Published online Jan 19, 2025. doi: 10.5498/wjp.v15.i1.102131
Revised: November 12, 2024
Accepted: December 2, 2024
Published online: January 19, 2025
Processing time: 69 Days and 21 Hours
This manuscript explores the recent study by Cui et al which assessed the interplay between inflammatory cytokines and brain-derived neurotrophic factor (BDNF) levels in first-episode schizophrenia patients. The study revealed that higher levels of interleukin-6 and tumor necrosis factor-α correlated with reduced BDNF levels and poorer cognitive performance. Schizophrenia is a severe psy
Core Tip: The recent research by Cui et al highlights the role of brain-derived neurotrophic factor and inflammatory markers, such as interleukin-6, in cognitive impairment, as well as in early diagnosis and treatment of schizophrenia. Thus, we emphasize the importance of personalized treatment that integrates pharmacological and non-pharmacological interventions, combined with continuous monitoring of cognitive function and neuroinflammatory markers, to optimize patient outcomes and address both symptomatic and cognitive challenges effectively.
- Citation: Okpete UE, Byeon H. Brain-derived neurotrophic factor alterations and cognitive decline in schizophrenia: Implications for early intervention. World J Psychiatry 2025; 15(1): 102131
- URL: https://www.wjgnet.com/2220-3206/full/v15/i1/102131.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i1.102131
Schizophrenia is a severe psychiatric disorder affecting approximately 1% of the global population. The clinical presentation is often categorized into positive and negative symptoms. Positive symptoms are additional experiences or behaviors that are not typically present in individuals without schizophrenia, including positive psychotic symptoms such as hallucinations, delusions, and disorganized behavior. In contrast, negative symptoms represent a decrease or loss in the ability to function normally, including diminished motivation, lack of energy, and cognitive impairments, particularly in executive function and memory[1]. Cognitive deficits are a core feature of schizophrenia, often persisting despite remission of psychotic symptoms, especially during the first episode. This impairment severely affects patients' social and occupational functioning, contributing to unemployment, isolation, and stigma. Furthermore, patients often suffer from comorbidities such as poor diet, substance use, and smoking, which is associated with a reduction in life expectancy by 13–15 years. Alarmingly, the lifetime risk of suicide among individuals with schizophrenia is 5%-10%[2].
The complexity of schizophrenia extends to its pathophysiology, with accumulating evidence suggesting that dysregulation in multiple neurotransmitter systems, including dopaminergic, glutamatergic, serotonergic, and gamma-aminobutyric acid pathways, contributes to the disorder. This dysregulation may disrupt the functioning of interneurons, which affects both micro-circuits and macro-circuits in the brain and may manifest as a combination of cognitive, behavioral, and social dysfunction. Schizophrenia’s etiology is multifaceted, with risk factors including genetic predispositions, birth complications, maternal health, and environmental stressors such as social isolation and cannabis use[2-6].
While the exact mechanisms underpinning cognitive dysfunction in schizophrenia remain elusive, emerging research suggests brain-derived neurotrophic factor (BDNF), a critical protein within the neurotrophin family that supports central nervous system function, and cytokines play crucial roles in schizophrenia pathophysiology, particularly in cognitive decline. Patients with schizophrenia exhibit altered BDNF and various cytokine levels, with BDNF and tumor necrosis factor-α (TNF-α) levels positively correlating with cognitive performance[7]. The interplay between BDNF and cytokines involves complex signaling pathways that are influenced by antipsychotic medications. BDNF has emerged as a promising biomarker for cognition in schizophrenia, with evidence linking it to cognitive recovery in patients undergoing cognitive remediation[8]. Understanding the relationship between BDNF and schizophrenia could provide new insights into the disease's pathophysiology, helping to identify biomarkers for early diagnosis and novel therapeutic targets. This manuscript reviews the recent findings regarding alterations in BDNF levels in patients experiencing their first episode of schizophrenia, highlighting the clinical implications and exploring potential interventions.
The recent research by Cui et al[9], published in the World Journal of Psychiatry, has investigated cognitive impairment in first-episode schizophrenia and its association with inflammatory cytokines and BDNF. This study involved 84 patients with first-episode schizophrenia and 80 healthy controls recruited from the Third People's Hospital of Huzhou City between August 2021 and September 2023. Inclusion criteria for the schizophrenia group included meeting the Diagnostic and Statistical Manual of Mental Disorders diagnostic criteria for schizophrenia, being aged 18-65 years, scoring ≥ 60 on the Positive and Negative Syndrome Scale (PANSS), and having no prior antipsychotic medication exposure. The exclusion criteria comprised organic brain disorders, significant physical illnesses, and a history of immune-related diseases.
This research is among the first to comprehensively evaluate the relationship of cognitive dysfunction with inflammatory cytokines and BDNF levels in first-episode schizophrenia patients utilizing clinical assessment and biological measurement. The study's design, which included psychiatric evaluations using the PANSS scale assessing for positive and negative symptoms, the Chinese Brief Cognitive Test to evaluate multiple cognitive domains and the evaluation of serum interleukin (IL)-1β, IL-6, and BDNF levels, addresses a significant gap in our understanding of how neuroinflammation and neurotrophic factors interplay in early-stage schizophrenia.
The study findings are remarkable: (1) Elevated levels of IL-6 and TNF-α were significantly associated with lower serum BDNF levels; and (2) Poorer performance on neuropsychological tests assessing executive function, attention, and working memory. This correlation suggests a critical inflammatory component contributing to cognitive deficits in the early stages of schizophrenia. Additionally, the observed reduction in BDNF levels in first-episode schizophrenia patients provides a biological rationale for the cognitive dysfunction commonly seen in schizophrenia. These insights suggest that IL-6 and IL-1β may contribute to cognitive dysfunction by exacerbating neuroinflammatory damage, while BDNF acts as a protective factor. The identification of these correlations opens new avenues for potential therapeutic strategies that could focus on modulating these biomarkers to alleviate cognitive impairments in schizophrenia patients.
Moreover, the study's longitudinal design revealed that patients with higher baseline inflammatory markers and lower BDNF levels were more likely to exhibit a deterioration in cognitive function over time. Such findings emphasize the importance of early identification and intervention in modulating inflammatory pathways, especially to reduce the duration of untreated psychosis and preserve cognitive function in schizophrenia.
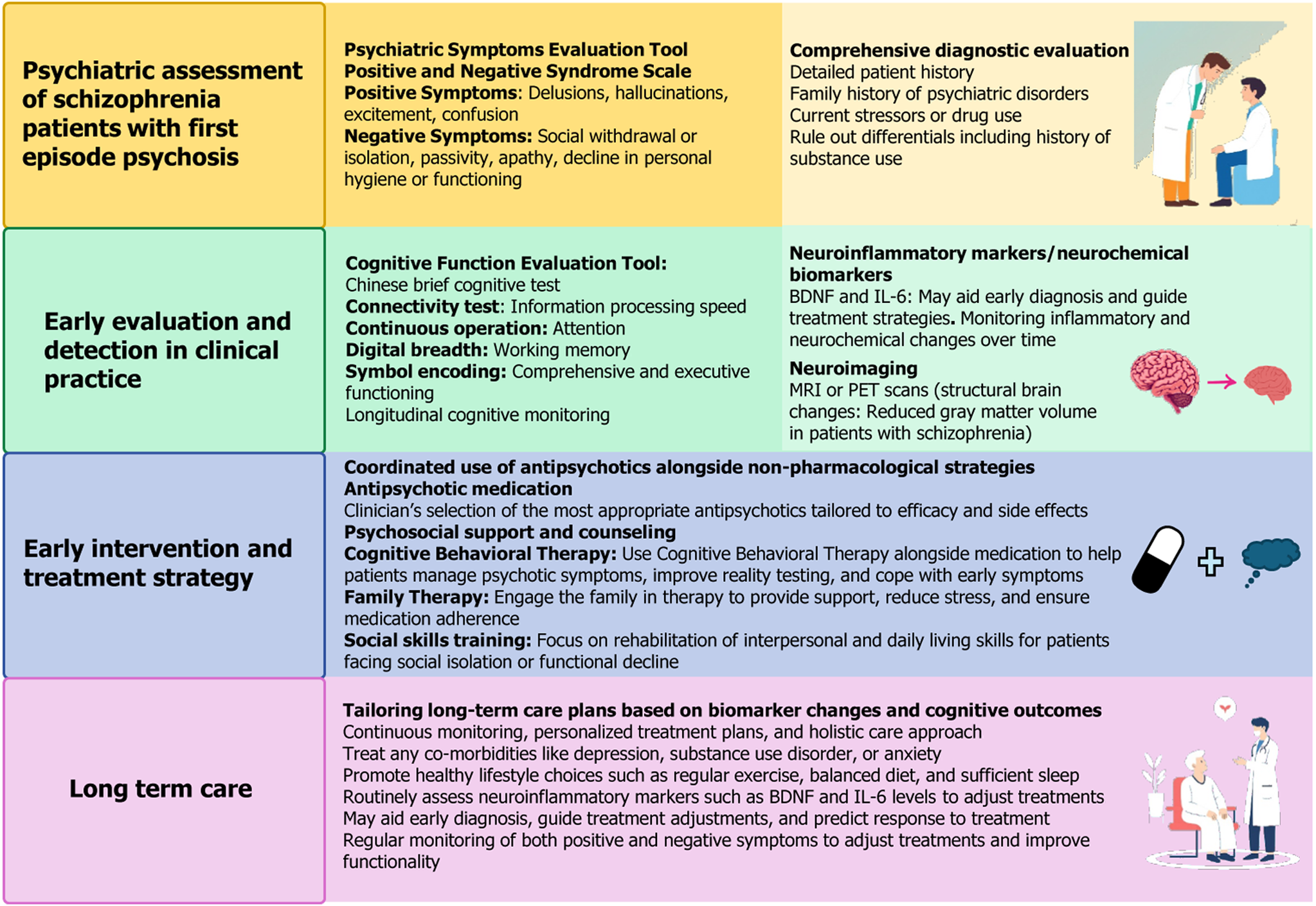
Treating first-episode schizophrenia presents various challenges and requires a personalized approach that combines medication, therapy, and patient support (Figure 1)[10]. The antipsychotics used are divided into first-generation antipsychotics (FGAs) and second-generation antipsychotics (SGAs). FGAs, such as haloperidol, primarily address positive symptoms (e.g., hallucinations) but are associated with significant side effects, including extrapyramidal symptoms and neuroleptic malignant syndrome. SGAs (e.g., risperidone) treat both positive and negative symptoms but carry the risk of metabolic side effects, including diabetes and weight gain.
Key challenges in treatment involve managing side effects, which can affect adherence, and determining the right medication and dose for each patient. Several studies examined the BDNF Val66Met variant in relation antipsychotic response in patients with schizophrenia, providing mixed results[11-13]. A meta-analysis of 11 studies (n = 3774) found no significant association between the BDNF Val66Met variant and antipsychotic response[13]. However, due to sample size limitations, small effects cannot be ruled out. This highlights the need for further research on how BDNF may influence antipsychotic response in schizophrenia. Cui et al’s findings suggest that neuroinflammation (marked by elevated IL-1β and IL-6) and reduced neurotrophic support (i.e., BDNF deficiency) may jointly contribute to cognitive impairment in first-episode schizophrenia[9]. Their finding aligns with existing research linking neuroimmune dysregulation and abnormal biochemical processes to cognitive deficits in schizophrenia. Integrating these elements into the treatment framework represents a forward-thinking approach, opening avenues for novel therapeutic targets, such as enhancing BDNF signaling or inhibiting inflammatory pathways, alongside early intervention strategies. However, further large-scale validation is required to integrate BDNF as a therapeutic target in clinical practice.
Non-pharmacological treatment also plays a crucial role. Psychotherapy, especially Cognitive Behavioral Therapy for Psychosis, supports patients by improving their coping mechanisms. Family involvement and support groups provide emotional backing, essential for long-term management. Combining medication with psychosocial interventions like psychoeducation, supported employment, and cognitive remediation helps enhance the patient's functioning and quality of life.
A holistic treatment plan should be continuously adjusted based on the individual's needs, monitoring for side effects and efficacy, and consider the patient's preferences and family in the decision-making process.
Standardized assessment tools are essential for evaluating treatment effectiveness and monitoring both cognitive and symptomatic outcomes in schizophrenia. The ASSESS battery, focusing on five critical domains (symptomatic remission, affective symptoms, cognitive functioning, treatment satisfaction, and personal and social functioning), significantly improved cognition, social functioning, and patient satisfaction[14,15]. Additionally, tools such as the Premorbid Adjustment Scale have been useful for assessing premorbid functioning in first-episode schizophrenia, although modifications may be necessary to make it more applicable to younger patients[15].
Incorporating biomarkers such as BDNF, IL-1β, and IL-6 into early assessment frameworks provides a more precise understanding of neurobiological changes, particularly in first-episode psychosis. For example, Cui et al’s findings[9], showing that lower BDNF levels correlate with cognitive impairment in patients with schizophrenia, open avenues for targeted interventions addressing not only symptomatic remission but also cognitive preservation.
Interventions targeting neuroinflammation and increasing BDNF levels show promise for cognitive remediation in schizophrenia. Emerging treatments, such as antioxidants (e.g., sulforaphane), demonstrated the potential to reverse cognitive deficits and reduce neuroinflammation in animal models[15]. These strategies, coupled with cognitive therapy, and pharmacological agents that modulate BDNF signaling or suppress inflammation, offer hope for the future development of treatments aimed at mitigating cognitive decline in schizophrenia.
The study by Cui et al[9] advances our understanding of cognitive impairment in first-episode schizophrenia by high
| 1. | Hany M, Rehman B, Rizvi A, Chapman J. Schizophrenia. 2024 Feb 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] |
| 2. | Marder SR, Cannon TD. Schizophrenia. N Engl J Med. 2019;381:1753-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 320] [Article Influence: 53.3] [Reference Citation Analysis (1)] |
| 3. | Kahn RS. On the Origins of Schizophrenia. Am J Psychiatry. 2020;177:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, O'Donovan M, Correll CU, Kane JM, van Os J, Insel TR. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 744] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 5. | Messias EL, Chen CY, Eaton WW. Epidemiology of schizophrenia: review of findings and myths. Psychiatr Clin North Am. 2007;30:323-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 193] [Article Influence: 10.7] [Reference Citation Analysis (4)] |
| 6. | Davis J, Eyre H, Jacka FN, Dodd S, Dean O, McEwen S, Debnath M, McGrath J, Maes M, Amminger P, McGorry PD, Pantelis C, Berk M. A review of vulnerability and risks for schizophrenia: Beyond the two hit hypothesis. Neurosci Biobehav Rev. 2016;65:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 7. | Zhang XY, Tan YL, Chen DC, Tan SP, Yang FD, Wu HE, Zunta-Soares GB, Huang XF, Kosten TR, Soares JC. Interaction of BDNF with cytokines in chronic schizophrenia. Brain Behav Immun. 2016;51:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Penadés R, López-Vílchez I, Catalán R, Arias B, González-Rodríguez A, García-Rizo C, Masana G, Ruíz V, Mezquida G, Bernardo M. BDNF as a marker of response to cognitive remediation in patients with schizophrenia: A randomized and controlled trial. Schizophr Res. 2018;197:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Cui LJ, Cai LL, Na WQ, Jia RL, Zhu JL, Pan X. Interaction between serum inflammatory cytokines and brain-derived neurotrophic factor in cognitive function among first-episode schizophrenia patients. World J Psychiatry. 2024;14:1804-1814. |
| 10. | Keshavan MS, Schooler NR. First-episode studies in schizophrenia: criteria and characterization. Schizophr Bull. 1992;18:491-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Nikolac Perkovic M, Nedic Erjavec G, Zivkovic M, Sagud M, Uzun S, Mihaljevic-Peles A, Kozumplik O, Muck-Seler D, Pivac N. Association between the brain-derived neurotrophic factor Val66Met polymorphism and therapeutic response to olanzapine in schizophrenia patients. Psychopharmacology (Berl). 2014;231:3757-3764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Huang E, Hettige NC, Zai G, Tomasi J, Huang J, Zai CC, Pivac N, Nikolac Perkovic M, Tiwari AK, Kennedy JL. BDNF Val66Met polymorphism and clinical response to antipsychotic treatment in schizophrenia and schizoaffective disorder patients: a meta-analysis. Pharmacogenomics J. 2019;19:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Ladea M, Barbu CM, Juckel G. Treatment effectiveness in patients with schizophrenia as measured by the ASSESS battery - first longitudinal data. Psychiatr Danub. 2015;27:364-370. [PubMed] |
| 14. | Green MF, Penn DL, Bentall R, Carpenter WT, Gaebel W, Gur RC, Kring AM, Park S, Silverstein SM, Heinssen R. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. 2008;34:1211-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 692] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 15. | van Mastrigt S, Addington J. Assessment of premorbid function in first-episode schizophrenia: modifications to the Premorbid Adjustment Scale. J Psychiatry Neurosci. 2002;27:92-101. [PubMed] |