Published online Jan 19, 2025. doi: 10.5498/wjp.v15.i1.97945
Revised: September 8, 2024
Accepted: October 28, 2024
Published online: January 19, 2025
Processing time: 155 Days and 20.7 Hours
Mild cognitive impairment (MCI) has a high risk of progression to Alzheimer’s disease. The disease is often accompanied by sleep disorders, and whether sleep disorders have an effect on brain function in patients with MCI is unclear.
To explore the near-infrared brain function characteristics of MCI with sleep disorders.
A total of 120 patients with MCI (MCI group) and 50 healthy subjects (control group) were selected. All subjects underwent the functional near-infrared spec
Compared with the control group, the FSS score of the MCI group was higher (t = 11.310), and the scores of Pittsburgh sleep quality index, sleep time, sleep efficiency, nocturnal sleep disturbance, and daytime dysfunction were higher (Z = -10.518, -10.368, -9.035, -10.661, -10.088). Subjective sleep quality and total sleep time scores were lower (Z = -11.592, -9.924). The sleep efficiency of the MCI group was lower, and the awakening frequency, rem sleep latency period, total sleep time, and oxygen desaturation index were higher (t = 5.969, 5.829, 2.887, 3.003, 5.937). The Oxy-Hb concentration at T0, T1, and T2 in the MCI group was lower (t = 14.940, 11.280, 5.721), and the peak time was higher (t = 18.800, 13.350, 9.827). In MCI patients, the concentration of Oxy-Hb during T0 was negatively correlated with the scores of Pittsburgh sleep quality index, sleep time, total sleep time, and sleep efficiency (r = -0.611, -0.388, -0.563, -0.356). It was positively correlated with sleep efficiency and total sleep time (r = 0.754, 0.650), and negatively correlated with oxygen desaturation index (r = -0.561) and FSS score (r = -0.526). All comparisons were P < 0.05.
Patients with MCI and sleep disorders have lower near-infrared brain function than normal people, which is related to sleep quality. Clinically, a comprehensive assessment of the near-infrared brain function of patients should be carried out to guide targeted treatment and improve curative effect.
Core Tip: Mild cognitive impairment (MCI) is a transitional state from normal cognitive development to dementia. Here, we analyzed 120 patients with MCI accompanied by sleep disorders and 50 healthy subjects and mainly observed the near-infrared brain function characteristics of the two groups and their relationship with the sleep status of patients with MCI. Through statistical analysis, we put forward the theory that the near-infrared brain function of MCI patients with sleep disorders has poor performance and is closely related to sleep quality and fatigue, which has a certain breakthrough.
- Citation: Liao H, Liao S, Gao YJ, Wang X, Guo LH, Zheng S, Yang W, Dai YN. Near-infrared brain functional characteristics of mild cognitive impairment with sleep disorders. World J Psychiatry 2025; 15(1): 97945
- URL: https://www.wjgnet.com/2220-3206/full/v15/i1/97945.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i1.97945
Mild cognitive impairment (MCI) is a common cognitive impairment with clinical manifestations between normal and dementia. It is a transitional state from normal cognitive development to dementia[1]. According to the survey, the incidence of MCI in the elderly is about 18.8%, increasing with the intensification of population ageing[2].
MCI, the main manifestation of memory loss, has not significantly affected the basic daily life and social activities of patients. However, MCI has a high risk of progression to Alzheimer’s disease (AD). Studies have shown that the incidence of AD in MCI patients is 10 times higher than that in healthy elderly people[3]. According to statistics, 10%-15% of MCI patients over 65 years old in China get worse and progress to AD every year[4]. In addition, MCI is often accompanied by a variety of sleep-wake disorders, including insomnia, sleep-related breathing disorders, rapid eye movement sleep behaviour disorders, circadian dysrhythmic sleep-wake disorders, excessive daytime sleepiness, restless leg syndrome, etc., which are mainly manifested by abnormal sleep structures such as reduced total sleep time, reduced sleep efficiency, and increased wake time and frequency. Studies have shown that the incidence of sleep disorders in patients with MCI is as high as 63%[5]. A large cohort study indicates that over 60% of MCI patients report sleep disorders, such as difficulty falling asleep, frequent awakenings during the night, and early awakenings. These sleep disorders not only severely impact the patients’ quality of life but may also accelerate further cognitive decline[6]. MCI patients exhibit significant changes in their sleep structure, including reduced deep sleep (slow-wave sleep) and increased light sleep and rapid eye movement sleep, which are closely linked to the extent of cognitive impairment[7]. Sleep disorders may affect neurotransmitter regulation, such as disrupting the balance of the cholinergic system; interfere with memory consolidation and hinder the brain’s storage of new information; cause neuroinflammatory responses and damage neurons[8]. These factors work together to lead to a more significant decline in cognitive ability. MCI accom
MCI is the early stage of the onset of AD and the optimal time window for treatment. At present, it is not clear whether sleep disorders have an impact on brain function in patients with MCI. Therefore, timely and comprehensive assessment of MCI with sleep disorders is of great significance for clinical intervention decision-making. Based on multiple indexes of deoxyhemoglobin and oxyhemoglobin (Oxy-Hb), functional near-infrared spectroscopy (fNIRS) can effectively detect brain functions related to neuronal activity, cellular energy metabolism, and hemodynamics[10]. However, there are few reports on the application of fNIRS in the assessment of MCI with sleep disorders. Based on this, this study intends to investigate the near-infrared brain functional characteristics of MCI with sleep disorders through fNIRS, to provide references for clinical research.
One hundred and twenty patients with MCI who were outpatients and inpatients of internal medicine in our hospital from August 2021 to July 2023 were selected and classified into the MCI group according to the designed inclusion and exclusion criteria. Inclusion conditions: (1) The patient complained of memory impairment and was diagnosed with MCI; (2) Accompanied by sleep disorders; (3) Memory impairment inconsistent with age and education level; (4) Overall cognitive function is normal and the ability to live daily was normal; (5) The treatment compliance was good; and (6) The diagnostic criteria for dementia have not been met. Exclusion criteria: (1) History of cerebrovascular disease; (2) Serious heart and lung, liver, and kidney diseases; (3) Suffering from diseases causing cognitive dysfunction such as AD, Parkinson’s disease, pulmonary cardiopathy, cardiac insufficiency, viral or autoimmune encephalitis, brain tumors, etc.; (4) Taking sleeping pills, excessive alcohol consumption; and (5) Depression was assessed according to the Hamilton Depression Scale[11].
In addition, 50 healthy persons matching the age, gender, and education level of the MCI group were selected and classified into the control group. MCI has no history of neurological disease, brain trauma, drug dependence, long-term alcohol consumption, or mental illness. This study protocol was reviewed and approved by the Medical Ethics Committee of our hospital. All subjects underwent fNIRS testing and had complete test records. We have obtained the consent of all subjects before accessing and accessing clinical data.
Refer to the MCI-related diagnostic criteria developed by the working group of the National Institute on Aging and the Alzheimer’s Association in 2011[12]: (1) The patient’s subjective feelings and (or) the informed person confirmed that there was a decline in cognitive function (mostly memory), and the course of the disease was more than 3 months; (2) Objective examination of cognitive decline. Mini-Mental State Examination (MMSE)[13] scores ≤ 26 points, Montreal Cognitive Assessment (MoCA) scale[14] scores ≤ 24 points (years of education ≤ 12 years plus 1 point); cognitive test scores are 1-1.5 standard deviations below the norm for age and literacy; (3) The diagnostic criteria for dementia have not been met. The rating of the global deterioration scale[15] is 2-3. Clinical dementia rating[16] = 0.5 points; MMSE scale score: Primary school level > 20 points, middle school level (including technical secondary school) > 22 points, university level (including junior college) > 23 points; and (4) The basic ability of daily living remains normal, and the ability of complex instrumental activity of daily living[17] may be slightly impaired.
Clinical data and indicators were collected: (1) Baseline data: Sex, age, body mass index, years of schooling, duration of MCI disease, systolic blood pressure, diastolic blood pressure; (2) MMSE, MoCA, fatigue severity scale (FSS), Pittsburgh sleep quality index (PSQI) scores; (3) Sleep parameters: Sleep efficiency, awakening frequency, rem sleep latency period, total sleep time, oxygen desaturation index; and (4) Oxy-Hb concentration and peak time of fNIRS test during the task period.
Assessment scale: (1) The MMSE scale assessed the cognitive function status of the subjects, with a total score of 30 points, covering the test items such as orientation, calculation, language function memory, etc. The lower the score, the more serious the cognitive function was impaired, and those with a score of 26 or more were considered to have normal cognitive function; (2) The MoCA scale includes 7 cognitive domains, including directed visual space, executive function, naming, memory, attention, language, abstract generalization, and various aspects of cognitive function such as orientation, executive ability, and memory. The highest total score is 30 points, and ≤ 26 points are cognitive dysfunction. If the subject’s years of schooling were less than 12 years, the positive education bias was measured by adding 1 point. The lower the score, the more serious the degree of cognitive function impairment, 19 to 25 is classified as mild cognitive function impairment; (3) The FSS[18] was used to evaluate the degree of fatigue. The scale consists of 9 rating items, each divided into 7 points, with 1 being “strongly disagree” and 7 being “strongly agree”. A higher total score indicates more severe fatigue; and (4) Sleep quality in the last 1 month was assessed by the PSQI[19]. The questionnaire consisted of 19 items with 6 components (subjective sleep quality, sleep time, total sleep time, sleep efficiency, nocturnal sleep, distur
Conventional Western medicine treatment: Donepezil hydrochloride tablets (Shaanxi Fangzhou Pharmaceutical Co., LTD., Sinopath H20030583, 5 mg) taken orally before bed, 5 mg/time, once/day, for 8 weeks. For those with mild sleep disorders, relaxation training, sleep restriction, and stimulation control are used. For those with mild and severe sleep disorders, drugs such as sleeping pills and hypnotics are used.
The principle behind measuring frontal lobe activity with fNIRS technology is primarily based on the tightly coupled relationship between neuronal activation and vascular response. fNIRS selects the frontal lobe as the measurement site due to several key reasons and principles: (1) The frontal lobe is crucial for cognitive functions, with the prefrontal cortex playing a pivotal role in advanced cognitive processes such as problem-solving, memory, judgment, and impulse control. These functions are essential for understanding human behavior and cognitive mechanisms. Additionally, the prefrontal cortex is implicated in emotional processing and decision-making, and is a primary affected area in numerous neurological and psychiatric disorders, including depression, anxiety, and schizophrenia; (2) Amenable to optical measurement. Located relatively shallow beneath the scalp, the prefrontal cortex is more accessible for optical mea
fNIRS tests were performed in the MCI group before treatment (T0) and at 4 weeks (T1) and 8 weeks (T2) after treatment, while fNIRS tests were performed in the control group during physical examination. The equipment is produced by Wuhan, China Zilian Hongkang, model BS-3000. The device consists of 16 light sources and 16 detectors, forming a total of 53 channels, arranged in a rectangle of 4 rows by 9 columns, the channel spacing is 3 cm, and the sampling frequency is 20 Hz. Localization was performed with reference to the international 10-20 system, covering bilateral frontal pole, bilateral frontal eye area, bilateral Broca area, and bilateral dorsolateral prefrontal lobe. Task method: The verbal fluency task (VFT) involves sitting in a chair with arms in a quiet environment, with eyes open and body relaxed. Patients were grouped with words of “day”, “cloud”, “learning”, and “Shang” respectively, 15 seconds for each group, 60 seconds in total, and 30 seconds for rest.
SPSS 23.0 statistical software package was used for statistical analysis. Measurement data conforming to normal distribution were represented by mean ± SD, an independent sample t-test was performed, and Pearson correlation analysis was used for correlation. The measurement data that did not conform to the normal distribution were described by P50 (P25, P75) and subjected to a non-parametric test (Z), and the correlation was analyzed by Spearmen correlation. Count data use case description, row χ2 test. Interpretation of Pearson or Spearmen correlation analysis results: |r| > 0.8, indicating a high correlation among the variables. 0.5 < |r|≤ 0.8 indicates a moderate correlation between the variables. 0.3 < |r|≤ 0.5 indicates a low correlation between the variables. P < 0.05 was considered to be statistically significant.
Compared with the control group, systolic and diastolic blood pressure were higher in the MCI group (P > 0.05) (Table 1).
| Group | MCI group (n = 120) | Control group (n = 50) | t | P value |
| Sex (male/female) | 67/53 | 30/20 | 0.250 | 0.617 |
| Age (years) | 65.39 ± 7.16 | 64.87 ± 8.03 | 0.416 | 0.678 |
| Body mass index (kg/m2) | 22.66 ± 2.18 | 22.74 ± 2.34 | 0.213 | 0.831 |
| Years of schooling (years) | 6.51 ± 2.02 | 6.39 ± 1.68 | 0.370 | 0.712 |
| Duration of MCI (months) | 4.38 ± 1.02 | 4.21 ± 1.13 | 0.959 | 0.339 |
| Systolic pressure (mmHg) | 138.21 ± 23.68 | 124.32 ± 10.15 | 3.992 | < 0.001 |
| Diastolic blood pressure (mmHg) | 86.20 ± 9.23 | 79.22 ± 6.69 | 4.840 | < 0.001 |
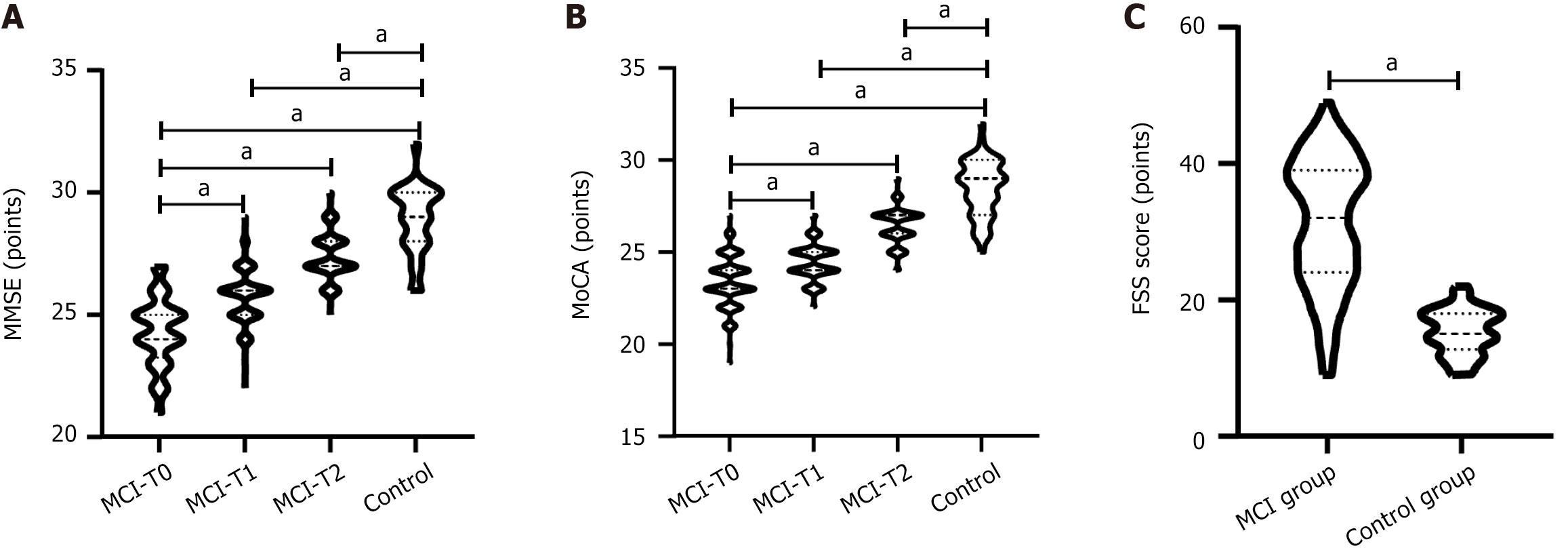
The MMSE scores at T0, T1, and T2 in the MCI group were 24.32 ± 1.41, 25.73 ± 1.08, and 27.38 ± 0.95, respectively, while those in the control group were 29.12 ± 1.68. MoCA scores at T0, T1, and T2 in the MCI group were 23.37 ± 1.40, 24.43 ± 1.00, and 26.42 ± 1.04, respectively, while those in the control group were 28.56 ± 1.58. In the MCI group, MMSE scores at T0, T1 and T2 were increased successively (t: T0 vs T1 = 8.696, T0 vs T2 = 19.720, T1 vs T2 = 12.570), and MoCA scores were increased successively (t: T0 vs T1 = 6.749, T0 vs T2 = 19.160, T1 vs T2 = 15.110); compared with the control group, MMSE scores at T0, T1, and T2 were all lower (t = 19.090, 15.680, 8.548) (Figure 1A), and MoCA scores were all lower (t = 26.930, 24.200, 12.390) (P < 0.001 for all) (Figure 1B). The FSS score in the MCI group was 31.25 ± 9.68 and that in the control group was 15.39 ± 3.25, and the FSS score in the MCI group was higher (t = 11.310, P < 0.001) (Figure 1C).
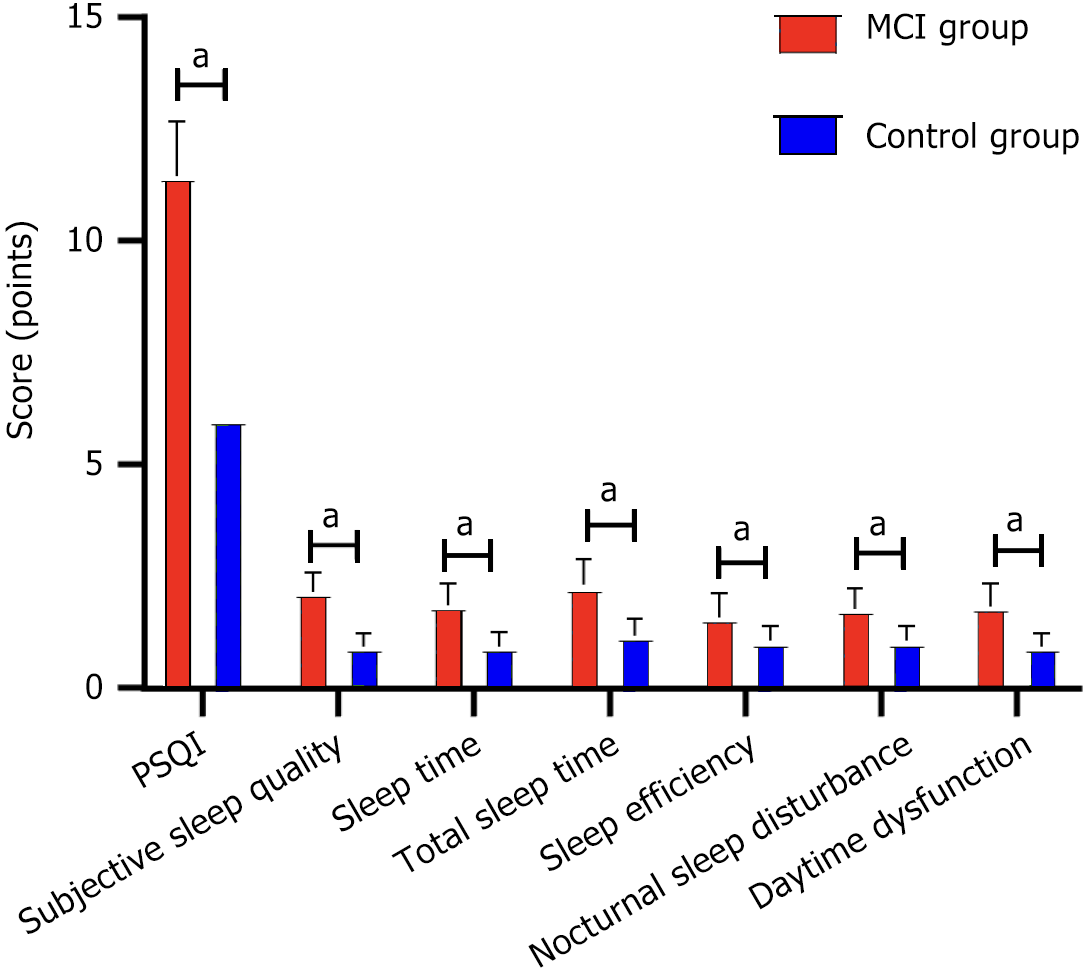
Compared with the control group, the scores of PSQI, sleeping time, sleep efficiency, night sleep disorder, daytime dysfunction, and other dimensions were higher in the MCI group, and the scores of subjective sleep quality and total sleep time were lower (P < 0.05) (Table 2 and Figure 2).
| Group | PSQI | Subjective sleep quality | Sleep time | Total sleep time | Sleep efficiency | Nocturnal sleep disturbance | Daytime dysfunction |
| MCI group (n = 120) | 6 (6, 6) | 2 (2, 2) | 0 (0, 1) | 2 (2, 3) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) |
| Control group (n = 50) | 9 | 1 (1, 1) | 2 (2, 2) | 1 (1, 1) | 2 (1, 2) | 2 (1, 2) | 2 (1.75, 2) |
| Z | -10.518 | -11.592 | -10.368 | -9.924 | -9.035 | -10.661 | -10.088 |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
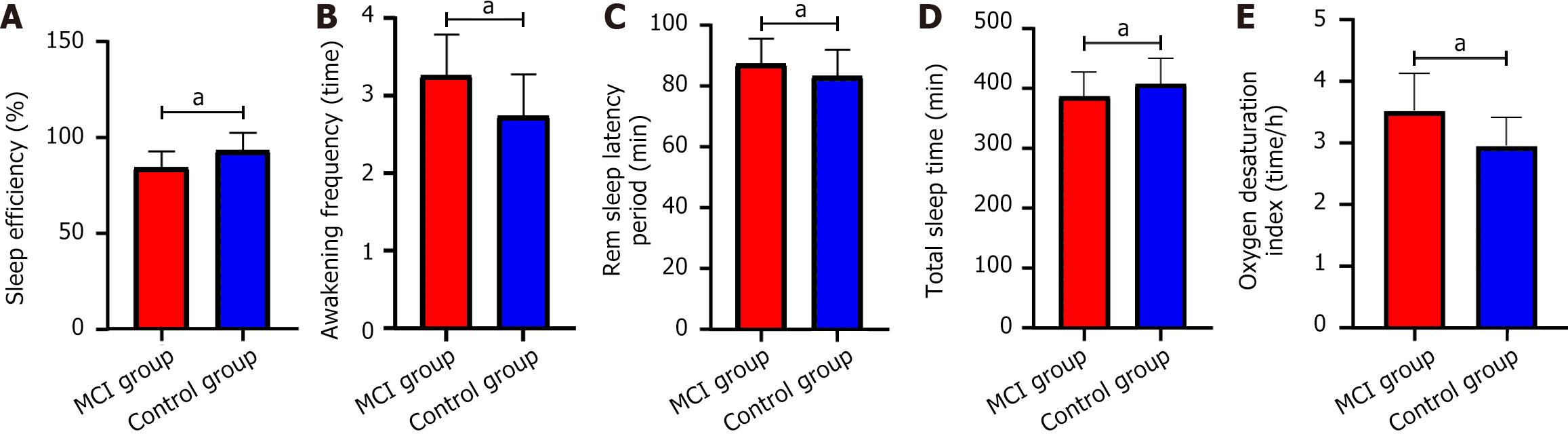
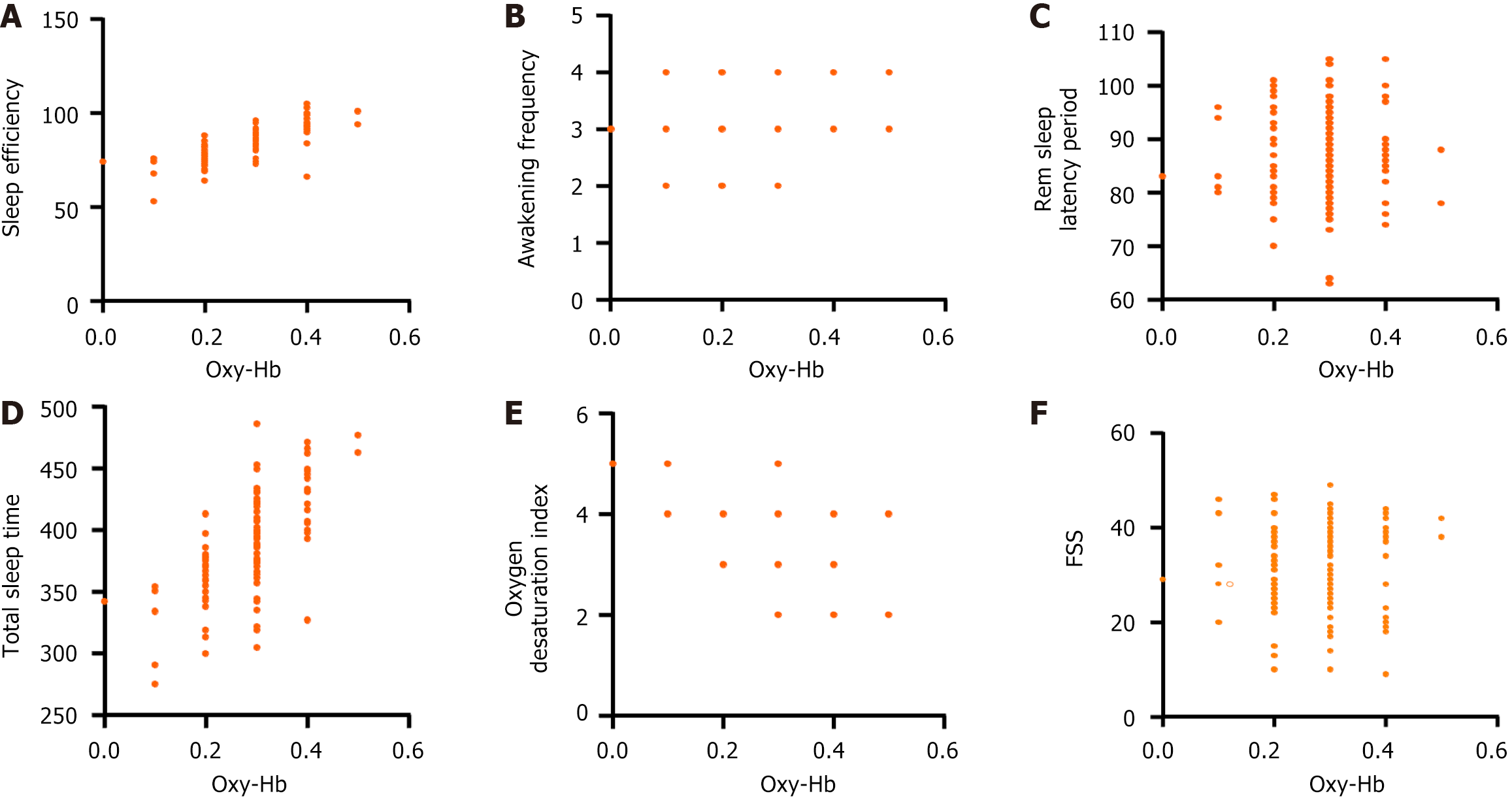
Sleep efficiency, awakening frequency, rem sleep latency period, total sleep time, oxygen desaturation index in the MCI group were 84.36% ± 8.52%, 3.26 ± 0.53 times, 87.35 ± 8.37 minutes, 386.58 ± 40.39 minutes, 3.52 ± 0.61 time/hour, respectively. The control group was 93.15% ± 9.28%, 2.74 ± 0.53 times, 83.22 ± 8.80 minutes, 407.26 ± 42.17 minutes, 2.95 ± 0.46 time/hour, respectively. The sleep efficiency of the MCI group was lower (t = 5.969, P < 0.001), and the awakening frequency (t = 5.829, P < 0.001), rem sleep latency period (t = 2.887, P = 0.004), total sleep time (t = 3.003, P = 0.003), and the oxygen desaturation index was higher (t = 5.937, P < 0.001) (Figure 3).
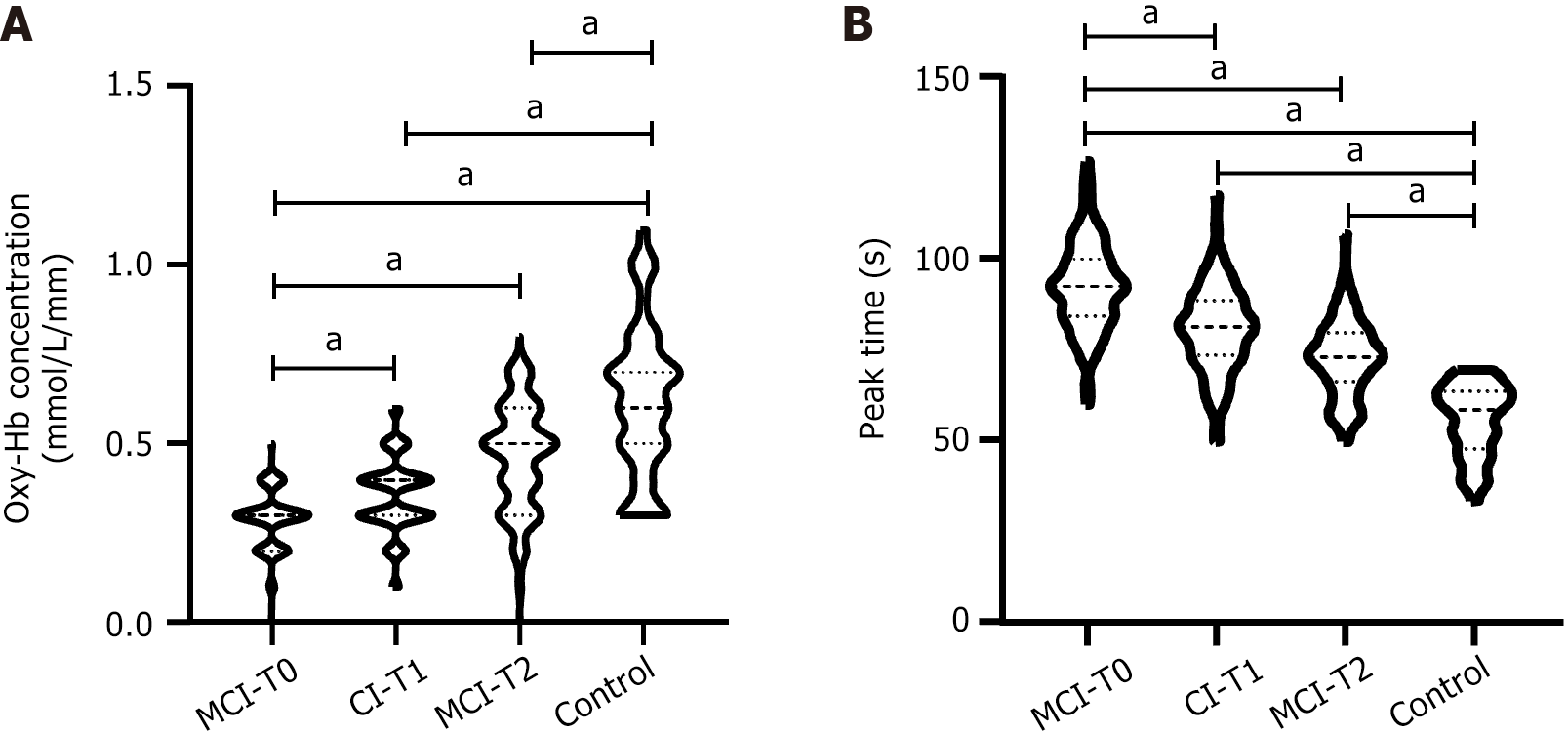
The functional Oxy-Hb concentrations of T0, T1, and T2 in MCI group were 0.30 ± 0.08 mmol/L/mm, 0.35 ± 0.11 mmol/L/mm, and 0.46 ± 0.15 mmol/L/mm, respectively, while those in control group were 0.62 ± 0.20 mmol/L/mm. The peak time at T0, T1, and T2 in the MCI group was 92.39 ± 12.23 seconds, 80.52 ± 11.47 seconds, and 72.58 ± 10.29 seconds, respectively, while that in the control group was 55.82 ± 9.74 seconds.
In the MCI group, Oxy-Hb concentration in T0, T1, and T2 during the task period increased successively (t: T0 vs T1 = 4.027, T0 vs T2 = 10.310, T1 vs T2 = 6.478), and the peak time decreased successively (t: T0 vs T1 = 7.755, T0 vs T2 = 13.580, T1 vs T2 = 5.645); compared with the control group, the Oxy-Hb concentration at T0, T1, and T2 was lower (t = 14.940, 11.280, 5.721), and the peak time was higher (t = 18.800, 13.350, 9.827) (all P < 0.001) (Figure 4).
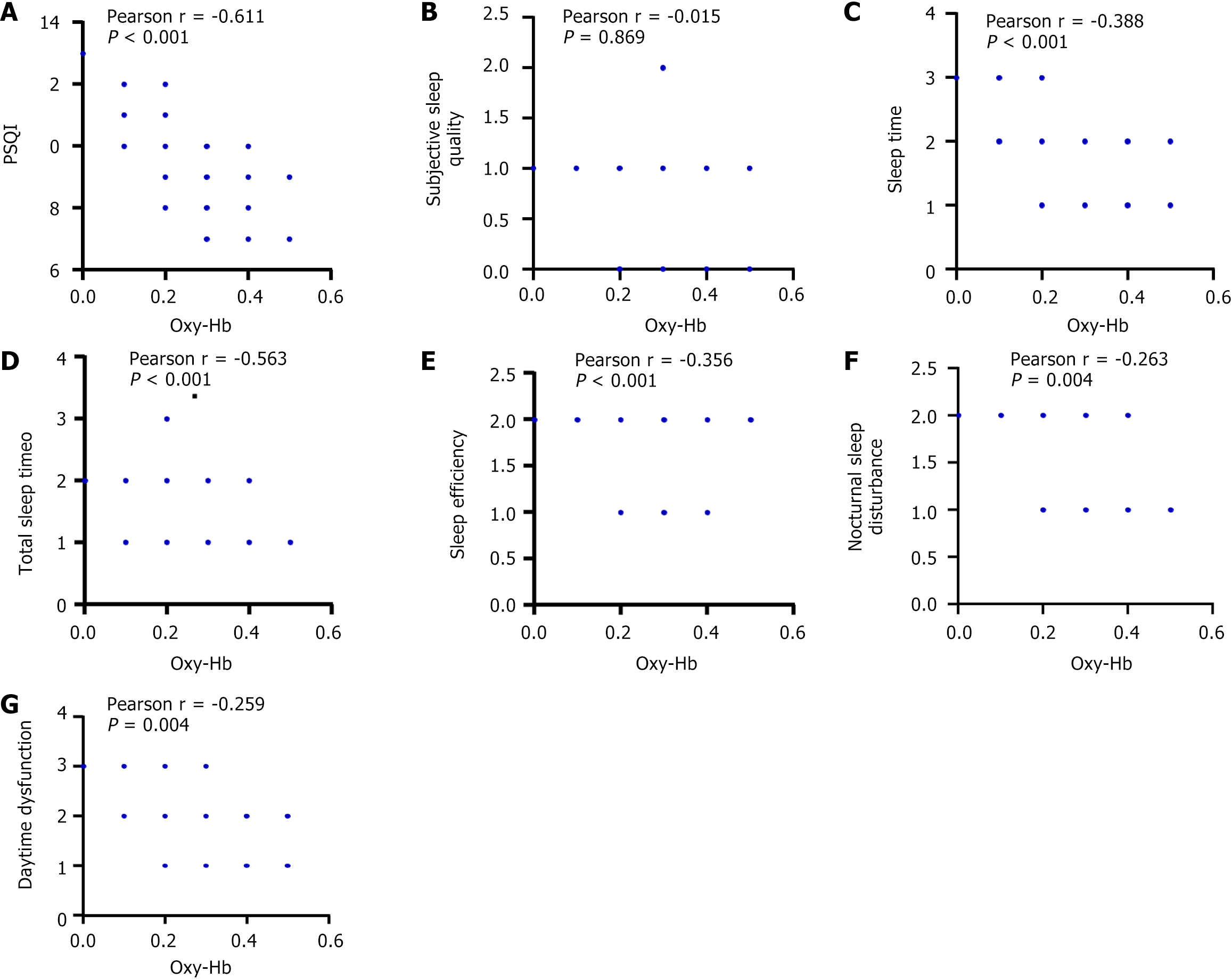
Oxy-Hb concentration during T0 was negatively correlated with the PSQI score and its dimensions such as sleep time, total sleep time, and sleep efficiency scores (r = -0.611, -0.388, -0.563, -0.356; all P < 0.001) (Figure 5). The functional Oxy-Hb concentration at T0 was positively correlated with sleep efficiency and total sleep time (r = 0.754, 0.650, both P < 0.001), and negatively correlated with oxygen depletion index (r = -0.561, P < 0.001) and FSS score (r = -0.526, P = 0.039) (Figure 6).
In the context of the accelerating ageing trend of the population, the number of MCI cases is increasing year by year, which has a serious impact on the quality of life of patients and their families. At present, how to accurately, scientifically and effectively evaluate MCI remains a challenge. Studies have found that factors such as high blood pressure, sleep and fatigue are risk factors for MCI[20]. MCI is commonly associated with sleep disorders, and the brain function status of these patients is unknown. fNIRS technology can detect the haemoglobin content in blood flow to evaluate the activity and characteristics of brain function[21]. Therefore, it is necessary to explore the near-infrared brain function and its relationship with sleep quality in patients with MCI accompanied by sleep disorders.
Sleep disorders can exacerbate MCI or cause more significant cognitive decline via various mechanisms, including neurotransmitter imbalances, activation of inflammatory responses, and abnormalities in brain energy metabolism[22]. These mechanisms, when combined, can impair the normal function and synaptic plasticity of brain neurons, thereby accelerating cognitive decline. Our study centers on the assessment of brain function, with the goal of using functional neuroimaging (fNIRS) to monitor and quantify these changes in real time, thereby providing a scientific basis for understanding the complex relationship between sleep and cognitive disorders. Through statistical analysis, we found that the MMSE and MoCA scores of MCI patients with sleep disorders were lower than those of normal people of the same age. However, after treatment, the lower range would become smaller and smaller and tend to the normal level. In addition, the patients’ FSS score was at a high level, indicating that fatigue was common in patients with MCI accom
To further explore the near-infrared brain functional characteristics of patients with MCI accompanied by sleep disorders, we used Pearson or Spearmen correlation analysis to analyze the relationship between Oxy-Hb concentration and sleep quality, parameters and fatigue index of patients during the task period. We found that Oxy-Hb concentration during T0 was negatively correlated with PSQI score and its dimensions such as sleep time, total sleep time and sleep efficiency scores, positively correlated with sleep efficiency and total sleep time, and negatively correlated with oxygen desaturation index and FSS scores. Among them, the Oxy-Hb concentration during the task period was closely related to PQSI score, sleep efficiency and total sleep time (R-value ranked the top 3, respectively -0.611, 0.754, and 0.650). Based on the matching mechanism between nerves and blood vessels, during the cognitive activity of the brain, the blood flow and oxygen carried in the brain area increase, and the concentration of Oxy-Hb in the blood increases accordingly[24]. Li et al[25] found that compared with patients with major depressive disorder who did not have insomnia, the dorsolateral prefrontal cortex of patients with insomnia, Oxy-Hb concentration was lower in dorsolateral prefrontal cortex and bilateral medial prefrontal cortex. It can be seen that cognitive function and sleep disorders simultaneously affect the concentration of Oxy-Hb in the brain and reduce the activity of brain function. In addition, lack of sleep also exacerbates cognitive dysfunction. Sleep deprivation (lack of sleep or sleep disorders) can cause a variety of physical and psychological changes that can lead to further cognitive decline. The process of sleep deprivation may be accompanied by the production of oxygen free radicals, thus inducing DNA damage, resulting in cell apoptosis or mitochondrial function damage, and finally leading to nerve cell function impairment, thus leading to cognitive function decline, which may be the possible mechanism of sleep deprivation causing cognitive function decline[26]. Miyata et al[27] showed that insufficient sleep at night would affect cognitive function during the day, accompanied by changes in cortical oxygenation response (decreased peak oxy-Hb levels in the left and right frontal lobes). Gao et al[28] found that the concentration of oxy-Hb in the 8th channel of the dorsolateral prefrontal cortex in patients with sleep disorders was significantly reduced, revealing that sleep disorders caused changes in brain function. These findings are basically consistent with the results of this study. However, at present, the specific mechanism of sleep disorders on near-infrared brain functional characteristics cannot be determined, and more in-depth and reliable studies are needed to further explore.
Oxygen desaturation index refers to the number of times during sleep that blood oxygen saturation drops by more than 4% per hour. Obstructive sleep apnea shows brain damage in areas responsible for autonomic, cognitive, and respiratory function. Sahib et al[29] showed that the degree of brain damage of obstructive sleep apnea partly depended on the apnea-hypopnea index and oxygen desaturation, which affected its continuous development from acute to chronic. Therefore, we speculate that the higher the hypoxic index means that the more times the blood oxygen saturation drops, the greater the brain damage, and the lower the brain function activity. FSS is an indicator of fatigue level. Cao et al[30] confirmed that fatigue is related to sleep disorders (changes in sleep structure). In addition, Dardin et al[31] proved that poor sleep quality would aggravate fatigue in patients with primary Sjogren’s syndrome. We hypothesize that fatigue in patients with MCI and sleep disorders may alter brain function (oxy-Hb concentration) by affecting sleep quality. But more research is needed. Our study analyzed near-infrared brain functional properties and their correlation with sleep quality in patients with MCI and sleep disorders, but there are limitations.
We propose some specific intervention measures and compliance for patients with MCI accompanied by sleep disorders. In terms of intervention measures, patients should establish a regular sleep schedule, improve sleep quality, and alleviate the impact of sleep disorders on cognitive function. In terms of diet, patients should also have a balanced diet to maintain brain health. Moderate exercise can promote blood circulation in the brain, increase the volume and quantity of dendritic cells, and enhance memory function. Participate in more brain stimulating learning activities to enhance memory function. In terms of compliance, patients’ awareness of MCI and sleep disorders can be improved through health education and other means, making them aware of the importance and necessity of intervention measures, thereby enhancing treatment compliance. At the same time, personalized intervention plans can be developed based on the specific situation of the patient, including intervention content, frequency, intensity, etc., to improve the pertinence and feasibility of the plan, thereby enhancing patient compliance.
The implications of our findings for clinical practice including: (1) Employing fNIRS to monitor brain function changes in MCI patients offers robust support for early diagnosis and intervention. Physicians can tailor personalized treatment plans based on brain function status to slow cognitive decline and enhance quality of life; (2) This study underscores the detrimental impact of sleep disorders on the brain function of MCI patients. Consequently, clinical practice should prioritize the management of sleep disorders in MCI patients by enhancing sleep quality through medication and psychological therapy; and (3) Fatigue is a common symptom in MCI patients, effectively managing it can alleviate cognitive load and enhance quality of life. Physicians can advise patients on organizing their schedules and engaging in moderate physical activities to alleviate fatigue. Future research directions include: (1) Multimodal integration research: Integrating fNIRS with other neuroimaging techniques, such as fMRI and electroencephalography, to conduct multimodal integration research that more thoroughly reveals brain function changes in MCI patients and their relationships with sleep quality and fatigue; (2) Longitudinal follow-up studies: Conducting longitudinal follow-up studies to explore the dynamic interplay between brain function, sleep quality, and fatigue in MCI patients and how they evolve over time; and (3) Evaluating intervention effectiveness: Employing randomized controlled trials and other study designs to assess the effectiveness of different interventions in enhancing brain function, sleep quality, and fatigue in MCI patients, thereby providing robust evidence to support clinical practice.
We used a retrospective analysis method, which may cause selection bias in the study. In addition, this study focused on statistical analysis of the changes in near-infrared brain function and its relationship with sleep quality in patients with MCI accompanied by sleep disorders, ignoring the effects of other factors such as hypertension and treatment methods on near-infrared brain function. Future prospective studies and data expansion are needed to verify the conclusions of this study. Although we have established a correlation, further research is needed to determine the causal relationship and whether these near-infrared brain functional features are unique to MCI. Therefore, we need to expand the sample size and in-depth analysis of patient clinical data in the future.
In summary, patients with MCI and sleep disorders have lower near-infrared brain function than normal people, which is related to sleep quality. Clinically, a comprehensive assessment of the near-infrared brain function of patients should be carried out to guide targeted treatment and improve curative effect.
| 1. | Hu Y, Peng W, Ren R, Wang Y, Wang G. Sarcopenia and mild cognitive impairment among elderly adults: The first longitudinal evidence from CHARLS. J Cachexia Sarcopenia Muscle. 2022;13:2944-2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 155] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 2. | Pieruccini-Faria F, Muir-Hunter SW, Montero-Odasso M. Do depressive symptoms affect balance in older adults with mild cognitive impairment? Results from the "gait and brain study". Exp Gerontol. 2018;108:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Zhao A, Jiao Y, Ye G, Kang W, Tan L, Li Y, Deng Y, Liu J; Alzheimer’s Disease Neuroimaging Initiative (ADNI). Soluble TREM2 levels associate with conversion from mild cognitive impairment to Alzheimer's disease. J Clin Invest. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 4. | Jia L, Du Y, Chu L, Zhang Z, Li F, Lyu D, Li Y, Li Y, Zhu M, Jiao H, Song Y, Shi Y, Zhang H, Gong M, Wei C, Tang Y, Fang B, Guo D, Wang F, Zhou A, Chu C, Zuo X, Yu Y, Yuan Q, Wang W, Li F, Shi S, Yang H, Zhou C, Liao Z, Lv Y, Li Y, Kan M, Zhao H, Wang S, Yang S, Li H, Liu Z, Wang Q, Qin W, Jia J; COAST Group. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. 2020;5:e661-e671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 924] [Article Influence: 184.8] [Reference Citation Analysis (0)] |
| 5. | McKinnon A, Terpening Z, Hickie IB, Batchelor J, Grunstein R, Lewis SJ, Naismith SL. Prevalence and predictors of poor sleep quality in mild cognitive impairment. J Geriatr Psychiatry Neurol. 2014;27:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Cogné É, Postuma RB, Chasles MJ, De Roy J, Montplaisir J, Pelletier A, Rouleau I, Gagnon JF. Montreal Cognitive Assessment and the Clock Drawing Test to Identify MCI and Predict Dementia in Isolated REM Sleep Behavior Disorder. Neurology. 2024;102:e208020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Wang Q, Xu S, Liu F, Liu Y, Chen K, Huang L, Xu F, Liu Y. Causal relationship between sleep traits and cognitive impairment: A Mendelian randomization study. J Evid Based Med. 2023;16:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Zielinski MR, Gibbons AJ. Neuroinflammation, Sleep, and Circadian Rhythms. Front Cell Infect Microbiol. 2022;12:853096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 9. | Yang H, Xu L, Qin W, Hu F, Li L, Chen C, Tang W. Gender differences in the modifying effect of living arrangements on the association of sleep quality with cognitive function among community-dwelling older adults: a cross-sectional study. Front Public Health. 2023;11:1142362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Huang X, Tang J, Luo J, Shu F, Chen C, Chen W. A Wearable Functional Near-Infrared Spectroscopy (fNIRS) System for Obstructive Sleep Apnea Assessment. IEEE Trans Neural Syst Rehabil Eng. 2023;31:1837-1846. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Xia X, Zhang T, Zhang C, Liu R, Yang Y, Liu S, Li X, Yue W. Relation between sleep disorders and post-stroke cognitive impairment. Front Aging Neurosci. 2023;15:1036994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 12. | Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8107] [Cited by in RCA: 7486] [Article Influence: 534.7] [Reference Citation Analysis (0)] |
| 13. | Bai W, Zhang J, Smith RD, Cheung T, Su Z, Ng CH, Zhang Q, Xiang YT. Inter-relationship between cognitive performance and depressive symptoms and their association with quality of life in older adults: A network analysis based on the 2017-2018 wave of Chinese Longitudinal Healthy Longevity Survey (CLHLS). J Affect Disord. 2023;320:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 58] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 14. | Jia X, Wang Z, Huang F, Su C, Du W, Jiang H, Wang H, Wang J, Wang F, Su W, Xiao H, Wang Y, Zhang B. A comparison of the Mini-Mental State Examination (MMSE) with the Montreal Cognitive Assessment (MoCA) for mild cognitive impairment screening in Chinese middle-aged and older population: a cross-sectional study. BMC Psychiatry. 2021;21:485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 347] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 15. | Rodríguez-Hidalgo E, García-Alba J, Novell R, Esteba-Castillo S. The Global Deterioration Scale for Down Syndrome Population (GDS-DS): A Rating Scale to Assess the Progression of Alzheimer's Disease. Int J Environ Res Public Health. 2023;20. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Li F, Harmer P, Eckstrom E, Fitzgerald K, Winters-Stone K. Clinical Effectiveness of Cognitively Enhanced Tai Ji Quan Training on Global Cognition and Dual-Task Performance During Walking in Older Adults With Mild Cognitive Impairment or Self-Reported Memory Concerns : A Randomized Controlled Trial. Ann Intern Med. 2023;176:1498-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Deardorff WJ, Barnes DE, Jeon SY, Boscardin WJ, Langa KM, Covinsky KE, Mitchell SL, Whitlock EL, Smith AK, Lee SJ. Development and External Validation of a Mortality Prediction Model for Community-Dwelling Older Adults With Dementia. JAMA Intern Med. 2022;182:1161-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Ju H, Kim HC, Jang SW, Won Y, Park SG, Leem JH. Relationship between fatigue severity scale and occupational injury in Korean workers. Ann Occup Environ Med. 2021;33:e15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 19. | Wang J, Zhou BY, Lian CL, Zhou P, Lin HJ, Wu SG. Evaluation of Subjective Sleep Disturbances in Cancer Patients: A Cross-Sectional Study in a Radiotherapy Department. Front Psychiatry. 2021;12:648896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Nagelhout G, Ebisch RM, Van Der Hel O, Meerkerk GJ, Magnée T, De Bruijn T, Van Straaten B. Is smoking an independent risk factor for developing cervical intra-epithelial neoplasia and cervical cancer? A systematic review and meta-analysis. Expert Rev Anticancer Ther. 2021;21:781-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Zhu J, Yang C, Song S, Wang R, Gu L, Chen Z. Classification of multiple cancer types by combination of plasma-based near-infrared spectroscopy analysis and machine learning modeling. Anal Biochem. 2023;669:115120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Vicente MC, Paneghini JL, Stabile AM, Amorim M, Anibal Silva CE, Patrone LGA, Cunha TM, Bícego KC, Almeida MC, Carrettiero DC, Gargaglioni LH. Inhibition of Pro-Inflammatory Microglia with Minocycline Improves Cognitive and Sleep-Wake Dysfunction Under Respiratory Stress in a Sporadic Model for Alzheimer's Disease. J Alzheimers Dis. 2023;95:317-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Terpening Z, Lewis SJ, Yee BJ, Grunstein RR, Hickie IB, Naismith SL. Association between Sleep-Disordered Breathing and Neuropsychological Performance in Older Adults with Mild Cognitive Impairment. J Alzheimers Dis. 2015;46:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Acevedo BP, Dattatri N, Le J, Lappinga C, Collins NL. Cognitive Training with Neurofeedback Using fNIRS Improves Cognitive Function in Older Adults. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 25. | Li Y, Li X, Zhaung W, Yu C, Wei S, Li Y, Liu J, Li J, Luorui C, Zheng C, Yu H, Zhou D, Zhang XY. Relationship between cognitive function and brain activation in major depressive disorder patients with and without insomnia: A functional near-infrared spectroscopy (fNIRS) study. J Psychiatr Res. 2024;169:134-141. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Khan MA, Al-Jahdali H. The consequences of sleep deprivation on cognitive performance. Neurosciences (Riyadh). 2023;28:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 27. | Miyata S, Noda A, Ozaki N, Hara Y, Minoshima M, Iwamoto K, Takahashi M, Iidaka T, Koike Y. Insufficient sleep impairs driving performance and cognitive function. Neurosci Lett. 2010;469:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Gao J, Zhang L, Zhu J, Guo Z, Lin M, Bai L, Zheng P, Liu W, Huang J, Liu Z. Prefrontal Cortex Hemodynamics and Functional Connectivity Changes during Performance Working Memory Tasks in Older Adults with Sleep Disorders. Brain Sci. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Sahib A, Roy B, Kang D, Aysola RS, Wen E, Kumar R. Relationships between brain tissue damage, oxygen desaturation, and disease severity in obstructive sleep apnea evaluated by diffusion tensor imaging. J Clin Sleep Med. 2022;18:2713-2721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Cao XY, Zhang JR, Shen Y, Mao CJ, Shen YB, Cao YL, Gu HY, Wang F, Liu CF. Fatigue correlates with sleep disturbances in Parkinson disease. Chin Med J (Engl). 2020;134:668-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Dardin LP, Garcia ABA, Gazoni FM, Santos FCD, Mello MT, Trevisani VFM. Correlation of sleep quality with fatigue and disease activity among patients with primary Sjögren's syndrome: a cross-sectional study. Sao Paulo Med J. 2020;138:146-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |