Published online Mar 19, 2025. doi: 10.5498/wjp.v15.i3.102643
Revised: December 23, 2024
Accepted: January 22, 2025
Published online: March 19, 2025
Processing time: 124 Days and 23.4 Hours
Depression is a prevalent mental disorder that affects numerous individuals, manifesting as persistent anhedonia, sadness, and hopelessness. Despite extensive research, the exact causes and optimal treatment approaches for depression remain unclear. Extracellular vesicles (EVs), which carry biological molecules such as proteins, lipids, nucleic acids, and metabolites, have emerged as crucial players in both pathological and physiological processes. EVs derived from various sources exert distinct effects on depression. Specifically, EVs released by neurons, astrocytes, microglia, oligodendrocytes, immune cells, stem cells, and even bacteria contribute to the pathogenesis of depression. Moreover, there is growing interest in potential of EVs as diagnostic and therapeutic tools for depression. This review provides a comprehensive overview of recent research on EVs from different sources, their roles in depression, and their potential clinical applications.
Core Tip: Depression is a prevalent mental disorder that affects numerous individuals, manifesting as persistent anhedonia, sadness, and hopelessness. Extracellular vesicles (EVs) are phospholipid membrane-enclosed structures that exhibit diversity in size, origin, activity, composition, and function. They encapsulate a myriad of biomolecules, including proteins, lipids, nucleic acids, and metabolites, contributing significantly to intercellular communication across various physiological and pathological progress. We herein summarize an overview of the latest research on EVs from different sources and their roles and potential applications in depression.
- Citation: Wu J, Lu J, Pan MZ, Gu XC, Dai L, Wang Y, Shen B, Zhang XB. Update on the roles and applications of extracellular vesicles in depression. World J Psychiatry 2025; 15(3): 102643
- URL: https://www.wjgnet.com/2220-3206/full/v15/i3/102643.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i3.102643
Depression is a psychiatric disorder characterized by persistent sadness and a significant loss of interest in daily activities. Symptoms of mild depression include sadness, anhedonia, and feelings of worthlessness. In its severe form, major depression is a leading cause of disability, morbidity, and mortality worldwide and is often associated with recurrent thoughts of suicide[1]. Depression is a global health priority. According to statistics from the World Health Organization, approximately 5% of adults worldwide suffer from depression, and over 700000 people die by suicide each year. Suicide is the fourth leading cause of death in 15-29-year-olds (https://www.who.int). Given its high rates of disability, mor
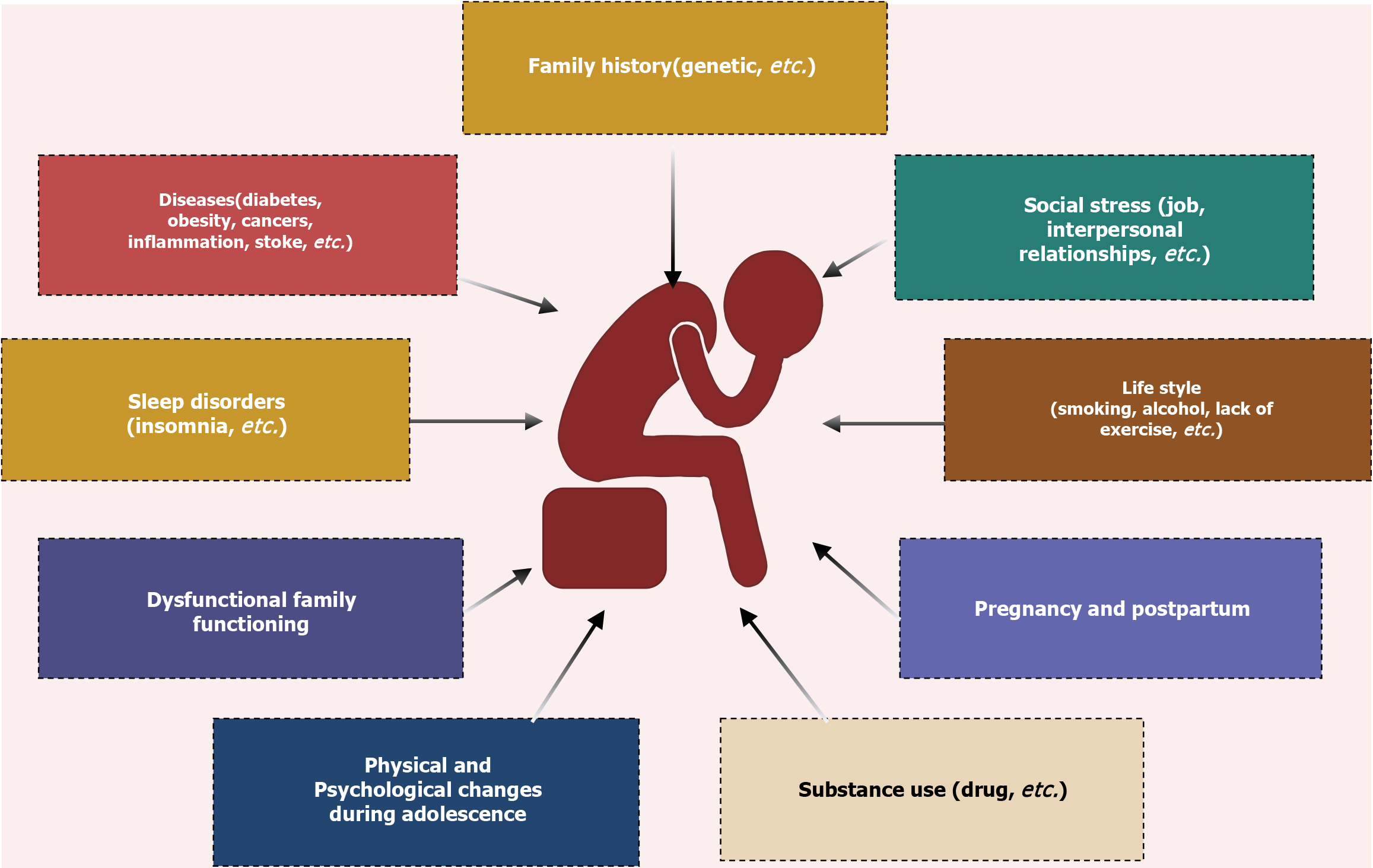
Depression is a complex and multifaceted condition influenced by biological, physiological, and environmental factors[2]. Its risk factors are diverse and include a family history of depression (with genetics accounting for approximately 35% of the risk), chronic diseases, social stress, unhealthy lifestyles, pregnancy and postpartum changes, substance use, sleep disorders, dysfunctional family, dynamics, and physical and psychological changes during adolescence[1,3-5] (Figure 1). Prolonged exposure to these risk factors can lead to the onset of depressive symptoms.
While research has identified several critical physiological pathways associated with depression, such as the hypo
Ongoing efforts aim to elucidate the pathogenesis of depression and identify reliable biomarkers to enable early detection and intervention. These efforts are critical to improving treatment outcomes and addressing this widespread and debilitating mental health condition.
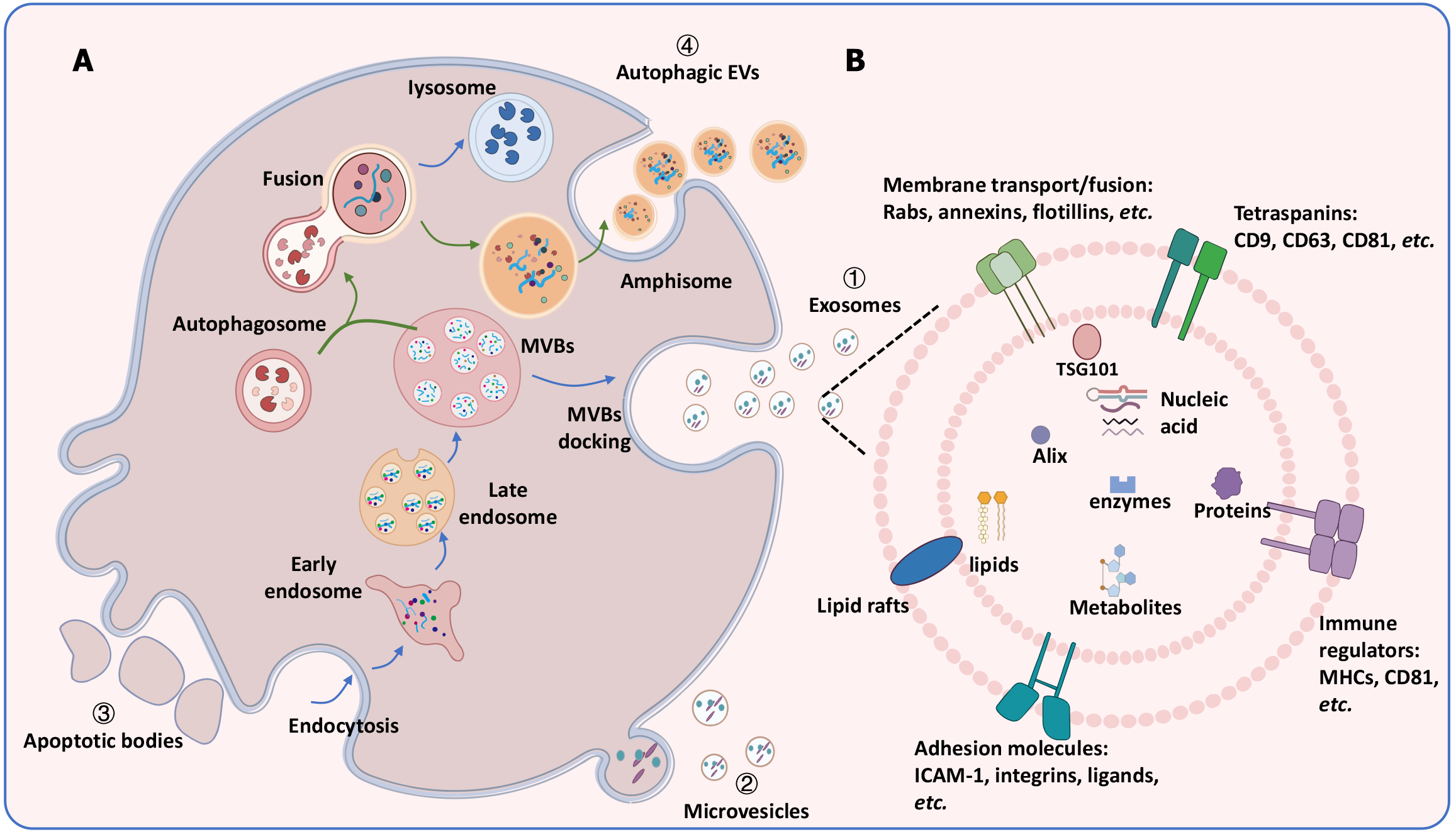
Extracellular vesicles (EVs) are phospholipid membrane-enclosed structures that exhibit diversity in size, origin, activity, composition, and function[4]. Traditionally, EVs are classified into three major categories based on their biogenesis mechanisms: Exosomes, microvesicles (MVs), and apoptotic bodies (ABs)[9] (Figure 2A). Recently, a new class known as autophagic EVs-has also been identified. Exosomes, with diameters ranging from 30 to 150 nm, originate from the inward budding of endosomal membranes and are released when multivesicular bodies (MVBs) fuse with the plasma membrane. MVs, which are larger (100-1000 nm), are formed through outward budding of the plasma membrane. ABs, ranging from 500 nm and 5 μm, are released during the final stages of apoptosis when cells fragment. Autophagy is a self-degradative process that maintains cellular homeostasis under stress stimuli through fusion with lysosomes or the plasma membrane. Recent studies indicate that the autophagy-mediated protein secretion mechanism is known as secretory autophagy[10]. However, this mechanism is not well-understood. During this process, autophagosomes fuse with late endosomes/MVBs to form amphisomes, which are subsequently released as autophagic EVs. The relationship between EVs biogenesis-particularly exosomes and autophagy is complex. Autophagy-related proteins contribute to exosomes production, while inhibiting lysosomal activity or autophagy can restore exosomes secretion. Conversely, an increase in autophagic EVs leads to a reduction in exosomes release[11,12].
A major challenge in EVs research lies in their heterogeneity and the methods used to isolate and purify distinct subpopulations. Various isolation techniques exploit the unique properties of EVs, such as size, density, composition, morphology, surface proteins, and charge. International guidelines for EV separation and characterization are now available and are regularly updated[13]. The current EVs separation methods include differential ultracentrifugation (dUC), density gradient/cushion, size exclusion chromatography (SEC), fluid flow-based separation, charge and molecular recognition-based separations. dUC enriches EVs subtypes based on sedimentation coefficient, proportional to their diameter and density. Density gradient/cushion separates certain contents, such as proteins, based on their characteristic densities. SEC facilitates size-based separation of particles and is an accessible technique. Fluid flow-based separation isolates EVs based on particle properties without relying on a stationary phase or matrix. Charge and molecular recognition-based separation captures EVs based on their surface charge or molecular composition. However, no existing isolation method separates EVs based on their specific origin. Therefore, the choice of isolation method should be guided by the heterogeneity of EVs, the scientific question being addressed, and downstream applications[14,15].
The International Society for EVs recommends that researchers explicitly describe the methods used for EVs collection and use precise terminology when referring to small vesicles. For consistency, this review will use the term “EVs” to broadly include all small EVs secreted with diameters less than 200 nm, while references that refer to exosomes will be encompassed within this category[16].
EVs encapsulate a wide range of biomolecules, including proteins, lipids, nucleic acids, and metabolites, and play critical roles in intercellular communication across physiological and pathological processes[17] (Figure 2B). They influence target cells by transporting and releasing their cargo, facilitating signal transduction, and modulating the behavior and function of recipient cells[18]. The growing interest in EVs is driven by their potential as non-invasive biomarkers for diagnosing and prognosticating various illnesses, including neurological and psychiatric disorders, due to their presence in bodily fluids. EVs cargo reflect the physiological and pathological conditions of the parent cells, providing insights into cellular dynamics under specific conditions[19]. Of particular importance is the emerging recognition of EVs as biological indicators of depression. EVs are implicated in critical pathways associated with depression, including neuroinflammation, neurogenesis, neuronal plasticity, and epigenetic modulation. These findings highlight the promise of EVs for improving diagnostic and therapeutic strategies in depression[20]. This review consolidates the current understanding of EVs derived from various sources and their roles in depression. It underscores the potential of EVs as diagnostic and therapeutic tools, providing a foundation for further investigation into EVs-associated mechanisms underlying depression.
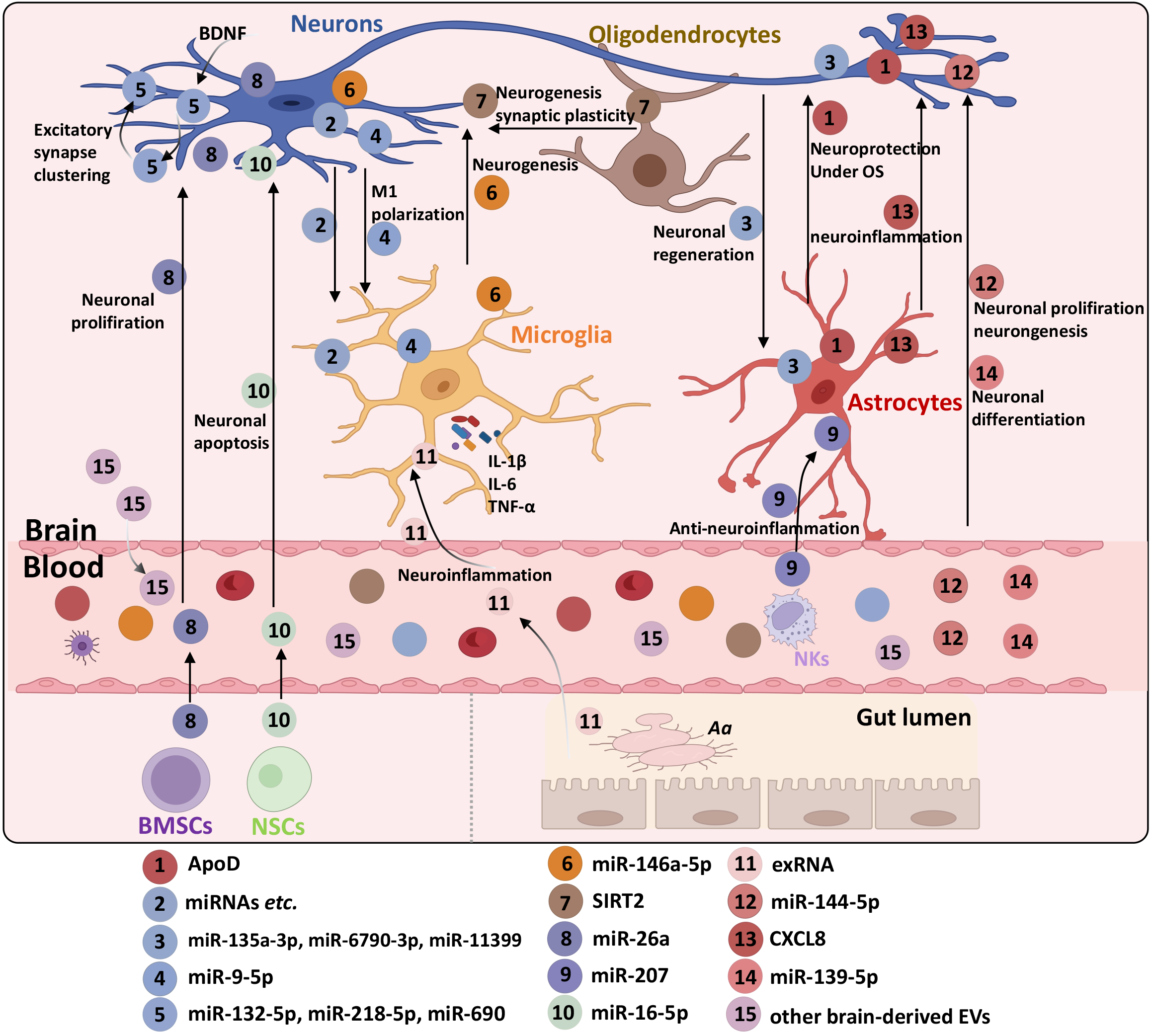
EVs derived from tissues, cells, blood, and even bacteria have been implicated in the development of depression. This section focuses on the roles of EVs from different sources in depression. Table 1 provides a comprehensive summary of these roles, while Figure 3 illustrates validated findings on the contributions of EVs derived from various sources in depression.
| Sources | EVs types | Cargos | Alternations | Roles/functions | Potential applications | Ref. |
| Cells-derived EVs | ||||||
| Astrocytes | Exosomes | ApoD | ↑ | Mediate neuronal survival upon oxidative stress | Therapeutic target | [25] |
| EVs | VEGF | ↑ | Maintaining the BBB permeability | Biomarkers | [27] | |
| EVs | Inflammatory markers (Interferon-γ, IL-12p70, IL-1β, IL-2, IL-4, IL-6, TNF-α, IL-17A) | ↑ | Promote neuroinflammation | Biomarkers | [29] | |
| Neurons | EVs | miR-135a-3p, miR-6790-3p, miR-11399 | ↑ | Suppress neuronal functions | - | [30] |
| Exosomes | miR-9-5p | ↑ | Promote microglia polarization through the SOCS2-STAT3 axis and neuronal injury | Therapeutic target | [31] | |
| EVs | miR-17-5p | ↑ | - | Diagnosis biomarkers | [32] | |
| EVs | miR-93 | ↓ | Lead to interoceptive processing dysfunctions through altered epigenetic modulation of insular function | Therapeutic target | [33] | |
| EVs | miR-132-5p, miR-218-5p, miR-690 | ↑ | Increase synaptic transmission and synchronous neuronal activity | - | [35] | |
| EVs | Mitochondrial proteins | ↑/↓ | Regulate neuronal metabolism and survival | Biomarkers and drug targets | [40] | |
| Exosomes | IRS-1 | ↑ | Regulate central insulin signaling | Therapeutic target | [38] | |
| EVs | miR-21-5p, miR-30d-5p, miR-486-5p | ↑ | - | Biomarker for ADT response | [36] | |
| Exosomes | IL-34/CD81, synaptophysin/CD81 | ↑ | Regulate neuroinflammation and synaptic functions | Biomarkers | [37] | |
| Microglia | EVs | miR-146a-5p | ↑ | Inhibit neurogenesis | Biomarkers and therapeutic target | [41] |
| Exosomes | VDBP | ↑ | - | Biomarker | [44] | |
| Oligodendrocytes | Exosomes | PLP, CNP, MBP, MOG | ↑ | Promote myelin synthesis and turnover | - | [46] |
| Exosomes | SIRT2 | ↑ | Restore hippocampal neurogenesis and synaptic plasticity via the AKT/GSK-3β pathway | Therapeutic target | [47] | |
| Anterior cingulate cortex | EVs | miR-92a-3p, miR-129-5p | ↑ | Involve neurotransmission and synaptic plasticity | - | [48] |
| Brain tissues | Exosomes | miR-210-5p, miR-143-5p, miR-574-5p, miR-551b-3p, let-7a-2-3p, etc. | ↑/↓ | Involve neuroplasticity, insulin resistance, and hypoxic response | Biomarkers | [49] |
| MSCs | Exosomes | miR-132 | ↑ | Reduce neuroinflammation and protect the integrity of BBB | Diagnosis and therapeutic target | [52] |
| BMSCs | Exosomes | miR-26a | ↑ | Inhibit oxidative stress and inflammation, boost neuron proliferation and restrain apoptosis | Therapeutic target | [53] |
| ASCs | EVs | - | - | Inhibition of ER stress-mediated apoptotic | Therapeutic target | [54] |
| NSCs | EVs | miR-16-5p | ↑ | Neuroprotection | - | [55] |
| Macro-phages | Exosomes | LFA-1, ICAM-1, BDNF | ↑ | Mediate its lateral migration and crossing the BBB, relieve neuroinflammation | Therapeutic target | [58] |
| DCs | Exosomes | - | - | Improve myelination and reduce oxidative stress | Therapeutic target | [59] |
| NKs | Exosomes | miR-207 | ↑ | Inhibit neuroinflammation | Therapeutic target | [60] |
| Bacteria-derived EVs | ||||||
| A. actinomycetemcomitans | OMV | exRNA | ↑ | Induce neuroinflammation | - | [68] |
| L. plantarum | EVs | - | - | Rescue the stress-induced decrease of BDNF, Sirt1 and MeCP2 | Treatment | [69] |
| B. subtilis | Rescue stress-induced reduction of BDNF, Nt3, Nt4/5, and Sirt1 | |||||
| A. muciniphila | ||||||
| Blood-derived EVs | ||||||
| Serum | sEVs | Aldolase C | ↑ | - | Biomarkers | [22] |
| Exosomes | miRNAs | ↑/↓ | Regulate neuronal functions involving MAPK, Wnt, and mTOR pathway | Biomarkers for the early diagnosis of depression | [75] | |
| EVs | miR-93-5p, miR-712-5p, miR-467b-3p, etc. | - | Modulate pro- or anti-inflammatory responses | Biomarkers | [76] | |
| Exosomes | miR-144-3p | ↓ | Involve synthesis, metabolism, and breakdown of fatty acids | Diagnostic biomarkers | [78] | |
| Exosomes | miR-144-5p | ↓ | Induce abnormal neurogenesis, neuronal apoptosis, altered synaptic plasticity, and neuroinflammation | Therapeutic target for MDD | [79] | |
| EVs | miR-450a-2-3p | ↑ | - | Diagnostic biomarkers | [80] | |
| EVs | miR-4433b-5p, miR-584-5p, miR-625-3p, miR-432-5p, miR-409-3p | ↓ | - | Diagnostic biomarkers | [81] | |
| EVs | miR-2277-3p miR-6813-3 | ↓ | Involve glucocorticoid and gamma-aminobutyric acid receptor signaling | Diagnostic biomarkers for severity | [84] | |
| Involve dopaminergic neural pathway | ||||||
| Exosomes | 15 metabolites | ↑ | - | Diagnostic biomarkers for BD and MDD | [95] | |
| Plasma | Exosomes | miR-16-5p, miR-129-5p, miR-363-3p, miR-92a-3p | ↓ | - | Diagnosis and treatment | [82] |
| Exosomes | miR-335-5p | ↑ | Involve the synaptic synthesis and transport of many neurotransmitters | Biomarkers of diagnosis and treatment for ADT | [83] | |
| miR-1292-3p | ↓ | |||||
| Exosomes | miR-30a-5p | ↑ | Inhibit osteogenic differentiation | Biomarker and therapeutic target for osteoporosis caused by depression | [86] | |
| Exosomes | CXCL8 | ↑ | Facilitate astrocyte-neuron communication | Therapeutic target for PTSD | [91] | |
| EVs | C3 | ↑ | - | Diagnosis biomarkers for MDD | [92] | |
| Exosomes | sigma-1R | ↑ | Ameliorate inflammation response | Treatment | [94] | |
| Blood/brain | Exosomes | miR-139-5p | ↑ | A negative regulator for neural stem cell proliferation and neuronal differentiation | Diagnosis and treatment | [85] |
| Blood | EVs | 13 mRNAs | - | Involve synaptic function and myelination | Diagnosis biomarkers for PPD | [88] |
| Blood | Exosomes | SERPINF1 | ↓ | A target of miR-186-5p | Biomarker for predicting MDD development | [89] |
In the central nervous system (CNS), cells, EVs derived from astrocytes, neurons, microglia, and oligodendrocytes serve as information carriers facilitating communication between adjacent and distant cells. These EVs mediate various physiological and pathological responses associated in depression.
Astrocytes: Astrocytes play a crucial role in EV-mediated responses linked to depression. Previous research has identified the glycolytic enzyme aldolase C presented in astrocytes was detected in the cerebrospinal fluid (CSF) of depressed mice[21]. Additionally, aldolase C tagged with green fluorescent protein has been recovered in serum small EVs, indicating that astrocyte-derived proteins can be detected in blood EVs[22]. Repeated or uncontrolled stress leads to region-specific changes in astrocyte density and morphology, which impacts EV formation, release, and molecular composition. When stimulated, astrocytes release EVs enriched with neuroprotective molecules such as heat shock proteins, synapsin 1, microRNAs (miRNAs), and glutamate transporters. In contrast, resting astrocytes release EVs containing factors like fibroblast growth factor-2, apolipoprotein-D (ApoD), and vascular endothelial growth factor (VEGF)[23]. Oxidative stress is a critical factor in the pathogenesis of depression[24]. Astrocyte-derived exosomes transport ApoD to neurons, providing neuroprotection under oxidative stress condition[25]. VEGF, a component of astrocyte-derived EVs (ADEVs), is known to increase the permeability of the blood-brain barrier (BBB) and modulate synaptic transmission, influencing hippocampal neurogenesis and depression development[26]. Elevated plasma levels of VEGF, along with ADEVs expressing AQP4 and GFAP, have been observed in individuals with stress-induced exhaustion disorder (SED), a subtype of depression[27]. Furthermore, increased leakage of ADEVs across the BBB has been reported in patients with SED and potentially in those with major depressive disorder (MDD)[28]. While the exact mechanisms are not fully understood, VEGF likely plays a central role in facilitating the transport of ADEVs across the BBB. Depressed individuals frequently exhibit elevated levels of pro-inflammatory cytokines, such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor α (TNF-α). These cytokines are partly derived from astrocytes and the EVs they release. Astrocytes with hyper-inflammatory responses generate EVs enriched with pro-inflammatory markers, contributing to the inflammatory processes observed in depression[29].
Neurons: Lund human mesencephalic cells (LUHMEs), human embryonic neuronal precursor cells capable of proliferation, have been widely studied for their role in EV production. Research by Nishi et al[30] demonstrated that EVs by LUHMEs upon IL-6 stimulation, carry miRNAs such as miR-135a-3p, miR-6790-3p, and miR-11399, which regulate neuroregeneration-related gene expression in astrocytes and microglia. In a cultured cell model, miR-9-5p in neuron-derived exosomes can mediate M1 microglial polarization via the SOCS2-STAT3 axis. M1-polarized microglia release pro-inflammatory factors that exacerbate neuronal damage and contribute to depression. Elevated levels of miR-9-5p have also been detected in serum EVs of MDD patients[31]. Strikingly, alterations in the levels of specific miRNAs, such as miR-17-5p and miR-93 in neuron-derived EVs (NDEVs), have been correlated with the severity of depressive symptoms, highlighting their potential as biomarkers for disease progression. Subthreshold depression (StD) is a depressive state that does not meet the criteria for MDD but carries a risk of progressing to MDD. miR-17-5p in NDEVs correlates positively with Patient Health Questionnaire-9 scores in patients with StD, a state that may progress to MDD[32]. In contrast, miR-93 expression is reduced in neuronal EVs of individuals with elevated serum levels of pro-inflammatory cytokines such as IL-1 receptor antagonist, IL-6, and TNF[33]. Brain-derived neurotrophic factor (BDNF) is a key regulator of neuronal dendrite morphogenesis[34] mediates synapse formation through the transfer of neuronal EVs and their miRNA cargo. BDNF-stimulated EVs, enriched with miR-132-5p, miR-218-5p, and miR-690, promote synaptic vesicle clustering at presynaptic terminals, enhance synaptic activity, and improve neuronal circuit connectivity[35]. Neuronal EVs expressing markers such as L1CAM and SNAP-25A (neuronal markers) can be isolated from plasma. Specific miRNA signatures in these exosomes, including miR-21-5p, miR-30d-5p, and miR-486-5p, may predict treatment response in depression[36]. In an exploratory pilot case-control study, SNAP-25A+ neuronal EVs were analyzed for neuroinflammatory and synaptic function markers. This study identified IL-34 and synaptophysin in CD81+ exosomes, suggesting their peripheral blood transport during depression[37]. Nasca et al[38] reported increased secretion of L1CAM+exosomes in MDD patients compared to age- and sex-matched controls. These exosomes exhibited elevated levels of insulin receptor substrate-1 (IRS-1), which was associated with suicidality and anhedonia. IRS-1 regulates insulin signaling pathways, which are critical for neuroplasticity. Mitochondrial dysfunction is a fundamental pathogenic mechanisms in depression[39]. Abnormal levels of functional mitochondrial proteins have been observed in plasma neuronal EVs of MDD patients. Interestingly, these protein levels were normalized in patients who responded to 8 weeks of treatment with selective serotonin reuptake inhibitors but remained unchanged in non-responders[40]. These findings highlight the intricate roles of neuronal EVs in neuroinflammation, neuroplasticity, and energy metabolism, revealing their potential as biomarkers and therapeutic targets in depression. Further exploration of these mechanisms may offer novel insights into the pathogenesis and treatment of depression.
Microglia: Microglial cells play a pivotal role in the progression of depression, particularly in cases driven by neuroinflammation. A notable study by Fan et al[41] identified an upregulation of miR-146a-5p in serum-derived exosomes from rats subjected to chronic unpredictable mild stress (CUMS). They demonstrated that miR-146a-5p is transferred from microglia to neurons via microglia-secreted exosomes. Downregulating of miR-146a-5p mitigated deficits in adult neurogenesis within the dentate gyrus regions and alleviated depression-like behaviors in rats by post-transcriptional regulating Krüppel-like factor 4. These findings align with earlier research by Jovičić et al[42], which identified microglia as the primary source of miR-146a-5p and suggested that serum EVs containing this miRNA originate from microglia. Recent investigations have also linked cerebral cortex thickening and overexpression of miR-146a-5p in EVs from medication-naïve major depression patients, highlighting the intricate relationship between EVs-associated miR-146a-5p and depression[43]. Currently, microglia-derived EVs (MDEVs) can be isolated using specific markers like TMEM119 through immunoprecipitation techniques. Zhang et al[44] reported a selective reduction in vitamin D-binding protein (VDBP) levels in MDEVs from the plasma of MDD patients compared to healthy controls (HCs), with VDBP levels inversely correlating with the Hamilton Depression Rating Scale scores. Their findings suggest that MDEV-associated VDBP may serve as a potential biomarker for MDD diagnosis.
Oligodendrocytes: Oligodendrocytes, the primary cells responsible for synthesizing and maintaining the myelin sheath in the CNS, play a critical role in providing electrical insulation and trophic support to axons. Genes related to myelination and synaptic plasticity have been implicated in the pathogenesis of depression[45]. Post-mortem studies of suicide victims with major depression revealed reduced oligodendrocyte density and notable demyelination in the white matter regions. Furthermore, oligodendroglial exosomes, stimulated by elevated intracellular Ca2+ levels, carry specific myelin components, including proteolipid protein, 2’3’-cyclic-nucleotide-phosphodiesterase, myelin basic protein, and myelin oligodendrocyte glycoprotein, suggesting the potential involvement of oligodendrocyte-derived EVs (ODEVs) in depression pathogenesis[46]. For example, oligodendrocyte-derived exosomes carrying sirtuin 2 were shown to alleviate depressive-like behaviors by restoring hippocampal neurogenesis and enhancing synaptic plasticity in depressed mice via the AKT/GSK-3β pathway[47]. This finding highlights a promising therapeutic avenue for depression treatment.
In addition to the role of CNS cell-derived EVs in depression discussed in the previous review, there have been reports highlighting the involvement of EVs derived from brain tissue. An analysis of small RNA profiles revealed that EVs from postmortem human anterior cingulate cortex tissue contain various RNA biotypes, with a notable down-regulation of miR-92a-3p and miR-129-5p. These miRNAs are thought to play significant roles in neurotransmission and synaptic plasticity, as indicated by in silico functional analysis[48]. Other studies have also highlighted the differential expression of miRNAs in brain tissue-derived EVs, suggesting their involvement in biological processes such as insulin resistance, neuroplasticity, and hypoxic response. These processes may influence brain functions and potentially contribute to depression-like behaviors[49]. In essence, a diverse array of EVs derived from CNS cells is implicated in signaling pathways crucial for neuroprotection, neuroinflammation, neurogenesis, synaptogenesis, and metabolism, collectively influencing the onset and progression of depression.
Stem cells are well known for their unique ability to self-renew and differentiate, and both stem cells and their derived EVs have garnered significant attention for their potential impact on depression[50]. Mesenchymal stromal cells (MSCs) improved depression-like behavior through their immunomodulatory and paracrine properties[51], with their derived EVs and miR-132 showing promise in improving the prognosis of epileptic patients and reducing the incidence of depression by reducing CNS inflammation[52]. Bone marrow MSCs (BMSCs)-derived EVs improve hippocampal neurons injury in rats with depression by upregulating miRNA-26a expression[53]. Corticosterone (CORT) induction increases neuronal apoptosis in brain tissue and induces depression-like behaviors. Adipose stem cells (ASCs)-derived EVs can ameliorate CORT-induced apoptosis in the cortical neurons by inhibiting endoplasmic reticulum stress[54], suggesting that treatment with ASCs-derived EVs might play an antidepressant role. Similarly, Min et al[55] discovered that neural stem cell-derived EVs reduce neuronal injury in CORT-induced depression rats via the miR-16-5p/MYB axis.
In depression research, the involvement of inflammatory processes in its pathophysiology has been well-established[56]. Dysregulation of both the innate and adaptive immune systems is frequently observed in individuals suffering from depression[57]. A variety of immune cells, including macrophages, dendritic cells (DCs), and natural killer cells (NKs), have been implicated in the pathological mechanisms of depression. Yuan et al[58] explored the role of macrophage-derived exosomes, highlighting the presence of lymphocyte function-associated antigen 1 and intercellular adhesion molecule 1 on macrophage-derived exosomes. These molecules facilitate the uptake of macrophage-derived exosomes by BBB cells. Moreover, macrophage-derived exosomes loaded with exogenous BDNF can be transported to the brain, where they accumulate in the inflammation sites. This suggests that macrophage-derived exosomes may serve as natural nanocarriers for delivering BDNF to inflamed regions in the brain, offering a promising therapeutic strategy for depression. In the context of migraine with aura, spreading depression is recognized as a primary cause of headache pain. The administration of interferon gamma-stimulated DCs EVs has been shown to reduce the susceptibility to spreading depression. This effect occurs by enhancing myelination and reducing oxidative stress both in vivo and in vitro, suggesting that DC-derived exosomes could be a potential therapeutic approach for migraines triggered by spreading depression[59]. Furthermore, NK-derived EVs, particularly those carrying miR-207, have been shown to inhibit NF-κB signaling pathway by directly targeting the toll-like receptor 4 interactor with leucine-rich repeats (Tril) in astrocytes. This action results in a reduction of pro-inflammatory cytokines and alleviates depression-like symptoms in animal models, underscoring the therapeutic potential of NK cell-derived EVs in modulating neuroinflammation and mitigating depressive behaviors[60].
Microbiota dysbiosis is a pathological feature of depression, contributing to subclinical inflammation, HPA axis imbalance, and alterations in neural, metabolic, and endocrine pathways[61,62]. Bacteria-derived EVs carrying nucleic acids, polysaccharides, and proteins are involved in the onset and progression of depression[63]. Outer membrane vesicles (OMVs) produced by the microbiota carry various signals that are crucial for spreading OMVs to distant organs through the bloodstream[64,65]. EVs produced by Gram-negative bacteria through the shedding of the outer membrane are a key factor in neuroinflammation-induced depression[66]. Lipopolysaccharides (LPS) from Gram-negative bacteria mediate systemic inflammatory responses and can be transported to the brain through EVs to trigger neuroinflammation by activating microglia and promoting the secretion of inflammatory cytokines such as TNF-α, IL-1β, and IL-6[67]. EVs derived from the periodontopathogen Aggregatibacter actinomycetemcomitans can infiltrate mouse brain monocytes and carry bacterial extracellular RNA to microglia, leading to a significant increase in NF-κB expression, stimulating IL-6 secretion, and ultimately triggering neuroinflammation[68]. On the other hand, EVs derived from probiotics have antidepressant effects. Gram-positive probiotics Bacillus subtilis and Lactobacillus plantarum, as well as the Gram-negative probiotic Bacteroides fragilis EVs, show antidepressant-like effects by varying degrees of restoring stress-induced alterations in BDNF, Sirtuin1, and MeCP2[69,70]. Dysregulation of serotonin is closely associated with the development of depression[71]. Research by Yaghoubfar et al[72] confirmed that Akkermansia muciniphila-derived EVs could affect the serotonergic system in the colon and hippocampus of mice. Serotonin, an intermediate product of tryptophan meta
In various physiological and pathological states, EVs carrying bioactive molecules can be found in biological fluids like blood, urine, milk, saliva, and amniotic fluid. The presence of these EVs provides valuable insights into disease conditions. By examining EVs in the bloodstream, researchers can gain a deeper understanding of the mechanisms involved in depression and explore novel diagnostic and therapeutic strategies. This section will focus on the significance of the contents within blood-derived EVs (blood-EVs) in relation to depression.
miRNAs: miRNAs are small endogenous RNAs, approximately 21 nucleotides in length, that regulate gene expression post-transcriptionally, serving as vital regulators and promising biomarkers[74]. Blood-EVs miRNAs have emerged as a focal point in depression biomarkers research. Extensive studies have been dedicated to exploring their potential in unraveling complexities of depression. Through miRNA sequencing in CUMS rat models, differentially expressed miRNAs in serum exosomes have been identified, showing intricate associations with key pathways such as MAPK, Wnt, and mTOR[75]. In a chronic social defeat stress (CSDS) mice model, miR-31-5p, miR-712-5p, miR-212-3p, miR-451a, miR-467b-3p, miR-193b-3p, and miR-93-5p found in serum EVs were shown to modulate pro- or anti-inflammatory responses, shedding light on the immune dysregulation observed in depression[76]. MiR-144-3p, differentially expressed in circulating blood from the CSDS model, has been identified as a predictor of MDD severity and ketamine response in humans[77]. Furthermore, miR-144 displays differential expression in serum EVs from depression patients. The expression level of exo-miR-144-3p was negatively correlated with the severity of depressive symptoms in heart failure patients[78]. In contrast, the knockdown of miR-144-5p in normal mice induced depression-like behaviors by promoting microglial M1-polarization. Moreover, upregulation of miR-144-5p ameliorated depression-like behavior and attenuated neuronal abnormalities by directly targeting TLR4 and PTEN/PI3K/Akt/FoxO-1 signaling pathway[79]. In studies of adolescent depression, miR-450a-2-3p, miR-556-3p, and miR-2115-3p in serum EVs were significantly upregulated in untreated adolescents with MDD[80], while miR-4433b-5p, miR-584-5p, miR-625-3p, miR-432-5p, and miR-409-3p in serum EVs were downregulated in adolescents with MDD[81]. Serum EV miR-450a-2-3p showed potential diagnostic and prognostic value in identifying and managing depression in younger populations[80]. The correlation between substance use and depression was further elucidated through the identification of plasma exosomal miRNAs associated with depressive symptoms in substance-dependent individuals. Plasma EV miR-92a-3p, miR-363-3p, miR-16-5p, and miR-129-5p exhibited a significant correlation with total HAM-D scores in methamphetamine-dependent patients[82]. In plasma EVs of treatment-resistant depression patients, miR-335-5p was significantly upregulated, while miR-1292-3p was significantly downregulated compared to HCs. These miRNAs play roles in the MAPK, Ras, and PI3K-AKT signaling pathways, affecting synaptic synthesis and neurotransmitter transport[83]. Downregulation of serum EV miR-2277-3p and miR-6813-3 were correlated with the severity of MDD. miR-2277-3p influences the dopaminergic neural pathway, while miR-6813-3p is linked to glucocorticoid receptor and gamma-aminobutyric acid receptor signaling. Notably, these two miRNAs are highly expressed in astrocytes, suggesting their potential astrocytic origin[84]. Elevated levels of miR-139-5p were identified in blood-EVs of MDD patients[85]. miR-139-5p, known to impair neural stem cell proliferation and neuronal differentiation, has been implicated in inducing depression-like behaviors in mice through the regulation of neurogenesis. Depression-induced osteoporosis, increasing with rising social stress, was investigated in a CUMS model, where plasma EV miR-30a-5p alleviated depressive osteoporosis by inhibiting the expression of osteogenic markers in BMSCs[86].
Messenger RNAs: Other RNA components in EVs, such as messenger RNAs (mRNAs), have also been reported to be associated with depression. mRNAs are the intermediate step between the translation of protein-encoding DNA and the production of proteins by ribosomes in the cytoplasm. The release of mRNA packaged in EVs into the cytosol of recipient cells initiates translation and protein expression[87]. Smirnova et al[88] demonstrated that blood-EVs carrying brain-specific mRNAs could serve as potential biomarkers for detecting gene expression changes in the female brain. Gene Ontology analysis revealed that these mRNAs are involved in synaptic function and myelination and are enrich in genes associated with mood disorders. A particularly notable finding was the correlation between the levels of 13 EV mRNAs and postpartum depression.
Proteins: Proteins found in EVs play a crucial role in depression and serve as important biological markers of depression. Research by Jiang et al[89] revealed that serpin family F member 1 is significantly diminished in the peripheral blood-derived exosomes of MDD patients compared to the HCs. EVs, essential in immune communication within the “gut-brain-immune axis”, are implicated in the pathophysiology of depression[90]. Plasma exosomes enhance communication between astrocytes and neurons by transmitting the immune factor C-X-C motif chemokine ligand 8 (CXCL8)[91], which astrocytes release to induce neuroinflammation. The exosomal marker CD63 regulates the packaging and delivery of CXCL8 into exosomes. Inhibiting CXCL8 has been shown to improve depression caused by post-traumatic stress disorder[91]. Complements are crucial immune regulatory factors, and complement C3 in plasma EVs has been found to be differentially expressed between MDD patients and HCs[92]. Sigma-1 receptor (sigma-1R), an upstream regulator of endoplasmic reticulum stress and signaling, has been linked to antidepressant effects[93]. Plasma exosomes from depressed individuals exhibit antidepressant-like effects through a sigma-1R-dependent mechanism in LPS-induced depression[94].
Metabolites: Metabolomics has emerged as a valuable tool in identifying potential biomarkers for diagnosing major neuropsychiatric diseases. Fifteen differentially expressed metabolites in EVs (Chenodeoxycholic Acid, and Lysope 18:0, Lysope 14:0, N-Acetylmethionine, 13-oxoODE, Glycine, 1-Naphthylacetic Acid, 2-Aminoethanesulfonic Acid, D-2-Aminobutyric Acid, Lysopc 18:0, Lysopc 20:1, Biopterin, Phosphoric Acid, Glucosamine, and PAF C-16) related to sugar metabolism in serum EVs have been found to distinguish bipolar disorder patients from those with MDD[95]. A recent study also highlighted differences in the contents of plasma EVs, including metabolites, proteins, and miRNAs, between non-depressed and depressed rats following traumatic spinal cord injury (TSCI), offering potential insights for diagnosing depression following TSCI[96]. These findings underscore the potential of metabolomics in providing valuable insights for the accurate diagnosis and management of depression.
The potential applications of EVs in depression are multifaceted and promising[97]. Firstly, EVs act as dynamic indicators of disease progression, offering real-time insights into the evolving state of depression. Secondly, their non-invasive extraction from biological fluids enables early detection, facilitating timely intervention. Moreover, the protective membranous structure of EVs preserves their contents, safeguarding valuable biomarkers over extended periods. Additionally, the traceable origin of EVs through cell surface markers enhances their diagnostic utility. Lastly, the ability of EVs to traverse the BBB opens avenues for accessing crucial information about CNS cells, which would otherwise require invasive procedures. These attributes highlight the significant clinical value of EVs in diagnosing, assessing treatment efficacy, and managing depression.
Currently, the diagnosis of depression primarily relies on clinical symptomatology and depression scales, which lack objective biochemical indicators. Detection based on EVs holds significant promise for the early diagnosis of depression. The positive correlation between the cargos found in EVs from blood and CSF suggests that EVs, as peripheral indicators, can potentially replace CSF for depression diagnosis[44]. While EVs isolated from CSF has been used in biomarker discovery studies for depression, EVs from the peripheral circulation are more easily accessible and non-invasive[98]. Specific surface molecular markers carried by EVs can be utilized to isolate cell-specific EVs-such as ADEVs, NDEVs, MDEVs, and ODEVs-from the bloodstream. Techniques like dUC, density gradients, precipitation, filtration, SEC, and immunoisolation can be employed to achieve this[19]. Analyzing the differential expression of cargos in these EVs provides an effective method for diagnosing depression and distinguishing it from other conditions[92,95].
miRNAs have emerged as the most common molecular tools for predicting and diagnosing depression among all EV cargo types. In the circulating EVs of depressed patients, miRNAs often exhibit significant change in expression and play a key role in the onset and progression of depression. They are closely associated with diagnosis, treatment assessment, and prognosis. In addition to miRNAs, protein and lipid cargos in EVs are hold crucial diagnostic potential. Recently, EVs-derived metabolites have been discovered and are emerging as new molecular tools for diagnosing depression.
Building on these advances, scientists are working to develop platforms for diagnosing depression. Topuzoğlu and Ilgın[99] are developing a rapid diagnostic platform, called the “Mentalexo” approach, which focuses on detection of EVs from CNS cells. They aim to apply this approach to identify suitable EV biomarkers for depression. Similarly, Shin et al[100] demonstrated a novel and precise method for diagnosing MDD using deep learning analysis and surface-enhanced Raman spectroscopy of plasma EVs, confirming that the diagnostic scores were correlated with the severity of depression.
EV-derived regulatory miRNAs are valuable parameters for assessing the response to ADT, including let-7e, miR-21-5p, miR-145, miR-146a, miR-155, miR-21-5p, miR-30d-5p, and miR-486-5p[36,101]. In patients with MDD, neuronal EV levels of MPs of all functional classes were normalized in those who responded to SSRI therapy, but not in those who failed to respond, as determined by psychiatric evaluation. This highlights the importance of neuronal EV contents in evaluating treatment response[40]. EV-derived BDNF can also serve as a biomarker for the efficacy of antidepressant drugs, as BDNF levels in serum and EVs fluctuate in opposite directions during antidepressant therapy[102]. Additionally, the red blood cell-specific miR-144-3p has shown potential in predicting the response to ketamine treatment in stress-susceptible mice and MDD patients[77].
As the substrates from the blood to the CNS are controlled by the BBB[103], effective drug transfer to the brain poses a challenge for treating CNS disorders like depression. However, EVs offer promising potential as drug carriers due to their nanoscale size, low immunogenicity, and stable structure, which enable them to cross the BBB and exert therapeutic effects. Lactobacillus-derived EVs can afford antidepressant-like effects in mice with stress-induced depression, with effects lasting for 30 days[69,104]. Further advancements in drug delivery include engineered RVG-circDYM-EVs, which effectively transport circDYM to the brain, inhibiting microglial cell activation, BBB leakage, peripheral immune cell infiltration, and alleviating chronic unpredictable stress-induced depression-like behavior[105]. Hu et al[106] developed a nanogel loaded with PACAP and estrogen (E2), sheathed with EVs and responsive to reactive oxygen species, referred to as HA NGs@EVs, These exhibit notable antioxidant and anti-inflammatory properties, and their intranasal application in a CUMS mouse model led to improved behavioral performance, demonstrating rapid-onset antidepressant effects for perinatal depression. In Traditional Chinese Medicine, herbal medicine and acupuncture focus on prevention and regulation, with Chinese herbal medicine showing antidepressant effects, though its action is slow and non-specific. Interestingly, research has shown that exogenous plant contents carried by EVs can be absorbed by intestinal enterocytes and stably exist in the gastrointestinal tract[107,108]. This discovery opens the possibility of using such EVs as a drug delivery system and for gene therapy via oral administration of herbal medicine to treat depression. Some experts also suggest that acupuncture may influence the distribution of exosomes in vivo, potentially using exosomes as carriers in acupuncture treatment of MDD in the future[109]. The enzyme neutral sphingomyelinase 2 (nSMase2), involved in the biogenesis of ceramide and EVs, is dysregulated in MDD. Zhu et al[110] reported that inhibiting nSMase2 could represent a new therapeutic strategy for HIV-associated MDD, as EcoHIV-infected mice exhibited increased levels of brain-derived EVs and altered miRNA cargo. Additionally, intravenous injection of ceramide-loaded exosomes induced MDD-like behavior in untreated mice, which was reversed by ex vivo pre-incubation of purified exosomes with anti-ceramide antibodies or ceramidase[111].
Although research is still limited, current literature supports the potential of EVs for diagnostic, assessment, and therapeutic purposes in depression, with transformative applications on the horizon.
Depression is a type of psychiatric disorder characterized by abnormal neuroinflammation, neurotransmitters, dysregulation of the HPA axis, and altered neural transmission. As key intercellular communication mediators, EVs play a crucial role in understanding the etiology, diagnosis, and treatment of depression. Previous studies have demonstrated that EVs from various sources have distinct roles in depression. This article reviews the contribution of EVs from different cells and biological sources to depression and briefly discusses their potential applications in diagnosing and treating the disorder. While the potential of EVs for diagnosing and treating depression is promising, several challenges remain. CNS cell-derived EVs show great potential as diagnostic biomarkers for depression that can be detected in peripheral blood. However, accurately and selectively isolating, identifying, and quantifying these brain-derived EVs remains challenging due to the incomplete development of EV preparation and purification methods. For example, conflicting reports exist regarding the use of L1CAM as a surface marker for isolating neuronal EVs. L1CAM expression is not only non-specific to neurons or the CNS but also occurs as a cleaved secretory protein. Furthermore, acquiring EVs requires a substantial volume of blood samples, which may hinder large-scale metabolomics and the assessment of multiple biomarkers. Thus, further advancements in EV separation methods are essential, particularly for obtaining higher concentrations of EVs from smaller sample volumes. Given the heterogeneity of depression, more extensive multisite prospective studies should be conducted, using robust data analysis techniques to improve the accuracy and feasibility of clinical findings. Studies on the role of EVs in depression have largely focused on the abundant contents of EVs, particularly miRNAs, which are the most widely studied biological molecules in EVs. However, miRNA alternation in EVs have shown inconsistent results, which may be attributed to methodological differences in extraction techniques, the complex and heterogeneous nature of depression, and unclear mechanisms of EV biosynthesis and release. Therefore, when determining the specific roles and potential applications of EVs, especially in miRNA-based diagnostic and therapeutic strategies for depression, it is critical to account for individual differences, methodological consistency, and other influencing factors. Additionally, many studies have only identified differentially expressed biomolecules, including miRNAs in EVs, without thoroughly investigating their specific functions and regulatory mechanisms. Hence, further exploration of the functions and mechanisms of bioactive substances in EVs is necessary. Besides miRNAs and proteins, other bioactive molecules, such as lipids and metabolites, should also be considered. Although recent studies have begun to address the roles of these molecules, more research is needed to fully understand their contribution. Moreover, the relationship between peripheral EVs and the brain, as well as the bidirectional transport mechanism of EVs across the BBB, needs further investigation. Understanding this mechanism is crucial for unraveling the pathogenesis of depression and could significantly advance the development of precise clinical diagnostic tools. In conclusion, future research should focus on developing more efficient, accurate and stable methods for isolating EVs, conducting in-depth studies of their biological mechanism, and undertaking clinical trials to facilitate the adoption of EV-based treatment strategies for depression.
| 1. | McCarron RM, Shapiro B, Rawles J, Luo J. Depression. Ann Intern Med. 2021;174:ITC65-ITC80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 277] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 2. | Bukh JD, Bock C, Vinberg M, Werge T, Gether U, Vedel Kessing L. Interaction between genetic polymorphisms and stressful life events in first episode depression. J Affect Disord. 2009;119:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Maier A, Riedel-Heller SG, Pabst A, Luppa M. Risk factors and protective factors of depression in older people 65+. A systematic review. PLoS One. 2021;16:e0251326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 4. | Shorey S, Ng ED, Wong CHJ. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br J Clin Psychol. 2022;61:287-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 570] [Article Influence: 142.5] [Reference Citation Analysis (0)] |
| 5. | Rosoff DB, Smith GD, Lohoff FW. Prescription Opioid Use and Risk for Major Depressive Disorder and Anxiety and Stress-Related Disorders: A Multivariable Mendelian Randomization Analysis. JAMA Psychiatry. 2021;78:151-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 6. | Nedic Erjavec G, Sagud M, Nikolac Perkovic M, Svob Strac D, Konjevod M, Tudor L, Uzun S, Pivac N. Depression: Biological markers and treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2021;105:110139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 7. | Liang S, Wu X, Hu X, Wang T, Jin F. Recognizing Depression from the Microbiota⁻Gut⁻Brain Axis. Int J Mol Sci. 2018;19:1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 8. | Dubovsky SL, Ghosh BM, Serotte JC, Cranwell V. Psychotic Depression: Diagnosis, Differential Diagnosis, and Treatment. Psychother Psychosom. 2021;90:160-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 9. | Sheta M, Taha EA, Lu Y, Eguchi T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology (Basel). 2023;12:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 102] [Reference Citation Analysis (0)] |
| 10. | Kim YH, Kwak MS, Lee B, Shin JM, Aum S, Park IH, Lee MG, Shin JS. Secretory autophagy machinery and vesicular trafficking are involved in HMGB1 secretion. Autophagy. 2021;17:2345-2362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 11. | Buratta S, Tancini B, Sagini K, Delo F, Chiaradia E, Urbanelli L, Emiliani C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int J Mol Sci. 2020;21:2576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 297] [Article Influence: 59.4] [Reference Citation Analysis (1)] |
| 12. | Wang Y, Ren L, Bai H, Jin Q, Zhang L. Exosome-Autophagy Crosstalk in Enveloped Virus Infection. Int J Mol Sci. 2023;24:10618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, Lan M, Chen Y. Minimal Information for Studies of Extracellular Vesicles (MISEV): Ten-Year Evolution (2014-2023). Pharmaceutics. 2024;16:1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 14. | Sahoo S, Adamiak M, Mathiyalagan P, Kenneweg F, Kafert-Kasting S, Thum T. Therapeutic and Diagnostic Translation of Extracellular Vesicles in Cardiovascular Diseases: Roadmap to the Clinic. Circulation. 2021;143:1426-1449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 15. | Han C, Yang J, Sun J, Qin G. Extracellular vesicles in cardiovascular disease: Biological functions and therapeutic implications. Pharmacol Ther. 2022;233:108025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 16. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 7687] [Article Influence: 1098.1] [Reference Citation Analysis (1)] |
| 17. | Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ. Reassessment of Exosome Composition. Cell. 2019;177:428-445.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1476] [Cited by in RCA: 2066] [Article Influence: 344.3] [Reference Citation Analysis (1)] |
| 18. | Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 1820] [Article Influence: 364.0] [Reference Citation Analysis (0)] |
| 19. | Kumar A, Nader MA, Deep G. Emergence of Extracellular Vesicles as "Liquid Biopsy" for Neurological Disorders: Boom or Bust. Pharmacol Rev. 2024;76:199-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 20. | Wang X, Yang H, Liu C, Liu K. A new diagnostic tool for brain disorders: extracellular vesicles derived from neuron, astrocyte, and oligodendrocyte. Front Mol Neurosci. 2023;16:1194210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Reference Citation Analysis (0)] |
| 21. | Ampuero E, Luarte A, Santibañez M, Varas-Godoy M, Toledo J, Diaz-Veliz G, Cavada G, Rubio FJ, Wyneken U. Two Chronic Stress Models Based on Movement Restriction in Rats Respond Selectively to Antidepressant Drugs: Aldolase C As a Potential Biomarker. Int J Neuropsychopharmacol. 2015;18:pyv038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Gómez-Molina C, Sandoval M, Henzi R, Ramírez JP, Varas-Godoy M, Luarte A, Lafourcade CA, Lopez-Verrilli A, Smalla KH, Kaehne T, Wyneken U. Small Extracellular Vesicles in Rat Serum Contain Astrocyte-Derived Protein Biomarkers of Repetitive Stress. Int J Neuropsychopharmacol. 2019;22:232-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Upadhya R, Zingg W, Shetty S, Shetty AK. Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders. J Control Release. 2020;323:225-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 184] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 24. | Bhatt S, Nagappa AN, Patil CR. Role of oxidative stress in depression. Drug Discov Today. 2020;25:1270-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 463] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 25. | Pascua-Maestro R, González E, Lillo C, Ganfornina MD, Falcón-Pérez JM, Sanchez D. Extracellular Vesicles Secreted by Astroglial Cells Transport Apolipoprotein D to Neurons and Mediate Neuronal Survival Upon Oxidative Stress. Front Cell Neurosci. 2018;12:526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 26. | Matsuno H, Tsuchimine S, O'Hashi K, Sakai K, Hattori K, Hidese S, Nakajima S, Chiba S, Yoshimura A, Fukuzato N, Kando M, Tatsumi M, Ogawa S, Ichinohe N, Kunugi H, Sohya K. Association between vascular endothelial growth factor-mediated blood-brain barrier dysfunction and stress-induced depression. Mol Psychiatry. 2022;27:3822-3832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 27. | Wallensten J, Mobarrez F, Åsberg M, Borg K, Beser A, Wilczek A, Nager A. Isoforms of soluble vascular endothelial growth factor in stress-related mental disorders: a cross-sectional study. Sci Rep. 2021;11:16693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Wallensten J, Nager A, Åsberg M, Borg K, Beser A, Wilczek A, Mobarrez F. Leakage of astrocyte-derived extracellular vesicles in stress-induced exhaustion disorder: a cross-sectional study. Sci Rep. 2021;11:2009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Xie XH, Lai WT, Xu SX, Di Forti M, Zhang JY, Chen MM, Yao LH, Wang P, Hao KK, Rong H. Hyper-inflammation of astrocytes in patients of major depressive disorder: Evidence from serum astrocyte-derived extracellular vesicles. Brain Behav Immun. 2023;109:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 30. | Nishi K, Izumi H, Tomonaga T, Nagano C, Morimoto Y, Horie S. IL-6-Mediated Upregulated miRNAs in Extracellular Vesicles Derived from Lund Human Mesencephalic (LUHMES) Cells: Effects on Astrocytes and Microglia. Biomolecules. 2023;13:718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 31. | Xian X, Cai LL, Li Y, Wang RC, Xu YH, Chen YJ, Xie YH, Zhu XL, Li YF. Neuron secrete exosomes containing miR-9-5p to promote polarization of M1 microglia in depression. J Nanobiotechnology. 2022;20:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 92] [Reference Citation Analysis (0)] |
| 32. | Mizohata Y, Toda H, Koga M, Saito T, Fujita M, Kobayashi T, Hatakeyama S, Morimoto Y. Neural extracellular vesicle-derived miR-17 in blood as a potential biomarker of subthreshold depression. Hum Cell. 2021;34:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Burrows K, Figueroa-Hall LK, Stewart JL, Alarbi AM, Kuplicki R, Hannafon BN, Tan C, Risbrough VB, McKinney BA, Ramesh R, Victor TA, Aupperle R, Savitz J, Teague TK, Khalsa SS, Paulus MP. Exploring the role of neuronal-enriched extracellular vesicle miR-93 and interoception in major depressive disorder. Transl Psychiatry. 2024;14:199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Wang L, Chang X, She L, Xu D, Huang W, Poo MM. Autocrine action of BDNF on dendrite development of adult-born hippocampal neurons. J Neurosci. 2015;35:8384-8393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 35. | Antoniou A, Auderset L, Kaurani L, Sebastian E, Zeng Y, Allahham M, Cases-Cunillera S, Schoch S, Gründemann J, Fischer A, Schneider A. Neuronal extracellular vesicles and associated microRNAs induce circuit connectivity downstream BDNF. Cell Rep. 2023;42:112063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 36. | Saeedi S, Nagy C, Ibrahim P, Théroux JF, Wakid M, Fiori LM, Yang J, Rotzinger S, Foster JA, Mechawar N, Kennedy SH, Turecki G. Neuron-derived extracellular vesicles enriched from plasma show altered size and miRNA cargo as a function of antidepressant drug response. Mol Psychiatry. 2021;26:7417-7424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 37. | Kuwano N, Kato TA, Mitsuhashi M, Sato-Kasai M, Shimokawa N, Hayakawa K, Ohgidani M, Sagata N, Kubo H, Sakurai T, Kanba S. Neuron-related blood inflammatory markers as an objective evaluation tool for major depressive disorder: An exploratory pilot case-control study. J Affect Disord. 2018;240:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Nasca C, Dobbin J, Bigio B, Watson K, de Angelis P, Kautz M, Cochran A, Mathé AA, Kocsis JH, Lee FS, Murrough JW, McEwen BS, Rasgon N. Insulin receptor substrate in brain-enriched exosomes in subjects with major depression: on the path of creation of biosignatures of central insulin resistance. Mol Psychiatry. 2021;26:5140-5149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 39. | Bansal Y, Kuhad A. Mitochondrial Dysfunction in Depression. Curr Neuropharmacol. 2016;14:610-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 40. | Goetzl EJ, Wolkowitz OM, Srihari VH, Reus VI, Goetzl L, Kapogiannis D, Heninger GR, Mellon SH. Abnormal levels of mitochondrial proteins in plasma neuronal extracellular vesicles in major depressive disorder. Mol Psychiatry. 2021;26:7355-7362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 41. | Fan C, Li Y, Lan T, Wang W, Long Y, Yu SY. Microglia secrete miR-146a-5p-containing exosomes to regulate neurogenesis in depression. Mol Ther. 2022;30:1300-1314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 140] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 42. | Jovičić A, Roshan R, Moisoi N, Pradervand S, Moser R, Pillai B, Luthi-Carter R. Comprehensive expression analyses of neural cell-type-specific miRNAs identify new determinants of the specification and maintenance of neuronal phenotypes. J Neurosci. 2013;33:5127-5137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 43. | Deng Y, Gong P, Han S, Zhang J, Zhang S, Zhang B, Lin Y, Xu K, Wen G, Liu K. Reduced cerebral cortex thickness is related to overexpression of exosomal miR-146a-5p in medication-free patients with major depressive disorder. Psychol Med. 2023;53:6253-6260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 44. | Zhang G, Li L, Kong Y, Xu D, Bao Y, Zhang Z, Liao Z, Jiao J, Fan D, Long X, Dai J, Xie C, Meng Z, Zhang Z. Vitamin D-binding protein in plasma microglia-derived extracellular vesicles as a potential biomarker for major depressive disorder. Genes Dis. 2024;11:1009-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 45. | Zhou B, Zhu Z, Ransom BR, Tong X. Oligodendrocyte lineage cells and depression. Mol Psychiatry. 2021;26:103-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 46. | Krämer-Albers EM, Bretz N, Tenzer S, Winterstein C, Möbius W, Berger H, Nave KA, Schild H, Trotter J. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin Appl. 2007;1:1446-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 406] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 47. | Zhang H, Xie XH, Xu SX, Wang C, Sun S, Song X, Li R, Li N, Feng Y, Duan H, Li D, Liu Z. Oligodendrocyte-derived exosomes-containing SIRT2 ameliorates depressive-like behaviors and restores hippocampal neurogenesis and synaptic plasticity via the AKT/GSK-3β pathway in depressed mice. CNS Neurosci Ther. 2024;30:e14661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 48. | Ibrahim P, Denniston R, Mitsuhashi H, Yang J, Fiori LM, Żurawek D, Mechawar N, Nagy C, Turecki G. Profiling Small RNA From Brain Extracellular Vesicles in Individuals With Depression. Int J Neuropsychopharmacol. 2024;27:pyae013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 49. | Zeng Q, Liao J, Ran LY, Shi L, Chen YJ, Zhang CY, Xiang JJ, Hong S, Kuang L. [Sequencing Analysis of miRNAs in Brain-Derived Exosomes of Adolescent Mice With Depression-Like Behaviors]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2023;54:316-321. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 50. | Sachdeva P, Ji S, Ghosh S, Ghosh S, Raghunath M, Kim H, Bhaskar R, Sinha JK, Han SS. Plausible Role of Stem Cell Types for Treating and Understanding the Pathophysiology of Depression. Pharmaceutics. 2023;15:814. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 51. | Lima MN, Oliveira HA, Fagundes PM, Estato V, Silva AYO, Freitas RJRX, Passos BABR, Oliveira KS, Batista CN, Vallochi AL, Rocco PRM, Castro-Faria-Neto HC, Maron-Gutierrez T. Mesenchymal stromal cells protect against vascular damage and depression-like behavior in mice surviving cerebral malaria. Stem Cell Res Ther. 2020;11:367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Wei N, Zhang H, Wang J, Wang S, Lv W, Luo L, Xu Z. The Progress in Diagnosis and Treatment of Exosomes and MicroRNAs on Epileptic Comorbidity Depression. Front Psychiatry. 2020;11:405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Guo H, Huang B, Wang Y, Zhang Y, Ma Q, Ren Y. Bone marrow mesenchymal stem cells-derived exosomes improve injury of hippocampal neurons in rats with depression by upregulating microRNA-26a expression. Int Immunopharmacol. 2020;82:106285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 54. | Hyun SA, Lee YJ, Jang S, Ko MY, Lee CY, Cho YW, Yun YE, Lee BS, Seo JW, Moon KS, Ka M. Adipose stem cell-derived extracellular vesicles ameliorates corticosterone-induced apoptosis in the cortical neurons via inhibition of ER stress. Stem Cell Res Ther. 2022;13:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 55. | Min XL, Liu HJ, Dou XK, Chen FX, Zhao Q, Zhao XH, Shi Y, Zhao QY, Sun SJ, Wang Z, Yu SH. Extracellular Vesicles from Neural Stem Cells Carry microRNA-16-5p to Reduce Corticosterone-induced Neuronal Injury in Depression Rats. Neuroscience. 2024;538:95-109. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 56. | Beurel E, Toups M, Nemeroff CB. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron. 2020;107:234-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 1250] [Article Influence: 250.0] [Reference Citation Analysis (0)] |
| 57. | Majd M, Saunders EFH, Engeland CG. Inflammation and the dimensions of depression: A review. Front Neuroendocrinol. 2020;56:100800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 58. | Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E, Kabanov AV. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 451] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 59. | Pusic KM, Won L, Kraig RP, Pusic AD. IFNγ-Stimulated Dendritic Cell Exosomes for Treatment of Migraine Modeled Using Spreading Depression. Front Neurosci. 2019;13:942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Li D, Wang Y, Jin X, Hu D, Xia C, Xu H, Hu J. NK cell-derived exosomes carry miR-207 and alleviate depression-like symptoms in mice. J Neuroinflammation. 2020;17:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 61. | Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM. The gut microbiota in anxiety and depression - A systematic review. Clin Psychol Rev. 2021;83:101943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 533] [Article Influence: 133.3] [Reference Citation Analysis (0)] |
| 62. | Liu L, Wang H, Zhang H, Chen X, Zhang Y, Wu J, Zhao L, Wang D, Pu J, Ji P, Xie P. Toward a Deeper Understanding of Gut Microbiome in Depression: The Promise of Clinical Applicability. Adv Sci (Weinh). 2022;9:e2203707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 63. | Díaz-Garrido N, Badia J, Baldomà L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J Extracell Vesicles. 2021;10:e12161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 169] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 64. | Bittel M, Reichert P, Sarfati I, Dressel A, Leikam S, Uderhardt S, Stolzer I, Phu TA, Ng M, Vu NK, Tenzer S, Distler U, Wirtz S, Rothhammer V, Neurath MF, Raffai RL, Günther C, Momma S. Visualizing transfer of microbial biomolecules by outer membrane vesicles in microbe-host-communication in vivo. J Extracell Vesicles. 2021;10:e12159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 65. | Chen S, Lei Q, Zou X, Ma D. The role and mechanisms of gram-negative bacterial outer membrane vesicles in inflammatory diseases. Front Immunol. 2023;14:1157813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 66. | Carlessi AS, Borba LA, Zugno AI, Quevedo J, Réus GZ. Gut microbiota-brain axis in depression: The role of neuroinflammation. Eur J Neurosci. 2021;53:222-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 67. | Fricke F, Gebert J, Kopitz J, Plaschke K. Proinflammatory Extracellular Vesicle-Mediated Signaling Contributes to the Induction of Neuroinflammation in Animal Models of Endotoxemia and Peripheral Surgical Stress. Cell Mol Neurobiol. 2021;41:1325-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 68. | Ha JY, Choi SY, Lee JH, Hong SH, Lee HJ. Delivery of Periodontopathogenic Extracellular Vesicles to Brain Monocytes and Microglial IL-6 Promotion by RNA Cargo. Front Mol Biosci. 2020;7:596366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 69. | Choi J, Kim YK, Han PL. Extracellular Vesicles Derived from Lactobacillus plantarum Increase BDNF Expression in Cultured Hippocampal Neurons and Produce Antidepressant-like Effects in Mice. Exp Neurobiol. 2019;28:158-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 70. | Choi J, Kwon H, Kim YK, Han PL. Extracellular Vesicles from Gram-positive and Gram-negative Probiotics Remediate Stress-Induced Depressive Behavior in Mice. Mol Neurobiol. 2022;59:2715-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 71. | Correia AS, Vale N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int J Mol Sci. 2022;23:8493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 147] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 72. | Yaghoubfar R, Behrouzi A, Ashrafian F, Shahryari A, Moradi HR, Choopani S, Hadifar S, Vaziri F, Nojoumi SA, Fateh A, Khatami S, Siadat SD. Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci Rep. 2020;10:22119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 73. | Brown SJ, Huang XF, Newell KA. The kynurenine pathway in major depression: What we know and where to next. Neurosci Biobehav Rev. 2021;127:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 74. | Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141:1202-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 1818] [Article Influence: 227.3] [Reference Citation Analysis (0)] |
| 75. | Fang K, Xu JX, Chen XX, Gao XR, Huang LL, Du AQ, Jiang C, Ge JF. Differential serum exosome microRNA profile in a stress-induced depression rat model. J Affect Disord. 2020;274:144-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 76. | Sakamoto S, Mallah D, Medeiros DJ, Dohi E, Imai T, Rose IVL, Matoba K, Zhu X, Kamiya A, Kano SI. Alterations in circulating extracellular vesicles underlie social stress-induced behaviors in mice. FEBS Open Bio. 2021;11:2678-2692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | van der Zee YY, Eijssen LMT, Mews P, Ramakrishnan A, Alvarez K, Lardner CK, Cates HM, Walker DM, Torres-Berrío A, Browne CJ, Cunningham A, Cathomas F, Kronman H, Parise EM, de Nijs L, Shen L, Murrough JW, Rutten BPF, Nestler EJ, Issler O. Blood miR-144-3p: a novel diagnostic and therapeutic tool for depression. Mol Psychiatry. 2022;27:4536-4549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 78. | Wang R, Huang K, Feng Y, Duan J, Ying H, Shi Q, Zhang Y, Jiang R, Yang L. Exo-miR-144-3p as a promising diagnostic biomarker for depressive symptoms in heart failure. Neurobiol Dis. 2024;192:106415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 79. | Wu X, Zhang Y, Wang P, Li X, Song Z, Wei C, Zhang Q, Luo B, Liu Z, Yang Y, Ren Z, Liu H. Clinical and preclinical evaluation of miR-144-5p as a key target for major depressive disorder. CNS Neurosci Ther. 2023;29:3598-3611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 80. | Ran LY, Kong YT, Xiang JJ, Zeng Q, Zhang CY, Shi L, Qiu HT, Liu C, Wu LL, Li YL, Chen JM, Ai M, Wang W, Kuang L. Serum extracellular vesicle microRNA dysregulation and childhood trauma in adolescents with major depressive disorder. Bosn J Basic Med Sci. 2022;22:959-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 81. | Honorato-Mauer J, Xavier G, Ota VK, Chehimi SN, Mafra F, Cuóco C, Ito LT, Ormond R, Asprino PF, Oliveira A, Bugiga AVG, Torrecilhas AC, Bressan R, Manfro GG, Miguel EC, Rohde LA, Pan PM, Salum GA, Pellegrino R, Belangero S, Santoro ML. Alterations in microRNA of extracellular vesicles associated with major depression, attention-deficit/hyperactivity and anxiety disorders in adolescents. Transl Psychiatry. 2023;13:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 82. | Chen F, Zou L, Dai Y, Sun J, Chen C, Zhang Y, Peng Q, Zhang Z, Xie Z, Wu H, Tian W, Yu X, Yu J, Wang K. Prognostic plasma exosomal microRNA biomarkers in patients with substance use disorders presenting comorbid with anxiety and depression. Sci Rep. 2021;11:6271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 83. | Li LD, Naveed M, Du ZW, Ding H, Gu K, Wei LL, Zhou YP, Meng F, Wang C, Han F, Zhou QG, Zhang J. Abnormal expression profile of plasma-derived exosomal microRNAs in patients with treatment-resistant depression. Hum Genomics. 2021;15:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 84. | Seki I, Izumi H, Okamoto N, Ikenouchi A, Morimoto Y, Horie S, Yoshimura R. Serum Extracellular Vesicle-Derived hsa-miR-2277-3p and hsa-miR-6813-3p Are Potential Biomarkers for Major Depression: A Preliminary Study. Int J Mol Sci. 2023;24:13902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 85. | Wei ZX, Xie GJ, Mao X, Zou XP, Liao YJ, Liu QS, Wang H, Cheng Y. Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Neuropsychopharmacology. 2020;45:1050-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 86. | Tan B, Jiang X, Chen L, Wang R, Wei H. Plasma exosomal miR-30a-5p inhibits osteogenic differentiation of bone marrow mesenchymal stem cells from a chronic unpredictable mild stress-induced depression rat model. Mol Cell Probes. 2024;75:101957. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 87. | Kirian RD, Steinman D, Jewell CM, Zierden HC. Extracellular vesicles as carriers of mRNA: Opportunities and challenges in diagnosis and treatment. Theranostics. 2024;14:2265-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 88. | Smirnova L, Modafferi S, Schlett C, Osborne LM, Payne JL, Sabunciyan S. Blood extracellular vesicles carrying brain-specific mRNAs are potential biomarkers for detecting gene expression changes in the female brain. Mol Psychiatry. 2024;29:962-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 89. | Jiang M, Gu YF, Cai JF, Wang A, He Y, Feng YL. MiR-186-5p Dysregulation Leads to Depression-like Behavior by De-repressing SERPINF1 in Hippocampus. Neuroscience. 2021;479:48-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Rajan EJE, Alwar SV, Gulati R, Rajiv R, Mitra T, Janardhanan R. Prospecting the theragnostic potential of the psycho-neuro-endocrinological perturbation of the gut-brain-immune axis for improving cardiovascular diseases outcomes. Front Mol Biosci. 2023;10:1330327. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 91. | Guo J, Chen S, Li F, Hou S, Guo M, Yuan X. CXCL8 delivered by plasma-derived exosomes induces the symptoms of post-traumatic stress disorder through facilitating astrocyte-neuron communication. J Psychiatr Res. 2023;161:261-272. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 92. | Xue T, Liu W, Wang L, Shi Y, Hu Y, Yang J, Li G, Huang H, Cui D. Extracellular vesicle biomarkers for complement dysfunction in schizophrenia. Brain. 2024;147:1075-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 93. | Voronin MV, Vakhitova YV, Seredenin SB. Chaperone Sigma1R and Antidepressant Effect. Int J Mol Sci. 2020;21:7088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 94. | Wang Y, Gao C, Gao T, Zhao L, Zhu S, Guo L. Plasma exosomes from depression ameliorate inflammation-induced depressive-like behaviors via sigma-1 receptor delivery. Brain Behav Immun. 2021;94:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 95. | Du Y, Dong JH, Chen L, Liu H, Zheng GE, Chen GY, Cheng Y. Metabolomic Identification of Serum Exosome-Derived Biomarkers for Bipolar Disorder. Oxid Med Cell Longev. 2022;2022:5717445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 96. | Wang Z, Xie Z, Zhang Z, Zhou W, Guo B, Li M. Multi-platform omics sequencing dissects the atlas of plasma-derived exosomes in rats with or without depression-like behavior after traumatic spinal cord injury. Prog Neuropsychopharmacol Biol Psychiatry. 2024;132:110987. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 97. | Kanninen KM, Bister N, Koistinaho J, Malm T. Exosomes as new diagnostic tools in CNS diseases. Biochim Biophys Acta. 2016;1862:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 98. | Saugstad JA, Lusardi TA, Van Keuren-Jensen KR, Phillips JI, Lind B, Harrington CA, McFarland TJ, Courtright AL, Reiman RA, Yeri AS, Kalani MYS, Adelson PD, Arango J, Nolan JP, Duggan E, Messer K, Akers JC, Galasko DR, Quinn JF, Carter BS, Hochberg FH. Analysis of extracellular RNA in cerebrospinal fluid. J Extracell Vesicles. 2017;6:1317577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 99. | Topuzoğlu A, Ilgın C. Mentalexo approach for diagnosis of psychiatric disorders. Med Hypotheses. 2020;143:109823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 100. | Shin H, Kang Y, Choi KW, Kim S, Ham BJ, Choi Y. Artificial Intelligence-Based Major Depressive Disorder (MDD) Diagnosis Using Raman Spectroscopic Features of Plasma Exosomes. Anal Chem. 2023;95:6410-6416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 101. | Hung YY, Chou CK, Yang YC, Fu HC, Loh EW, Kang HY. Exosomal let-7e, miR-21-5p, miR-145, miR-146a and miR-155 in Predicting Antidepressants Response in Patients with Major Depressive Disorder. Biomedicines. 2021;9:1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 102. | Gelle T, Samey RA, Plansont B, Bessette B, Jauberteau-Marchan MO, Lalloué F, Girard M. BDNF and pro-BDNF in serum and exosomes in major depression: Evolution after antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 103. | Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 961] [Article Influence: 192.2] [Reference Citation Analysis (0)] |
| 104. | Wu Q, Duan WZ, Chen JB, Zhao XP, Li XJ, Liu YY, Ma QY, Xue Z, Chen JX. Extracellular Vesicles: Emerging Roles in Developing Therapeutic Approach and Delivery Tool of Chinese Herbal Medicine for the Treatment of Depressive Disorder. Front Pharmacol. 2022;13:843412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 105. | Yu X, Bai Y, Han B, Ju M, Tang T, Shen L, Li M, Yang L, Zhang Z, Hu G, Chao J, Zhang Y, Yao H. Extracellular vesicle-mediated delivery of circDYM alleviates CUS-induced depressive-like behaviours. J Extracell Vesicles. 2022;11:e12185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 106. | Hu Y, Zhao M, Wang H, Guo Y, Cheng X, Zhao T, Wang H, Zhang Y, Ma Y, Tao W. Exosome-sheathed ROS-responsive nanogel to improve targeted therapy in perimenopausal depression. J Nanobiotechnology. 2023;21:261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 107. | Ito Y, Taniguchi K, Kuranaga Y, Eid N, Inomata Y, Lee SW, Uchiyama K. Uptake of MicroRNAs from Exosome-Like Nanovesicles of Edible Plant Juice by Rat Enterocytes. Int J Mol Sci. 2021;22:3749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 108. | Fujita D, Arai T, Komori H, Shirasaki Y, Wakayama T, Nakanishi T, Tamai I. Apple-Derived Nanoparticles Modulate Expression of Organic-Anion-Transporting Polypeptide (OATP) 2B1 in Caco-2 Cells. Mol Pharm. 2018;15:5772-5780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 109. | Lyu Q, Zhou X, Shi LQ, Chen HY, Lu M, Ma XD, Ren L. Exosomes may be the carrier of acupuncture treatment for major depressive disorder. Front Behav Neurosci. 2023;17:1107265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 110. | Zhu X, Hollinger KR, Huang Y, Borjabad A, Kim BH, Arab T, Thomas AG, Moniruzzaman M, Lovell L, Turchinovich A, Witwer KW, Volsky DJ, Haughey NJ, Slusher BS. Neutral sphingomyelinase 2 inhibition attenuates extracellular vesicle release and improves neurobehavioral deficits in murine HIV. Neurobiol Dis. 2022;169:105734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 111. | Schumacher F, Carpinteiro A, Edwards MJ, Wilson GC, Keitsch S, Soddemann M, Wilker B, Kleuser B, Becker KA, Müller CP, Kornhuber J, Gulbins E. Stress induces major depressive disorder by a neutral sphingomyelinase 2-mediated accumulation of ceramide-enriched exosomes in the blood plasma. J Mol Med (Berl). 2022;100:1493-1508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |