Published online Mar 19, 2025. doi: 10.5498/wjp.v15.i3.102117
Revised: December 30, 2024
Accepted: January 21, 2025
Published online: March 19, 2025
Processing time: 96 Days and 20.1 Hours
Postoperative delirium (POD) is a prevalent complication, particularly in elderly patients with hip fractures (HFs). It significantly affects recovery, length of hos
To investigate POD incidence in elderly patients with HF and the independent risk factors, according to which a nomogram prediction model was developed and validated.
This retrospective study included elderly patients with HF who were surgically treated in Dongying People’s Hospital from April 2018 to April 2022. The endpoint event includes POD. They were categorized into the modeling and validation cohorts in a 7:3 ratio by randomization. Both cohorts were further classified into the delirium and normal (non-delirium) groups according to the presence or absence of the endpoint event. The incidence of POD was calculated, and logistic multivariate analysis was conducted to determine the independent risk factors. The calibration curve and the Hosmer-Lemeshow test as well as the net benefit threshold probability interval by the decision curve were utilized to statistically validate the accuracy of the nomogram prediction model, developed according to each factor’s influence intensity.
This study included 532 elderly patients with HF, with an overall POD incidence of 14.85%. The comparison of baseline data with perioperative indicators revealed statistical differences in age (P < 0.001), number of co
This study reveals cognitive impairment history, American Society of Anesthesiologists grade of > 2, RBC transfusion of ≥ 2 units, postoperative intensive care unit care, and preoperative hemoglobin level as independent risk factors for POD in elderly patients with HF. The developed nomogram model demonstrates excellent accuracy and stability in predicting the risk of POD, which is recommended to be applied in clinical practice to optimize postoperative management and reduce delirium incidence.
Core Tip: This study integrates advanced machine learning techniques, including Lasso regression, support vector machine, and random forest, to determine independent risk factors for postoperative delirium. A nomogram prediction model was developed and demonstrated high accuracy and stability in both training and validation cohorts. The decision curve analysis confirmed its clinical use within a risk threshold range of 8%-35%. This tool provides valuable guidance for the early determination of patients at high-risk and personalized postoperative management to reduce postoperative delirium in
- Citation: Li L, Sheng WW, Song LJ, Cheng S, Cui EG, Zhang YB, Yu XZ, Liu YL. Developing a nomogram for postoperative delirium in elderly patients with hip fractures. World J Psychiatry 2025; 15(3): 102117
- URL: https://www.wjgnet.com/2220-3206/full/v15/i3/102117.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i3.102117
Hip fracture (HF), a disease that seriously affects the health of the elderly, has emerged as a significant global public health issue[1]. The number of patients with HF is projected to reach 2.6 million by 2025 globally, and this figure may increase to between 7 million and 20 million by the middle of this century[2,3]. HFs pose a considerable health threat to the elderly and are among the leading causes of disability and death. A considerable number of studies have revealed that the vast majority of elderly patients with HF have difficulty reverting to their original functional state after injury[4-6]. They frequently lose the ability to care for themselves, require long-term bed rest or admission to care institutions, and demonstrate a relatively high mortality rate.
Surgical treatment has significantly improved the prognosis of patients with HFs, but postoperative complications, particularly postoperative delirium (POD), remain one of the main challenges faced by clinicians and patients[7]. POD is pervasive among older adults, with its incidence closely associated with the patient’s underlying disease, preoperative status, and surgical procedure[8,9]. Symptoms of delirium are complex and different, potentially including confusion of consciousness, inattention, disordered thinking, etc. The disease course is typically short, but the onset is rapid and fluctuating, frequently making the diagnosis and management in the early stage challenging[10]. Research indicates that POD not only prolongs patients’ hospital stays and increases medical costs, but also significantly decreases their quality of life and survival[11]. Currently, certain studies focus on the risk factors of POD, but they are often confined to single-factor analysis or have a relatively small sample size, which lack systematic multi-factor analysis and prediction models[12]. This makes the early determination and prevention of delirium difficult to implement in clinical practice, thereby warranting more systematic research to fill the gap in this field.
Machine learning, as an advanced data analysis tool, has gained increasingly extensive applications in medical research[13]. Machine learning automatically identifies complex patterns and associations from a large number of data and is particularly suitable for handling medical data with multiple variables and nonlinear relationships compared with traditional statistical methods[14]. Lasso regression, support vector machine (SVM), and random forest are the predominantly used machine learning modalities at present. These methods can not only effectively screen out the key features affecting disease occurrence but also develop high precision prediction models[15]. The application of machine learning assists in understanding more comprehensively the effect of various potential factors on the occurrence of delirium in the research of POD after HFs, thereby providing a basis for establishing individualized prevention and treatment plans.
The novelty of this study lies in the combination of multiple machine learning methods for the first time to systematically screen out the independent risk factors of POD in elderly patients with HF and the development of a nomogram prediction model. The model not only integrates traditional clinical variables but also considers the comprehensive influence of various preoperative and intraoperative factors on delirium incidence. This study provides a more accurate risk prediction model than before and conducts a comprehensive assessment of the model’s performance in internal and external validations by applying machine learning techniques based on a large sample size. Furthermore, the research results will provide clinicians with a practical instrument to determine high-risk populations early, thereby developing better prevention and management strategies.
Shaw et al[16] and Hughes et al[17] reported POD incidence of 11%-51%. We calculated the required sample size for the two extremes of incidence based on the sample size calculation formula: N = Z2 × [P × (1-P)]/E2. The required sample size is 151 and 385 for an incidence rate of 11% and 51%, respectively. This study collected data from 532 patients, which exceeds the theoretically required sample size. This not only ensures sufficient statistical power for the study but also improves the reliability and applicability of the results.
This retrospective analysis included elderly patients with HF who were surgically treated at Dongying People’s Hospital from April 2018 to April 2022.
Inclusion criteria were: (1) First diagnosis of femoral neck fracture or intertrochanteric fracture; (2) Age of ≥ 65 years; and (3) Treatment with procedures, such as artificial femoral head replacement, total hip replacement, or closed or open reduction and internal fixation.
Exclusion criteria were: (1) Pathological fractures; (2) Open fractures; (3) Presence of multiple injuries; (4) Clear tumor history; (5) No surgical treatment; and (6) Loss to follow-ups or incomplete information. The specific operation flow is the same as the first part.
The endpoint event was POD occurrence, and the observation period was from the end of the operation to 3 months postoperatively. Delirium is defined based on the fifth revision of the Diagnostic and Statistical Manual of Mental Disorders[18] as acute cognitive dysfunction, including attention disorders, perception disorders (such as hallucinations and auditory hallucinations), orientation disorders (i.e., confusion in time and space), abnormal thinking (delusions, fears, logical confusion, etc.), and mood swings. Symptoms are transient or recurrent.
The participants were categorized based on POD occurrence into the delirium (n = 79) and non-delirium groups (n = 453). All the included patients were randomized into training and validation groups following the principle of random allocation for developing and validating the prediction model. The training group was utilized for establishing the model and feature screening, containing approximately 70% of the total sample (n = 372). The remaining 30% (n = 160) were included in the validation group to assess the model’s prediction performance and stability.
The following clinical characteristic data of patients were collected through the digital electronic medical record management system browser of the medical record management office in the information department of our hospital: Delirium status (present or absent), gender, fracture type (femoral neck fracture or intertrochanteric fracture), cognitive impairment history, smoking history, preoperative comorbidities, number of comorbidities (≥ 2 or < 2), hypertension, diabetes, chronic obstructive pulmonary disease (COPD), walking ability before injury (normal, mild limitation, and inability to walk), American Society of Anesthesiologists (ASA) grading, anesthesia mode (intravertebral/nerve block/local anesthesia or general anesthesia), red blood cell (RBC) transfusion of ≥ 2 units, postoperative intensive care unit (ICU) care, age, body mass index (BMI), preoperative RBC count, preoperative white blood cell count (WBC), pre
Data analysis was conducted with Statistical Package for the Social Sciences version 26.0. First, the Kolmogorov-Smirnov test was conducted to investigate the normality of the measurement data. Data conforming to the normal distribution was presented as mean ± SD, and the t-test, specifically the independent sample t-test, was used for inter-group comparison. Non-normally distributed data was represented by the quartile P50 [P25, P75] and analyzed with the rank sum test. Count data analysis was conducted with the χ2-test. The Delong test was used for comparing the area under the receiver operating characteristic (ROC) curve (AUC). The Lasso model was developed via the Lasso and Elastic-Net Regularized Generalized Linear Models package in R language (version 4.3.2), and the Gaussian was selected as the model con
This study enrolled 532 patients, with a POD incidence of 14.85%. The comparison of patients’ baseline data revealed significant differences in age (P < 0.001), number of comorbidities (P = 0.042), and ASA grading (P = 0.004) between the delirium and non-delirium groups. We revealed no statistical between-group differences in gender (P = 0.548), fracture type (P = 0.742), BMI (P = 0.093), cognitive impairment history (P = 0.152), smoking history (P = 0.292), preoperative comorbidities (P = 0.467), hypertension (P = 0.453), diabetes (P = 0.766), COPD (P = 0.812), and walking ability before injury (P = 0.294) (Table 1).
| Variable | Total | Non-delirium group (n = 453) | Delirium group (n = 79) | χ2/t | P value |
| Age, years | 78.00 [74.00, 82.00] | 77.68 ± 4.84 | 81.33 ± 4.72 | 6.313 | < 0.001 |
| Gender | |||||
| Male | 359 | 308 | 51 | 0.362 | 0.548 |
| Female | 173 | 145 | 28 | ||
| Fracture type | |||||
| Femoral neck fracture | 312 | 267 | 45 | 0.109 | 0.742 |
| Femoral intertrochanteric fracture | 220 | 186 | 34 | ||
| Body mass index, kg/m2 | 23.44 ± 2.30 | 23.36 ± 2.17 | 23.94 ± 2.93 | 1.695 | 0.093 |
| History of cognitive impairment | |||||
| With | 19 | 14 | 5 | 2.049 | 0.152 |
| Without | 513 | 439 | 74 | ||
| Smoking history | |||||
| With | 224 | 195 | 29 | 1.108 | 0.292 |
| Without | 308 | 258 | 50 | ||
| Preoperative comorbidities | |||||
| With | 317 | 267 | 50 | 0.529 | 0.467 |
| Without | 215 | 186 | 29 | ||
| Number of comorbidities | |||||
| ≥ 2 | 473 | 408 | 65 | 4.138 | 0.042 |
| < 2 | 59 | 45 | 14 | ||
| Hypertension | |||||
| With | 222 | 186 | 36 | 0.563 | 0.453 |
| Without | 310 | 267 | 43 | ||
| Diabetes | |||||
| With | 73 | 63 | 10 | 0.089 | 0.766 |
| Without | 459 | 390 | 69 | ||
| COPD | |||||
| With | 51 | 45 | 6 | 0.056 | 0.812 |
| Without | 481 | 408 | 73 | ||
| Walking ability before injury | |||||
| Normal | 372 | 322 | 50 | 2.446 | 0.294 |
| Mild limitation | 85 | 68 | 17 | ||
| Inability to walk | 75 | 63 | 12 | ||
| ASA grading | |||||
| 1-2 | 381 | 335 | 46 | 8.182 | 0.004 |
| 3-4 | 151 | 118 | 33 |
The incidence of delirium was 85.15% of the 532 patients selected. The comparison of patients’ preoperative and intraoperative indexes revealed that preoperative RBC count (P < 0.001), preoperative ALB (P < 0.001), preoperative Hb (P < 0.001), preoperative PLT (P < 0.001), IBL (P < 0.001), RBC transfusion of ≥ 2 units (P = 0.001), and postoperative ICU care (P < 0.001) were statistically different between the delirium and non-delirium groups. No statistical significance was observed in other factors, including preoperative WBC (P = 0.914), preoperative SCr (P = 0.442), time from admission to surgery (P = 0.11), mode of anesthesia (P = 0.216), and OT (P = 0.767) (Table 2).
| Variable | Total | Non-delirium group (n = 453) | Delirium group (n = 79) | Z/χ2/t | P value |
| Preoperative RBC count, × 109/L | 3.78 ± 0.45 | 3.81 ± 0.44 | 3.59 ± 0.50 | -3.614 | < 0.001 |
| Preoperative WBC, × 109/L | 8.21 ± 1.73 | 8.21 ± 1.74 | 8.23 ± 1.72 | 0.108 | 0.914 |
| Preoperative albumin, g/L | 36.00 [33.00, 38.00] | 36.00 [34.00, 38.00] | 34.00 [31.00, 36.00] | -5.158 | < 0.001 |
| Preoperative serum creatinine, μmol/L | 66.53 ± 10.68 | 66.38 ± 10.64 | 67.40 ± 10.95 | 0.772 | 0.442 |
| Preoperative hemoglobin, g/L | 115.90 ± 12.88 | 116.81 ± 13.17 | 110.71 ± 9.65 | -4.883 | < 0.001 |
| Preoperative PLT, × 109/L | 173.96 ± 34.33 | 176.61 ± 33.22 | 158.73 ± 36.79 | -4.041 | < 0.001 |
| Time from admission to surgery, days | 6.00 [5.00, 6.00] | 6.00 [5.00, 6.00] | 6.00 [5.00, 7.00] | 1.533 | 0.11 |
| Mode of anesthesia | |||||
| Intravertebral/nerve block/local anesthesia | 443 | 381 | 62 | 1.528 | 0.216 |
| General anesthesia | 89 | 72 | 17 | ||
| Operation time, minutes | 137.00 [123.00, 160.00] | 137.00 [123.00, 160.00] | 137.00 [126.00, 158.00] | 0.296 | 0.767 |
| Intraoperative blood loss, mL | 200.00 [100.00, 300.00] | 200.00 [100.00, 300.00] | 300.00 [200.00, 300.00] | 3.238 | < 0.001 |
| RBC transfusion ≥ 2 units | |||||
| With | 255 | 204 | 51 | 10.274 | 0.001 |
| Without | 277 | 249 | 28 | ||
| Postoperative ICU care | |||||
| With | 88 | 63 | 25 | 15.332 | < 0.001 |
| Without | 444 | 390 | 54 |
This study assigned 532 patients to the training group (n = 372) and a validation group (n = 160) by random allocation. Comparisons were performed on the baseline features and preoperative and intraoperative indicators of the two groups. Most of the factors demonstrated no significant inter-group differences, including delirium status (P = 0.551), gender (P = 0.108), fracture type (P = 0.678), cognitive impairment history (P = 0.512), smoking history (P = 0.519), preoperative comorbidities (P = 0.608), number of comorbidities (P = 0.705), hypertension (P = 0.535), diabetes (P = 0.990), COPD (P = 0.832), walking ability before injury (P = 0.07), ASA grading (P = 0.613), anesthesia mode (P = 0.483), RBC transfusion of ≥ 2 units (P = 0.61), postoperative ICU care (P = 0.696), age (P = 0.558), BMI (P = 0.629), preoperative RBC count (P = 0.799), preoperative WBC (P = 0.088), preoperative ALB (P = 0.372), preoperative SCr (P = 0.681), preoperative Hb (P = 0.871), preoperative PLT (P = 0.355), time from admission to surgery (P = 0.824), OT (P = 0.296), and IBL (P = 0.86). These results indicate no significant inter-group differences in baseline characteristics and preoperative and intraoperative indicators (Table 3).
| Variable | Total | Training group (n = 372) | Validation group (n = 160) | Z/χ2/t | P value |
| Delirium status | |||||
| With | 79 | 53 | 26 | 0.355 | 0.551 |
| Without | 453 | 319 | 134 | ||
| Gender | |||||
| Male | 359 | 259 | 100 | 2.587 | 0.108 |
| Female | 173 | 113 | 60 | ||
| Fracture type | |||||
| Femoral neck fracture | 312 | 216 | 96 | 0.173 | 0.678 |
| Femoral intertrochanteric fracture | 220 | 156 | 64 | ||
| History of cognitive impairment | |||||
| With | 19 | 12 | 7 | 0.429 | 0.512 |
| Without | 513 | 360 | 153 | ||
| Smoking history | |||||
| With | 224 | 160 | 64 | 0.416 | 0.519 |
| Without | 308 | 212 | 96 | ||
| Preoperative comorbidities | |||||
| With | 317 | 219 | 98 | 0.263 | 0.608 |
| Without | 215 | 153 | 62 | ||
| Number of comorbidities | |||||
| ≥ 2 | 59 | 40 | 19 | 0.143 | 0.705 |
| < 2 | 473 | 332 | 141 | ||
| Hypertension | |||||
| With | 222 | 152 | 70 | 0.384 | 0.535 |
| Without | 310 | 220 | 90 | ||
| Diabetes | |||||
| With | 73 | 51 | 22 | < 0.001 | 0.990 |
| Without | 459 | 321 | 138 | ||
| COPD | |||||
| With | 51 | 35 | 16 | 0.045 | 0.832 |
| Without | 481 | 337 | 144 | ||
| Walking ability before injury | |||||
| Normal | 75 | 57 | 18 | 5.307 | 0.07 |
| Mild limitation | 85 | 66 | 19 | ||
| Inability to walk | 372 | 249 | 123 | ||
| ASA grading | |||||
| 1-2 | 381 | 264 | 117 | 0.256 | 0.613 |
| 3-4 | 151 | 108 | 43 | ||
| Mode of anesthesia | |||||
| Intravertebral/nerve block/local anesthesia | 443 | 307 | 136 | 0.491 | 0.483 |
| General anesthesia | 89 | 65 | 24 | ||
| RBC transfusion ≥ 2 units | |||||
| With | 255 | 181 | 74 | 0.259 | 0.61 |
| Without | 277 | 191 | 86 | ||
| Postoperative ICU care | |||||
| With | 88 | 60 | 28 | 0.152 | 0.696 |
| Without | 444 | 312 | 132 | ||
| Age, years | 78.00 [74.00, 82.00] | 78.00 [74.00, 82.00] | 78.34 ± 4.51 | 0.585 | 0.558 |
| Body mass index, kg/m2 | 23.44 ± 2.30 | 23.47 ± 2.28 | 23.37 ± 2.36 | -0.483 | 0.629 |
| Preoperative RBC count, × 109/L | 3.78 ± 0.45 | 3.78 ± 0.46 | 3.77 ± 0.45 | -0.255 | 0.799 |
| Preoperative WBC, × 109/L | 8.21 ± 1.73 | 8.13 ± 1.73 | 8.41 ± 1.73 | 1.714 | 0.088 |
| Preoperative albumin, g/L | 36.00 [33.00, 38.00] | 36.00 [33.00, 38.00] | 35.89 ± 3.99 | 0.89 | 0.372 |
| Preoperative serum creatinine, μmol/L | 66.53 ± 10.68 | 66.41 ± 10.76 | 66.82 ± 10.52 | 0.412 | 0.681 |
| Preoperative hemoglobin, g/L | 115.90 ± 12.88 | 115.97 ± 12.66 | 115.76 ± 13.44 | -0.162 | 0.871 |
| Preoperative PLT, × 109/L | 173.96 ± 34.33 | 174.88 ± 33.84 | 171.81 ± 35.46 | -0.927 | 0.355 |
| Time from admission to surgery, days | 6.00 [5.00, 6.00] | 6.00 [5.00, 6.00] | 6.00 [5.00, 6.00] | 0.214 | 0.824 |
| Operation time, minutes | 137.00 [123.00, 160.00] | 137.00 [123.00, 157.00] | 139.00 [123.75, 164.25] | 1.044 | 0.296 |
| Intraoperative blood loss, mL | 200.00 [100.00, 300.00] | 200.00 [100.00, 300.00] | 200.00 [100.00, 300.00] | -0.169 | 0.86 |
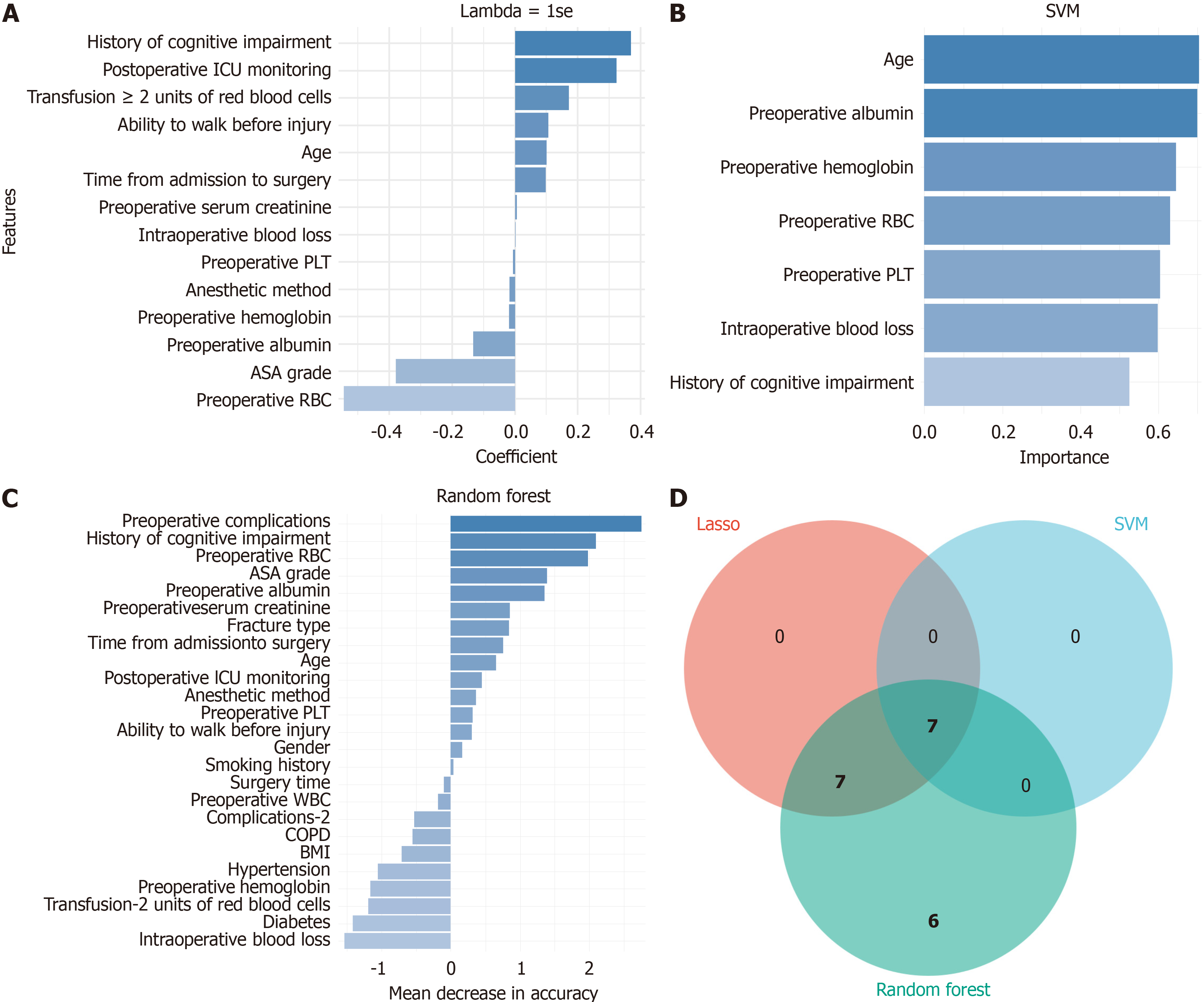
Feature screening was conducted according to the factors with differences in the entire collection, and the key features for predicting POD occurrence were identified through three different feature screening methods (Lasso regression, SVM, and random forest). The features screened out by Lasso regression included age, cognitive impairment history, walking ability before injury, ASA grading, preoperative RBC count, preoperative ALB, preoperative SCr, preoperative Hb, preoperative PLT, time from admission to surgery, IBL, anesthesia mode, RBC transfusion of ≥ 2 units, and postoperative ICU care (Figure 1A). The SVM method determined age, preoperative ALB, preoperative PLT, preoperative RBC count, preoperative Hb, IBL, and cognitive impairment history as significant features (Figure 1B). Age, BMI, gender, cognitive impairment history, preoperative RBC count, preoperative ALB, preoperative comorbidities, number of comorbidities of ≥ 2, diabetes, walking ability before injury, ASA grading, preoperative Hb, preoperative PLT, time from admission to surgery, OT, IBL, anesthesia mode, RBC transfusion of ≥ 2 units, and postoperative ICU care were screened out by the random forest method (Figure 1C). Seven common features were screened out by the three methods based on the Venn diagram analysis, namely age, cognitive impairment history, preoperative RBC count, preoperative ALB, preoperative Hb, preoperative PLT, and IBL (Figure 1D).
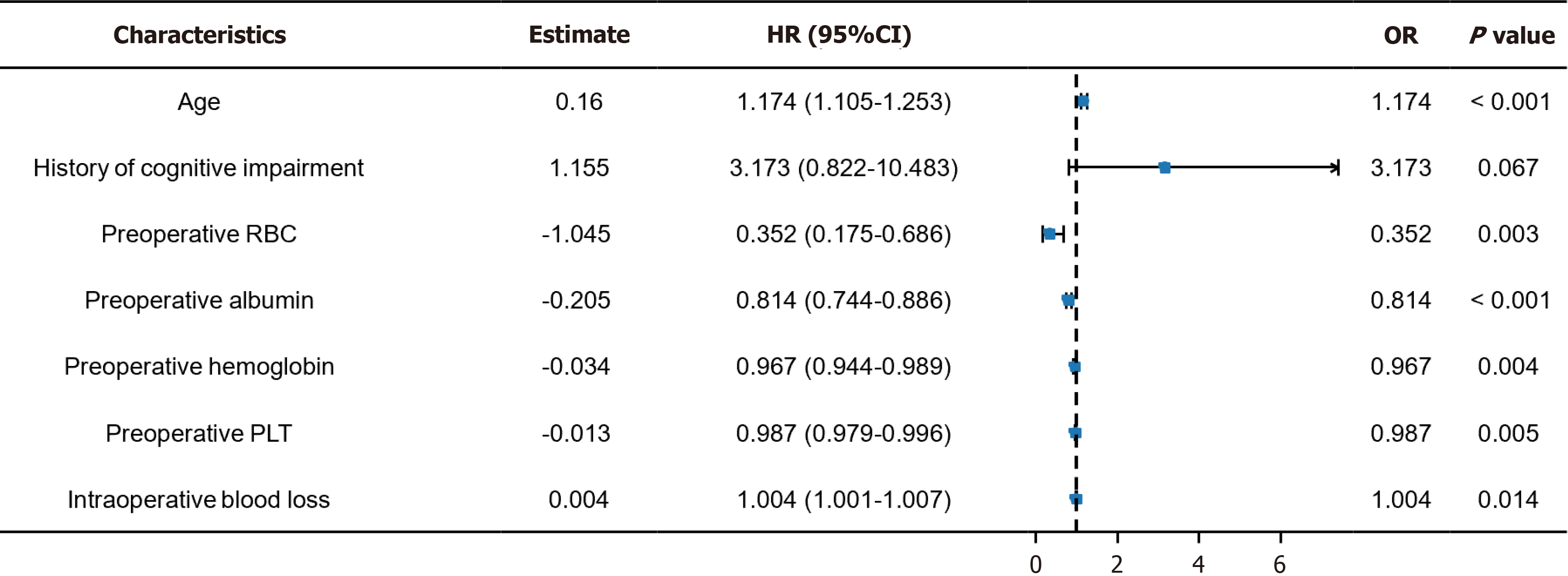
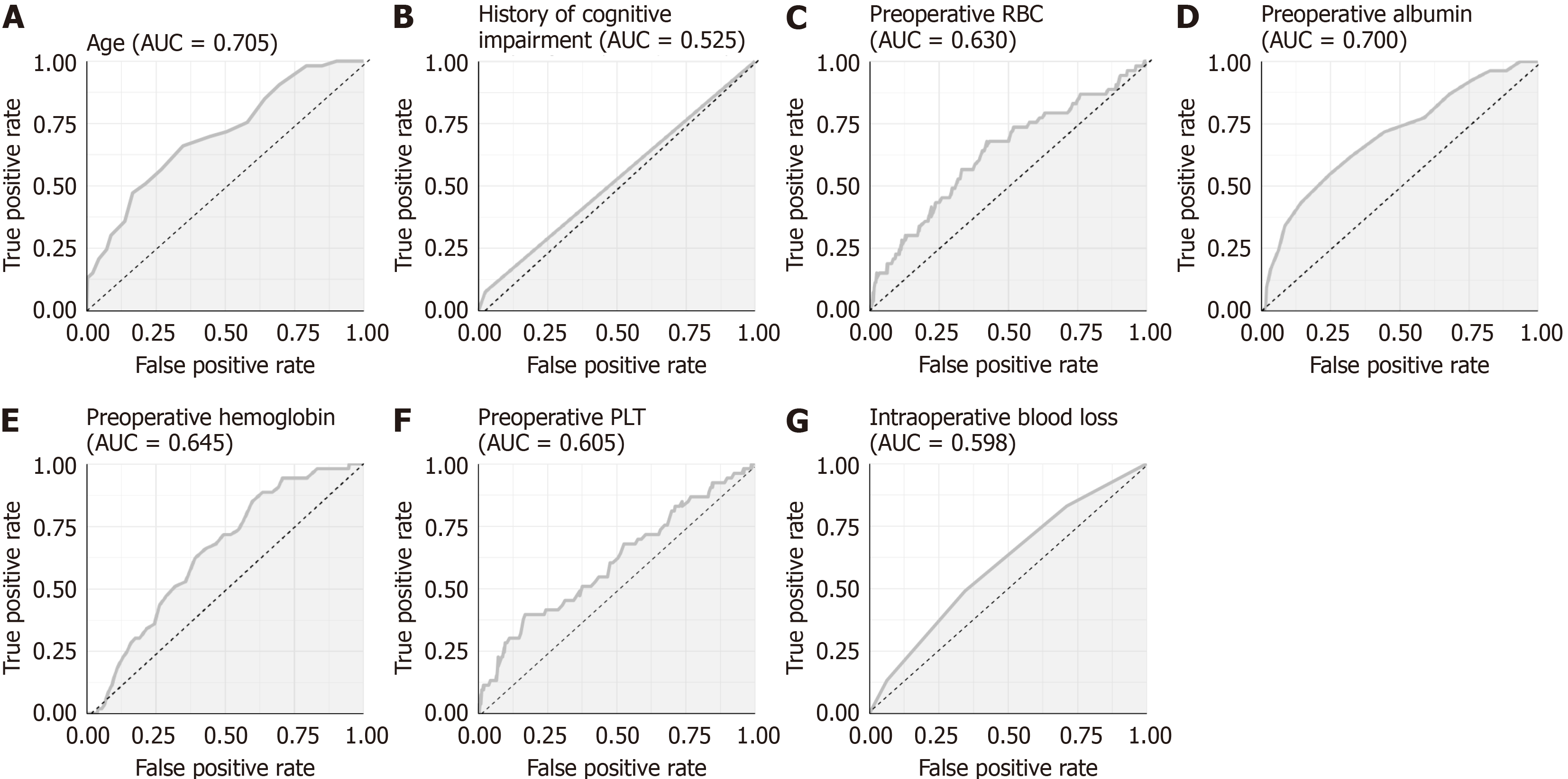
This study conducted a risk factor analysis on seven characteristic factors based on the Venn diagram results. Univariate analysis revealed age [P < 0.001, odds ratio (OR) = 1.174], preoperative RBC count (P = 0.003, OR = 0.352), preoperative ALB (P < 0.001, OR = 0.814), preoperative Hb (P = 0.004, OR = 0.967), preoperative PLT (P = 0.005, OR = 0.987), and IBL (P = 0.014, OR = 1.004) as risk factors for POD in patients (Figure 2). Subsequently, we drew ROC curves for each of the seven characteristic factors. The results indicated that only the AUC of age and preoperative ALB exceeded 0.7, whereas the other indicators demonstrated a relatively lower value in predicting POD in patients (Figure 3 and Table 4). Mul
| Marker | AUC | 95%CI | Specificity | Sensitivity | Youden index | Cut off |
| Age | 0.705 | 0.629-0.781 | 65.20% | 66.04% | 31.24% | 79.5 |
| History of cognitive impairment | 0.525 | 0.488-0.562 | 97.49% | 7.55% | 5.04% | 0.5 |
| Preoperative RBC count | 0.63 | 0.544-0.717 | 57.68% | 67.92% | 25.60% | 3.725 |
| Preoperative albumin | 0.7 | 0.621-0.779 | 75.24% | 54.72% | 29.95% | 33.5 |
| Preoperative hemoglobin | 0.645 | 0.574-0.717 | 36.36% | 88.68% | 25.04% | 121.5 |
| Preoperative PLT | 0.605 | 0.518-0.693 | 83.07% | 39.62% | 22.69% | 145.5 |
| Intraoperative blood loss | 0.598 | 0.519-0.677 | 65.52% | 49.06% | 14.57% | 250 |
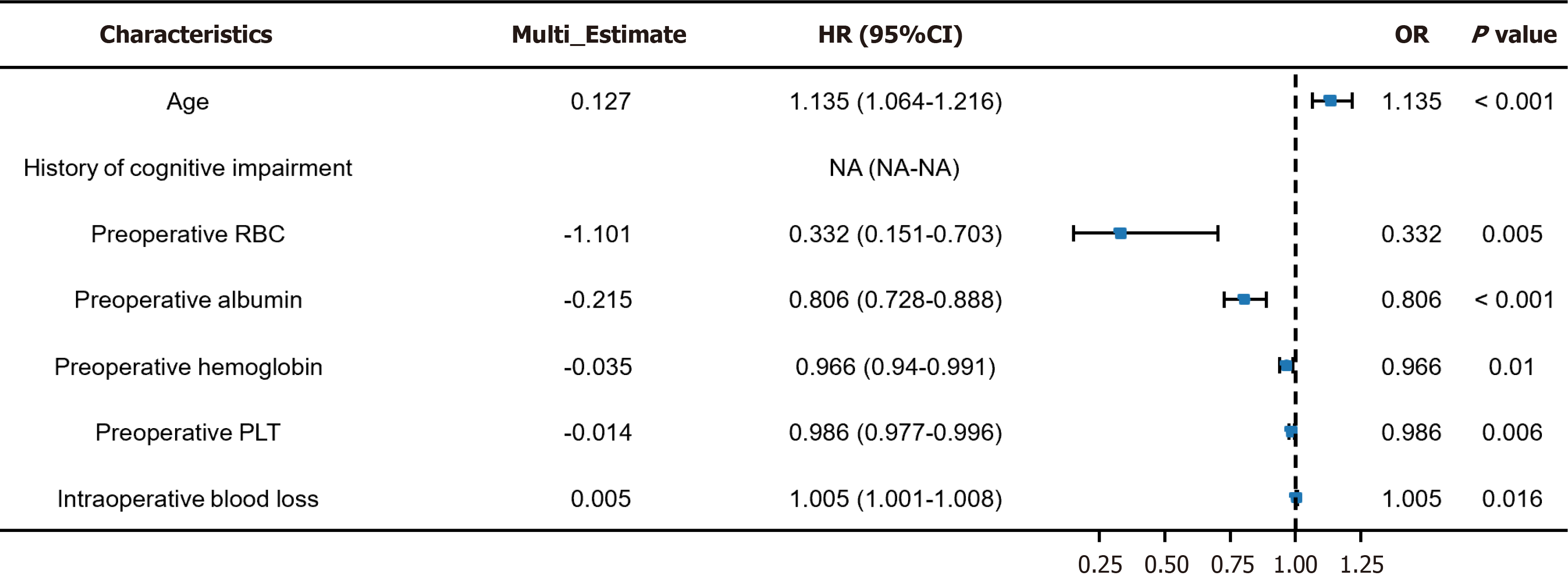
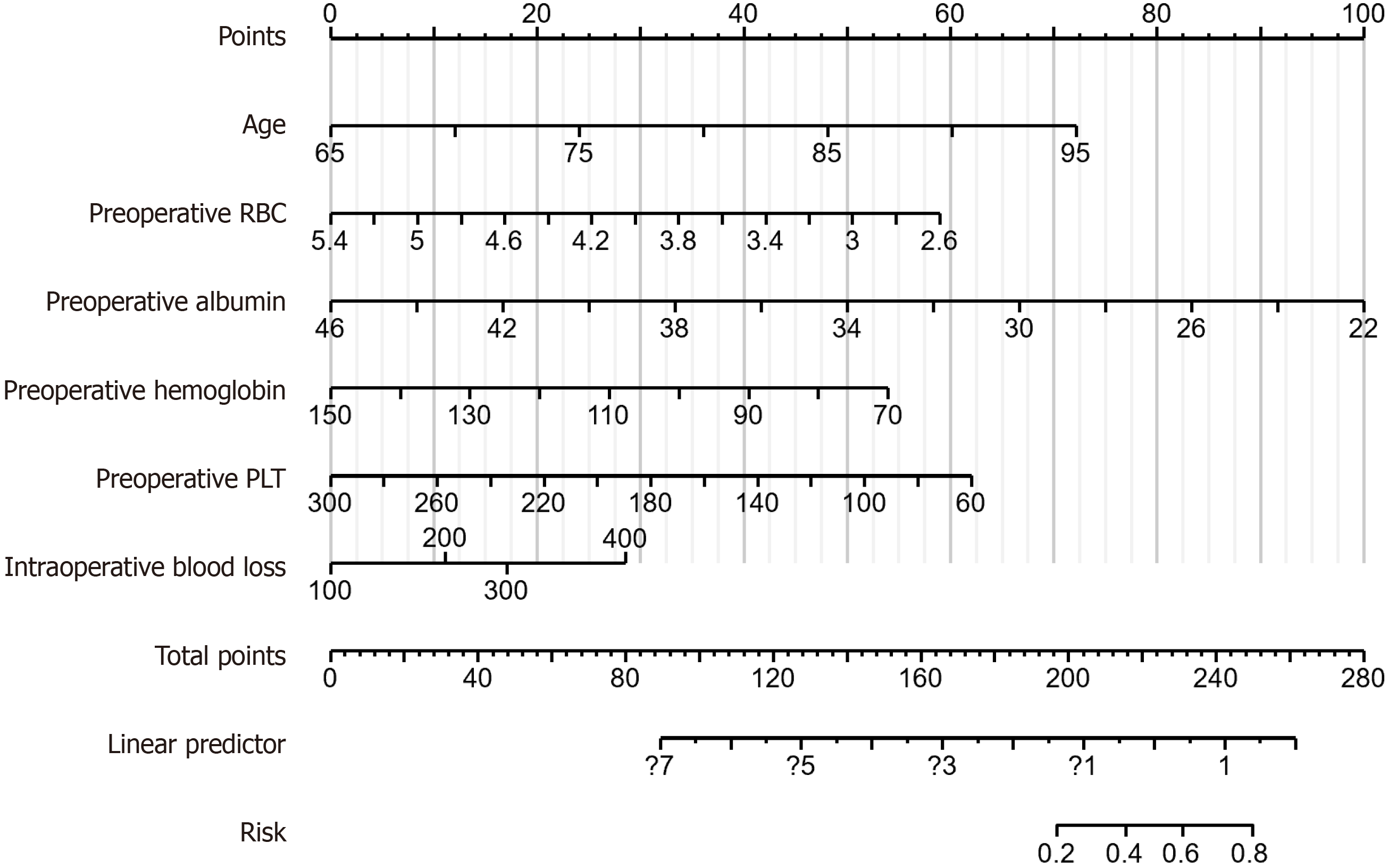
We developed a model for predicting the POD risk according to six characteristic factors: Age, preoperative RBC count, preoperative ALB, preoperative Hb, preoperative PLT, and IBL. The risk score calculation formula for this model is: 5.461 + age × 0.126 + preoperative RBC count × -1.101 + preoperative ALB × -0.218 + preoperative Hb × -0.035 + preoperative PLT × -0.014 + IBL 200 × 0.582 + IBL 300 × 0.891 + IBL 400 × 1.494. The model demonstrated that preoperative Hb was strongly correlated with POD in patients, whereas IBL was weakly associated with POD in patients (Figure 5).
The risk model developed in this study was comprehensively confirmed in both the training and validation groups. We first validated it in the training group. The AUC value was 0.833, with a 95% confidence interval of 0.774-0.888, indicating the model’s excellent discriminatory capacity (Figure 6A). The precision-recall curve further exhibited the model’s precision at different true positive rates, and the model maintained a relatively high precision at higher recall rates (Figure 6B). The calibration curve revealed a favorable consistency between the predicted probability and the actual incidence rate (Figure 6C). The DCA curve indicated a certain benefit in the clinical application of the model within the risk threshold range of 0%-30% (Figure 6D). The constructed risk model was further verified in the validation group. The AUC value of the ROC curve was 0.850 (95% confidence interval = 0.718-0.982), indicating that the model still had relatively good discriminatory capacity in the validation group (Figure 6E). The precision-recall curve demonstrated that the model maintained a relatively stable precision at higher recall rates (Figure 6F). The calibration curve revealed a good agreement between the model’s predicted probability and the actual incidence (Figure 6G). The DCA curve analysis indicated that the application of the model could bring certain benefits to the clinical practice within the risk threshold range of 0%-36% (Figure 6H).
The primary results of this research indicate that POD in elderly patients with HF is closely associated with several independent risk factors, including a cognitive impairment history, ASA grade of > 2, RBC transfusion of ≥ 2 units, postoperative ICU care, and preoperative Hb level. These factors all demonstrated significant predictive capabilities in the multivariate analysis, indicating their potential crucial role in the pathophysiological process of delirium. A no
This study confirmed age as an important independent risk factor for POD (P < 0.001, OR = 1.135). The likelihood of developing delirium significantly rises as patients age, possibly related to reduced cognitive reserve, increased neuroinflammatory response, and decreased physiological tolerance to surgical trauma in older adults[20,21]. Leigheb et al[22] support this conclusion, indicating that advanced age is a crucial factor causing POD. Furthermore, the team of Wang et al[23] discovered that the probability of POD was significantly increased in elderly patients aged ≥ 75 years after or
Several key preoperative indicators in this study revealed significant correlations with POD occurrence. A lower preoperative RBC count was negatively associated with POD occurrence (P = 0.005, OR = 0.332). A low RBC level may reflect the presence of anemia in patients postoperatively, which could cause insufficient oxygen supply to the brain, thereby increasing the risk of POD. Wang et al[23] did not directly investigate the association between RBC and delirium, but revealed that blood parameters have a significant effect on the cognitive state, indicating that blood indicators may play a certain role in the pathophysiological mechanism of delirium. A low preoperative ALB level is an independent risk factor for POD (P < 0.001, OR = 0.806). A low ALB level is typically related to malnutrition or chronic diseases, and these conditions may weaken the patient’s recovery ability and heighten the risk of postoperative complications[24,25]. Further, Hawley et al[26] indicated malnutrition as an important risk factor for POD, consistent with our results. The preoperative Hb level was strongly related to POD (P = 0.010, OR = 0.966). Low Hb may cause insufficient oxygen supply to the brain, augmenting the risk of delirium[27]. Wang et al[28] indicated a potential association between low Hb levels and delirium occurrence, although their research focused on other blood indicators. Additionally, this study affirmed the association between preoperative PLT and the risk of POD (P = 0.006, OR = 0.986). Relatively few current studies focused on the association between PLT and delirium, but the results of this study indicate that PLT may indirectly affect the occurrence of delirium by affecting the risk of postoperative thrombosis or bleeding.
IBL was considered a significant correlation with POD occurrence (P = 0.016, OR = 1.005). A large amount of IBL may cause insufficient blood volume and reduce cerebral perfusion, thereby increasing the risk of delirium. Callan et al[29], who revealed a strong correlation between IBL and postoperative complications, supported our results. Additionally, Rong et al[30] demonstrated that IBL is a risk factor for POD in patients. All these emphasize the importance of effective bleeding control during surgery to reduce the occurrence of POD and other complications.
The nomogram prediction model was constructed according to six independent risk factors, namely age, preoperative RBC count, preoperative ALB, preoperative Hb, preoperative PLT, and IBL, exhibiting excellent predictive performance and stability. The AUC of the model reached 0.833 and 0.850 in the training and validation groups, respectively, indicating that the model performs consistently and demonstrates good predictive ability across different datasets. The POD prediction nomogram constructed by Zhang et al[31] exhibited AUCs of 0.836 and 0.817 in the training and validation sets, respectively, indicating that both models have similar performance in predicting POD. Further, the AUC of the model constructed by Chen et al[32] using the machine learning method reaches 0.94, demonstrating high pre
The developed nomogram prediction model exhibits relatively high accuracy and stability, particularly in predicting the risk of POD for elderly patients with HF. The model integrates multiple independent risk factors and can provide comprehensive risk assessment for clinical practice. However, this model has certain limitations. First of all, the relatively limited samples and the single-center study design may cause the restricted generalization ability of the model. Second, the observation period is three months postoperatively, which may be insufficient to capture all delirium events, es
This study provides new information and tools for the early prevention and management of POD by determining the independent risk factors of POD and developing a predictive model. Doctors determine high-risk patients earlier and take appropriate intervention measures by applying these results in clinical practice, thereby improving patients’ postoperative prognosis, reducing the incidence of delirium, and enhancing their overall quality of life.
| 1. | Li T, Li J, Yuan L, Wu J, Jiang C, Daniels J, Mehta RL, Wang M, Yeung J, Jackson T, Melody T, Jin S, Yao Y, Wu J, Chen J, Smith FG, Lian Q; RAGA Study Investigators. Effect of Regional vs General Anesthesia on Incidence of Postoperative Delirium in Older Patients Undergoing Hip Fracture Surgery: The RAGA Randomized Trial. JAMA. 2022;327:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 216] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 2. | Parker M, Johansen A. Hip fracture. BMJ. 2006;333:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 344] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 3. | Mehta SP, Ellis AP, Meadows S, Lu S, Bullock M, Oliashirazi A. Rasch Analysis of Joint Replacement Version for Hip Disability and Osteoarthritis Outcome in Individuals With Advanced Osteoarthritis of Hip Awaiting Total Hip Arthroplasty Surgery. Arch Phys Med Rehabil. 2023;104:2084-2091. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Özel M, Altıntaş M, Tatlıparmak AC. Predictors of one-year mortality following hip fracture surgery in elderly. PeerJ. 2023;11:e16008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Zhang P, Li X, Yuan Y, Li X, Liu X, Fan B, Yang M, Wu X. Risk factor analysis for in-hospital death of geriatric hip fracture patients. Saudi Med J. 2022;43:197-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | George J, Sharma V, Farooque K, Trikha V, Mittal S, Malhotra R. Excess mortality in elderly hip fracture patients: An Indian experience. Chin J Traumatol. 2023;26:363-368. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Hua Y, Yuan Y, Wang X, Liu L, Zhu J, Li D, Tu P. Risk prediction models for postoperative delirium in elderly patients with hip fracture: a systematic review. Front Med (Lausanne). 2023;10:1226473. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Lim EJ, Koh WU, Kim H, Kim HJ, Shon HC, Kim JW. Regional Nerve Block Decreases the Incidence of Postoperative Delirium in Elderly Hip Fracture. J Clin Med. 2021;10:3586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Han Y, Zhang W, Liu J, Song Y, Liu T, Li Z, Wang X, Yang N, Li Y, Han D, Mi X, Zhou Y, Li M, Guo X, Zhong L, Wang G, Yuan Y. Metabolomic and Lipidomic Profiling of Preoperative CSF in Elderly Hip Fracture Patients With Postoperative Delirium. Front Aging Neurosci. 2020;12:570210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Zhang X, Tong DK, Ji F, Duan XZ, Liu PZ, Qin S, Xu KH, Di-Li XT. Predictive nomogram for postoperative delirium in elderly patients with a hip fracture. Injury. 2019;50:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Zhu R, Yang F, Li C, Zhu H, Lin L, Zhao X. Effect of Enhanced Recovery After Surgery on the Prognosis of Patients With Hip Fractures: A Systematic Review and Meta-Analysis. J Trauma Nurs. 2023;30:271-281. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Tang L, Fang P, Fang Y, Lu Y, Xu G, Liu X. Comparison of Effects between Combined Lumbar-Sacral Plexus Block plus General Anesthesia and Unilateral Spinal Anesthesia in Elderly Patients Undergoing Hip Fracture Surgery: A Pilot Randomized Controlled Trial. Evid Based Complement Alternat Med. 2021;2021:6685497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Guo J, He Q, Peng C, Dai R, Li W, Su Z, Li Y. Machine learning algorithms to predict risk of postoperative pneumonia in elderly with hip fracture. J Orthop Surg Res. 2023;18:571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 14. | Yosibash Z, Trabelsi N, Buchnik I, Myers KW, Salai M, Eshed I, Barash Y, Klang E, Tripto-Shkolnik L. Hip Fracture Risk Assessment in Elderly and Diabetic Patients: Combining Autonomous Finite Element Analysis and Machine Learning. J Bone Miner Res. 2023;38:876-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 15. | Uragami M, Matsushita K, Shibata Y, Takata S, Karasugi T, Sueyoshi T, Masuda T, Nakamura T, Tokunaga T, Hisanaga S, Yugami M, Sugimoto K, Yonemitsu R, Ideo K, Fukuma Y, Takata K, Arima T, Kawakami J, Maeda K, Yoshimura N, Matsunaga H, Kai Y, Tanimura S, Shimada M, Tateyama M, Miyamoto K, Kubo R, Tajiri R, Tian X, Homma F, Morinaga J, Yamanouchi Y, Takebayashi M, Kajitani N, Uehara Y; Kumamoto Stop OsteoPorotic Hip Fractures (K-STOP) Group, Miyamoto T. A machine learning-based scoring system and ten factors associated with hip fracture occurrence in the elderly. Bone. 2023;176:116865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 16. | Shaw JF, Budiansky D, Sharif F, McIsaac DI. The Association of Frailty with Outcomes after Cancer Surgery: A Systematic Review and Metaanalysis. Ann Surg Oncol. 2022;29:4690-4704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Hughes CG, Boncyk CS, Culley DJ, Fleisher LA, Leung JM, McDonagh DL, Gan TJ, McEvoy MD, Miller TE; Perioperative Quality Initiative (POQI) 6 Workgroup. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Postoperative Delirium Prevention. Anesth Analg. 2020;130:1572-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 18. | McCabe GA, Smith MM, Widiger TA. Psychopathy and antisocial personality disorder in the fifth edition of the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders: An attempted replication of Wygant et al. (2016). Personal Disord. 2023;14:636-648. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Quaranta D, L'Abbate F, Pelosi A, Arighi A, Asoni G, Bagattini C, Bessi V, Bonanni L, Bortoletto M, Bruni AC, Cagnin A, Cappa SF, Giubilei F, Guarino M, Iavarone A, Isella V, Luca A, Monastero R, Pellegrini FF, Perini M, Piccoli T, Rainero I, Tedeschi G, Marra C, Caffarra P. Itel MMSE: a short phone screening test for cognitive decline. Italian Validation study by the SINdem Neuropsychology Working Group. Neurol Sci. 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Oren RL, Kim EJ, Leonard AK, Rosner B, Chibnik LB, Das S, Grodstein F, Crosby G, Culley DJ. Age-dependent differences and similarities in the plasma proteomic signature of postoperative delirium. Sci Rep. 2023;13:7431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Pinho C, Cruz S, Santos A, Abelha FJ. Postoperative delirium: age and low functional reserve as independent risk factors. J Clin Anesth. 2016;33:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Leigheb M, De Sire A, Zeppegno P, Forni F, Sgreccia M, Gagliardi VP, Pogliacomi F, Sabbatini M. Delirium risk factors analysis post proximal femur fracture surgery in elderly. Acta Biomed. 2022;92:e2021569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Wang J, Li Z, Yu Y, Li B, Shao G, Wang Q. Risk factors contributing to postoperative delirium in geriatric patients postorthopedic surgery. Asia Pac Psychiatry. 2015;7:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Dong B, Wang J, Li P, Li J, Liu M, Zhang H. The impact of preoperative malnutrition on postoperative delirium: a systematic review and meta-analysis. Perioper Med (Lond). 2023;12:55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Onuma H, Inose H, Yoshii T, Hirai T, Yuasa M, Kawabata S, Okawa A. Preoperative risk factors for delirium in patients aged ≥75 years undergoing spinal surgery: a retrospective study. J Int Med Res. 2020;48:300060520961212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Hawley S, Inman D, Gregson CL, Whitehouse M, Johansen A, Judge A. Risk Factors and 120-Day Functional Outcomes of Delirium After Hip Fracture Surgery: A Prospective Cohort Study Using the UK National Hip Fracture Database (NHFD). J Am Med Dir Assoc. 2023;24:694-701.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 27. | Kong C, Zhang Y, Wang C, Wang P, Li X, Wang W, Wang Y, Shen J, Ren X, Wang T, Zhao G, Lu S. Comprehensive geriatric assessment for older orthopedic patients and analysis of risk factors for postoperative complications. BMC Geriatr. 2022;22:644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 28. | Wang LH, Jin TT, Zhang XW, Xu GH. Risk factors and prevention for postoperative delirium after orthopedic surgery. Indian J Psychiatry. 2021;63:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Callan KT, Donnelly M, Lung B, McLellan M, DiGiovanni R, McMaster W, Yang S, Stitzlein R. Risk factors for postoperative delirium in orthopaedic hip surgery patients: a database review. BMC Musculoskelet Disord. 2024;25:71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 30. | Rong X, Ding ZC, Yu HD, Yao SY, Zhou ZK. Risk factors of postoperative delirium in the knee and hip replacement patients: a systematic review and meta-analysis. J Orthop Surg Res. 2021;16:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 31. | Zhang Y, Xie LJ, Wu RJ, Zhang CL, Zhuang Q, Dai WT, Zhou MX, Li XH. Predicting the Risk of Postoperative Delirium in Elderly Patients Undergoing Hip Arthroplasty: Development and Assessment of a Novel Nomogram. J Invest Surg. 2024;37:2381733. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Chen D, Wang W, Wang S, Tan M, Su S, Wu J, Yang J, Li Q, Tang Y, Cao J. Predicting postoperative delirium after hip arthroplasty for elderly patients using machine learning. Aging Clin Exp Res. 2023;35:1241-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |