Published online Mar 19, 2025. doi: 10.5498/wjp.v15.i3.101142
Revised: December 23, 2024
Accepted: January 11, 2025
Published online: March 19, 2025
Processing time: 173 Days and 22.7 Hours
This is an invited commentary on the paper by Zou et al, accepted for publication in World Journal of Psychiatry. It reflects the findings of the authors in the broader context of the search for scientifically sound and evidence based nomothetic system for diagnosis and treatment in psychiatry, with a special focus on the application of translational neuroimaging in that effort.
Core Tip: The advances in the field of translational neuroscience over the past decade have facilitated the introduction of nomothetic network knowledge, which is a key prerequisite for any medical discipline. Neuroimaging in its various modalities has pivotal contribution in that context, and particularly for the better explanation and management of depression.
- Citation: Najar D, Stoyanov D. Scientific psychiatry within technical reach. World J Psychiatry 2025; 15(3): 101142
- URL: https://www.wjgnet.com/2220-3206/full/v15/i3/101142.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i3.101142
Eleven years before our program article[1] and after the publication of the seminal Oxford University Press volume on psychiatric validation[2], there has been a substantial advance in the field of translational applications of neuroscience in psychiatry. Those applications neither challenge nor undermine the efforts to understand the values and comprehensive experiences of the mentally ill[3], which supervene neurobiological me
The pursuit of objective biomarkers in psychiatric research has been significant in recent years, driven by the need for such tools in clinical practice. A primary research focus is developing objective diagnostic criteria that align with the current gold standard in psychiatry, the clinical interview. Despite the advancements in science-based medicine, psy
Magnetic resonance spectroscopy is one of the techniques applied in neuropsychiatric research, aiming to investigate the biochemical changes associated with psychopathology[5]. It is considered to potentially serve as a valuable tool for identifying biomarkers that could be of use in the diagnostic and therapeutic process.
Zou et al[1] explored this topic using biochemical and neuroimaging techniques in depressed adolescents. They looked for metabolic alterations in the prefrontal white matter (PWM), anterior cingulate, gray matter and thalami using proton magnetic resonance spectroscopy (H-MRS), with the target metabolites being N-acetyl aspartate (NAA), choline (Cho) complexes, Creatine (Cr) and myo-inositol (mI). They calculated NAA/Cr, Cho/Cr, and MI/Cr ratios. The Hamilton Depression Scale-24 item (HAMD-24) and Wechsler Memory Scale (WMS) scores served as both diagnostic criteria and confirmatory metrics in the analysis of the depressed adolescent and control groups. A cross-validation approach enhances the reliability of the neuroimaging data.
The study's primary findings suggest a correlation between PWM and grey matter biochemical imbalances in adolescents and the development of depressive symptoms, particularly those associated with cognitive impairment. PWM has been a focus of research in adolescent neuropsychiatry. Studies exploring its role in the development of de
| NAA/Cr | Cho/Cr | mI/Cr | |
| Bilateral PWM | DA < HA | DA < HA | Similar |
| Bilateral ACGM | No difference | ||
| Bilateral thalami | DA < HA | No difference | DA < HA |
Considering the extensive body of research on adults with depression, this study provides a valuable contribution to the field. Therefore, in contrast to studies in adolescents-the prefrontal cortex in depressed adults has been well-researched. Shifting our studies on adults-some are showing the importance of prefrontal structural abnormalities in patients even more-linking it to suicidal ideation and attempts[7]. Findings of low Cho/Cr ratios in the PWM of major depressive disorders (MDD) patients, which increased following SSRI treatment, suggest that these metabolic alterations may play a role in the pathophysiology of MDD[8]. Given the findings of this study, there may be assumed a connection between the observed metabolic alterations and their potential impact on the neurodevelopment and function of this region in the adult brain.
Considering the prefrontal cortex's well-established role in cognitive functions and its association with depressive symptoms, the metabolic alterations identified in this study could offer valuable insights into the neurobiological underpinnings of depression in adolescents[9]. This shows a possibility to find an explanation for the occurrence of cognitive symptoms in patients, such as altered concentration, memory impairments, slowed processing speed, and deficits in social cognition. By examining the developmental trajectories of these regions in depressed individuals com
Furthermore, bilateral thalamic Cho/Cr and mI/Cr values were reported to be lower in the patient group. It's proposed that this result could be linked to a metabolic disorder of the membranes of neurons in the thalamus. There is data on structural changes, specifically reduced volume, of the bilateral thalami in adults with depression[10]. Findings of reduced thalamic volume and altered metabolism provide converging evidence for the thalamus as a critical region involved in the pathophysiology of depression. The observed abnormalities of thalamic metabolism could potentially be interpreted in two primary hypotheses: Both structural and metabolic changes in the thalamic region are responsible for some of the clinical symptoms, or metabolic alterations in adolescents could lead to structural abnormalities in the thalamic regions of adult patients. As mentioned in the study, the thalamus is a core component of the reward circuit and is linked to anhedonia. Therefore, abnormalities in this area have yielded meaningful results as an approach to a neurotopical explanation of clinical symptoms[11].
To a great extent, the reported results are also consistent with earlier findings of our group, with structural magnetic resonance imaging (MRI) and multivariate analysis combining various MRI modalities for precision diagnostics in psychiatry[12,13].
Through studying the interplay between metabolic, structural, and functional brain changes, a deeper understanding of the underlying mechanisms of mental disorders could be gained, and potentially more targeted treatments developed[13]. The authors should consider applying this methodology to larger populations and addressing the possible effect of medication interpreting results. While metabolic alterations could potentially serve as reliable biomarkers in psychiatry, as they do in other medical fields, it's crucial to consider the translational context. The brain regions affected by these alterations correspond to key cognitive functions, emphasizing the need to interpret these findings considering clinical presentation. This approach aims to bridge the gap between traditional clinical evaluation methods in psychiatry and a novel biological perspective. While challenging, this strategy represents a promising avenue for unraveling the com
Establishing and confirming the significance and underlying mechanisms of these metabolic alterations and their impact on neurodevelopment could identify potential therapeutic targets on a metabolic level. This may offer avenues for interventions that could help mitigate the occurrence of structural alterations in adulthood. A biomarker derived from this data could support personalized medicine by enabling diagnostic and treatment plans tailored to the specific needs of each patient.
A promising avenue for future research would involve a multi-faceted approach. A longitudinal cohort study exa
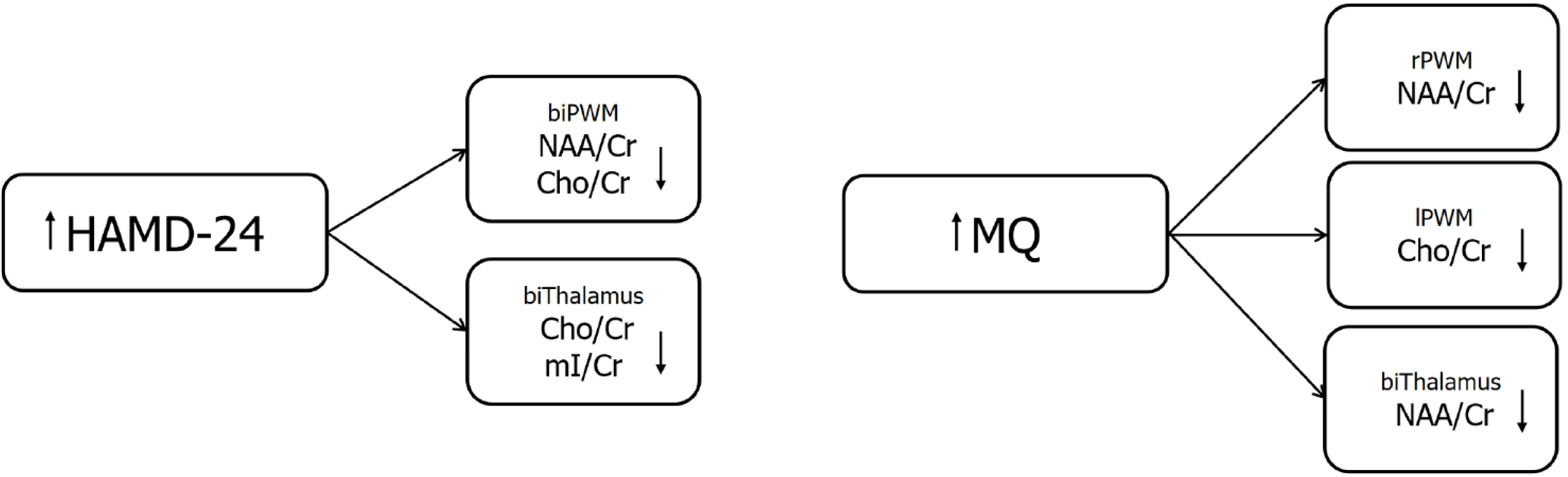
This study revealed significant correlations between clinical interview findings and neuroimaging data. Findings revealed an inverse relationship between depression severity (HAMD-24) and metabolite levels (NAA/Cr, Cho/Cr, and mI/Cr) in both the PWM and thalamus. Conversely, the MQ exhibited positive correlations with these metabolites in specific brain regions (right PWM NAA/Cr, left PWM Cho/Cr, and bilateral thalamus Cho/Cr and mI/Cr). These results suggest that lower metabolite levels may contribute to both increased depression severity and cognitive impairment in these brain regions (Figure 1).
The core take-home message from this study is that metabolic abnormalities in the PWM may play a significant role in the development of depressive symptoms, particularly those related to cognitive impairment. However, it is essential to acknowledge the complex interplay between biological factors and clinical presentation in psychiatric disorders. The study's findings highlight the need for further research to clarify the specific mechanisms underlying this relationship and to explore the potential impact of medications and other factors on the development and course of depression. These results present intriguing possibilities that warrant further exploration and could significantly contribute to the development of contemporary psychiatric diagnostic tools.
Based on the entire scope of such findings, scientific psychiatry is currently within technical reach.
| 1. | Zou Y, Wu YQ, Han YJ, He XM, Zhao J. Application of proton magnetic resonance spectroscopy in metabolic alterations of prefrontal white and gray matter in depression adolescents. World J Psychiatry. 2024;14:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 2. | Zachar P, Stoyanov DS, Aragona M, Jablensky A (Eds. ). Alternative perspectives on psychiatric validation: DSM, IDC, RDoC, and Beyond. International Perspectives in Philosophy & Psychiatry. Oxford. 2014. [DOI] [Full Text] |
| 3. | Stoyanov D, Fulford B, Stanghellini G, Van Staden W, Wong MT (Eds. ). International perspectives in values-based mental health practice. Case studies and commentaries. 2021. Available from: https://link.springer.com/book/10.1007/978-3-030-47852-04. |
| 4. | Stoyanov D, Maes MH. How to construct neuroscience-informed psychiatric classification? Towards nomothetic networks psychiatry. World J Psychiatry. 2021;11:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (2)] |
| 5. | Dager SR, Corrigan NM, Richards TL, Posse S. Research applications of magnetic resonance spectroscopy to investigate psychiatric disorders. Top Magn Reson Imaging. 2008;19:81-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Kaya S, McCabe C. What Role Does the Prefrontal Cortex Play in the Processing of Negative and Positive Stimuli in Adolescent Depression? Brain Sci. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Wang L, Zhao Y, Edmiston EK, Womer FY, Zhang R, Zhao P, Jiang X, Wu F, Kong L, Zhou Y, Tang Y, Wei S. Structural and Functional Abnormities of Amygdala and Prefrontal Cortex in Major Depressive Disorder With Suicide Attempts. Front Psychiatry. 2019;10:923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Zhang Y, Han Y, Wang Y, Zhang Y, Li L, Jin E, Deng L, Watts B, Golden T, Wu N. A MRS study of metabolic alterations in the frontal white matter of major depressive disorder patients with the treatment of SSRIs. BMC Psychiatry. 2015;15:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Straub J, Brown R, Malejko K, Bonenberger M, Grön G, Plener PL, Abler B. Adolescent depression and brain development: evidence from voxel-based morphometry. J Psychiatry Neurosci. 2019;44:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Zhang Y, Zhang Y, Ai H, Van Dam NT, Qian L, Hou G, Xu P. Microstructural deficits of the thalamus in major depressive disorder. Brain Commun. 2022;4:fcac236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Heshmati M, Russo SJ. Anhedonia and the brain reward circuitry in depression. Curr Behav Neurosci Rep. 2015;2:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 12. | Kandilarova S, Stoyanov D, Sirakov N, Maes M, Specht K. Reduced grey matter volume in frontal and temporal areas in depression: contributions from voxel-based morphometry study. Acta Neuropsychiatr. 2019;31:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Stoyanov D, Kandilarova S, Aryutova K, Paunova R, Todeva-Radneva A, Latypova A, Kherif F. Multivariate Analysis of Structural and Functional Neuroimaging Can Inform Psychiatric Differential Diagnosis. Diagnostics (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |