Published online Feb 19, 2025. doi: 10.5498/wjp.v15.i2.101807
Revised: November 25, 2024
Accepted: December 18, 2024
Published online: February 19, 2025
Processing time: 109 Days and 4.7 Hours
Depression is a prevalent affective disorder, but its pathophysiology remains unclear. Dysfunction in the gamma-aminobutyric acid (GABA)-ergic system may contribute to its onset. Recently, antidepressants (e.g., brexanolone, zuranolone) targeting the GABA-A receptor were introduced. The zona incerta (ZI), an inhi
To explore the relationship between GABAergic neurons in the ZI and depre
A chronic restraint stress (CRS) model was utilized to induce depression in mice. Whole-cell patch-clamp recordings assessed the excitability changes of GABAergic neurons in the ZI. Additionally, chemogenetic techniques were employed to modulate ZI GABAergic neurons. The performance of the mice in behavioral tests for depression and anxiety was observed.
The findings indicated that GABAergic neurons in the ZI were closely associated with depression-like behaviors in mice. Twenty-eight days after the CRS model was established, depression-like and anxiety-like behaviors were observed in the mice. The excitability of GABAergic neurons in the ZI was reduced. Chemogenetic activation of these neurons alleviated CRS-induced depression-like and anxiety-like behaviors. Conversely, inhibition of GABAergic neurons in the ZI led to changes in emotion-related behavioral outcomes in mice.
Activity of GABAergic neurons in the ZI was closely associated with depression-like phenotypes in mice, suggesting that these neurons could be a potential therapeutic target for treating depression.
Core Tip: Gamma-aminobutyric acid (GABA)-ergic dysfunction may play a role in the onset of depression. Case reports have indicated that deep brain stimulation of the zona incerta (ZI), which is abundant in GABAergic neurons, can modify depressive and anxiety symptoms. However, direct evidence linking ZI GABAergic neurons to depression-related behaviors in rodents is limited. This study demonstrated chronic resistant stress induced depression-like and anxiety-like behaviors in mice and reduced neuronal excitability. Chemogenetic activation alleviated these symptoms, whereas inhibition altered the behavior of normal mice. These findings suggested that ZI GABAergic neurons may serve as potential therapeutic targets for depression.
- Citation: Chen SH, Lan B, Zhang YY, Li GH, Qian YL, Hu MX, Tian YL, Zang WD, Cao J, Wang GH, Wang YG. Activation of zona incerta gamma-aminobutyric acid-ergic neurons alleviates depression-like and anxiety-like behaviors induced by chronic restraint stress. World J Psychiatry 2025; 15(2): 101807
- URL: https://www.wjgnet.com/2220-3206/full/v15/i2/101807.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i2.101807
Depression is a common affective disorder, characterized by a diverse combination of symptoms. The primary manifestations include persistent low mood, loss of interest, anhedonia, difficulty concentrating, sleep disturbances, fatigue, suicidal ideation, and impaired physical functioning[1]. Depression is the leading contributor to the global mental health disease burden and a major cause of disability worldwide. Recent reports revealed that it currently affects 280 million individuals and accounts for over 47 million disability-adjusted life years[2,3]. As one of the primary causes of disability, depression significantly diminishes quality of life and imposes a substantial socioeconomic burden worldwide[4].
The mechanistic understanding of depression and psychiatry as a whole has been dominated by the monoamine hypothesis. This theory posits that central deficiencies in monoaminergic neurotransmitters, such as serotonin, noradrenaline, and dopamine, play a critical role in the pathophysiology of depression[5]. It originated from observations that medications influencing serotonin and noradrenaline levels could affect mood. This model has persisted due to consistent supporting evidence from in vivo and postmortem studies examining neurotransmitters and their metabolites. This evidence has demonstrated the involvement of monoaminergic systems in mood regulation across various biological contexts. Furthermore, the continued relevance of the model has been supported by the clinical effectiveness of selective drugs, such as autoreceptor antagonists (e.g., mirtazapine, which targets the adrenergic system) and serotonin agonists (e.g., gepirone), both of which have demonstrated antidepressant properties[6,7]. However, the significant delay between the modulation of monoamine pathways by antidepressants, which occurs within hours, and the onset of their clinical effects, which often takes weeks, and the high variability in treatment response and symptom presentation among individuals with depression indicate that additional mechanisms are involved[8].
Research on the etiology and treatment of depression has increasingly focused on the primary amino acid neurotransmitters, gamma-aminobutyric acid (GABA) and glutamate. These neurotransmitters are hypothesized to have a more direct influence on neuronal activity than monoamines, offering fresh perspectives on depression mechanisms and therapeutic strategies[9,10]. Glutamate, the primary excitatory neurotransmitter of the brain, exerts its effects mainly via glutamate-gated cation channels. Conversely, GABA, the principal inhibitory neurotransmitter, functions predominantly by activating GABA-gated chloride channels, known as GABA-A receptors. GABA and glutamate, along with their receptors, tightly regulate neuronal activity and functional plasticity. Even minor alterations in the function of these neurotransmitters or their receptors can profoundly and rapidly affect emotional and cognitive behaviors[9,11].
GABA was initially studied in the context of depression decades ago[12,13], but its potential in treating depression was largely overlooked after the discovery of benzodiazepines, which are the most widely used anxiolytics. However, patients with depression often exhibit reduced GABAergic function, including decreased GABA levels in the brain and altered GABA-A receptor density. This dysfunction may disrupt the balance between excitation and inhibition in the brain, potentially leading to mood disorders[12,14,15].
In recent years, the United States Food and Drug Administration approved brexanolone and zuranolone, which are novel positive allosteric modulators of GABA-A receptors, for the treatment of postpartum depression (PPD)[16,17]. Brexanolone, a synthetic formulation of allopregnanolone, has shown rapid efficacy in treating PPD via intravenous administration. It likely restores GABAergic inhibition disrupted by postpartum neurosteroid withdrawal. Similarly, zuranolone, an oral neuroactive steroid, offers a convenient and effective treatment option for both PPD and major depressive disorder by enhancing GABA-A receptor activity and reestablishing inhibitory tone within neural circuits[9,17,18]. These advancements underscore the potential clinical value of modulating GABAergic neurotransmission in the treatment of depression.
The zona incerta (ZI) is a subthalamic region predominantly composed of GABAergic neurons. It can be broadly divided into the following four regions: Rostral; dorsal; ventral; and caudal[19]. Recent studies have suggested that the ZI is one of the integrative nodes of the brain for global behavioral modulation and plays a crucial role in the regulation of emotional processes such as fear memory and anxiety[19,20]. Case reports have indicated that deep brain stimulation (DBS) of the subthalamic nucleus and adjacent areas (including the caudal ZI region) could induce transient depressive states[21]. Another DBS study in patients with Parkinson’s disease has demonstrated that shifting the stimulation target from the subthalamic nucleus to areas near or within the ZI reduce subjective anxiety and depression[22]. Evidence from animal studies has shown that circuit plasticity between the ZI and ventral tegmental area (VTA) is altered in depressive states[23,24]. Taken together, these findings suggest that the ZI may be involved in the regulation of depression. However, direct evidence linking the ZI to depression-like behaviors in animals is lacking. It remains unclear whether the abundant GABAergic neurons within the ZI play a role in the onset and development of depression.
We utilized a chronic restraint stress (CRS) model to induce depression in mice. Then we employed whole-cell patch-clamp recordings to assess the excitability changes of GABAergic neurons in the ZI. Additionally, we utilized chemo
Experiments were conducted using adult male 6-8-week-old C57BL/6 mice (Liaoning Changsheng Biological Co., Ltd., Shenyang, China). The mice were housed under a 12-hour light-dark cycle in a temperature-controlled environment maintained at 25 ± 1 °C with free access to food and water. All aspects of animal care and use adhered strictly to institutional guidelines and governmental regulations. Additionally, all protocols were approved by the Animal Care and Use Committee of Zhengzhou University, with appropriate measures taken to minimize animal suffering during surgical procedures.
To induce CRS, mice were placed in narrow restraint tubes (3 cm in diameter, 10 cm in length). These tubes were designed to restrict forward, backward, and lateral movement while minimizing additional discomfort. Restraint sessions lasted 6 hour/day from 10:00-16:00 over a period of 28 consecutive days. Mice in the control group were subjected to no additional treatments other than food and water deprivation during the restraint treatment period for the mice in the CRS group. A total of 50 mice underwent CRS modeling treatment, 13 mice received control treatment, and 31 mice did not undergo any modeling treatment.
The mice were anesthetized using isoflurane inhalation (induction at 2%-3%; maintenance at 1%-2%) and positioned on a stereotaxic apparatus (RWD Life Science, Shenzhen, China). Viruses were then injected using a glass micropipette connected to a microsyringe. The injection was performed at a controlled rate of 10-15 nL/minute. After the injection, the needle remained in place for an additional 10 minutes before retraction to ensure proper diffusion.
The injection coordinates for the ZI were determined based on a brain atlas as follows: Anterior-posterior, -2.3 mm; medial-lateral, 1.68 mm; and dorsal-ventral, -4.25 mm. All viral vectors were obtained from BrainVTA Co., Ltd. (Wuhan, China). For the ex vivo electrophysiology experiment, rAAV2/9-VGAT1-EGFP-WPRE-pA (120 nL, > 2 × 10¹² vg/mL, PT-3176) was injected bilaterally into the unilateral ZI of the mice. For chemogenetic manipulation, rAAV2/9-VGAT1-hM3D (Gq)-mCherry-WPRE-hGH polyA (120 nL, > 2 × 10¹² vg/mL, PT-0489), rAAV2/9-VGAT1-hM4D (Gi)-mCherry-WPRE-hGH polyA (120 nL, > 2 × 10¹² vg/mL, PT-0488), or rAAV2/9-VGAT1-mCherry-WPRE-pA (120 nL, > 2 × 10¹² vg/mL, PT-0325) was bilaterally injected into the bilateral ZI.
The animals were anesthetized with an overdose of pentobarbital sodium (100 mg/kg, i.p.) and underwent transcardial perfusion with 50 mL of 0.01 M PBS (pH 7.4), followed by 150 mL of 4% paraformaldehyde prepared in 0.1 M phosphate buffer (pH 7.4) to preserve the tissue. The brains were carefully extracted and post-fixed in 4% paraformaldehyde for 2-4 hours, then transferred to a solution of 30% sucrose in 0.1 M phosphate buffer until they settled at the bottom of the containers, indicating full saturation. Subsequent to embedding, the brains were sectioned transversely into 30 µm slices using a cryostat (CM1950; Leica, Heidelberg, Germany) and stored in PBS at 4 °C until further processing. For immunostaining, all sections were first blocked with 5% goat serum for 30 minutes and then incubated with primary antibodies (rabbit-anti-GABA, 1:500, A2052; Sigma-Aldrich, St. Louis, MO, United States) at room temperature for 16-24 hours, followed by incubation in the secondary antibody (Alexa Fluor 488–conjugated goat anti-rabbit, 1:300, 115-545-003; Jackson ImmunoResearch, West Grove, PA, United States) for an additional 4-6 hours at room temperature.
Open field test: For the open field test (OFT), mice were placed in the center of an open field apparatus, which was a gray Plexiglas box (40 cm × 40 cm × 50 cm). Their behavior was recorded for 5 minutes using a computer-connected camera. The time spent in the central squares and the total distance traveled within the box were measured and analyzed using the Smart v3.0 video tracking system (Harvard Apparatus; Panlab, Barcelona, Spain). After each test, the open-field arena was cleaned with 75% ethanol. The total distance traveled was used as a measure of locomotor activity, while the time spent in the center zone served as an indicator of anxiety levels.
Elevated plus maze test: The elevated plus maze (EPM) was positioned 60 cm above the floor and consisted of a central platform (5 cm × 5 cm), two open arms (30 cm × 6 cm), and two closed arms (30 cm × 6 cm × 15 cm). During the test session, each mouse was placed on the central platform facing one of the open arms and allowed to explore freely for 5 minutes in a dimly lit room. The total distance traveled and the time spent in the open arms were recorded using the Smart v3.0 software (Harvard Apparatus; Panlab). After each trial, the maze was cleaned with 70% ethanol. Time spent in the open arms was used as a measure of anxiety levels.
Sucrose preference test: For the sucrose preference test (SPT), mice were individually housed and acclimated to 1% sucrose and water for 2 days. The positions of the bottles alternated every 12 hours. On the testing day, the mice were deprived of both food and water for 12 hours. Then, they were presented with pre-weighed identical bottles for a 4-hour period. One bottle contained water, and the other bottle contained 1% sucrose. Sucrose preference was determined by dividing the amount of sucrose consumed by the total liquid intake (water and sucrose).
Tail suspension test: For the tail suspension test (TST), mice were suspended by their tails (proximately 2 cm from the tip, proximately 30 cm above the ground) using a piece of medical tape. The movements of the mice were recorded from the side for 6 minutes. The duration of immobility during the final 4 minutes of the test was quantified.
Forced swimming test: For the forced swimming test (FST), mice were placed in a cylindrical container filled with water (temperature: 23-25 °C; dimensions: 15 cm diameter, 30 cm height) for 6 min. The water level was set to prevent the mice from touching the bottom with their limbs or tails. During the test, immobility was assessed during the final 4 minutes. Mice were considered immobile when they floated motionless or made only the minimal movements necessary to keep their heads above the water.
The mice that underwent virus injection into the ZI were used. Brain slices containing the ZI were prepared following procedures similar to those described previously[25]. The 300 μm-thick brain slices were prepared using a vibratome (VT 1200s; Leica) in ice-cold, oxygenated sucrose-based artificial cerebrospinal fluid (95% O2/5% CO2), containing 252 mmol/L sucrose, 2.5 mmol/L KCl, 6 mmol/L MgSO4·7H2O, 1.2 mmol/L NaH2PO4, 26 mmol/L NaHCO3, 0.5 mmol/L CaCl2, and 10 mmol/L D-glucose. The slices were then transferred to normal sodium-based artificial cerebrospinal fluid, which consisted of 124 mmol/L NaCl, 2 mmol/L CaCl2·H2O, 2.5 mmol/L KCl, 1 mmol/L MgSO4, 1 mmol/L NaH2PO4, and 37 mmol/L D-glucose. Recordings of ZI GABAergic neurons, identified as bright cells under infrared differential interference contrast microscopy, were performed in current-clamp modes using a Multiclamp 700B amplifier (Axon Instruments, Foster City, CA, United States). The data were analyzed using pCLAMP v.10.3 software. Whole-cell patch-clamp was achieved after forming a giga-seal (> 1 GΩ) between the pipette tip (3-7 MΩ) and the neuronal membrane. Series resistance was continuously monitored, and recordings were discarded if resistance changed by more than 15%. During recordings, cells were perfused with a bath solution at room temperature (22-25 °C). The current clamp (holding current I = 0 mA) was used to measure resting membrane potential and to record neuronal responses to injected currents. For action potential number testing, the protocol was configured as stepwise 400 milliseconds current injections ranging from 0 to 100 pA in 10 pA increments. For rheobase current testing, the protocol was configured as stepwise 30 milliseconds current injections starting from 0 pA with 5 pA increments until an action potential was elicited.
Data are expressed as the mean ± SEM and were analyzed using GraphPad Prism 8 software (GraphPad Software, Inc., La Jolla, CA, United States). For comparisons between two groups, an unpaired Student’s t-test was employed. For comparisons involving more than two groups, one-way or two-way analysis of variance followed by Tukey’s multiple comparisons test was used. Statistical significance was set at P < 0.05.
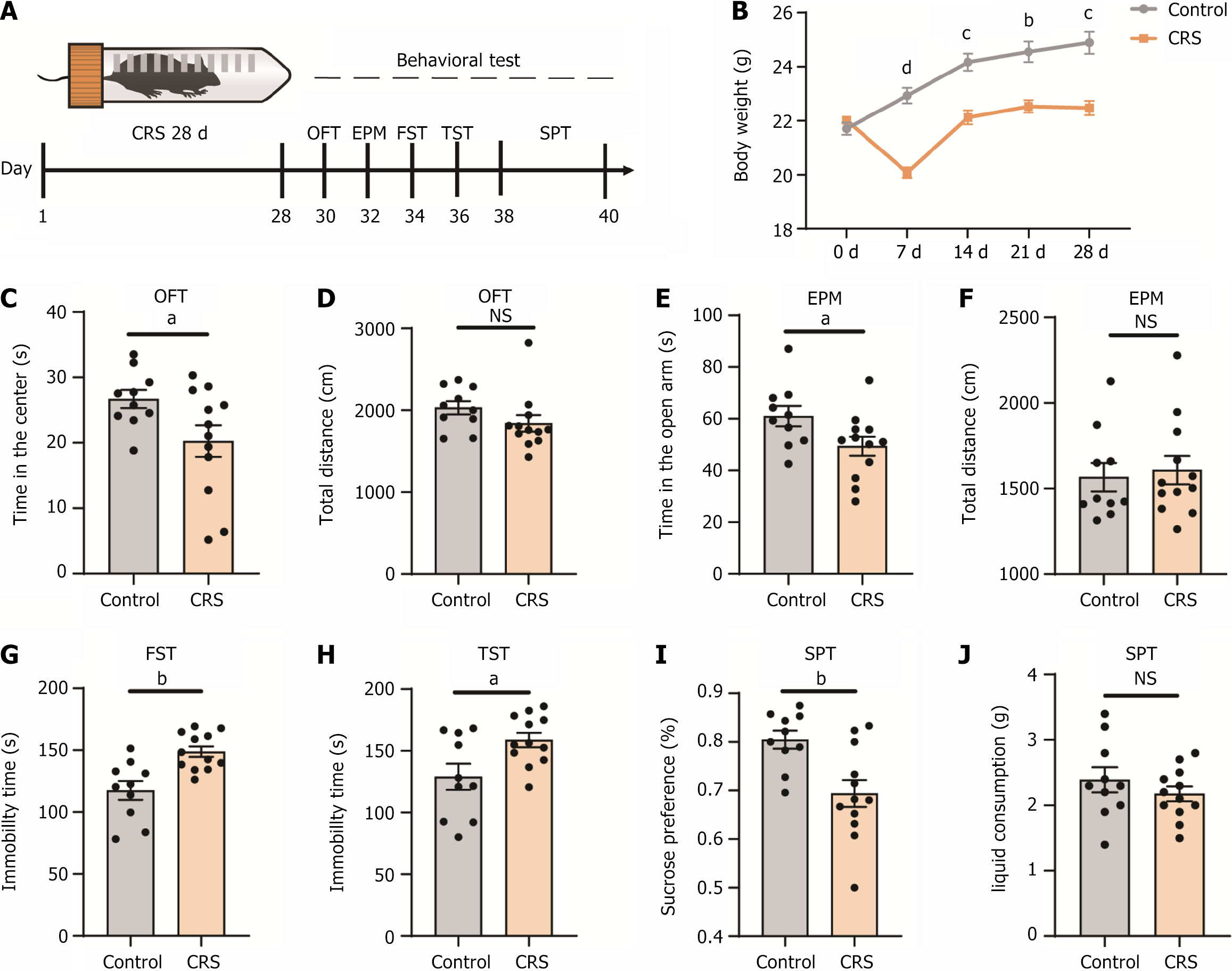
To examine the effects of chronic stress on behavior, we subjected C57BL/6 mice to the CRS model protocol for 28 consecutive days, with each session lasting 6 hour/day. Following the completion of the stress protocol, various behavioral tests were performed to evaluate emotional responses in the mice (Figure 1A). The body weight of mice in the CRS group initially decreased within the 1st week of stress exposure and gradually approached baseline levels thereafter. Body weight remained significantly lower in the CRS group compared to the control group (Figure 1B). Anxiety-like behaviors were assessed using both the OFT and EPM. In the OFT, mice in the CRS group spent less time in the center of the field, while their total distance traveled was similar to that of the control group (Figure 1C and D). Similarly, in the EPM, mice in the CRS group spent less time in the open arms, although their total distance traveled was comparable to the control group (Figure 1E and F). We then subjected the mice to the FST, TST, and SPT to evaluate depressive symptoms, specifically despair and anhedonia. The results demonstrated that mice in the CRS group exhibited significantly increased immobility time in both the FST and TST when compared to the control group (Figure 1G and H), indicating higher levels of behavioral despair. Additionally, mice in the CRS group showed a lower sucrose preference index in the SPT, reflecting anhedonia (Figure 1I), while their total liquid consumption remained unaffected (Figure 1J). These findings confirmed that CRS induced depression-like and anxiety-like behaviors in mice.
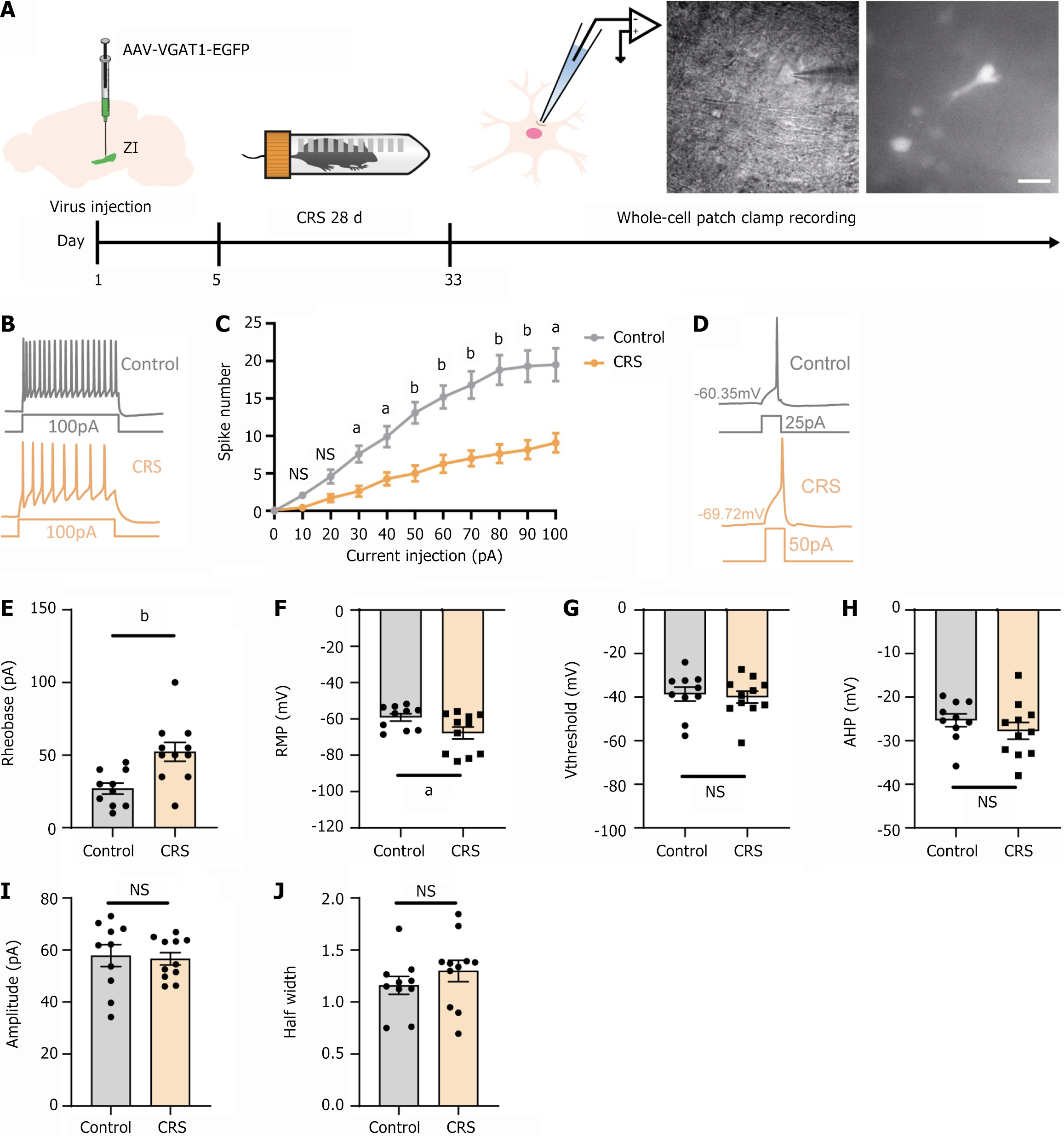
To further investigate how CRS affected the electrophysiological properties of GABAergic neurons in the ZI, we injected rAAV-VGAT1-EGFP into the ZI to label the GABAergic neurons. Following completion of the CRS model protocol, whole-cell patch-clamp recordings were performed on EGFP-positive neurons in both the CRS and control groups. The experimental workflow and the EGFP-positive neurons observed under the microscope during whole-cell patch-clamp recordings are illustrated in Figure 2A. The firing patterns of these neurons are shown in Figure 2B. Upon current injection, neurons from the CRS group exhibited a significantly reduced firing frequency compared to those from the control group (Figure 2C). We also observed an increased threshold for current intensity required to evoke action potentials (Figure 2D and E). The resting membrane potential of these neurons was hyperpolarized (Figure 2F). However, other intrinsic properties, such as action potential threshold, afterhyperpolarization amplitude, action potential amplitude, and action potential half-width, showed no significant differences between the groups (Figure 2G-J). These results indicated that CRS altered the electrophysiological characteristics of GABAergic neurons in the ZI, leading to reduced neuronal excitability.
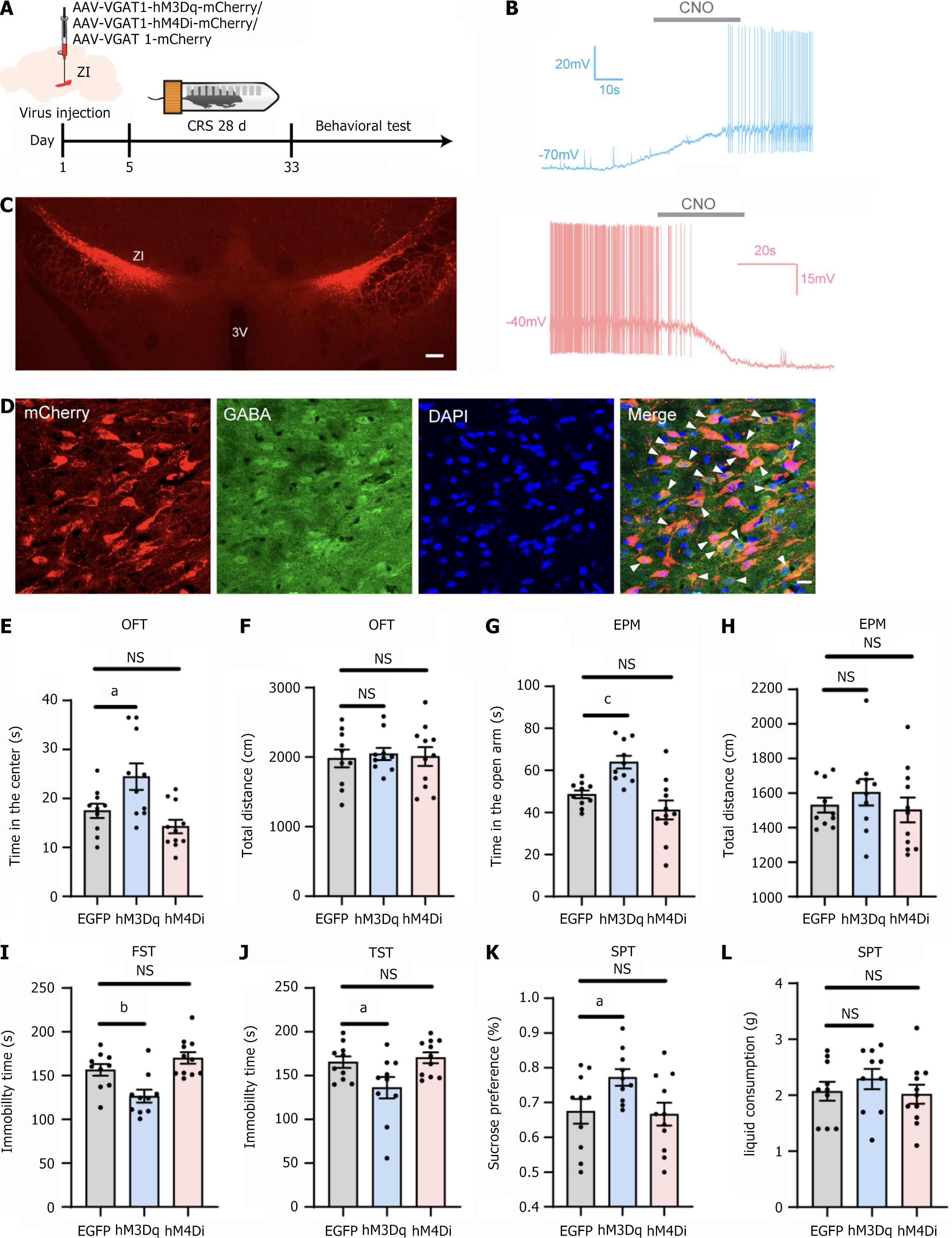
To evaluate whether activation of ZI GABAergic neurons could mitigate the affective behaviors induced by CRS, we employed chemogenetic techniques. Mice received bilateral injections of either rAAV-VGAT1-hM3Dq-mCherry or rAAV-VGAT1-hM4Di-mCherry into the ZI. Mice in the control group were injected with rAAV-VGAT1-mCherry. After a 5-day recovery period, the CRS model protocol was initiated. Behavioral tests were conducted as previously described, with clozapine-N-oxide (CNO) (5 mg/kg) administered intraperitoneally 30 min prior to each test (Figure 3A). Whole-cell patch-clamp recordings were performed to confirm the functionality of the chemogenetic viral vector. During current-clamp recordings, the application of CNO (10 μM) elicited changes in neuronal firing activity (Figure 3B).
The injections were predominantly localized within the ZI, with slight diffusion to the adjacent zone (Figure 3C). A representative image of the virus-infected neurons co-labeled with GABA-immunoreactive cells is presented in Figure 3D. The majority of mCherry-positive neurons overlapped with GABA-positive neurons. Injection sites from one mouse in each of the three groups were selected for statistical analysis. The proportion of mCherry/GABA double-positive neurons among mCherry-positive neurons was 90.48%, 87.76%, and 89.36%, respectively, confirming the specificity of the viral promoter.
Chemogenetic activation of GABAergic neurons in the ZI increased the time mice spent in the center of the OFT and the open arms of the EPM, while locomotor activity remained unaffected (Figure 3E-H). Additionally, during the assessment of depressive-like behaviors, neuronal activation reduced immobility time in the FST and TST (Figure 3I and J), increased sucrose consumption in the SPT (Figure 3K), but did not affect total liquid consumption (Figure 3L). In contrast, chemogenetic inhibition of ZI GABAergic neurons showed a trend towards increased anxiety-like and depressive-like behaviors in the OFT, EPM, and FST. However, these changes did not reach statistical significance (Figure 3E, G and I). These results demonstrated that chemogenetic activation of ZI GABAergic neurons effectively reduced CRS-induced anxiety-like and depression-like behaviors.
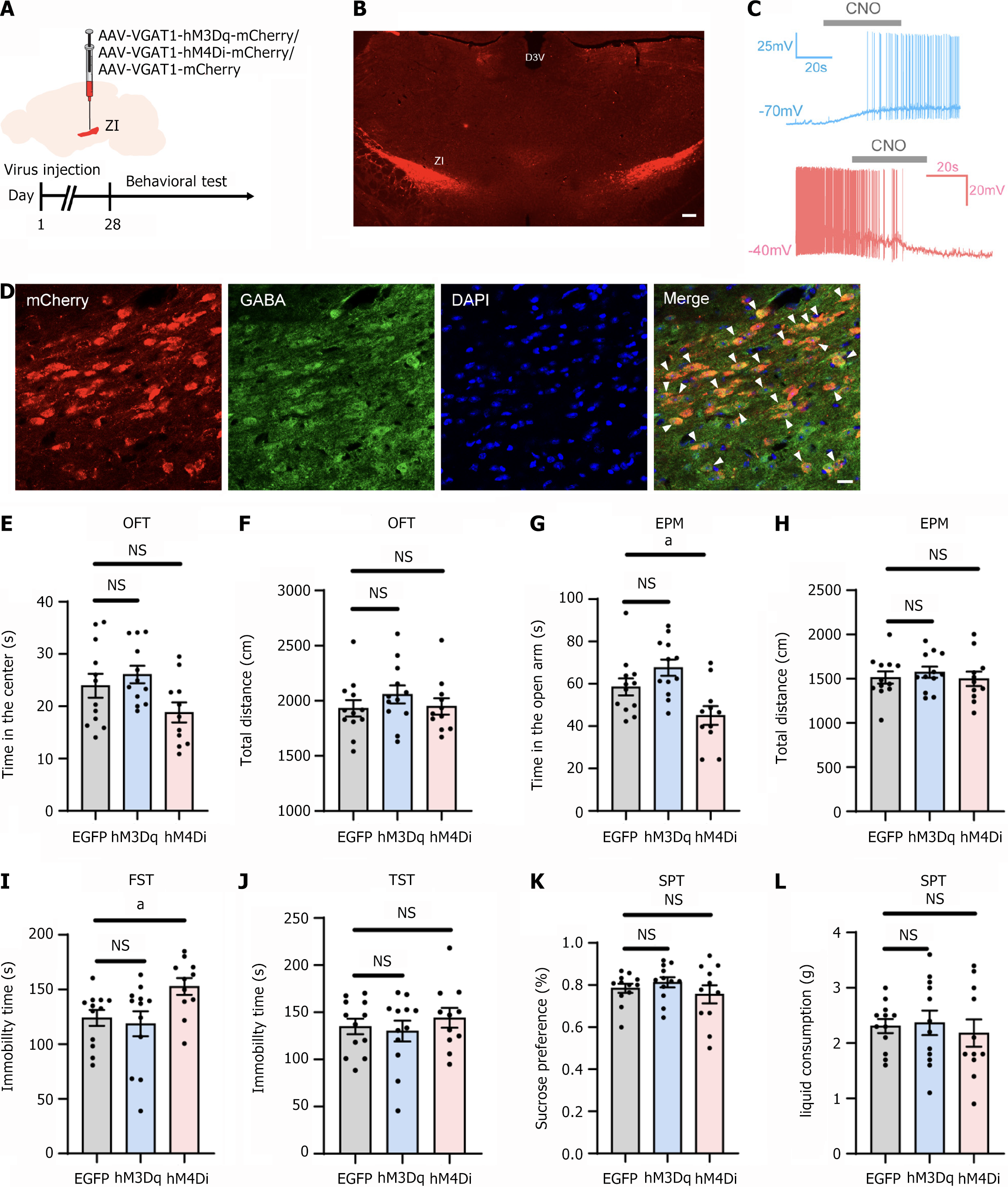
To examine whether modulation of GABAergic neurons in the ZI induced behavioral abnormalities in normal mice, we bilaterally injected rAAV-VGAT1-hM3Dq-mCherry or rAAV-VGAT1-hM4Di-mCherry into the ZI, with control mice receiving rAAV-VGAT1-mCherry. Following a 28-day period for viral expression, behavioral tests were conducted. CNO (5 mg/kg) was administered intraperitoneally 30 minutes before each test (Figure 4A). The injections were primarily localized within the ZI with minor diffusion into the adjacent zone (Figure 4B). Whole-cell patch-clamp recordings were conducted to verify the functionality of the chemogenetic viral vector. Under current-clamp conditions, the application of CNO (10 μM) induced alterations in neuronal firing activity (Figure 4C). The colocalization of virus-infected neurons and GABA-immunoreactive cells is depicted in Figure 4D. The majority of mCherry-positive neurons were co-labeled with GABA-positive neurons. Injection sites from one mouse in each of the three groups were selected for statistical analysis. The mCherry/GABA double-positive neurons accounted for 88.00%, 93.44%, and 84.21% of the mCherry-positive neurons, respectively, thereby confirming the specificity of the viral promoter.
The results indicated that neither activation nor inhibition of ZI GABAergic neurons significantly altered the time spent in the center of the OFT or the total distance traveled (Figure 4E and F). However, inhibition of these neurons led to an increase in time spent in the open arms of the EPM, while locomotor activity remained unaffected (Figure 4G and H), suggesting heightened anxiety-like behavior. Furthermore, inhibition of ZI GABAergic neurons increased immobility time in the FST (Figure 4I), indicative of pronounced depressive-like symptoms. However, no significant differences were observed in the TST or SPT (Figure 4J-L).
Through a combination of ex vivo electrophysiology, chemogenetic manipulation, and behavioral analysis, we de
We first validated the effects of CRS on inducing depression-like behaviors in mice. The duration of the CRS model protocol varies significantly across different studies, with total lengths ranging from 7-28 days and daily restraint times between 2-6 hours[26-30]. Shorter durations of the CRS model protocol are generally believed to induce anxiety-like behaviors in rodents and are primarily used to study the physiological mechanisms of anxiety[26,27]. Longer durations of the CRS model protocol can effectively induce depression-like behaviors in rodents while retaining some anxiety-like phenotypes. This method is more suitable for studying the physiological mechanisms of depression[27,29,30].
We selected the OFT and EPM to assess anxiety-like behaviors, and the TST, FST, and SPT to evaluate depression-like behaviors. The choice of these behavioral paradigms was based on their widespread application and reliability in prior research[31,32]. The OFT and EPM are sensitive to capturing responses of the animals to stressors such as novel environments or elevated spaces, reflecting their anxiety levels[33,34]. The OFT uses the time spent in the central area as an indicator, while the EPM evaluates anxiety by recording the time spent in the open arms.
The TST and FST assess depression-like behaviors by measuring behavioral despair, such as immobility time when escape is impossible. Their validity has been extensively confirmed, and they are effective in distinguishing the effects of antidepressants. Additionally, the SPT evaluates anhedonia, a core symptom of depression, by observing changes in preference for a sucrose reward. The combined use of these paradigms provides multidimensional behavioral infor
Our results indicated that the long-term CRS model protocol (6 hours of daily restraint for 28 days) effectively induced depression-like and anxiety-like behaviors in mice, which was consistent with previous reports[27,29]. Using whole-cell patch-clamp techniques, we found that CRS led to a reduction in the number of action potentials fired by ZI GABAergic neurons in response to current injection, increased threshold current intensity, and hyperpolarization of the membrane potential. These findings supported the notion that the excitability of ZI GABAergic neurons was reduced when mice are in a depressive state.
To further establish the causal relationship between ZI GABAergic neurons and depression-like behaviors, we combined chemogenetic manipulation with behavioral testing. Our results demonstrated that activation of ZI GABAergic neurons alleviated CRS-induced depression-like and anxiety-like behaviors. This effect is potentially mediated by projections to distinct brain regions. A previous study suggested that activation of ZI GABAergic neurons reduced generalized fear responses following fear conditioning through projections to the thalamic nucleus reuniens[35]. Fear generalization is characterized by an inability to effectively distinguish between threatening and non-threatening stimuli. It closely aligns with hypervigilance and avoidance behaviors observed in anxiety disorders such as generalized anxiety disorder and post-traumatic stress disorder.
Research on social buffering effects in mice with learned helplessness revealed significant changes in fiber connections between the ZI and the VTA[23,24]. The bidirectional pathway between the ZI and VTA likely plays a critical role in the development and alleviation of depression-like behaviors. The ZI functions as an integrative hub for global behavioral regulation and has extensive connections throughout the brain. Its role in specific emotional processes likely depends on the circuits engaged[20]. Furthermore, the distinct anatomical subdivisions of the ZI may differentially contribute to the regulation of emotional states. This field of research requires further investigation.
While GABAergic neurons are abundant and widely distributed across the ZI, the presence of markers such as somatostatin, calretinin, calbindin, parvalbumin, and vasopressin highlight significant heterogeneity within the GABAergic population[36]. This diversity suggests that distinct subtypes of GABAergic neurons may have specialized roles in regulating various emotional processes. Detailed exploration of their specific contributions is warranted.
Our findings also showed that inhibition of ZI GABAergic neurons led to partial induction of behavioral despair and anxiety-like phenotypes. The mice increased immobility time in the FST and decreased time spent in the open arms of the EPM. However, no significant changes were observed in the TST, OFT, or SPT. Although ZI GABAergic neuron activity modulates performance in specific despair-related behavioral paradigms, these results highlight that its role as a primary determinant in the development of depression remains unclear.
The differences in the sensitivity and focus of the different behavioral tests may explain these findings. Both the FST and TST assess despair under acute stress. However, the water-based environment of the FST imposes a higher stress load than the physical suspension in the TST[37]. Some researchers argue that immobility in the FST may also reflect psychomotor retardation, which represents a low-level sensorimotor symptom of depression[38]. Similarly, the EPM offers a more straightforward assessment of anxiety characteristics that are less influenced by locomotor activity compared to the OFT[39]. However, the OFT is often regarded as a comprehensive test that captures multiple dimensions of behavior, including fear, exploration, emotional states, and anxiety-like responses[39,40]. These differences in sensitivity and focus underscore the complexity of the role of ZI GABAergic neurons in emotional regulation.
The neurochemical diversity of ZI GABAergic neurons further complicates interpretations. Distinct subpopulations within the rostral region of the ZI have been shown to exert opposing effects on anxiety-like behaviors in normal mice. For instance, activation of somatostatin-positive neurons reduced exploration in the open arms of the EPM. This suggests heightened anxiety, whereas activation of calretinin-positive neurons induces anxiolytic effects[41]. These observations underscore the need for further research to delineate the specific contributions of ZI GABAergic subpopulations in modulating anxiety and emotional behaviors.
Our study was the first to establish a direct relationship between ZI GABAergic neurons and depression-like behaviors in rodents, highlighting a potential therapeutic target for depression. This finding provides new insights into the neural mechanisms of depression and offers experimental data to support the development of antidepressant drugs targeting the GABAergic system. Furthermore, clinical DBS targeting the ZI may represent a potential therapeutic approach to modulate ZI GABAergic neuron activity and restore the excitation/inhibition balance in the brains of individuals suffering from depression. However, further experimental validation and clinical studies are needed to confirm this hypothesis.
| 1. | Marx W, Penninx BWJH, Solmi M, Furukawa TA, Firth J, Carvalho AF, Berk M. Major depressive disorder. Nat Rev Dis Primers. 2023;9:44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 202] [Reference Citation Analysis (0)] |
| 2. | Pearce M, Garcia L, Abbas A, Strain T, Schuch FB, Golubic R, Kelly P, Khan S, Utukuri M, Laird Y, Mok A, Smith A, Tainio M, Brage S, Woodcock J. Association Between Physical Activity and Risk of Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2022;79:550-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 546] [Article Influence: 182.0] [Reference Citation Analysis (0)] |
| 3. | GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11327] [Cited by in RCA: 9637] [Article Influence: 1927.4] [Reference Citation Analysis (35)] |
| 4. | Friedrich MJ. Depression Is the Leading Cause of Disability Around the World. JAMA. 2017;317:1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 491] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 5. | Bhatt S, Devadoss T, Manjula SN, Rajangam J. 5-HT(3) receptor antagonism a potential therapeutic approach for the treatment of depression and other disorders. Curr Neuropharmacol. 2021;19:1545-1559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Willner P, Scheel-Krüger J, Belzung C. The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev. 2013;37:2331-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 342] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 7. | Asgharian P, Quispe C, Herrera-Bravo J, Sabernavaei M, Hosseini K, Forouhandeh H, Ebrahimi T, Sharafi-Badr P, Tarhriz V, Soofiyani SR, Helon P, Rajkovic J, Durna Daştan S, Docea AO, Sharifi-Rad J, Calina D, Koch W, Cho WC. Pharmacological effects and therapeutic potential of natural compounds in neuropsychiatric disorders: An update. Front Pharmacol. 2022;13:926607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 2479] [Article Influence: 354.1] [Reference Citation Analysis (0)] |
| 9. | Luscher B, Maguire JL, Rudolph U, Sibille E. GABA(A) receptors as targets for treating affective and cognitive symptoms of depression. Trends Pharmacol Sci. 2023;44:586-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 10. | Sarawagi A, Soni ND, Patel AB. Glutamate and GABA Homeostasis and Neurometabolism in Major Depressive Disorder. Front Psychiatry. 2021;12:637863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 11. | Engin E, Benham RS, Rudolph U. An Emerging Circuit Pharmacology of GABA(A) Receptors. Trends Pharmacol Sci. 2018;39:710-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 12. | Della Vecchia A, Arone A, Piccinni A, Mucci F, Marazziti D. GABA System in Depression: Impact on Pathophysiology and Psychopharmacology. Curr Med Chem. 2022;29:5710-5730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Lloyd KG, Zivkovic B, Scatton B, Morselli PL, Bartholini G. The gabaergic hypothesis of depression. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:341-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Duman RS, Sanacora G, Krystal JH. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron. 2019;102:75-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 635] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 15. | Gerner RH, Hare TA. CSF GABA in normal subjects and patients with depression, schizophrenia, mania, and anorexia nervosa. Am J Psychiatry. 1981;138:1098-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 139] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Kargbo RB. Neurosteroids and Postpartum Depression: The Mechanism, Efficacy, and Approval of Brexanolone and Zurzuvae. ACS Med Chem Lett. 2023;14:1326-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 17. | Cerne R, Lippa A, Poe MM, Smith JL, Jin X, Ping X, Golani LK, Cook JM, Witkin JM. GABAkines - Advances in the discovery, development, and commercialization of positive allosteric modulators of GABA(A) receptors. Pharmacol Ther. 2022;234:108035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 18. | Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 412] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 19. | Fratzl A, Hofer SB. The caudal prethalamus: Inhibitory switchboard for behavioral control? Neuron. 2022;110:2728-2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 20. | Wang X, Chou XL, Zhang LI, Tao HW. Zona Incerta: An Integrative Node for Global Behavioral Modulation. Trends Neurosci. 2020;43:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 21. | Tommasi G, Lanotte M, Albert U, Zibetti M, Castelli L, Maina G, Lopiano L. Transient acute depressive state induced by subthalamic region stimulation. J Neurol Sci. 2008;273:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Burrows AM, Ravin PD, Novak P, Peters ML, Dessureau B, Swearer J, Pilitsis JG. Limbic and motor function comparison of deep brain stimulation of the zona incerta and subthalamic nucleus. Neurosurgery. 2012;70:125-130; discussion 130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Cai H, Zhang P, Qi G, Zhang L, Li T, Li M, Lv X, Lei J, Ming J, Tian B. Systematic Input-Output Mapping Reveals Structural Plasticity of VTA Dopamine Neurons-Zona Incerta Loop Underlying the Social Buffering Effects in Learned Helplessness. Mol Neurobiol. 2022;59:856-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Tong Y, Cho S, Coenen VA, Döbrössy MD. Input-output relation of midbrain connectomics in a rodent model of depression. J Affect Disord. 2024;345:443-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Li JN, Wu XM, Zhao LJ, Sun HX, Hong J, Wu FL, Chen SH, Chen T, Li H, Dong YL, Li YQ. Central medial thalamic nucleus dynamically participates in acute itch sensation and chronic itch-induced anxiety-like behavior in male mice. Nat Commun. 2023;14:2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 26. | Liu WZ, Zhang WH, Zheng ZH, Zou JX, Liu XX, Huang SH, You WJ, He Y, Zhang JY, Wang XD, Pan BX. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat Commun. 2020;11:2221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 247] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 27. | Ye F, Dong MC, Xu CX, Jiang N, Chang Q, Liu XM, Pan RL. Effects of different chronic restraint stress periods on anxiety- and depression-like behaviors and tryptophan-kynurenine metabolism along the brain-gut axis in C57BL/6N mice. Eur J Pharmacol. 2024;965:176301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 28. | Zhang YF, Wu J, Wang Y, Johnson NL, Bhattarai JP, Li G, Wang W, Guevara C, Shoenhard H, Fuccillo MV, Wesson DW, Ma M. Ventral striatal islands of Calleja neurons bidirectionally mediate depression-like behaviors in mice. Nat Commun. 2023;14:6887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 29. | Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 462] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 30. | Wang M, Li P, Li Z, da Silva BS, Zheng W, Xiang Z, He Y, Xu T, Cordeiro C, Deng L, Dai Y, Ye M, Lin Z, Zhou J, Zhou X, Ye F, Cunha RA, Chen J, Guo W. Lateral septum adenosine A(2A) receptors control stress-induced depressive-like behaviors via signaling to the hypothalamus and habenula. Nat Commun. 2023;14:1880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 31. | Acikgoz B, Dalkiran B, Dayi A. An overview of the currency and usefulness of behavioral tests used from past to present to assess anxiety, social behavior and depression in rats and mice. Behav Processes. 2022;200:104670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Markov DD. Sucrose Preference Test as a Measure of Anhedonic Behavior in a Chronic Unpredictable Mild Stress Model of Depression: Outstanding Issues. Brain Sci. 2022;12:1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 33. | Olney JJ, Marshall SA, Thiele TE. Assessment of depression-like behavior and anhedonia after repeated cycles of binge-like ethanol drinking in male C57BL/6J mice. Pharmacol Biochem Behav. 2018;168:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Bouguiyoud N, Roullet F, Bronchti G, Frasnelli J, Al Aïn S. Anxiety and Depression Assessments in a Mouse Model of Congenital Blindness. Front Neurosci. 2021;15:807434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Venkataraman A, Hunter SC, Dhinojwala M, Ghebrezadik D, Guo J, Inoue K, Young LJ, Dias BG. Incerto-thalamic modulation of fear via GABA and dopamine. Neuropsychopharmacology. 2021;46:1658-1668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Arena G, Londei F, Ceccarelli F, Ferrucci L, Borra E, Genovesio A. Disentangling the identity of the zona incerta: a review of the known connections and latest implications. Ageing Res Rev. 2024;93:102140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Thierry B, Stéru L, Simon P, Porsolt RD. The tail suspension test: ethical considerations. Psychopharmacology (Berl). 1986;90:284-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Canbeyli R. Sensorimotor modulation of mood and depression: an integrative review. Behav Brain Res. 2010;207:249-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Gencturk S, Unal G. Rodent tests of depression and anxiety: Construct validity and translational relevance. Cogn Affect Behav Neurosci. 2024;24:191-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 40. | Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2247] [Article Influence: 102.1] [Reference Citation Analysis (0)] |
| 41. | Li Z, Rizzi G, Tan KR. Zona incerta subpopulations differentially encode and modulate anxiety. Sci Adv. 2021;7:eabf6709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |