Published online Mar 19, 2025. doi: 10.5498/wjp.v15.i3.103321
Revised: December 27, 2024
Accepted: January 8, 2025
Published online: March 19, 2025
Processing time: 102 Days and 19 Hours
Major depressive disorder (MDD), a psychiatric disorder characterized by func
Core Tip: Major depressive disorder (MDD), especially in adolescents, poses considerable diagnostic and therapeutic challenges owing to its heterogeneity and the subjective nature of traditional assessment methods. Recent advances in neuroimaging, combined with machine learning (ML) technologies, have led to the development of promising biomarkers and diagnostic tools for MDD. However, these challenges can be addressed through improved data privacy protection measures, advanced encryption and anonymization techniques, greater model transparency, stricter data quality control, and the establishment of clear ethical and legal frameworks. Such efforts are crucial to ensuring the safe, reliable, and compliant application of ML technologies in MDD diagnosis.
- Citation: Yin SQ, Li YH. Advancing the diagnosis of major depressive disorder: Integrating neuroimaging and machine learning. World J Psychiatry 2025; 15(3): 103321
- URL: https://www.wjgnet.com/2220-3206/full/v15/i3/103321.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i3.103321
Major depressive disorder (MDD) has become one of the leading causes of mental illness and physical disability worldwide[1]. Data suggest that over the past three decades, the prevalence of depression has considerably increased, with a trend toward affecting younger populations[2-4]. Adolescence is a pivotal period for individual development, marked by considerable physiological, emotional, and sociopsychological changes that considerably increase the susceptibility of adolescents to mental disorders[5]. Adolescent depression can lead to severe long-term consequences, including more severe depression in adulthood, lower educational attainment, unemployment, reduced social support, early marriage, and early childbearing—all of which are concerning psychosocial issues[6,7]. Moreover, depression is closely linked to substance abuse, nonsuicidal self-injury, academic difficulties, and physical health problems. The most severe consequence of untreated depression is the increased risk of suicide[8]. Therefore, early diagnosis and intervention are crucial.
Diagnosing MDD presents multifaceted challenges stemming from both the inherent heterogeneity of the disorder and the subjectivity embedded in current diagnostic approaches. MDD is a highly heterogeneous condition, marked by considerable interindividual variation in its etiology, pathophysiological mechanisms, symptomatology, and disease severity. Clinically, the assessment of mental health disorders predominantly depends on psychiatrists’ observations and semistructured interviews focused on symptom intensity, along with standardized self-assessment scales[9]. This diagnostic approach relies heavily on clinicians’ interpretation of individual symptom profiles and patient narratives, making it vulnerable to subjective biases. The convergence of heterogeneity and subjectivity not only complicates diagnosis but also increases the risk of misdiagnosis—a considerable concern because approximately one-quarter of individuals with bipolar disorder are mistakenly diagnosed with MDD[10,11]. Therefore, there is an urgent need to thoroughly examine individual differences and clarify the specific biological mechanisms underlying depressive dis
This editorial comments on a study recently published in World Journal of Psychiatry titled “Resting-state functional magnetic resonance imaging and support vector machines for the diagnosis of major depressive disorder in adolescents”[12]. The study used functional magnetic resonance imaging (fMRI) and support vector machine (SVM) analysis to identify abnormal functional connectivity (FC) values in the right lingual gyrus as potential neuroimaging biomarkers for distinguishing MDD patients. The editorial summarizes the potential of neuroimaging and machine learning (ML) in the diagnosis of MDD and discusses the ethical, technical, and practical challenges, along with future application prospects.
Gray and white matter are essential for regulating various complex behaviors and cognitive functions, including emotions, perception, attention, and social interactions[13,14]. Disruptions in these brain structures may underlie psychiatric disorders, with deficits in areas like the prefronto-limbic system and corpus callosum often seen in MDD[15-17]. Identifying measurable brain activity markers for MDD is critical for optimizing diagnosis. Advances in neuroimaging technology, particularly fMRI, have enabled the detection of brain activity and structural changes in MDD patients.
FMRI, based on blood-oxygen-level-dependent signals, is widely used to monitor brain activity by detecting changes in blood flow[18]. It includes resting-state functional MRI (rs-fMRI) and task functional MRI (task-fMRI). Studies have shown that patients with MDD predominantly exhibit lower task-related activation in task-fMRI[19]. However, there are also cases of enhanced activation in certain brain regions, such as the prefrontal and anterior temporal areas, indicating the difficulties experienced by patients with MDD in executive functioning and emotional regulation[20]. Unlike task-fMRI, which requires a task design, rs-fMRI offers the convenience of not requiring specific tasks and is highly sensitive to intrinsic brain network activity, making it particularly suitable for patients who cannot comply with tasks or for special populations. The current analysis methods related to rs-fMRI data mainly include regional homogeneity (ReHo), amplitude of low-frequency fluctuations (ALFF), fractional ALFF (fALFF), and FC analysis. ReHo analysis revealed significant increases in the left dorsal medial prefrontal gyrus and the left anterior lobe of the cerebellum, whereas significant decreases were observed in the frontal, temporal, parietal, and occipital cortices[21-23]. Similarly, ALFF and fALFF analyses revealed abnormalities in the intensity of spontaneous neural activity in the brains of patients with MDD, such as decreases in ALFF in the bilateral cerebellum, bilateral precuneus, and left occipital cortex, whereas increases in ALFF occurred in the right superior frontal gyrus, bilateral insula extending into the striatum, and left supramarginal gyrus[24,25]. Additionally, fALFF analysis showed that fALFF in the left frontal region of patients with MDD was significantly lower than that in healthy controls[26].
In contrast to ReHo, ALFF, and fALFF, which reflect spontaneous neural brain activity, FC is quantified by calculating the Pearson correlation coefficients between different brain regions, providing a direct measure of their functional interactions[27]. In patients with MDD, FC is often abnormally enhanced between the default mode network (DMN), central executive network, and salience network, which is closely associated with the pathological characteristics of the disease[28]. Existing research shows a trend toward diversification and combination in neuroimaging feature selection, including the use of FC features and dynamic functional network connectivity, along with low-frequency oscillation features (e.g., ALFF, fALFF), ReHo, and network properties (e.g., degree centrality) to describe the complex variations in brain activity and network structure[29-31]. The integration of multiple features, combined with ML techniques, enables the processing of vast amounts of information and facilitates the classification or prediction of data based on specific characteristics. This approach significantly enhances the diagnostic potential for MDD.
Integrating ML with clinical decision-making helps clinicians identify MDD patients and optimize treatment by providing personalized recommendations. ML methods like SVM and deep learning (DL) are trained to recognize disease-specific imaging features, distinguishing MDD patients from healthy controls or different disease subtypes[32-34]. These methods can also predict treatment outcomes, improving early risk identification and therapeutic efficacy[35].
Moreover, traditional ML is still the dominant approach in existing studies. Some existing studies have also explored other methods, such as logistic regression (LR), random forest, Extreme Gradient Boosting (XGBoost), AdaBoost, sparse LR, maximum margin clustering (MMC)-based classifiers, and linear discriminant analysis[36-40]. DL algorithms have also been introduced to enhance classification performance[40]. These studies also employed different performance evaluation metrics, such as accuracy, area under the curve, and consistency. Cross-validation techniques, such as leave-one-out cross-validation and k-fold cross-validation, were employed to ensure the generalizability and robustness of the models[36-40].
Although DL applications in MDD are less common than traditional ML methods, they have demonstrated promising results. For instance, an unsupervised ML method based on MMC effectively segmented voxels in the perigenual cingulate cortex into two subregions, leveraging distinct functional connection patterns in the resting state to successfully distinguish patients with MDD from healthy controls[40]. Convolutional neural networks have been employed for whole-brain FC analysis, revealing the key role of visual and sensorimotor networks in MDD through SHapley additive exPlanations (SHAP) feature exploration[41]. Additionally, spectral graph convolutional networks, which integrate effective connectivity with nonimaging phenotypic data, have proven effective in analyzing complex neuroimaging data[42].
As a potential auxiliary diagnostic tool, the effectiveness of ML classification methods in the diagnosis of MDD is influenced by various factors, such as the selection of neuroimaging features and classifiers[31,36,43-45]. Studies have demonstrated that using data lacking discriminative features can result in poor classification performance. For instance, a study involving 360 patients with MDD and 360 healthy controls demonstrated that using FC as a feature for SVM classification achieved accuracies close to chance levels (52.5%–52.8%) for both binary and ternary classifications[36]. Another study demonstrated that without feature selection, the SVM classifier’s accuracy remained at random levels in a cohort of 360 patients with MDD and 360 healthy controls[46]. Alternatively, a study using five distinct features and four feature selection methods achieved a higher accuracy of 90.2%. This highlights the considerable influence of the methodology and feature selection on the classification performance[29]. In contrast, incorporating highly discriminative features can considerably enhance the classification performance. For example, a combination of MiR-9 levels, Childhood Trauma Questionnaire scores, and FC in the amygdala–prefrontal–limbic regions yielded an optimal accuracy of 85.1%. This accuracy is considerably higher than that achieved using nondiscriminative features[44]. Another study also reported strong discriminative features in the prefrontal and DMN regions, showing considerable differences between patients with MDD and healthy controls, with classification accuracies reaching 90%[47]. Furthermore, the selection of classification algorithms also impacts accuracy. Although SVM has been widely used, other classifiers can yield comparable results. A study comparing six classifiers [SVM, LR, decision tree (DT), ridge regression, least absolute shrinkage and selection operator, and XGBoost] using FC data from 1021 patients with MDD and 1100 healthy controls demonstrated that XGBoost achieved the highest accuracy of 72.8%[45]. In another study, researchers improved the model’s ability to differentiate between MDD and healthy controls using various feature extraction algoristhms and optimizing SVM parameters, achieving an accuracy of 66%[31].
Although the early results of ML methods in MDD diagnosis are encouraging, their full potential lies in optimizing feature selection and classification algorithms. This will lead to earlier and more accurate diagnoses. Future ML models that integrate multimodal neuroimaging features with individualized data could pave the way for personalized treatment plans and considerably improve early intervention capabilities for MDD in clinical settings[48].
The use of ML in healthcare presents significant challenges, particularly concerning informed consent and privacy protection. ML algorithms require large patient datasets for training, raising concerns about how patient data will be used. Obtaining informed consent is complicated because patients may not fully understand terms like "ML models" and "data algorithms", which can lead to the "fallacy of consent"[49]. The current legal framework fails to clearly define data ownership, and the resulting ambiguity complicates the challenge of balancing research accessibility with patient privacy protection[50,51]. Although continuous accumulation of medical data is crucial for improving ML algorithms, the repeated use of data without proper consent may not align with patient expectations[52]. Patients often lose control over their personal information once it is integrated into databases, and even if data use appears harmless or patients are unaware of it, the erosion of ownership and control, along with the lack of transparency regarding data usage, can still infringe upon individual privacy. Additionally, some medical data are uploaded and stored online, considerably increasing the risk of data breaches. Patient information uploaded on the internet may be vulnerable to hacker attacks or database vulnerabilities. Notably, long-term sensitive medical data, such as genetic and specific disease information, can lead to persistent privacy violations and potential discrimination if leaked[53]. The increasing aggregation and analysis of data increases the risk of reidentification in large online databases[54]. Despite traditional deidentification methods, health data remain susceptible to reidentification, raising concerns about privacy breaches[55]. Balancing data use while protecting patient privacy is a critical challenge in medical ML. However, data scarcity in adolescents with MDD can lead to overfitting and reduced diversity. This can compromise the model’s ability to generalize and perform well in real-world settings[56]. Algorithmic bias is also a significant ethical issue in healthcare, as imbalanced or unrepresentative training data can lead to unfair treatment of certain groups, such as women and people of different races and ethnicities[57]. For example, using data from predominantly white populations to predict cardiovascular risk in nonwhite populations may result in overestimating or underestimating the risk, which can affect diagnostic and treatment decisions[58].
Another important consideration is the inconsistency of research results, which is influenced by data quality and volume, as well as model performance. Despite the expansion of datasets, high-quality data cannot be guaranteed because of the variations in imaging preprocessing, acquisition techniques, and patient demographics across different institutions. Factors like the number of head coil channels, inconsistent subject positioning, varying image contrast, differences in magnetic resonance scanner manufacturers and field strengths, and physiological factors like heart rate, cardiopulmonary function, age, and gender, contribute to the variability in MRI quality[59-62]. This heterogeneity complicates data integration and analysis[63]. ML models require sufficient sample sizes for effective training. However, data scarcity in adolescents with MDD can lead to overfitting and reduced diversity. This can compromise the model’s ability to generalize and perform well in real-world settings[56]. Additionally, the effectiveness of these models heavily depends on the selection of neuroimaging features and the choice of algorithms. Features used for training must be representative and discriminative. Otherwise, classification accuracy may be close to random, considerably diminishing the model’s practical value and its ability to provide meaningful diagnostic assistance in clinical settings[36]. The choice of algorithm plays a crucial role in evaluating model performance. An unsuitable algorithm can compromise the stability and accuracy of the results, leading to unreliable outcomes or limited applicability to new datasets[31]. Moreover, studies using the same algorithm, such as the SVM classifier, have reported considerably varied results, highlighting the instability of models across different datasets and feature selection criteria[31,44,47].
The limitations in the integration of ML into clinical practice are also important, especially in terms of model explainability, decision-making authority, and practical application challenges. The most critical issue is model explainability, particularly the enigmatic “black-box” characteristic of certain ML models. The primary concern lies in the inherent complexity of these models. DL architectures, which have extensive parameter counts and intricate multilayered nonlinear configurations, render decision-making processes opaque and challenging to elucidate. The unpredictability of the training process poses another major challenge because these models are trained on heterogeneous and complex datasets, with the optimization procedure navigating a high-dimensional solution space to find the optimal outcome. This complexity makes it difficult to predict and interpret the patterns and decisions that the model learns, and the lack of transparency may reduce the confidence of healthcare practitioners, potentially affecting clinical decision-making[64-66]. A further concern is the potential weakening of the decision-making authority of clinicians. Overreliance on ML-driven diagnostic and therapeutic recommendations may weaken the trust-based doctor-patient relationship, reduce clinicians’ decision-making authority, and lead to negative consequences, such as diminished trust, dehumanized decision-making, and decreased job satisfaction for doctors[66]. Furthermore, this reliance blurs the lines of accountability. When errors or misjudgments occur, it becomes unclear whether the doctor, model developer, or medical institution should be held responsible. This ambiguity poses considerable challenges to accountability and hinders the widespread adoption of ML models in medical settings[49]. Finally, the clinical feasibility of ML for the diagnosis of MDD faces resource and tech
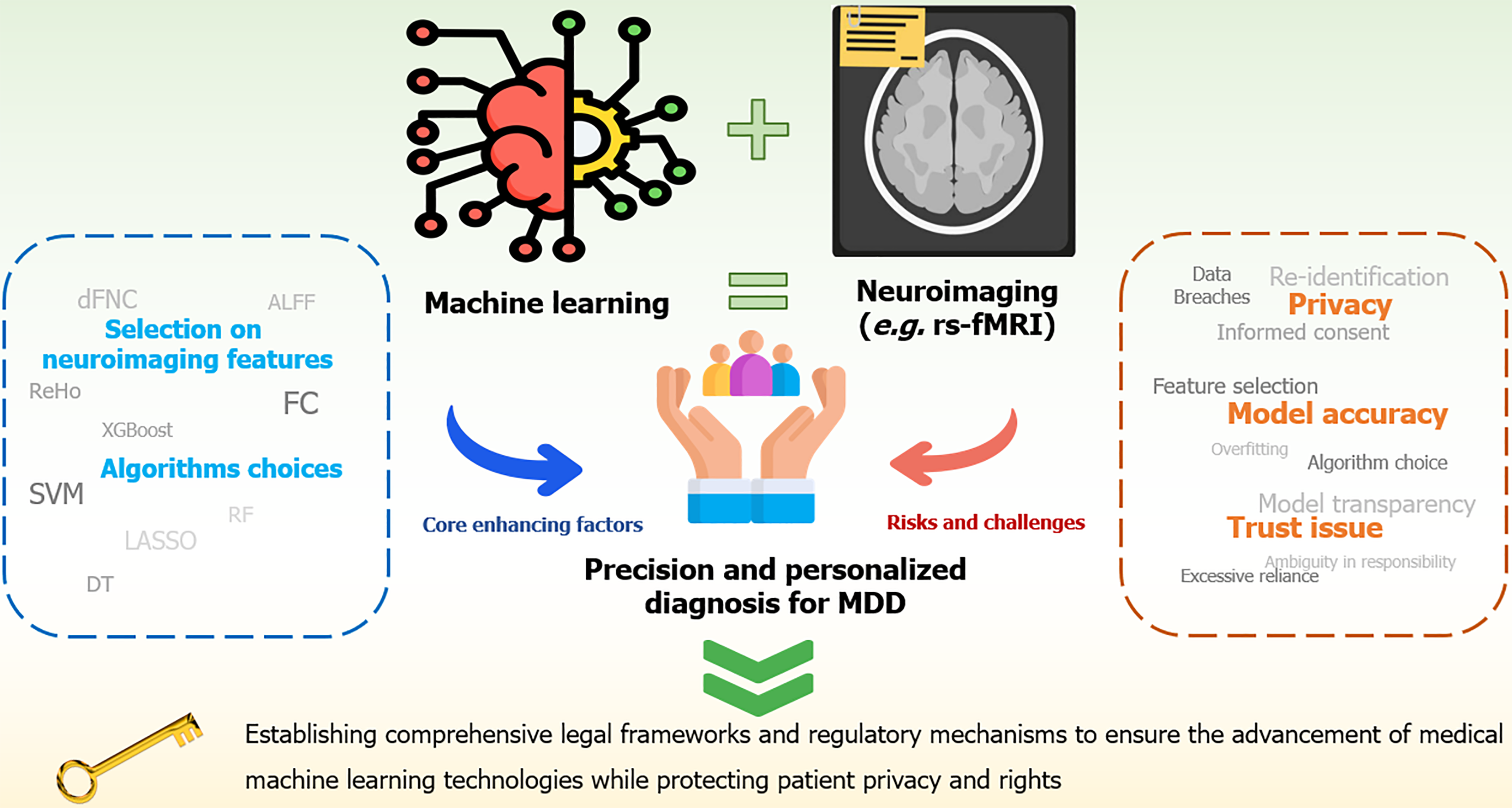
To overcome the challenges hindering the seamless application of medical ML, future efforts should prioritize developing a comprehensive framework that guarantees data compliance, enhances model accuracy, and fosters trust (Figure 1).
First, to protect data privacy, stricter regulations and secure storage solutions must be implemented to ensure the safe custody and transmission of medical data, minimizing the risk of breaches and misuse[68]. Moreover, continuous research and development of advanced encryption technologies will provide additional technical support to safeguard data privacy[69]. With the increasing adoption of ML, it is essential to develop simplified and transparent informed consent mechanisms. Using plain language and interactive models can help patients understand how their data will be used, thus narrowing the knowledge gap between healthcare providers and patients[70]. Exploring dynamic and continuously updated consent models is essential to keep patients informed and actively participate in decisions about the use of their data during the process[71]. It is also essential to recognize the complexity of “ownership” in clinical data because it is not generated or controlled by patients but is the result of contributions from multiple stakeholders[51]. Therefore, the solution requires the collaborative involvement of governments, healthcare institutions, technology platforms, healthcare providers, and patients, to build a flexible and comprehensive data security governance framework that ensures balanced protection of all parties’ interests.
The issues related to heterogeneity in data sources and sample size can be addressed using the following methods. First, standardizing data acquisition processes and unifying scanning protocols can help reduce confounding factors; using standardized software packages, preprocessing settings, task contrasts, seed masks, etc., can help overcome heterogeneity in fMRI data during preprocessing, feature extraction, and statistical testing. Second, applying statistical methods, such as stratified analysis to adjust for physiological variables can control for potential biological interference and improve data accuracy and consistency. Additionally, conducting large-scale multi-center studies with diverse par
To improve model accuracy, integrating data from diverse populations and using techniques, such as data aug
Building trust requires enhancing algorithmic explainability. For simpler models like regression models and DTs, their natural transparency and simplicity make them inherently interpretable, directly reflecting the decision-making logic. However, for more complex models, such as DL, their inherent complexity often requires post-hoc explainability techniques, such as local interpretable model-agnostic explanations and SHAP, to improve the oversight of decision-making processes[78,79]. This strengthens their oversight of the decision-making process[80]. Furthermore, a collaborative human–machine decision-making framework should be established to minimize the overreliance on ML models. This framework should clearly define the supportive role of models in clinical decision-making. To address accountability, healthcare institutions and regulatory agencies must establish clear guidelines outlining the responsibilities of model developers, healthcare providers, and institutions. This ensures the safe and transparent implementation of medical ML systems[49].
To address accessibility issues, data sharing platforms and synthetic data generation techniques can help overcome data scarcity. Second, online learning and various training methods can equip doctors and healthcare professionals with fundamental ML knowledge and its application in diagnosis while simplifying tools to make them easier to use. Additionally, policy support and financial investment can promote the widespread adoption of these technologies.
Future studies should focus on strengthening patient data privacy protection, optimizing model reliability, and fostering trust among healthcare providers. Establishing a standardized and regulated ecosystem for ML applications in healthcare is crucial to facilitate its widespread and safe adoption in clinical settings.
The convergence of neuroimaging and ML presents immense possibilities for the early detection of MDD, paving the way for more accurate diagnostic tools and individualized treatment plans. However, integrating ML models with neu
| 1. | Herrman H, Kieling C, McGorry P, Horton R, Sargent J, Patel V. Reducing the global burden of depression: a Lancet-World Psychiatric Association Commission. Lancet. 2019;393:e42-e43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 2. | Gore FM, Bloem PJ, Patton GC, Ferguson J, Joseph V, Coffey C, Sawyer SM, Mathers CD. Global burden of disease in young people aged 10-24 years: a systematic analysis. Lancet. 2011;377:2093-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1306] [Cited by in RCA: 1338] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 3. | Shorey S, Ng ED, Wong CHJ. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br J Clin Psychol. 2022;61:287-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 553] [Article Influence: 138.3] [Reference Citation Analysis (0)] |
| 4. | Clayborne ZM, Varin M, Colman I. Systematic Review and Meta-Analysis: Adolescent Depression and Long-Term Psychosocial Outcomes. J Am Acad Child Adolesc Psychiatry. 2019;58:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 422] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 5. | Johnson D, Dupuis G, Piche J, Clayborne Z, Colman I. Adult mental health outcomes of adolescent depression: A systematic review. Depress Anxiety. 2018;35:700-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 299] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 6. | Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, Klier CM, Ryan ND, Dahl RE, Wickramaratne P. Depressed adolescents grown up. JAMA. 1999;281:1707-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 558] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 7. | Bodden DHM, Stikkelbroek Y, Dirksen CD. Societal burden of adolescent depression, an overview and cost-of-illness study. J Affect Disord. 2018;241:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Grossberg A, Rice T. Depression and Suicidal Behavior in Adolescents. Med Clin North Am. 2023;107:169-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 9. | Paykel ES. Basic concepts of depression. Dialogues Clin Neurosci. 2008;10:279-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Ghaemi SN, Ko JY, Goodwin FK. The bipolar spectrum and the antidepressant view of the world. J Psychiatr Pract. 2001;7:287-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS; National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095-3105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5389] [Cited by in RCA: 5367] [Article Influence: 244.0] [Reference Citation Analysis (0)] |
| 12. | Yu ZH, Yu RQ, Wang XY, Ren WY, Zhang XQ, Wu W, Li X, Dai LQ, Lv YL. Resting-state functional magnetic resonance imaging and support vector machines for the diagnosis of major depressive disorder in adolescents. World J Psychiatry. 2024;14:1696-1707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Serra-Blasco M, Portella MJ, Gómez-Ansón B, de Diego-Adeliño J, Vives-Gilabert Y, Puigdemont D, Granell E, Santos A, Alvarez E, Pérez V. Effects of illness duration and treatment resistance on grey matter abnormalities in major depression. Br J Psychiatry. 2013;202:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | van Velzen LS, Kelly S, Isaev D, Aleman A, Aftanas LI, Bauer J, Baune BT, Brak IV, Carballedo A, Connolly CG, Couvy-Duchesne B, Cullen KR, Danilenko KV, Dannlowski U, Enneking V, Filimonova E, Förster K, Frodl T, Gotlib IH, Groenewold NA, Grotegerd D, Harris MA, Hatton SN, Hawkins EL, Hickie IB, Ho TC, Jansen A, Kircher T, Klimes-Dougan B, Kochunov P, Krug A, Lagopoulos J, Lee R, Lett TA, Li M, MacMaster FP, Martin NG, McIntosh AM, McLellan Q, Meinert S, Nenadić I, Osipov E, Penninx BWJH, Portella MJ, Repple J, Roos A, Sacchet MD, Sämann PG, Schnell K, Shen X, Sim K, Stein DJ, van Tol MJ, Tomyshev AS, Tozzi L, Veer IM, Vermeiren R, Vives-Gilabert Y, Walter H, Walter M, van der Wee NJA, van der Werff SJA, Schreiner MW, Whalley HC, Wright MJ, Yang TT, Zhu A, Veltman DJ, Thompson PM, Jahanshad N, Schmaal L. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol Psychiatry. 2020;25:1511-1525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 15. | Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 387] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 16. | Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, Simmons JG, Yücel M, Pantelis C, McGorry P, Allen NB. Structural brain development and depression onset during adolescence: a prospective longitudinal study. Am J Psychiatry. 2014;171:564-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 17. | Veer IM, Beckmann CF, van Tol MJ, Ferrarini L, Milles J, Veltman DJ, Aleman A, van Buchem MA, van der Wee NJ, Rombouts SA. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010;4:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 358] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 18. | Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868-9872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4233] [Cited by in RCA: 3756] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 19. | Alders GL, Davis AD, MacQueen G, Strother SC, Hassel S, Zamyadi M, Sharma GB, Arnott SR, Downar J, Harris JK, Lam RW, Milev R, Müller DJ, Ravindran A, Kennedy SH, Frey BN, Minuzzi L, Hall GB; CAN-BIND Investigator Team. Escitalopram ameliorates differences in neural activity between healthy comparison and major depressive disorder groups on an fMRI Emotional conflict task: A CAN-BIND-1 study. J Affect Disord. 2020;264:414-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Wan L, Pei P, Zhang Q, Gao W. Specificity in the commonalities of inhibition control: using meta-analysis and regression analysis to identify the key brain regions in psychiatric disorders. Eur Psychiatry. 2024;67:e69. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Lai CH. The neural markers of MRI to differentiate depression and panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Kong XM, Xu SX, Sun Y, Wang KY, Wang C, Zhang J, Xia JX, Zhang L, Tan BJ, Xie XH. Electroconvulsive therapy changes the regional resting state function measured by regional homogeneity (ReHo) and amplitude of low frequency fluctuations (ALFF) in elderly major depressive disorder patients: An exploratory study. Psychiatry Res Neuroimaging. 2017;264:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Wang L, Li K, Zhang Q, Zeng Y, Dai W, Su Y, Wang G, Tan Y, Jin Z, Yu X, Si T. Short-term effects of escitalopram on regional brain function in first-episode drug-naive patients with major depressive disorder assessed by resting-state functional magnetic resonance imaging. Psychol Med. 2014;44:1417-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Wang M, Ju Y, Lu X, Sun J, Dong Q, Liu J, Zhang L, Zhang Y, Zhang S, Wang Z, Liu B, Li L. Longitudinal changes of amplitude of low-frequency fluctuations in MDD patients: A 6-month follow-up resting-state functional magnetic resonance imaging study. J Affect Disord. 2020;276:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Gong J, Wang J, Qiu S, Chen P, Luo Z, Wang J, Huang L, Wang Y. Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: voxel-based meta-analysis. Transl Psychiatry. 2020;10:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 26. | Huang M, Lu S, Yu L, Li L, Zhang P, Hu J, Zhou W, Hu S, Wei N, Huang J, Weng J, Xu Y. Altered fractional amplitude of low frequency fluctuation associated with cognitive dysfunction in first-episode drug-naïve major depressive disorder patients. BMC Psychiatry. 2017;17:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Smitha KA, Akhil Raja K, Arun KM, Rajesh PG, Thomas B, Kapilamoorthy TR, Kesavadas C. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. Neuroradiol J. 2017;30:305-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 424] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 28. | Tian S, Chattun MR, Zhang S, Bi K, Tang H, Yan R, Wang Q, Yao Z, Lu Q. Dynamic community structure in major depressive disorder: A resting-state MEG study. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Gu L, Huang L, Yin F, Cheng Y. Classification of Depressive Disorder Based on RS-fMRI Using Multivariate Pattern Analysis with Multiple Features. 2017 4th IAPR Asian Conference on Pattern Recognition (ACPR). China: IEEE, 2017: 61-66. [DOI] [Full Text] |
| 30. | Sendi MSE, Zendehrouh E, Sui J, Fu Z, Zhi D, Lv L, Ma X, Ke Q, Li X, Wang C, Abbott CC, Turner JA, Miller RL, Calhoun VD. Abnormal Dynamic Functional Network Connectivity Estimated from Default Mode Network Predicts Symptom Severity in Major Depressive Disorder. Brain Connect. 2021;11:838-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Ramasubbu R, Brown MR, Cortese F, Gaxiola I, Goodyear B, Greenshaw AJ, Dursun SM, Greiner R. Accuracy of automated classification of major depressive disorder as a function of symptom severity. Neuroimage Clin. 2016;12:320-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Ni S, Peng T, Gao S, Ling C, Wu F, Jiang J, Sun J, Xiao C, Xu X. Altered brain regional homogeneity, depressive symptoms, and cognitive impairments in medication-free female patients with current depressive episodes in bipolar disorder and major depressive disorder. BMC Psychiatry. 2024;24:892. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Xu K, Long D, Zhang M, Wang Y. The efficacy of topological properties of functional brain networks in identifying major depressive disorder. Sci Rep. 2024;14:29453. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Guo Y, Chu T, Li Q, Gai Q, Ma H, Shi Y, Che K, Dong F, Zhao F, Chen D, Jing W, Shen X, Hou G, Song X, Mao N, Wang P. Diagnosis of Major Depressive Disorder Based on Individualized Brain Functional and Structural Connectivity. J Magn Reson Imaging. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 35. | Chekroud AM, Zotti RJ, Shehzad Z, Gueorguieva R, Johnson MK, Trivedi MH, Cannon TD, Krystal JH, Corlett PR. Cross-trial prediction of treatment outcome in depression: a machine learning approach. Lancet Psychiatry. 2016;3:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 411] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 36. | Feder S, Sundermann B, Wersching H, Teuber A, Kugel H, Teismann H, Heindel W, Berger K, Pfleiderer B. Sample heterogeneity in unipolar depression as assessed by functional connectivity analyses is dominated by general disease effects. J Affect Disord. 2017;222:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Jin J, Huang L. A Region-Based Feature Extraction Method for Rs-fMRI of Depressive Disorder Classification. 2020 International Conference on Computer Vision, Image and Deep Learning (CVIDL). China: IEEE, 2020: 707-710. [DOI] [Full Text] |
| 38. | Yu H, Li ML, Li YF, Li XJ, Meng Y, Liang S, Li Z, Guo W, Wang Q, Deng W, Ma X, Coid J, Li DT. Anterior cingulate cortex, insula and amygdala seed-based whole brain resting-state functional connectivity differentiates bipolar from unipolar depression. J Affect Disord. 2020;274:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Nakano T, Takamura M, Ichikawa N, Okada G, Okamoto Y, Yamada M, Suhara T, Yamawaki S, Yoshimoto J. Enhancing Multi-Center Generalization of Machine Learning-Based Depression Diagnosis From Resting-State fMRI. Front Psychiatry. 2020;11:400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Zeng LL, Shen H, Liu L, Hu D. Unsupervised classification of major depression using functional connectivity MRI. Hum Brain Mapp. 2014;35:1630-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 41. | Chun JY, Sendi MSE, Sui J, Zhi D, Calhoun VD. Visualizing Functional Network Connectivity Difference between Healthy Control and Major Depressive Disorder Using an Explainable Machine-learning Method. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:1424-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Jun E, Na KS, Kang W, Lee J, Suk HI, Ham BJ. Identifying resting-state effective connectivity abnormalities in drug-naïve major depressive disorder diagnosis via graph convolutional networks. Hum Brain Mapp. 2020;41:4997-5014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Gao J, Heng F, Yuan Y, Liu Y. A novel machine learning method for multiaxial fatigue life prediction: Improved adaptive neuro-fuzzy inference system. Int J Fatigue. 2024;178:108007. [DOI] [Full Text] |
| 44. | He C, Bai Y, Wang Z, Fan D, Wang Q, Liu X, Zhang H, Zhang H, Zhang Z, Yao H, Xie C. Identification of microRNA-9 linking the effects of childhood maltreatment on depression using amygdala connectivity. Neuroimage. 2021;224:117428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 45. | Shi Y, Zhang L, Wang Z, Lu X, Wang T, Zhou D, Zhang Z. Multivariate Machine Learning Analyses in Identification of Major Depressive Disorder Using Resting-State Functional Connectivity: A Multicentral Study. ACS Chem Neurosci. 2021;12:2878-2886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 46. | Sundermann B, Feder S, Wersching H, Teuber A, Schwindt W, Kugel H, Heindel W, Arolt V, Berger K, Pfleiderer B. Diagnostic classification of unipolar depression based on resting-state functional connectivity MRI: effects of generalization to a diverse sample. J Neural Transm (Vienna). 2017;124:589-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Wei M, Qin J, Yan R, Li H, Yao Z, Lu Q. Identifying major depressive disorder using Hurst exponent of resting-state brain networks. Psychiatry Res. 2013;214:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Wu J, Lu AD, Zhang LP, Zuo YX, Jia YP. [Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia]. Zhonghua Xue Ye Xue Za Zhi. 2019;40:52-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 125] [Reference Citation Analysis (0)] |
| 49. | Mennella C, Maniscalco U, De Pietro G, Esposito M. Ethical and regulatory challenges of AI technologies in healthcare: A narrative review. Heliyon. 2024;10:e26297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 90] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 50. | Liddell K, Simon DA, Lucassen A. Patient data ownership: who owns your health? J Law Biosci. 2021;8:lsab023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 51. | Ballantyne A. How should we think about clinical data ownership? J Med Ethics. 2020;46:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 52. | Murdoch B. Privacy and artificial intelligence: challenges for protecting health information in a new era. BMC Med Ethics. 2021;22:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 238] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 53. | Thapa C, Camtepe S. Precision health data: Requirements, challenges and existing techniques for data security and privacy. Comput Biol Med. 2021;129:104130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 54. | Rocher L, Hendrickx JM, de Montjoye YA. Estimating the success of re-identifications in incomplete datasets using generative models. Nat Commun. 2019;10:3069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 55. | Na L, Yang C, Lo CC, Zhao F, Fukuoka Y, Aswani A. Feasibility of Reidentifying Individuals in Large National Physical Activity Data Sets From Which Protected Health Information Has Been Removed With Use of Machine Learning. JAMA Netw Open. 2018;1:e186040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 56. | Rajput D, Wang WJ, Chen CC. Evaluation of a decided sample size in machine learning applications. BMC Bioinformatics. 2023;24:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 109] [Reference Citation Analysis (0)] |
| 57. | Cahan EM, Hernandez-Boussard T, Thadaney-Israni S, Rubin DL. Putting the data before the algorithm in big data addressing personalized healthcare. NPJ Digit Med. 2019;2:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 58. | Char DS, Shah NH, Magnus D. Implementing Machine Learning in Health Care - Addressing Ethical Challenges. N Engl J Med. 2018;378:981-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 645] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 59. | Krueger G, Granziera C, Jack CR Jr, Gunter JL, Littmann A, Mortamet B, Kannengiesser S, Sorensen AG, Ward CP, Reyes DA, Britson PJ, Fischer H, Bernstein MA. Effects of MRI scan acceleration on brain volume measurement consistency. J Magn Reson Imaging. 2012;36:1234-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Caramanos Z, Fonov VS, Francis SJ, Narayanan S, Pike GB, Collins DL, Arnold DL. Gradient distortions in MRI: characterizing and correcting for their effects on SIENA-generated measures of brain volume change. Neuroimage. 2010;49:1601-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Preboske GM, Gunter JL, Ward CP, Jack CR Jr. Common MRI acquisition non-idealities significantly impact the output of the boundary shift integral method of measuring brain atrophy on serial MRI. Neuroimage. 2006;30:1196-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Lee H, Nakamura K, Narayanan S, Brown RA, Arnold DL; Alzheimer's Disease Neuroimaging Initiative. Estimating and accounting for the effect of MRI scanner changes on longitudinal whole-brain volume change measurements. Neuroimage. 2019;184:555-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 63. | Winter NR, Blanke J, Leenings R, Ernsting J, Fisch L, Sarink K, Barkhau C, Emden D, Thiel K, Flinkenflügel K, Winter A, Goltermann J, Meinert S, Dohm K, Repple J, Gruber M, Leehr EJ, Opel N, Grotegerd D, Redlich R, Nitsch R, Bauer J, Heindel W, Gross J, Risse B, Andlauer TFM, Forstner AJ, Nöthen MM, Rietschel M, Hofmann SG, Pfarr JK, Teutenberg L, Usemann P, Thomas-Odenthal F, Wroblewski A, Brosch K, Stein F, Jansen A, Jamalabadi H, Alexander N, Straube B, Nenadic I, Kircher T, Dannlowski U, Hahn T. A Systematic Evaluation of Machine Learning-Based Biomarkers for Major Depressive Disorder. JAMA Psychiatry. 2024;81:386-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 64. | Ribeiro MT, Singh S, Guestrin C. "Why Should I Trust You?". Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. United States: Association for Computing Machinery, 2016: 1135-1144. [DOI] [Full Text] |
| 65. | Gastounioti A, Kontos D. Is It Time to Get Rid of Black Boxes and Cultivate Trust in AI? Radiol Artif Intell. 2020;2:e200088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 66. | Reyes M, Meier R, Pereira S, Silva CA, Dahlweid FM, von Tengg-Kobligk H, Summers RM, Wiest R. On the Interpretability of Artificial Intelligence in Radiology: Challenges and Opportunities. Radiol Artif Intell. 2020;2:e190043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 198] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 67. | Rajkomar A, Dean J, Kohane I. Machine Learning in Medicine. N Engl J Med. 2019;380:1347-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1580] [Article Influence: 263.3] [Reference Citation Analysis (0)] |
| 68. | Yigzaw KY, Olabarriaga SD, Michalas A, Marco-ruiz L, Hillen C, Verginadis Y, de Oliveira MT, Krefting D, Penzel T, Bowden J, Bellika JG, Chomutare T. Health data security and privacy: Challenges and solutions for the future. In: Hovenga E, Grain H, editor. Roadmap to Successful Digital Health Ecosystems. Netherlands: Elsevier, 2022: 335-362. [DOI] [Full Text] |
| 69. | Khalid N, Qayyum A, Bilal M, Al-Fuqaha A, Qadir J. Privacy-preserving artificial intelligence in healthcare: Techniques and applications. Comput Biol Med. 2023;158:106848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 90] [Reference Citation Analysis (0)] |
| 70. | Skelton E, Drey N, Rutherford M, Ayers S, Malamateniou C. Electronic consenting for conducting research remotely: A review of current practice and key recommendations for using e-consenting. Int J Med Inform. 2020;143:104271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 71. | Kaye J, Whitley EA, Lund D, Morrison M, Teare H, Melham K. Dynamic consent: a patient interface for twenty-first century research networks. Eur J Hum Genet. 2015;23:141-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 346] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 72. | Schmaal L, Pozzi E, C Ho T, van Velzen LS, Veer IM, Opel N, Van Someren EJW, Han LKM, Aftanas L, Aleman A, Baune BT, Berger K, Blanken TF, Capitão L, Couvy-Duchesne B, R Cullen K, Dannlowski U, Davey C, Erwin-Grabner T, Evans J, Frodl T, Fu CHY, Godlewska B, Gotlib IH, Goya-Maldonado R, Grabe HJ, Groenewold NA, Grotegerd D, Gruber O, Gutman BA, Hall GB, Harrison BJ, Hatton SN, Hermesdorf M, Hickie IB, Hilland E, Irungu B, Jonassen R, Kelly S, Kircher T, Klimes-Dougan B, Krug A, Landrø NI, Lagopoulos J, Leerssen J, Li M, Linden DEJ, MacMaster FP, M McIntosh A, Mehler DMA, Nenadić I, Penninx BWJH, Portella MJ, Reneman L, Rentería ME, Sacchet MD, G Sämann P, Schrantee A, Sim K, Soares JC, Stein DJ, Tozzi L, van Der Wee NJA, van Tol MJ, Vermeiren R, Vives-Gilabert Y, Walter H, Walter M, Whalley HC, Wittfeld K, Whittle S, Wright MJ, Yang TT, Zarate C Jr, Thomopoulos SI, Jahanshad N, Thompson PM, Veltman DJ. ENIGMA MDD: seven years of global neuroimaging studies of major depression through worldwide data sharing. Transl Psychiatry. 2020;10:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 73. | Shorten C, Khoshgoftaar TM. A survey on Image Data Augmentation for Deep Learning. J Big Data. 2019;6:60. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3677] [Cited by in RCA: 2214] [Article Influence: 553.5] [Reference Citation Analysis (0)] |
| 74. | Ichikawa N, Lisi G, Yahata N, Okada G, Takamura M, Hashimoto RI, Yamada T, Yamada M, Suhara T, Moriguchi S, Mimura M, Yoshihara Y, Takahashi H, Kasai K, Kato N, Yamawaki S, Seymour B, Kawato M, Morimoto J, Okamoto Y. Primary functional brain connections associated with melancholic major depressive disorder and modulation by antidepressants. Sci Rep. 2020;10:3542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 75. | Shimizu Y, Yoshimoto J, Toki S, Takamura M, Yoshimura S, Okamoto Y, Yamawaki S, Doya K. Toward Probabilistic Diagnosis and Understanding of Depression Based on Functional MRI Data Analysis with Logistic Group LASSO. PLoS One. 2015;10:e0123524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 76. | Frost C, Kallis C. Reply: A plea for confidence intervals and consideration of generalizability in diagnostic studies. Brain. 2009;132:e103; author reply e102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 77. | Diaz O, Kushibar K, Osuala R, Linardos A, Garrucho L, Igual L, Radeva P, Prior F, Gkontra P, Lekadir K. Data preparation for artificial intelligence in medical imaging: A comprehensive guide to open-access platforms and tools. Phys Med. 2021;83:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 78. | Nordin N, Zainol Z, Mohd Noor MH, Chan LF. An explainable predictive model for suicide attempt risk using an ensemble learning and Shapley Additive Explanations (SHAP) approach. Asian J Psychiatr. 2023;79:103316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 79. | Hassan SU, Abdulkadir SJ, Zahid MSM, Al-Selwi SM. Local interpretable model-agnostic explanation approach for medical imaging analysis: A systematic literature review. Comput Biol Med. 2024;185:109569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |