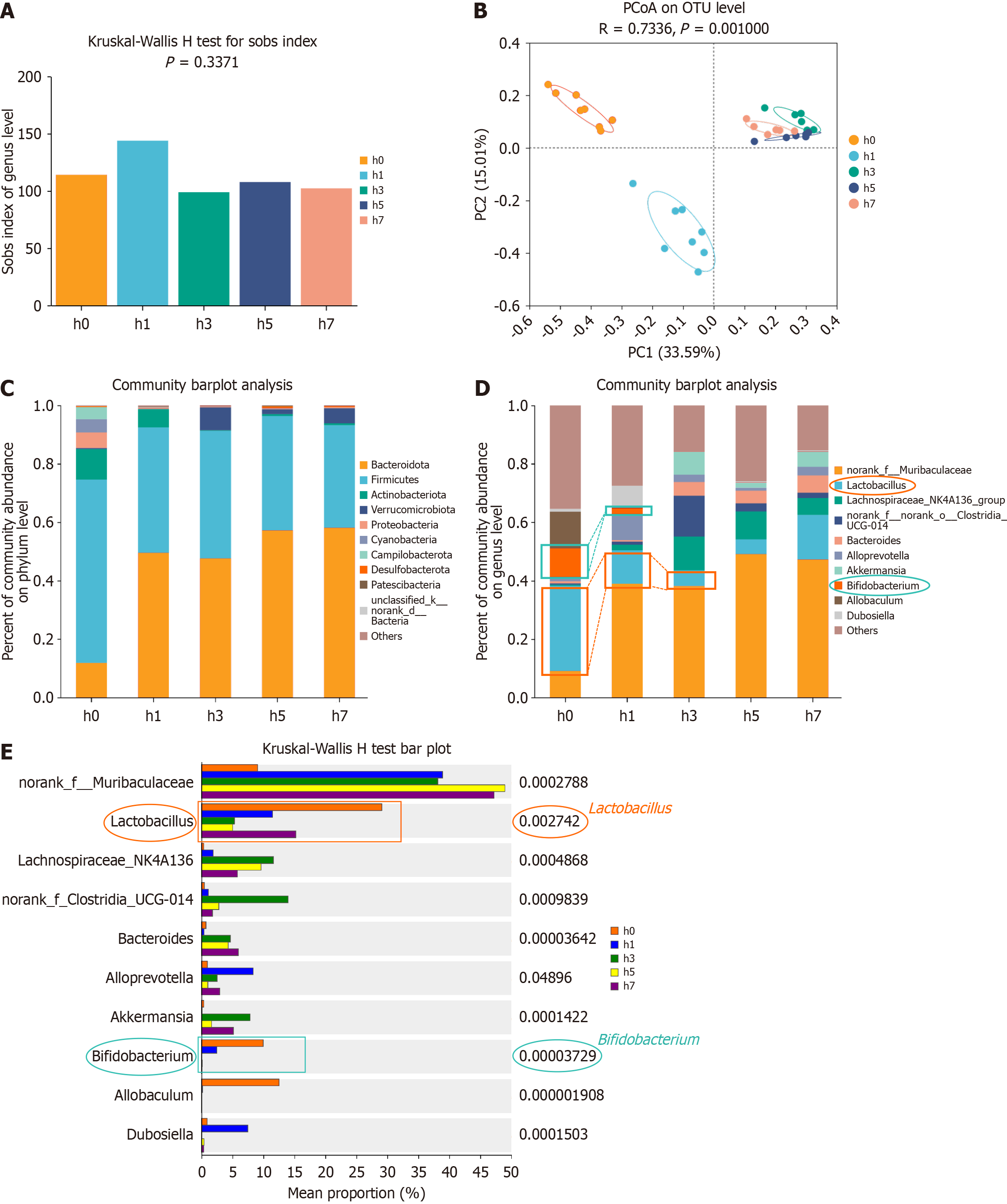
The Sobs index analysis indicated that the α-diversity of the intestinal microbiota in mice was not significantly affected by HHE for 1, 3, 5, or 7 days. Although the Sobs index of the fecal microbiota in the HH1 group slightly increased, it was not statistically significant (Figure 6). No significant fluctuations were found in the other groups, and the microbiota remained relatively stable (Figure 6B; P = 0.3371), suggesting no significant difference in the species richness of the fecal microbiota between the Con and HHE groups (Figure 6G). PCoA based on Bray-Curtis distances revealed a distinct structural separation of the intestinal microbiota in the Con, HH1, HH3, HH5, and HH7 groups, although the HH3, HH5, and HH7 groups were not completely separated (Figure 6C; r = 0.7336, P = 0.001000). The predominant phyla in the fecal microbiota were Bacteroidetes, Firmicutes, and Proteobacteria (Figure 6K). A significant reduction in the abundance of Lactobacillus and Bifidobacterium was identified in the fecal microbiota of mice exposed to HH1 and HH3 (Figure 6D). Furthermore, differential analysis of species composition confirmed that compared with the Con group, the fecal microbiota of HH1 and HH3 mice exhibited a significant reduction in the abundance of Lactobacillus and Bifidobacterium (Figure 6E; P = 0.002742).
Previous results indicated that HH1 mice exhibited the most notable anxiety- and depression-like behaviors in behavioral tests (Figure 4D-K). Concurrently, the 16S sequencing analysis of oral microbiota revealed a significant decrease in the abundance of Lactobacillus at the genus level in the oral cavity of HH1 mice (Figure 5D), suggesting a potential link between the observed behavioral changes and alterations in the oral microbiota. Therefore, Lactobacillus was administered orally to HH1 mice (HH1 + Lac), and behavioral tests were conducted 24 hours later (Figure 7). The results revealed a statistically significant amelioration in anxiety- and depression-like behaviors in the HH1 + Lac group compared with that in the HH1 group. In the OFT, while no significant difference was identified in the total distance (Figure 7C), the HH1 + Lac group exhibited increased center distance and time spent at the center area compared with the HH1 group (Figure 7D-E). In the EPM, the HH1 + Lac group also spend a longer time in the open arms, less time in the close arm and entered into the open arms more times (Figure 7F-H). During the FST, the HH1 + Lac group exhibited longer immobility latency and shorter immobility time compared with the HH1 group (Figure 7I-J), indicating that the time to cease struggling was extended, while the duration of struggling was reduced. These observations suggest that HH1 + Lac mice were less likely to reach a state of “despair” than HH1 mice. The results demonstrate that the oral administration of Lactobacillus alleviated anxiety-like behaviors in mice exposed to HH to a certain extent.
The present study indicated that HHE induced mood disorders in mice and caused significant variations in the composition of their oral and fecal microbiota. The oral administration and gavage of Lactobacillus significantly alleviated anxiety- and depression-like behaviors in mice.
In this study's behavioral assessments, mice exposed to 1 day of HH showed reduced activity in the central zone in the OFT, increased anxiety in the EPM, and longer immobility in the FST. These findings indicate anxiety- and depression-like behaviors, being consistent with prior research. For example, female rats exposed to 4500 or 10000 ft altitudes for 1 week displayed enhanced anxiety- and depression-like behaviors[21]. Another study reported that exposure to moderate-high altitude [5430 ft (1655 m)] has been shown to induce depressive-like behaviors in Long-Evans rats. Specifically, these rats exhibited extended immobility in the modified Porsolt FST and reduced sucrose preference, indicating anhedonia[22]. These data suggest that high-altitude, low-pressure, and low-oxygen environments pose a risk for anxiety and depression. However, daily intermittent of HH at 5000 m for 4 hours over 2 weeks alleviated these behavioral changes, indicating a neuroprotective effect against unpredictable chronic mild stress-induced depression-like behaviors[23].
IF analysis of brain tissue revealed activation in multiple nuclei, including the ACC, PV, LHb, and PVH, after 1 day of HHE in mice. The cingulate cortex, a component of the limbic system, comprises the ACC, midcingulate cortex (MCC), and posterior cingulate cortex (PCC). The PCC is primarily associated with visuospatial processing, memory retrieval, and internally directed cognition, functioning as a key node in the default mode network. In contrast, the ACC and MCC are involved in emotion and cognition. The ACC plays a central role in emotion regulation, decision-making, and conflict monitoring, integrating emotional and cognitive information. The MCC is implicated in processing negative emotions, pain perception, and behavioral responses to threats[24]. Zhang et al. identified robust c-Fos expression in the ACC in both lipopolysaccharide (LPS)-induced depression and chronic restraint stress models, indicating ACC activation in response to stress. This supports the hypothesis that HHE may similarly activate the ACC, potentially impairing emotional regulation and exacerbating negative emotions in both humans and animals. Additionally, the PV nucleus, a key relay and integration site, influences arousal[25], addiction[26], reward, and fear memory. Its activation has also been found in dextran sulfate sodium-induced depression-like behaviors in mice[27].
The LHb, located near the dorsal third ventricle and centrally positioned in coronal brain sections, serves as a critical hub for integrating diverse signals and regulating activities, particularly via its influence on dopaminergic and serotonergic systems. Extensive research in both animal models and human subjects has firmly established the LHb as a critical node implicated in various mental disorders, particularly major depression[28-34]. Preliminary clinical trials have demonstrated that LHb inactivation can lead to significant improvement or even complete remission of depressive symptoms[35-39]. The hypothalamus, comprising multiple nuclei and fiber tracts, plays a notable role in regulating endocrine and autonomic functions, including thermoregulation, feeding behavior, sleep, and emotional processing. Lesions in specific hypothalamic nuclei are known to result in significant behavioral dysregulation. Immunofluorescence results from the present study revealed that HHE elicited activation in the PVH, LH, and Dorsomedial hypothalamic nucleus. Recent evidence has identified a population of NPY-positive neurons in the dorsal raphe nucleus/ventrolateral periaqueductal gray (vlPAG) of male mice with anxiolytic properties; inhibition of their projections to the PVT and LH produces corresponding anxiolytic effects[40]. These findings implicate both the LH and vlPAG in the modulation of anxiety-like behaviors. Furthermore, the observed activation of the anterior PAG (aPAG) in HHE-exposed mice suggests that brain regions, such as the LH and PAG, may contribute to the progression of anxiety- and depression-like behaviors under low-pressure and hypoxic conditions.
Research into activated nuclei indicates that HHE functions as a stressor, inducing complex behavioral changes in mice through intricate, non-linear pathways. Further studies are needed to clarify these mechanisms.
The 16S rRNA sequencing results for oral microbiota revealed that low-pressure, low-oxygen exposure slightly impacted microbial diversity. The oral microbiota in the HH1, HH3, HH5, and HH7 groups showed minor fluctuations before stabilizing. However, a notable genus-level decline in Lactobacillus was found in the HH1 and HH3 groups, with the sharpest drop in the HH1 group. The abundance of Lactobacillus began to recover in the HH5 group but declined slightly again in HH7. In contrast, HH1 mice showed a notable increase in Achromobacter and Rosenbergiella. Achromobac
The 16S rRNA gene sequencing revealed a significant reduction in the abundance of Bacillus on days 1, 3, and 5 post-HHE. Previous research has linked Corynebacterium durum, an oral commensal bacterium, to Veillonella, Neisseria, Rothia, Paludibacter, Capnocytophaga, and Kingella, indicative of healthy periodontal status[41]. Fecal microbiota analysis via 16S sequencing revealed stable α-diversity over 1, 3, 5, and 7 days, despite significant shifts in specific bacterial populations. Notably, the abundance of Lactobacillus in fecal microbiota decreased by day 3 post-HHE, mirroring its decline in oral microbiota by day 1. By day 7, Lactobacillus levels in fecal samples had returned to baseline, reflecting the gut microbiota's resilience to short-term disruptions. This temporal relationship suggests interconnectedness between the oral cavity and intestinal microbiota, with oral changes preceding intestinal alterations.
In the fecal microbiota, a significant reduction was found in the abundance of Allobaculum and Helicobacter in the treatment group. Allobaculum, a key SCFA producer, plays a role in gut-brain signaling and energy metabolism, demonstrating that its decline could disrupt SCFA-mediated pathways under HH. Similarly, the suppression of Helicobacter, which contributes to immune regulation and gut homeostasis, may result from changes in the gut microenvironment, such as increased oxidative stress or inflammation induced by hypoxia. The potential role of Lactobacillus supplementation in these reductions, through mechanisms, such as competitive exclusion or altered metabolic interactions, highlights the complex interaction among microbial populations in response to environmental stress and therapeutic interventions.
Probiotic supplementation has been recognized as a significant strategy for modulating microbial homeostasis, particularly in the adjunctive treatment of anxiety and depression. Previous research indicated that administering L. rhamnosus GG (LGG) during early life could mitigate anxiety-like behaviors in adulthood, potentially by elevating acetate level subsequent to LGG treatment[42]. A subsequent study demonstrated that pre- and post-stress oral supplementation with L. rhamnosus NK33 effectively inhibited LPS-induced NF-κB activation in BV-2 cells, thereby significantly mitigating the onset and progression of anxiety and depression[43]. An 8-week randomized, double-blind, placebo-controlled clinical trial demonstrated that treatment with NVP-1704, a combination of L. reuteri NK33 and B. adolescentis NK98, significantly reduced serum interleukin-6 (IL-6) level and enhanced the presence of Bifidobacterium and Lactobacillus in the gut microbiota compared with those in the placebo group. These changes correlated with the alleviation of the symptoms of depression and anxiety were found at 4 and 8 weeks of treatment[44], indicating that supplementation with traditional Lactobacillus strains could alleviate symptoms of depression and anxiety.
Probiotic supplementation has emerged as an effective strategy for regulating microbial homeostasis and managing anxiety and depression. Early-life administration of LGG has been exhibited to reduce anxiety-like behaviors in adulthood, likely by increasing acetate level[45]. Lactobacillus species have been shown to produce SCFAs, particularly acetate, propionate, and butyrate, which can cross the BBB and modulate synthesis and release of neurotransmitters, including serotonin, dopamine, and gamma-aminobutyric acid[40]. These neurotransmitters play critical roles in emotional regulation and stress responses. Additionally, Lactobacillus can modulate systemic inflammation by reducing the levels of pro-inflammatory cytokines, such as IL-6 and tumor necrosis factor-alpha, thereby alleviating neuroinflammation associated with anxiety and depression[21].
Conversely, Fusobacterium nucleatum, a common oral pathogen, has been implicated in promoting inflammation and disrupting immune homeostasis. It can induce the production of pro-inflammatory cytokines and chemokines, exacerbating systemic inflammation and potentially compromising the integrity of the BBB[45]. This inflammatory cascade can negatively impact neurotransmitter balance and neuronal function, contributing to the pathogenesis of mood disorders. Understanding these distinct mechanisms emphasizes the importance of maintaining a balanced oral microbiota and highlights potential therapeutic targets for mitigating psychiatric disorders induced by environmental stressors, such as HH.
The present study demonstrated that oral administration of a probiotic formulation (≥ 5 billion CFUs per bottle) containing L. rhamnosus DSM17648[45], L. acidophilus DDS-1, and L. rhamnosus UALR-06 effectively alleviated anxiety- and depression-like behaviors in HH1 mice. Probiotic administration involves direct delivery of the solution into the oral cavity, followed by a one-hour restriction of food and water intake to enhance interaction among the probiotics, their bioactive components, and the resident oral microbiota. This targeted approach, adapted from the principles of gut microbiota transplantation but specifically tailored for the oral environment, represents a novel methodological feature of the present study.
However, the study has certain limitations. Firstly, the observed activation of brain nuclei highlights the complexity of HHE-induced changes in the central nervous system, and these nuclei are linked to anxiety and depression. Nonetheless, further research is required to clarify their cooperative roles in exacerbating or mitigating these conditions. Future studies will employ trans-synaptic viral tracing to map the functional connectivity among these regions, providing deeper insight into their interactions.
Secondly, while 16S sequencing and behavioral analyses indicated that reduced Lactobacillus abundance under HHE could influence anxiety- and depression-like behaviors, the specific metabolic products and pathways mediating these effects remain elusive. Integrating 16S sequencing with metabolomics in future research will help identify the key molecules through which Lactobacillus exerts its effects, providing stronger evidence for targeted probiotic therapy.
HHE led to significant alterations in the composition of both oral and gut microbiota and activated specific brain nuclei in mice. Lactobacillus supplementation alleviated anxiety- and depression-like behaviors. These findings suggest that modulating the oral microbiota may serve as a promising strategy for mitigating psychiatric disorders induced by HHE.
We gratefully acknowledge Dr. Xiang Cheng and Ms. Xiu-Fang Jang for their support with equipment during the experimental phase. Appreciation is also extended to the anonymous reviewers for their insightful comments and suggestions, which greatly enhanced the quality of this manuscript.
| 1. | Bouak F, Vartanian O, Hofer K, Cheung B. Acute Mild Hypoxic Hypoxia Effects on Cognitive and Simulated Aircraft Pilot Performance. Aerosp Med Hum Perform. 2018;89:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Gamboa JL, Caceda R, Arregui A. Is depression the link between suicide and high altitude? High Alt Med Biol. 2011;12:403-4; author reply 405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Zegarra-Rodríguez CA, Plasencia-Dueñas NR, Failoc-Rojas VE. Disparities in the prevalence of screened depression at different altitudes in Peru: A retrospective analysis of the ENDES 2019. PLoS One. 2022;17:e0278947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Bardwell WA, Ensign WY, Mills PJ. Negative mood endures after completion of high-altitude military training. Ann Behav Med. 2005;29:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Bogdanova OV, Abdullah O, Kanekar S, Bogdanov VB, Prescot AP, Renshaw PF. Neurochemical alterations in frontal cortex of the rat after one week of hypobaric hypoxia. Behav Brain Res. 2014;263:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Olugbemide AS, Ben-Azu B, Bakre AG, Ajayi AM, Femi-Akinlosotu O, Umukoro S. Naringenin improves depressive- and anxiety-like behaviors in mice exposed to repeated hypoxic stress through modulation of oxido-inflammatory mediators and NF-kB/BDNF expressions. Brain Res Bull. 2021;169:214-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Kumari P, Wadhwa M, Chauhan G, Alam S, Roy K, Kumar Jha P, Kishore K, Ray K, Kumar S, Nag TC, Panjwani U. Hypobaric hypoxia induced fear and extinction memory impairment and effect of Ginkgo biloba in its amelioration: Behavioral, neurochemical and molecular correlates. Behav Brain Res. 2020;387:112595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Han Y, Xu J, Yan Y, Zhao X. Dynamics of the gut microbiota in rats after hypobaric hypoxia exposure. PeerJ. 2022;10:e14090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 9. | Jia Z, Zhao X, Liu X, Zhao L, Jia Q, Shi J, Xu X, Hao L, Xu Z, Zhong Q, Yu K, Cui S, Chen H, Guo J, Li X, Han Y, Song X, Zhao C, Bo X, Tian Y, Wang W, Xie G, Feng Q, He K. Impacts of the Plateau Environment on the Gut Microbiota and Blood Clinical Indexes in Han and Tibetan Individuals. mSystems. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Bai X, Liu G, Yang J, Zhu J, Wang Q, Zhou Y, Gu W, La L, Li X. Changes in the Gut Microbiota of Rats in High-Altitude Hypoxic Environments. Microbiol Spectr. 2022;10:e0162622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 11. | van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O'Sullivan O, Clarke G, Stanton C, Dinan TG, Cryan JF. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. 2018;596:4923-4944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 509] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 12. | Bhatt S, Kanoujia J, Mohana Lakshmi S, Patil CR, Gupta G, Chellappan DK, Dua K. Role of Brain-Gut-Microbiota Axis in Depression: Emerging Therapeutic Avenues. CNS Neurol Disord Drug Targets. 2023;22:276-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 13. | Dicks LMT. Our Mental Health Is Determined by an Intrinsic Interplay between the Central Nervous System, Enteric Nerves, and Gut Microbiota. Int J Mol Sci. 2023;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 14. | Ferrari S, Mulè S, Parini F, Galla R, Ruga S, Rosso G, Brovero A, Molinari C, Uberti F. The influence of the gut-brain axis on anxiety and depression: A review of the literature on the use of probiotics. J Tradit Complement Med. 2024;14:237-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 15. | Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Gut microbiota's effect on mental health: The gut-brain axis. Clin Pract. 2017;7:987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 316] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 16. | Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM. The gut microbiota in anxiety and depression - A systematic review. Clin Psychol Rev. 2021;83:101943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 533] [Article Influence: 133.3] [Reference Citation Analysis (0)] |
| 17. | Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1521] [Article Influence: 126.8] [Reference Citation Analysis (0)] |
| 18. | Tao K, Yuan Y, Xie Q, Dong Z. Relationship between human oral microbiome dysbiosis and neuropsychiatric diseases: An updated overview. Behav Brain Res. 2024;471:115111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Wingfield B, Lapsley C, McDowell A, Miliotis G, McLafferty M, O'Neill SM, Coleman S, McGinnity TM, Bjourson AJ, Murray EK. Variations in the oral microbiome are associated with depression in young adults. Sci Rep. 2021;11:15009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 20. | Li C, Chen Y, Wen Y, Jia Y, Cheng S, Liu L, Zhang H, Pan C, Zhang J, Zhang Z, Yang X, Meng P, Yao Y, Zhang F. A genetic association study reveals the relationship between the oral microbiome and anxiety and depression symptoms. Front Psychiatry. 2022;13:960756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 21. | Sheth C, Ombach H, Olson P, Renshaw PF, Kanekar S. Increased Anxiety and Anhedonia in Female Rats Following Exposure to Altitude. High Alt Med Biol. 2018;19:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Nguyen KT, Gates CA, Hassell JE Jr, Foxx CL, Salazar SN, Luthens AK, Arnold AL, Elam BL, Elsayed AI, Leblanc M, Adams SC, Lowry CA, Reuter JD. Evaluation of the effects of altitude on biological signatures of inflammation and anxiety- and depressive-like behavioral responses. Prog Neuropsychopharmacol Biol Psychiatry. 2021;111:110331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Kushwah N, Jain V, Deep S, Prasad D, Singh SB, Khan N. Neuroprotective Role of Intermittent Hypobaric Hypoxia in Unpredictable Chronic Mild Stress Induced Depression in Rats. PLoS One. 2016;11:e0149309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Oane I, Barborica A, Mindruta IR. Cingulate Cortex: Anatomy, Structural and Functional Connectivity. J Clin Neurophysiol. 2023;40:482-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Ren S, Wang Y, Yue F, Cheng X, Dang R, Qiao Q, Sun X, Li X, Jiang Q, Yao J, Qin H, Wang G, Liao X, Gao D, Xia J, Zhang J, Hu B, Yan J, Wang Y, Xu M, Han Y, Tang X, Chen X, He C, Hu Z. The paraventricular thalamus is a critical thalamic area for wakefulness. Science. 2018;362:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 255] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 26. | Zhou K, Zhu Y. The paraventricular thalamic nucleus: A key hub of neural circuits underlying drug addiction. Pharmacol Res. 2019;142:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Chen Y, Zheng D, Wang H, Zhang S, Zhou Y, Ke X, Chen G. Lipocalin 2 in the Paraventricular Thalamic Nucleus Contributes to DSS-Induced Depressive-Like Behaviors. Neurosci Bull. 2023;39:1263-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Hu H, Cui Y, Yang Y. Circuits and functions of the lateral habenula in health and in disease. Nat Rev Neurosci. 2020;21:277-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 316] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 29. | Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphé nuclei following tryptophan depletion. Neuroimage. 1999;10:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Strotmann B, Kögler C, Bazin PL, Weiss M, Villringer A, Turner R. Mapping of the internal structure of human habenula with ex vivo MRI at 7T. Front Hum Neurosci. 2013;7:878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Schmidt FM, Schindler S, Adamidis M, Strauß M, Tränkner A, Trampel R, Walter M, Hegerl U, Turner R, Geyer S, Schönknecht P. Habenula volume increases with disease severity in unmedicated major depressive disorder as revealed by 7T MRI. Eur Arch Psychiatry Clin Neurosci. 2017;267:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Caldecott-Hazard S, Mazziotta J, Phelps M. Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. J Neurosci. 1988;8:1951-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 143] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Mirrione MM, Schulz D, Lapidus KA, Zhang S, Goodman W, Henn FA. Increased metabolic activity in the septum and habenula during stress is linked to subsequent expression of learned helplessness behavior. Front Hum Neurosci. 2014;8:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 34. | Andalman AS, Burns VM, Lovett-Barron M, Broxton M, Poole B, Yang SJ, Grosenick L, Lerner TN, Chen R, Benster T, Mourrain P, Levoy M, Rajan K, Deisseroth K. Neuronal Dynamics Regulating Brain and Behavioral State Transitions. Cell. 2019;177:970-985.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 35. | Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, Henn FA, Meyer-Lindenberg A. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9-e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 464] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 36. | Zhang C, Kim SG, Li D, Zhang Y, Li Y, Husch A, Hertel F, Yan F, Voon V, Sun B. Habenula deep brain stimulation for refractory bipolar disorder. Brain Stimul. 2019;12:1298-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Fan T, Zhang Y, Wang Z, Yi M, Liu N, Hu C, Luo L. Effects of lateral habenula and ventral medial prefrontal cortex deep brain stimulation in rats. J Neurorestoratol. 2022;10:43-51. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 961] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 39. | Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 436] [Cited by in RCA: 410] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 40. | Zhang Y, Shen J, Xie F, Liu Z, Yin F, Cheng M, Wang L, Cai M, Herzog H, Wu P, Zhang Z, Zhan C, Liu T. Feedforward inhibition of stress by brainstem neuropeptide Y neurons. Nat Commun. 2024;15:7603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 41. | Cai Z, Lin S, Hu S, Zhao L. Structure and Function of Oral Microbial Community in Periodontitis Based on Integrated Data. Front Cell Infect Microbiol. 2021;11:663756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 42. | Zhou B, Jin G, Pang X, Mo Q, Bao J, Liu T, Wu J, Xie R, Liu X, Liu J, Yang H, Xu X, Wang B, Cao H. Lactobacillus rhamnosus GG colonization in early life regulates gut-brain axis and relieves anxiety-like behavior in adulthood. Pharmacol Res. 2022;177:106090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 43. | Jang HM, Lee KE, Kim DH. The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 44. | Lee HJ, Hong JK, Kim JK, Kim DH, Jang SW, Han SW, Yoon IY. Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 45. | Mehling H, Busjahn A. Non-viable Lactobacillus reuteri DSMZ 17648 (Pylopass™) as a new approach to Helicobacter pylori control in humans. Nutrients. 2013;5:3062-3073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |