Published online Jan 19, 2025. doi: 10.5498/wjp.v15.i1.101182
Revised: October 21, 2024
Accepted: November 7, 2024
Published online: January 19, 2025
Processing time: 103 Days and 0.5 Hours
Acute ischemic stroke (AIS) is an abrupt blood flow cessation to a specific brain region within a vascular zone, causing a subsequent decline in neurological capabilities. Stent thrombectomy is a recently established technique for treating AIS. It provides the benefits of being a relatively simple and safe procedure, capable of partially enhancing a patient’s condition. However, some patients may experience endothelial damage and recurrent thrombosis, with clinical outcomes that are not always satisfactory. Hence, the efficacy of this method remains unclear.
To survey the association of stent thrombectomy vs standard treatment with neurological function protection, complications, and short-term prognosis in patients diagnosed with AIS.
This study assigned 90 patients with AIS to the observation and control groups (n = 45 patients) from December 2020 to December 2022. Stent thrombectomy was conducted in the observation group, whereas routine treatment was provided to the control group. The study assessed the therapeutic outcomes of two groups, including a comparison of their neurological function, living ability, anxiety and depression status, plaque area, serum inflammatory factors, serum Smur100 β protein, neuron-specific enolase (NSE), homocysteine (Hcy), and vascular endo
The total effective rate of treatment was 77.78% and 95.56% in the control and observation groups, respectively. After 8 weeks of treatment, the scores on the National Institutes of Health Stroke Scale, Hamilton Anxiety Scale, and Hamilton Depression Scale decreased remarkably; the Barthel index increased remarkably, with better improvement effects of the scores in the observation group (P < 0.05); total cholesterol, triglyceride, C-reactive protein, and plaque area lessened remarkably, with fewer patients in the observation group (P < 0.05); S-100β protein, NSE, and Hcy levels lessened remarkably, with fewer patients in the observation group
Stent thrombectomy appeared to provide more remarkable neuroprotective effects in patients with AIS compared to the intravenous thrombolysis regimen. Additionally, it has effectively improved the neurological function, daily activities, and vascular endothelial function of patients, while reducing the incidence of complications and improving short-term prognosis.
Core Tip: Acute ischemic stroke (AIS) disrupts blood flow within the blood supply area of the local brain tissue, which causes ischemic necrosis of the affected brain tissue. Stent thrombectomy is a novel treatment with medicine and material science development in recent years, and it demonstrates a good effect in acute cerebral infarction treatment. This study aims to confirm the association of stent thrombectomy with neurological protection, complications, and short-term prognosis in patients with AIS.
- Citation: Yang KJ, Huang JJ, Xuan CX. Association of stent thrombectomy and conventional treatment with neuroprotection, complications, anxiety, and depression in acute ischemic stroke patients. World J Psychiatry 2025; 15(1): 101182
- URL: https://www.wjgnet.com/2220-3206/full/v15/i1/101182.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i1.101182
Acute ischemic stroke (AIS), accounting for 70%-80% of all cerebrovascular diseases, is the most prevalent type of stroke. Acute cerebrovascular occlusion, causing disrupted blood flow within the blood supply area of the local brain tissue, is the primary factor of AIS. This disorder in blood supply causes ischemic necrosis of the affected brain tissue[1,2]. Contemporary medical research has confirmed that timely thrombus removal within the effective window, blood flow reestablishment in occluded vessels, blood and oxygen supply restoration to brain tissue, and blood reperfusion promotion are crucial in AIS management[3]. Intravenous thrombolysis, simple arterial thrombolysis, mechanical thrombolysis, and stent thrombectomy are predominantly used in the clinical treatment of AIS, among which intravenous thrombolysis is a popular and effective method. Patients with acute cerebral infarction within 3 hours are treated with recombinant human tissue plasminogen activator approved by the Food and Drug Administration (FDA). Intravenous thrombolysis using recombinant human tissue plasminogen activator was effective within 3-4.5 hours after an acute stroke, according to the European Cooperative Study on Acute Stroke[4,5]. Furthermore, most patients in China have missed the best treatment time window (3.0-4.5 hours), causing poor effect of intravenous thrombolysis and increasing the risk of intracranial hemorrhage. The administration of arterial thrombolysis poses a challenge in patients with ischemic stroke having TICI perfusion grades 0-1. Only a small amount of thrombolytic drugs are delivered to the proximal end of the thrombus occlusion, whereas a substantial portion of the thrombolytic agents bypass or reflux into normal blood vessels, causing a poor treatment outcome and an increased intracranial hemorrhage incidence[6,7].
Stent thrombectomy is a novel treatment with medicine and material science advancement in recent years. It demonstrates a good effect when treating acute cerebral infarction. The FDA approved the first generation of mechanical thrombectomy equipment in 2004 and Solitaire and Trevo stent thrombectomy devices in 2012. Currently, stent thrombectomy is a predominantly adopted approach to treat acute cerebral infarction[8]. The technique entails stent transportation to the occlusive segment of the thrombus via a catheter, followed by delivering a reusable stent to the thrombus segment, which is then released to fully cover the thrombus segment. The stent and delivery catheter are withdrawn to the guide catheter and, subsequently, removed from the patient’s body after closing the valve bypass. Repeated thrombectomy directly removes most of the thrombus in vitro, opens the occlusive vessels, restores the blood supply of infarcted vessels, ensures the blood vessels patency, and shortens the recanalization time. Stent thrombectomy prevents drug-induced intracranial hemorrhage compared with intravenous thrombolysis, arterial thrombolysis, and arterial mechanical thrombolysis. Silva et al[9] revealed a 90% vascular recanalization rate of stent thrombectomy when treating acute cerebral infarction, with no cardio-cerebrovascular events[9]. Fischer et al[10] demonstrated that Solitaire AB stent thrombectomy improved the vascular recanalization rate in patients with acute cerebral infarction. The procedure is characterized by its simplicity, ease, and safety. However, some patients may experience reinfarction after the intervention[10].
Mechanical thrombectomy exhibits certain therapeutic advantages, but some patients still have endothelial damage and subsequent thrombosis, with less than satisfactory clinical findings. This indicates that the clinical value of stent thrombectomy in patients with AIS remains somewhat controversial. Further prospective studies are warranted for this technique to be widely disseminated in clinical practice. This study aimed to further confirm the association of stent thrombectomy with neurological protection, complications, and short-term prognosis in patients with AIS.
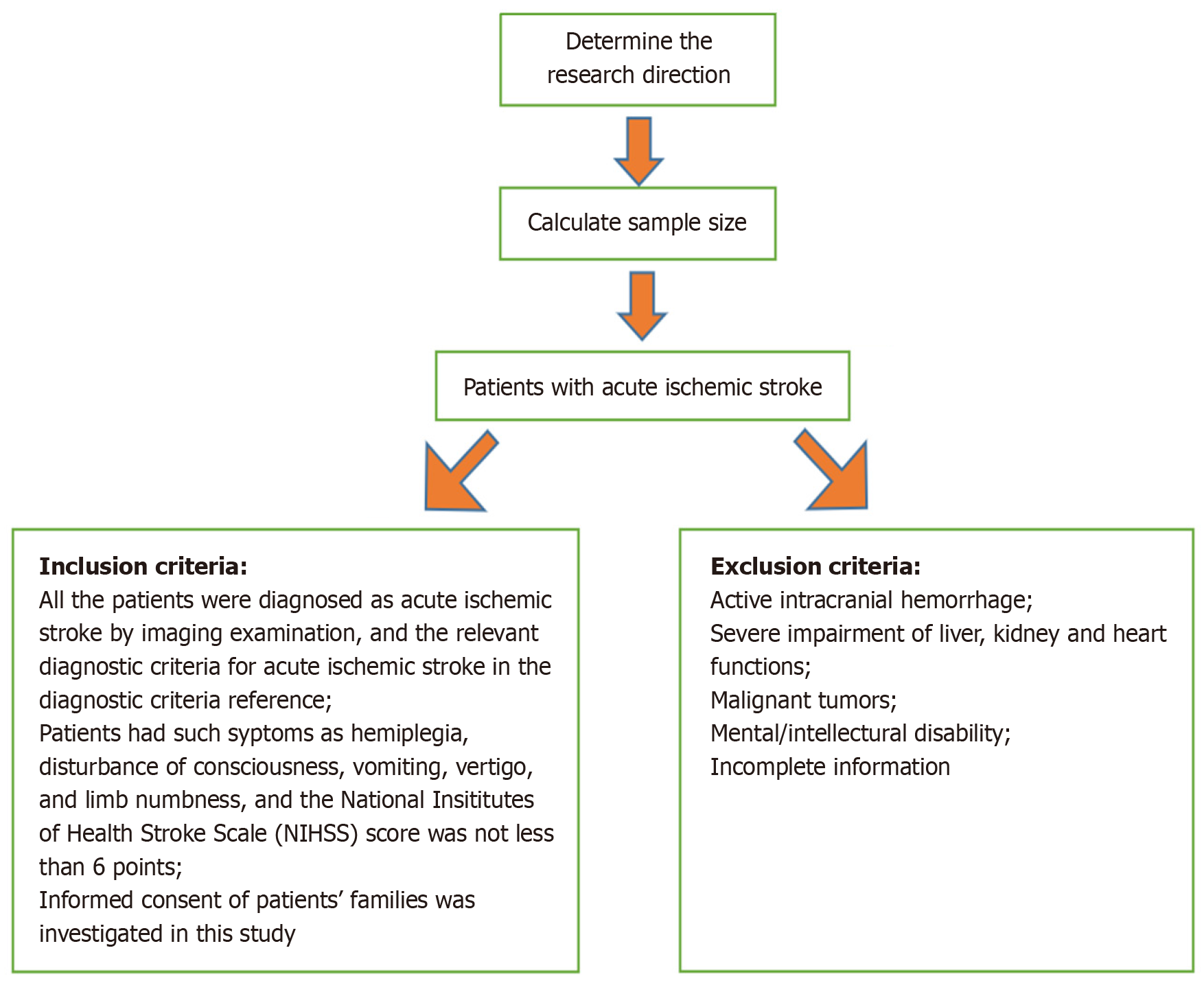
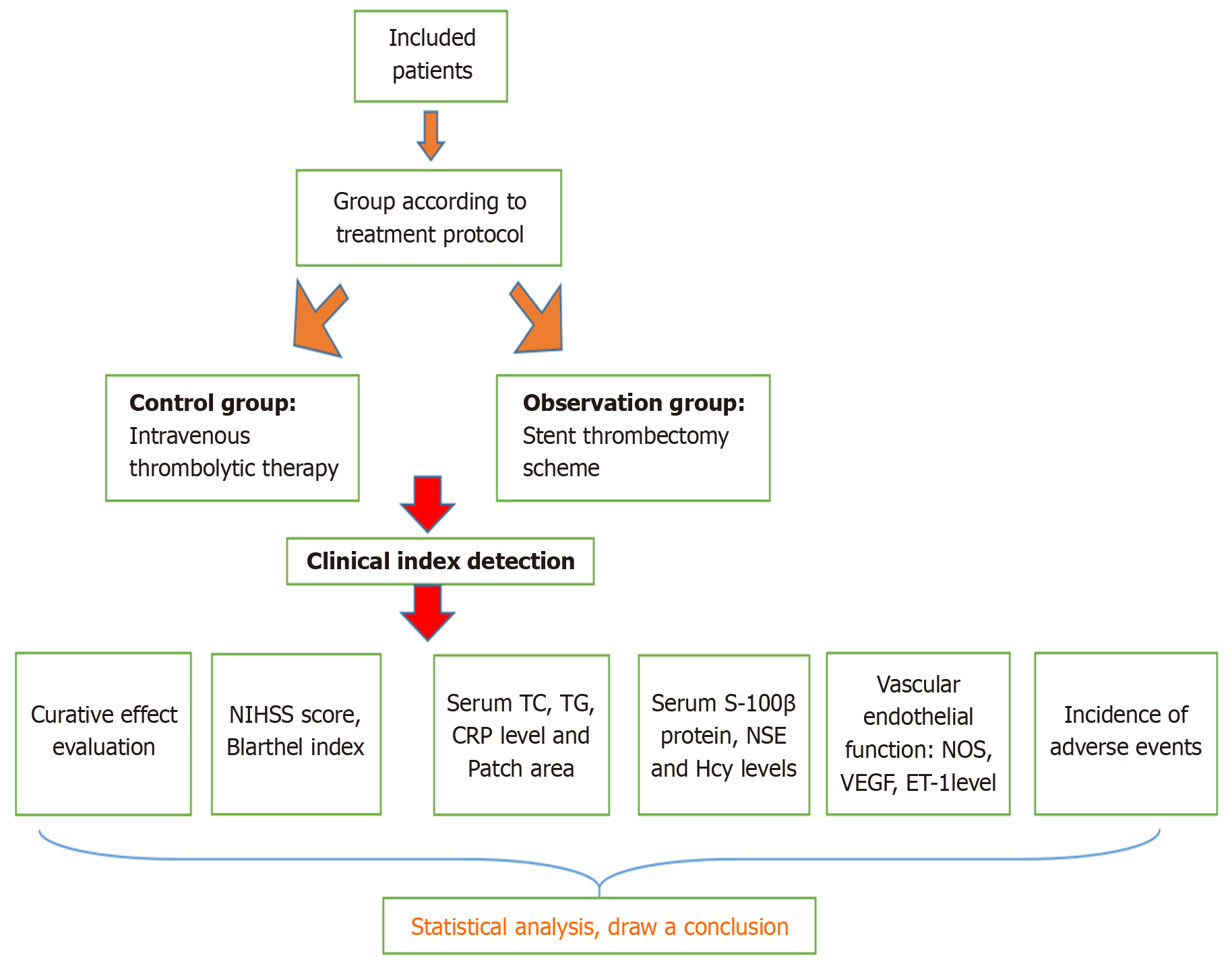
This study assigned 90 patients with AIS from the Department of Neurology, Qinzhou Second People’s Hospital from December 2020 to December 2022 into the observation and control groups (n = 45 patients in each group). The observation group underwent stent thrombectomy, whereas the control group received routine treatment. See Figure 1 for the screening flow chart.
The observation group consisted of 26 men and 19 women, with an age of 50-71 years and a mean age of 53.31 ± 6.87 years. The height and body mass index (BMI) of the participants were 20.04-28.92 kg/m2, with a mean BMI of 23.52 ± 5.36 kg/m2. The area of cerebral infarction was 1.25-5.30 cm2, with a mean value of 3.62 ± 0.89 cm2. The time from onset to medical consultation was 1-14 hours, with a mean time of 5.78 ± 1.34 hours. Among the study group, 20 participants reported concomitant hypertension, 17 indicated concomitant hyperlipidemia, and 8 exhibited concomitant diabetes.
The control group included 28 men and 17 women, with an age of 51-71 years and a mean age of 54.53 ± 7.71 years. The BMI index was 20.81-28.04 kg/m2, with a mean BMI of 23.81 ± 4.81 kg/m2. The area of cerebral infarction was 1.25-5.30 cm2, with a mean value of 3.62 ± 0.89 cm2. The time from onset to medical consultation was 1-16 hours, with a mean time of 6.31 ± 1.04 hours. The control group consisted of 22 participants with concomitant hypertension, 15 with concomitant hyperlipidemia, and 8 with concomitant diabetes. No statistically remarkable differences were observed in the general characteristics (P > 0.05).
Inclusion criteria: (1) All patients diagnosed with AIS by imaging examination and the relevant diagnostic criteria of AIS in reference[11]; (2) Hemiplegia, consciousness disturbance, vomiting, dizziness, limb numbness, and other symptoms, with the National Institutes of Health Stroke Scale (NIHSS) score of ≥ 6; and (3) Patients and their families provided informed consent to this study.
Exclusion criteria: (1) Patients with intracranial active hemorrhage; (2) Patients with severe heart, liver, and kidney damage; (3) Patients with malignant tumors; (4) Patients with mental/mental retardation; and (5) Patients with incomplete clinical data.
Calculation formula of sample size: The total effective rate was selected with a significance level (α) of 0.05 and a statistical power (β) of 0.20 for the primary outcome measure. According to relevant literature and previous studies[12], the estimated probability of success (P1) was 0.95, and the probability of success in the control group (P2) was 0.75. Sample size calculations were conducted using these values, with a dropout rate of 10%. Additionally, 41 participants were required in each group. Approximately 45 patients were included in each group, with a total of 90 patients, considering the potential for dropouts.
The control group received intravenous thrombolysis treatment, particularly intravenous thrombolysis with recombinant human tissue plasminogen activator (Guangzhou Mingkang Biological Engineering Co., Ltd., S20150001). The ob
Curative effect evaluation. Neurological deficit evaluation was conducted with the NIHSS[13] to assess the therapeutic effect on patients after 8 weeks of treatment. The treatment outcomes were categorized based on the following criteria: (1) Cure: NIHSS score reduction of > 90% compared to baseline; (2) Marked improvement: NIHSS score reduction of > 45% but not > 90%; and (3) Improvement: NIHSS score reduction of > 18% but not > 45%; Treatment ineffective: NIHSS score reduction was ≤ 18%, or if the score elevated, or if the patient died.
Total effective rate = (cured cases + markedly effective cases + effective cases)/total cases × 100%.
Anxiety and depression status valuation. The anxiety and depression status of the patients were evaluated by the Hamilton Anxiety Scale (HAMA) and Hamilton Depression Scale (HAMD) before and after 8 weeks of treatment. The HAMA is an other-rated scale utilized to rate the severity of anxiety symptoms. It consists of 14 items, which are rated on a scale of 0 (asymptomatic) to 4 (very severe), with a total score range of 0-56, in which a total score of ≥ 14 is evaluated as the presence of anxiety. HAMD is an alternative rating scale used to examine the state of depression and its severity. It consists of 24 items, of which 14 were rated on a scale of 0 (asymptomatic) to 4 (extremely severe), 10 of which were rated on a scale of 0 (asymptomatic) to 2 (severe), and the total score ranged of 0-76, with a total score of ≥ 8 being evaluated as the presence of depression.
The neurological function and living ability were assessed with NIHSS score[13] and Basle index (BI)[14] before and 8 weeks after treatment, respectively. The NIHSS score ranges from 0 to 42. Nerve damage becomes more severe with a higher score. An NIHSS score of < 6 indicated that the patient recovered well. The BI is a scale used to evaluate an individual’s functional ability in performing activities of daily living. It includes various activities, including feeding, grooming, transferring, toileting, etc. The BI is scored on a scale of 0-100, where a higher score indicates a higher independence level in performing activities of daily living.
Blood lipids total cholesterol (TC), triglyceride (TG), C-reactive protein (CRP), and plaque area were compared before and 8 weeks after treatment. Venous blood samples of 10 mL were collected from the fasting patients and serum was separated through centrifugation at a rate of 3000 rotations/min. TC and TG levels were determined with a blood lipid detector, whereas the CRP level was identified through immunoturbidimetry. The plaque area was detected through cervical vascular color Doppler ultrasonography, with the patient in a supine position, with raised shoulders, and the head in a median or lateral position. The common carotid artery and its bifurcation were evaluated from the patient’s cervical root, followed by the internal carotid artery and external carotid artery. The presence of plaque formation was identified based on vascular morphology, intimal changes, and color blood flow signals, among other factors. A local thickness of > 1.2 mm indicated plaque formation. The plaque area was calculated as the product of the length and width of the detected plaque.
Fasting venous blood of 10 mL was collected before and after 8 weeks of treatment, and the serum was centrifuged (3000 r/min, 10 minutes). The serum S-100β protein and neuron-specific enolase (NSE) levels were assessed by enzyme-linked immunosorbent assay with an automatic biochemical analyzer (BS-380 from Shenzhen Mindray Company), and the serum levels were identified by direct and indirect methods.
Vascular endothelial function. The serum nitric oxide synthase (NOS), vascular endothelial growth factor (VEGF), and endothelin-1 (ET-1) levels were detected by chemical colorimetry before and 8 weeks after treatment. Shanghai Zhenao Biotechnology Co., Ltd. provided the reagents, with batch numbers 2017BS241, 2018BS017, and 2018BS446.
Complication incidence. The common complications observed were vascular reocclusion, brain edema, reperfusion injury, secondary hemorrhage of the infarct focus, intracranial hemorrhage, and other similar conditions based on the evaluation conducted within 48 h after treatment. The total incidence of complications = the sum of all kinds of complications/the total number of cases × 100%.
Vascular reocclusion is a post-treatment complication involving blood vessel blockage recurrence after successful recanalization. Patients may present with symptoms, including limb dysfunction, dizziness, headache, aphasia, nausea, and vomiting. Improved cerebrovascular vessel computed tomography (CT) helps in diagnosis, and a lack or reduction of blood flow in the previously opened vessel may demonstrate vascular reocclusion.
Conversely, brain edema is a pathological phenomenon characterized by an increase in water content within the brain, causing an increase in brain volume. This is a response of the brain to various pathogenic factors and causes intracranial hypertension and brain tissue injury. Diagnosis may involve observation of the patient’s symptoms and signs, as well as laboratory evaluations. Patients may demonstrate vital sign fluctuations, nausea and vomiting, somnolence, consciou
Secondary hemorrhage in the focus of infarction is the occurrence of cerebral hemorrhage in the infarcted area during cerebral infarction caused by blood vessel reperfusion during the ischemic period. This condition is confirmed by cerebral CT examination, where a CT plain scan demonstrates two different types of lesions in the same focus. It is diagnosed as a secondary hemorrhage in the infarcted focus, characterized by spots, patches, and patches, based on the infarcted focus. Intracranial hemorrhage is the occurrence of non-traumatic cerebral parenchymal hemorrhage or subarachnoid hemorrhage. It is a medical emergency and causes neurological deficits and even death. Intracranial hemorrhage is usually diagnosed based on clinical presentation, imaging studies, including CT and MRI scans, and laboratory tests. Patients may exhibit symptoms, such as hemiplegia, aphasia, and sensory disturbance, through clinical observation, diagnostic imaging, and other means of examination. Head CT scans may reveal biconvex lenses in the epidural space and skull, which confirms the presence of extradural bleeding or high-density foci in the brain. Additionally, cerebral hemorrhage is determined in the thalamus or frontal lobe. A crescent shape visible under the dura may indicate the occurrence of subdural hemorrhage.
Statistical Package for the Social Sciences version 22.0 statistical software was used for data analyses and processing. We used the one-sample Kolmogorov-Smirnov test to investigate the normality of continuous variables. The mean ± SD is utilized to indicate measurements with a normal distribution or approximate normal distribution. Differences in continuous variables were compared using Student’s t-test. The count data in this study were presented as n (%) and analyzed with the χ2 test. P-values of < 0.05 indicated statistical significance.
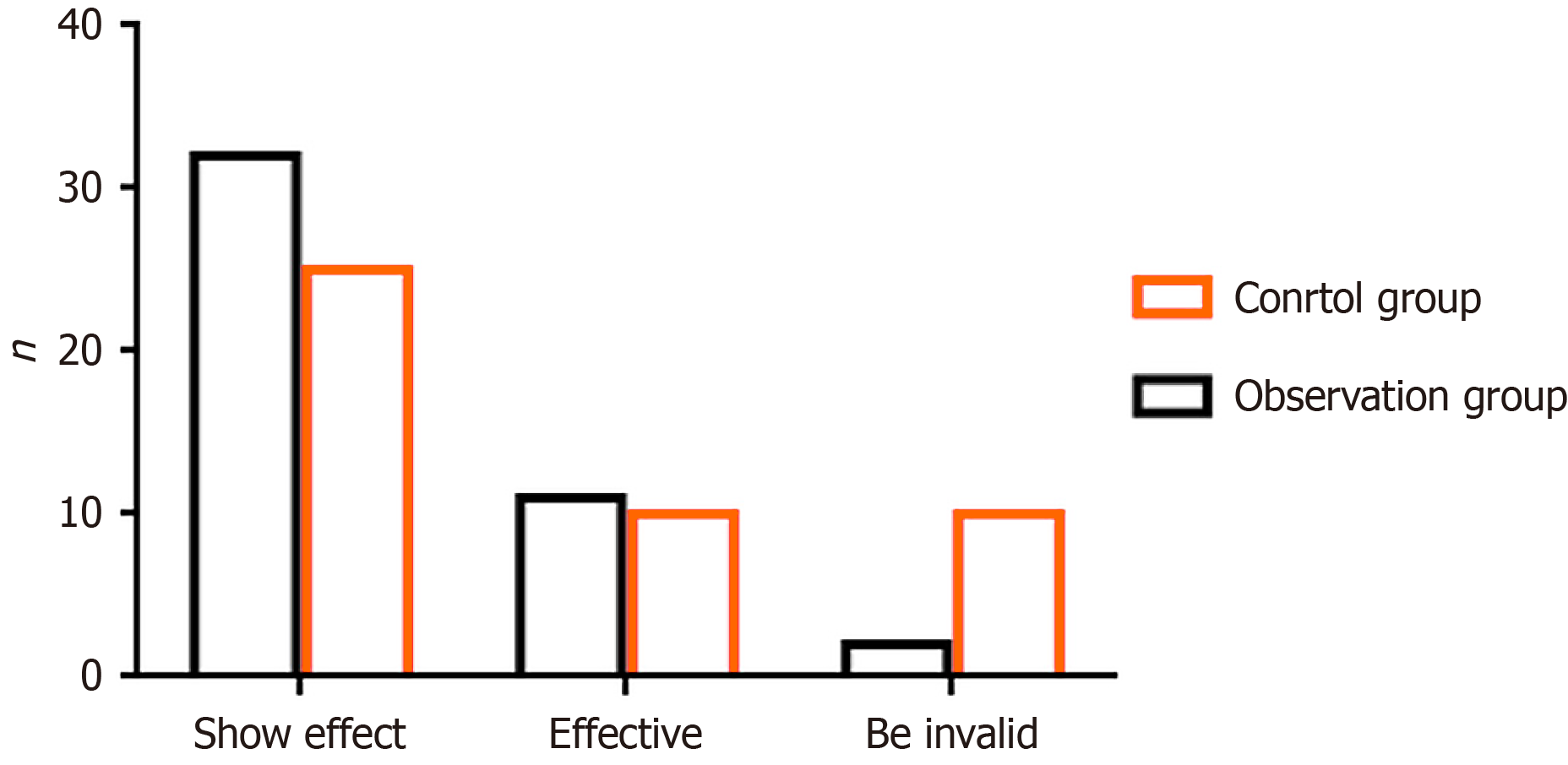
Figure 3 shows that out of the total cases in the observation group, 32 were classified as markedly effective, 11 as effective, and 2 as ineffective. The total effective rate of treatment in this group was calculated as 95.56%. Of the cases in the control group, 25 were classified as markedly effective, 10 as effective, and 10 as ineffective, causing a total effective rate of 77.78% (P < 0.05).
No remarkable difference in the NIHSS and BI scores before treatment (P > 0.05) (Table 1). The NIHSS score lessened remarkably after 8 weeks of treatment, and the BI score elevated remarkably. The observation group demonstrated a better improvement effect of each score (P < 0.05).
| Group | NIHSS scoring | Barthel index score | ||
| Before treatment | After 8 weeks of treatment | Before treatment | After 8 weeks of treatment | |
| Observation group | 13.42 ± 3.71 | 4.08 ± 2.81a | 27.04 ± 9.92 | 59.83 ± 11.45a |
| Control group | 12.84 ± 3.61 | 8.14 ± 3.24b | 27.41 ± 10.04 | 45.25 ± 12.08b |
| t value | 0.752 | 6.350 | 0.176 | 5.876 |
| P value | > 0.05 | < 0.05 | > 0.05 | < 0.05 |
No remarkable difference in HAMA and HAMD scores was found before treatment (P > 0.05) (Table 2). The HAMA and HAMD scores lessened remarkably after 8 weeks of treatment.
No remarkable difference was observed in TC, TG, CRP, and plaque area (P > 0.05) before treatment (Table 3). TC, TG, CRP, and plaque area levels lessened remarkably after 8 weeks of treatment, with fewer patients in the observation group (P < 0.05).
| Group | TC (mmol/L) | TG (mmol/L) | CRP (mg/L) | Patch area (mm2) | ||||
| Before treatment | After 8 weeks of treatment | Before treatment | After 8 weeks of treatment | Before treatment | After 8 weeks of treatment | Before treatment | After 8 weeks of treatment | |
| Observation group | 5.73 ± 0.42 | 3.42 ± 0.21a | 1.89 ± 0.62 | 1.43 ± 0.15a | 8.45 ± 1.35 | 4.52 ± 1.56a | 26.54 ± 10.38 | 19.52 ± 7.35a |
| Control group | 5.81 ± 0.53 | 4.25 ± 0.25b | 1.93 ± 0.57 | 1.08 ± 0.12b | 8.38 ± 1.61 | 3.14 ± 1.37b | 27.12 ± 10.45 | 24.56 ± 7.01b |
| t value | 0.794 | 17.053 | 0.319 | 12.223 | 0.223 | 4.459 | 0.264 | 3.329 |
| P value | > 0.05 | < 0.05 | > 0.05 | < 0.05 | > 0.05 | < 0.05 | > 0.05 | < 0.05 |
No remarkable difference was found in S-100β protein, NES, and homocysteine (Hcy) levels before treatment (P > 0.05) (Table 4). S-100β protein, NES, and Hcy levels lessened remarkably after 8 weeks of treatment, with fewer patients in the observation group (P < 0.05).
| Group | NSE (μg/L) | S-100β (μg/L) | Hcy (μmol/L) | |||
| Before treatment | After 8 weeks of treatment | Before treatment | After 8 weeks of treatment | Before treatment | After 8 weeks of treatment | |
| Observation group | 14.35 ± 2.09 | 6.73 ± 1.25a | 1.54 ± 0.35 | 0.31 ± 0.09a | 31.54 ± 5.29 | 17.24 ± 3.51a |
| Control group | 14.25 ± 2.24 | 8.56 ± 1.44b | 1.38 ± 0.47 | 0.74 ± 0.19b | 30.89 ± 5.47 | 21.73 ± 4.88b |
| t value | 0.219 | 6.438 | 1.832 | 13.720 | 0.573 | 5.011 |
| P value | > 0.05 | < 0.05 | > 0.05 | < 0.05 | > 0.05 | < 0.05 |
No remarkable difference was found in serum VEGF, NOS, and ET-1 levels before treatment (P > 0.05) (Table 5). The serum VEGF and NOS increased remarkably after 8 weeks of treatment, whereas the ET-1 level decreased. The improvement effect of the observation group was better (P < 0.05).
| Group | VEGF (ng/L) | NOS (ng/L) | ET-1 (U/mL) | |||
| Before treatment | After 8 weeks of treatment | Before treatment | After 8 weeks of treatment | Before treatment | After 8 weeks of treatment | |
| Observation group | 298.54 ± 35.25 | 418.84 ± 42.15a | 24.84 ± 6.15 | 37.98 ± 6.41a | 91.58 ± 7.54 | 50.65 ± 5.48a |
| Control group | 297.73 ± 34.91 | 315.24 ± 39.58b | 24.75 ± 6.48 | 25.44 ± 6.05b | 92.05 ± 8.01 | 64.25 ± 6.51b |
| t value | 0.110 | 12.019 | 0.068 | 9.544 | 0.287 | 10.721 |
| P value | > 0.05 | < 0.05 | > 0.05 | < 0.05 | > 0.05 | < 0.05 |
Complications occurred in 8.88% of the observation group, compared with 33.33% in the control group (P < 0.05) (Table 6).
| Group | n | Vascular re-occlusion | Brain edema | Reperfusion injury | Intracranial hemorrhage | Secondary hemorrhage of infarct focus | Total incidence rate |
| Observation group | 45 | 1 (2.22) | 2 (4.44) | 0 (0.00) | 1 (2.22) | 0 (0.00) | 4 (8.88) |
| Control group | 45 | 4 (8.88) | 3 (6.67) | 2 (4.44) | 4 (8.88) | 2 (4.44) | 15 (33.33) |
| χ² value | 8.073 | ||||||
| P value | < 0.05 | ||||||
AIS is when cerebral atherosclerosis causes vascular thickening, lumen stenosis, local blockage, or thrombosis. A thrombus in a large artery dislodges and travels to the brain, thereby causing additional stenosis and cerebral vasculature occlusion, resulting in localized brain tissue necrosis and subsequent loss of neurological function in the affected area[15-17]. Currently, the mechanical thrombectomy with arterial stent technique is a predominantly used approach for treating AIS. Clinical studies have revealed that this therapy is effective in removing thrombi from occluded vessels and facilitating collateral circulation establishment in the affected vessels, causing an improvement in cerebral blood perfusion and a reduction in cerebral ischemia[18]. However, mechanical thrombectomy may damage the local endothelium of blood vessels, causing vascular stenosis or dissection, secondary to new thrombosis, intraoperative thrombus fragmentation, or microthrombus flow into distal small vessels with blood flow, resulting in distal or another vascular occlusion[19,20]. Thus, further clinical evidence is warranted to support the application value of stent thrombectomy in patients with AIS.
The results of this study indicated that the total effective rate was 77.78% and 95.56% in the control and observation groups, respectively. Stent embolectomy is more effective compared with routine intravenous thrombolysis. The NIHSS score, TC, TG, CRP level, and plaque area were remarkably reduced after 8 weeks of treatment, whereas the BI score was remarkably elevated. The observed improvement in patient scores in the treatment group was remarkably better, indicating that stent embolectomy is a valuable intervention in the clinical management of patients with AIS. This procedure effectively protects neurological function, reduces patient inflammation, decreases infarct size, and improves patients’ daily living abilities. Stent thrombectomy exerted a neuroprotective effect by promoting local angiogenesis, increasing cerebral blood flow and oxygen supply, and decreasing the infarct area. This is achieved by deploying a retrievable stent, which is transported through a catheter to the occlusion site, and then fully expanded to cover the thrombus segment and occlude the valve bypass before being withdrawn[21]. This approach enables multiple thrombus removal attempts, thereby allowing the direct extraction of a remarkable portion of the thrombus, re-establishing blood flow in the occluded vessels, and restoring blood supply to the affected vessels. This technique is more effective in controlling drug-induced intracranial hemorrhage by shortening the recanalization time compared to mechanical thrombolysis, arterial thrombolysis, and intravenous thrombolysis[22,23]. Stent embolectomy improves the vascular recanalization rate of patients and protects the related brain cells in the penumbra of cerebral ischemia to better enhance the neurological deficit and prognosis of patients[24].
The monitoring of serum markers has become a crucial means to assess the condition, curative effect, and prognosis of neurological diseases in recent years. NSE and S-100β proteins are specific nerve cell proteins, which are mainly distributed in nerve tissues. NSE and S-100β proteins leak into the bloodstream from the brain when the integrity of the blood-brain barrier is compromised. Hence, NSE and S-100β protein levels in the serum increase, indicating the presence of blood-brain barrier damage. The NSE and S-100β protein levels are directly proportional to the degree of neurological impairment and can be utilized as biological markers of the severity and prognosis of AIS[25]. Hcy is a metabolic intermediate of sulfur-containing amino acids in the human body. Increased Hcy levels have mediated the production of inflammatory factors and oxidative free radicals, damaged vascular endothelial cells, and caused inflammatory infiltration. Consequently, Hcy has been a causal factor in cardiovascular disease, including atherosclerosis[26]. Hcy demonstrates a remarkable positive correlation with the degree of neurological deficit in patients with AIS, which can be utilized as a reference index for the condition and prognosis[27]. The results revealed that NSE, S-100β protein, and Hcy in the observation group were lower after treatment. It is indicated that stent thrombectomy further reduces the levels of serum NSE, Smur100 β protein, and Hcy in patients with AIS. Stent thrombectomy remarkably alleviates neurological impairment and protects neurological function is confirmed by objective indexes.
Extensive clinical research has demonstrated that vascular endothelial cells exhibit a strong association with cardiovascular and cerebrovascular disorders. These cells are crucial in sustaining the firmness, elasticity, and tautness of blood vessels, as well as regulating vascular wall permeability[28,29]. ET-1 is an important index to assess vascular endothelial function. NOS reduces the injury after acute cerebral ischemia and promotes its recovery by improving the cerebral blood flow around ischemic tissues. Damaged endothelial cells destroy the balance of NOS and ET-1, causing cerebral vasospasm, aggravating atherosclerosis formation, and causing cerebral ischemic changes[30]. VEGF is a growth factor that acts on vascular endothelial cells, promoting endothelial cell division, proliferation, and metastasis, increasing vascular wall permeability, and promoting blood vessel growth and collateral circulation establishment. The results of this study reveal that intravenous thrombolysis alone is limited in reducing serum ET-1 Levels and exhibits no remarkable effect on serum VEGF and NOS levels, whereas stent embolectomy remarkably improves all three markers. The underlying mechanism may be related to the ability of the combination therapy to decrease serum ET-1 Levels and increase NOS levels, thereby maintaining a dynamic equilibrium between ET-1 and NOS synthesis and release, preserving the normal vascular endothelium physiological function, promoting endothelial cell te proliferation, regulating neuronal autophagy after cerebral ischemia, exerting neuroprotective effects, and enhancing long-term outcomes in patients. The observation group demonstrated a remarkably lower complication incidence, indicating stent thrombectomy as a safer procedure. This could be associated with the fact that stent embolectomy causes minimal damage to normal physiological tissue and is less traumatic. Therefore, it remarkably decreases the risk of complications in patients undergoing this treatment.
This study still has some limitations. First, the sample size was too small, which may be biased, because this was a single-center retrospective study. Second, the study focuses on short-term prognosis. Thus, a long-term follow-up component is warranted to provide a more comprehensive understanding of the sustained effects of stent thrombectomy on neurological function and quality of life. Third, the pathway mechanism of stent thrombectomy has not been further investigated. Fourth, stent thrombectomy may have different effects on different subgroups of patients with AIS, such as stroke severity or comorbid conditions. Therefore, a well-designed, multi-center, large sample size with a long-term follow-up study is needed to further confirm the conclusion of this study.
In summary, stent thrombectomy is a potent treatment for patients with AIS. It effectively improves the severity of early neurological deficits, reduces the inflammatory response, improves daily living abilities, and decreases complication incidences. This procedure is safe and feasible and deserves to be predominantly used in clinical practice. The limitations of this study include the small number of participants, the absence of regional variation, and the lack of feedback. More cross-regional, multi-center, and large sample studies should be conducted in the future to provide more precise evidence that helps improve clinical practice.
| 1. | Saceleanu VM, Toader C, Ples H, Covache-Busuioc RA, Costin HP, Bratu BG, Dumitrascu DI, Bordeianu A, Corlatescu AD, Ciurea AV. Integrative Approaches in Acute Ischemic Stroke: From Symptom Recognition to Future Innovations. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 2. | Mendelson SJ, Prabhakaran S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA. 2021;325:1088-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 451] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 3. | Rabinstein AA. Update on Treatment of Acute Ischemic Stroke. Continuum (Minneap Minn). 2020;26:268-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 4. | Rajan JE, Kannath SK, Sabarish S, Arun K, P Varma PR, Sreedharan SE, Sukumaran S, Sylaja PN. Mechanical Thrombectomy in Acute Ischemic Stroke: Angiographic Predictors of Outcome. Neurol India. 2022;70:1407-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Mohammaden MH, Haussen DC, Perry da Camara C, Pisani L, Olive Gadea M, Al-Bayati AR, Liberato B, Rangaraju S, Frankel MR, Nogueira RG. Hyperdense vessel sign as a potential guide for the choice of stent retriever versus contact aspiration as first-line thrombectomy strategy. J Neurointerv Surg. 2021;13:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Azzam AY, Ghozy S, Kallmes KM, Adusumilli G, Heit JJ, Hassan AE, Kadirvel R, Kallmes DF. Aspiration thrombectomy versus stent retriever thrombectomy alone for acute ischemic stroke: evaluating the overlapping meta-analyses. J Neurointerv Surg. 2023;15:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Feske SK. Ischemic Stroke. Am J Med. 2021;134:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 422] [Article Influence: 105.5] [Reference Citation Analysis (0)] |
| 8. | Lapergue B, Blanc R, Costalat V, Desal H, Saleme S, Spelle L, Marnat G, Shotar E, Eugene F, Mazighi M, Houdart E, Consoli A, Rodesch G, Bourcier R, Bracard S, Duhamel A, Ben Maacha M, Lopez D, Renaud N, Labreuche J, Gory B, Piotin M; ASTER2 Trial Investigators. Effect of Thrombectomy With Combined Contact Aspiration and Stent Retriever vs Stent Retriever Alone on Revascularization in Patients With Acute Ischemic Stroke and Large Vessel Occlusion: The ASTER2 Randomized Clinical Trial. JAMA. 2021;326:1158-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 9. | Silva GS, Nogueira RG. Endovascular Treatment of Acute Ischemic Stroke. Continuum (Minneap Minn). 2020;26:310-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Fischer U, Kaesmacher J, S Plattner P, Bütikofer L, Mordasini P, Deppeler S, Cognard C, Pereira VM, Siddiqui AH, Froehler MT, Furlan AJ, Chapot R, Strbian D, Wiesmann M, Bressan J, Lerch S, Liebeskind DS, Saver JL, Gralla J; SWIFT DIRECT study investigators. SWIFT DIRECT: Solitaire™ With the Intention For Thrombectomy Plus Intravenous t-PA Versus DIRECT Solitaire™ Stent-retriever Thrombectomy in Acute Anterior Circulation Stroke: Methodology of a randomized, controlled, multicentre study. Int J Stroke. 2022;17:698-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Chalos V, van der Ende NAM, Lingsma HF, Mulder MJHL, Venema E, Dijkland SA, Berkhemer OA, Yoo AJ, Broderick JP, Palesch YY, Yeatts SD, Roos YBWEM, van Oostenbrugge RJ, van Zwam WH, Majoie CBLM, van der Lugt A, Roozenbeek B, Dippel DWJ; MR CLEAN Investigators. National Institutes of Health Stroke Scale: An Alternative Primary Outcome Measure for Trials of Acute Treatment for Ischemic Stroke. Stroke. 2020;51:282-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Zhang QQ, Fu C, Wang L, Zhang GQ, Cao PW, Chen GF, Fu XM. Clinical efficacy of tirofiban combined with a Solitaire stent in treating acute ischemic stroke. Braz J Med Biol Res. 2019;52:e8396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Farooq S, Shkirkova K, Villablanca P, Sanossian N, Liebeskind DS, Starkman S, Avila G, Sharma L, Kim-Tenser M, Gasparian S, Eckstein M, Conwit R, Hamilton S, Saver JL. National Institutes of Health Stroke Scale Correlates Well with Initial Intracerebral Hemorrhage Volume. J Stroke Cerebrovasc Dis. 2022;31:106348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 14. | Uchinaka EI, Hanaki T, Morimoto M, Murakami Y, Tomoyuki M, Yamamoto M, Tokuyasu N, Sakamoto T, Hasegawa T, Fujiwara Y. The Barthel Index for Predicting Postoperative Complications in Elderly Patients Undergoing Abdominal Surgery: A Prospective Single-center Study. In Vivo. 2022;36:2973-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 15. | Grotta JC. Fifty Years of Acute Ischemic Stroke Treatment: A Personal History. Cerebrovasc Dis. 2021;50:666-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Herpich F, Rincon F. Management of Acute Ischemic Stroke. Crit Care Med. 2020;48:1654-1663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 483] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 17. | AlRuwaili R, Al-Kuraishy HM, Alruwaili M, Khalifa AK, Alexiou A, Papadakis M, Saad HM, Batiha GE. The potential therapeutic effect of phosphodiesterase 5 inhibitors in the acute ischemic stroke (AIS). Mol Cell Biochem. 2024;479:1267-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Rodríguez-López A, Gimeno I, Vila S, Seoane A, Ballenilla F, Ramos A, Hilario A, Núñez N, Camacho A, Simón R. Mechanical Thrombectomy for Acute Stroke in a 2-Month-Old Patient and Review of the Literature in Infancy. Neuropediatrics. 2022;53:376-380. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Okuda T, Arimura K, Matsuo R, Tokunaga S, Hara K, Yamaguchi S, Yoshida H, Kurogi R, Kameda K, Ito O, Tsumoto T, Iihara K, Mizokami T, Uwatoko T, Nishimura A, Iwaki K, Mizoguchi M; QNET investigators. Efficacy of combined use of a stent retriever and aspiration catheter in mechanical thrombectomy for acute ischemic stroke. J Neurointerv Surg. 2022;14:892-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Jolugbo P, Ariëns RAS. Thrombus Composition and Efficacy of Thrombolysis and Thrombectomy in Acute Ischemic Stroke. Stroke. 2021;52:1131-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 279] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 21. | Renú A, Millán M, San Román L, Blasco J, Martí-Fàbregas J, Terceño M, Amaro S, Serena J, Urra X, Laredo C, Barranco R, Camps-Renom P, Zarco F, Oleaga L, Cardona P, Castaño C, Macho J, Cuadrado-Godía E, Vivas E, López-Rueda A, Guimaraens L, Ramos-Pachón A, Roquer J, Muchada M, Tomasello A, Dávalos A, Torres F, Chamorro Á; CHOICE Investigators. Effect of Intra-arterial Alteplase vs Placebo Following Successful Thrombectomy on Functional Outcomes in Patients With Large Vessel Occlusion Acute Ischemic Stroke: The CHOICE Randomized Clinical Trial. JAMA. 2022;327:826-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 197] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 22. | Yang JL, Yang YR, Chen SD. The potential of drug repurposing combined with reperfusion therapy in cerebral ischemic stroke: A supplementary strategy to endovascular thrombectomy. Life Sci. 2019;236:116889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Sila D, Lenski M, Vojtková M, Elgharbawy M, Charvát F, Rath S. Efficacy of Mechanical Thrombectomy using Penumbra ACE(TM) Aspiration Catheter Compared to Stent Retriever Solitaire(TM) FR in Patients with Acute Ischemic Stroke. Brain Sci. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Bhatti A, Huded V, Vyas D, Mushtaq M, Kekatpure M, Hiremath S, Sapare A, Aggarwal R. Mechanical Thrombectomy Using Retrievable Stents in Pediatric Acute Ischemic Stroke. Indian Pediatr. 2019;56:571-575. [PubMed] |
| 25. | Altintas Kadirhan O, Kucukdagli OT, Gulen B. The effectiveness of serum S100B, TRAIL, and adropin levels in predicting clinical outcome, final infarct core, and stroke subtypes of acute ischemic stroke patients. Biomedica. 2022;42:55-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 26. | Walter K. What Is Acute Ischemic Stroke? JAMA. 2022;327:885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 129] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 27. | Pagiola I, Amaral B, Saito C, Nalli D, Carrete Junior H, Frudit ME. Solitaire stent retriever failure during thrombectomy: is there any image that can predict non-recanalization? Arq Neuropsiquiatr. 2021;79:90-91. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Kim KA, Kim D, Kim JH, Shin YJ, Kim ES, Akram M, Kim EH, Majid A, Baek SH, Bae ON. Autophagy-mediated occludin degradation contributes to blood-brain barrier disruption during ischemia in bEnd.3 brain endothelial cells and rat ischemic stroke models. Fluids Barriers CNS. 2020;17:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 29. | Green TL, McNair ND, Hinkle JL, Middleton S, Miller ET, Perrin S, Power M, Southerland AM, Summers DV; American Heart Association Stroke Nursing Committee of the Council on Cardiovascular and Stroke Nursing and the Stroke Council. Care of the Patient With Acute Ischemic Stroke (Posthyperacute and Prehospital Discharge): Update to 2009 Comprehensive Nursing Care Scientific Statement: A Scientific Statement From the American Heart Association. Stroke. 2021;52:e179-e197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 30. | Wassélius J, Arnberg F, von Euler M, Wester P, Ullberg T. Endovascular thrombectomy for acute ischemic stroke. J Intern Med. 2022;291:303-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |