Published online Sep 19, 2024. doi: 10.5498/wjp.v14.i9.1386
Revised: August 15, 2024
Accepted: August 22, 2024
Published online: September 19, 2024
Processing time: 156 Days and 16 Hours
Musical hallucinations (MH) involve the false perception of music in the absence of external stimuli which links with different etiologies. The pathomechanisms of MH encompass various conditions. The etiological classification of MH is of particular importance and offers valuable insights to understand MH, and further to develop the effective treatment of MH. Over the recent decades, more MH cases have been reported, revealing newly identified medical and psychiatric causes of MH. Functional imaging studies reveal that MH activates a wide array of brain regions. An up-to-date analysis on MH, especially on MH comorbid psychiatric conditions is warranted.
To propose a new classification of MH; to study the age and gender differences of MH in mental disorders; and neuropathology of MH.
Literatures searches were conducted using keywords such as “music hallucina
Among the 357 yielded publications, 294 MH cases were collected. The average age of MH cases was 67.9 years, with a predominance of females (66.8% females vs 33.2% males). MH was classified into eight groups based on their etiological mechanisms. Statistical analysis of MH cases indicates varying associations with psychiatric diagnoses.
We carried out a more comprehensive review of MH studies. For the first time according to our knowledge, we demonstrated the psychiatric conditions linked and/or associated with MH from statistical, biological and molecular point of view.
Core Tip: There has been a noteworthy increase in musical hallucination (MH) publications over the last several years warranting a more recent and updated analysis of MH. In our study, we engaged in a more comprehensive review of these MH publications and studies. Our study and findings are unique in that we demonstrated the psychiatric conditions linked and/or associated with MH from a statistical perspective. The neurobiological processes associated with psychiatric disorders comorbid with MH were analyzed, categorized and summarized. Our study also found that the recent biological and molecular studies indicate possible mechanisms that cause neuron disruptions in MH.
- Citation: Lian X, Song W, Si TM, Lian NZ. Classification of musical hallucinations and the characters along with neural-molecular mechanisms of musical hallucinations associated with psychiatric disorders. World J Psychiatry 2024; 14(9): 1386-1396
- URL: https://www.wjgnet.com/2220-3206/full/v14/i9/1386.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i9.1386
Musical hallucination (MH) represents a heterogeneous and relatively infrequent phenomenon, constituting a complex form of auditory hallucination characterized by the perception of music without any external sources. The initial recorded case of MH dates back to 1846 when it was reported by Jules Baillarger[1]. Due to its rarity, epidemiological data on the lifetime prevalence of MH in the general population is lacking. Nevertheless, such data is available for specific groups, including individuals in inpatient settings, the elderly with hearing impairments, or those with mental illnesses. MH has been frequently reported among individuals with hearing impairments. Cole et al[2] observed a significant prevalence of 32.8 percent of MH in elderly patients attending an audiology clinic. Teunisse and Olde Rikkert[3] reported a 3.6% prevalence of MH among patients referred for audiometric testing. In addition, MH is commonly reported in psychiatric populations. Fukunishi et al[4] noted a prevalence of 0.16 percent in general hospital settings, rising to 20 percent in psychiatric settings. Hermesh et al[5] reported a MH prevalence of 26.8 percent in a psychiatric hospital, where 190 patients were diagnosed with various psychiatric disorders, including but not limited to obsessive-compulsive disorder (OCD), social phobia, panic disorder, schizophrenia, schizoaffective disorder, bipolar disorder, and major depressive disorder (MDD). Their study revealed that patients with OCD had the highest prevalence of MH at 41%. In an exa
Hearing impairment is commonly identified as the primary predisposing condition for MH. However, Cole’s study[2] revealed that moderate to severe hearing impairment does not necessarily lead to distinct pathophysiological mecha
Individuals with MH may worry that it could be a symptom of a psychiatric condition, such as schizophrenia, where hearing voices are common. However, it is exceedingly rare to experience MH, and there is no known connection between MH and schizophrenia. Some studies have reported that MH is relatively common in people with OCD, and depression may play a contributory role in the occurrence of such hallucinations[8].
Medical factors are often implicated as precipitating agents. For example, Bhatt and Carpentier[9] described a case in which MH occurred in a patient following a low-speed traffic accident resulting in whiplash[9]. Additionally, Calabrò et al[10] reported a case where right temporal lobe ischemic stroke was associated with the onset of MH.
On the theoretical front, various explanatory models for MH have been formulated, especially with the advancement of new technologies. Recently, neuroimaging techniques have been employed to investigate the essential neuro networks involved in the generation of MH. This has heightened interest in understanding the pathomechanisms of MH at the neurofunctional level. Functional imaging studies reveal that MHs activate a wide array of brain regions, including the motor cortex, visual areas, basal ganglia, brainstem, pons, tegmentum, cerebellum, hippocampi, amygdala, and the central and peripheral auditory system. Network science is likely to provide a more suitable framework for a comprehensive MH mechanism model.
The etiological classification of MH is of particular importance and offers valuable insights. In a review study, E Fischer et al[11] summarized hearing loss, brain disease, advanced age, and social isolation as major factors in the etiology of MH. Evers and Ellger[6] compiled a database of 132 MH cases through systematic reviews. They categorized the etiological factors of these cases into five main groups: Hypoacusis, psychiatric disorders, focal brain lesions, epilepsy, and intoxication. Over the past decade, with increasing interest in MH, more MH cases have been reported, revealing newly identified medical and psychiatric causes of MH. For example, some MH cases were reported following electroconvulsive therapy in the context of Parkinson’s disease[12,13], and one MH case was reported after the subject contracted Lyme disease[14]. These newly reported cases do not fit into the categories described by Evers and Ellger[6]. Simultaneously, a broader spectrum of psychiatric disorders, beyond psychosis, has been found to be associated with MH, including adjustment disorder, attention deficit hyperactivity disorder (ADHD), and anxiety[15,16]. Consequently, a more comprehensive profiling of MH, incorporating the wealth of new findings, is warranted.
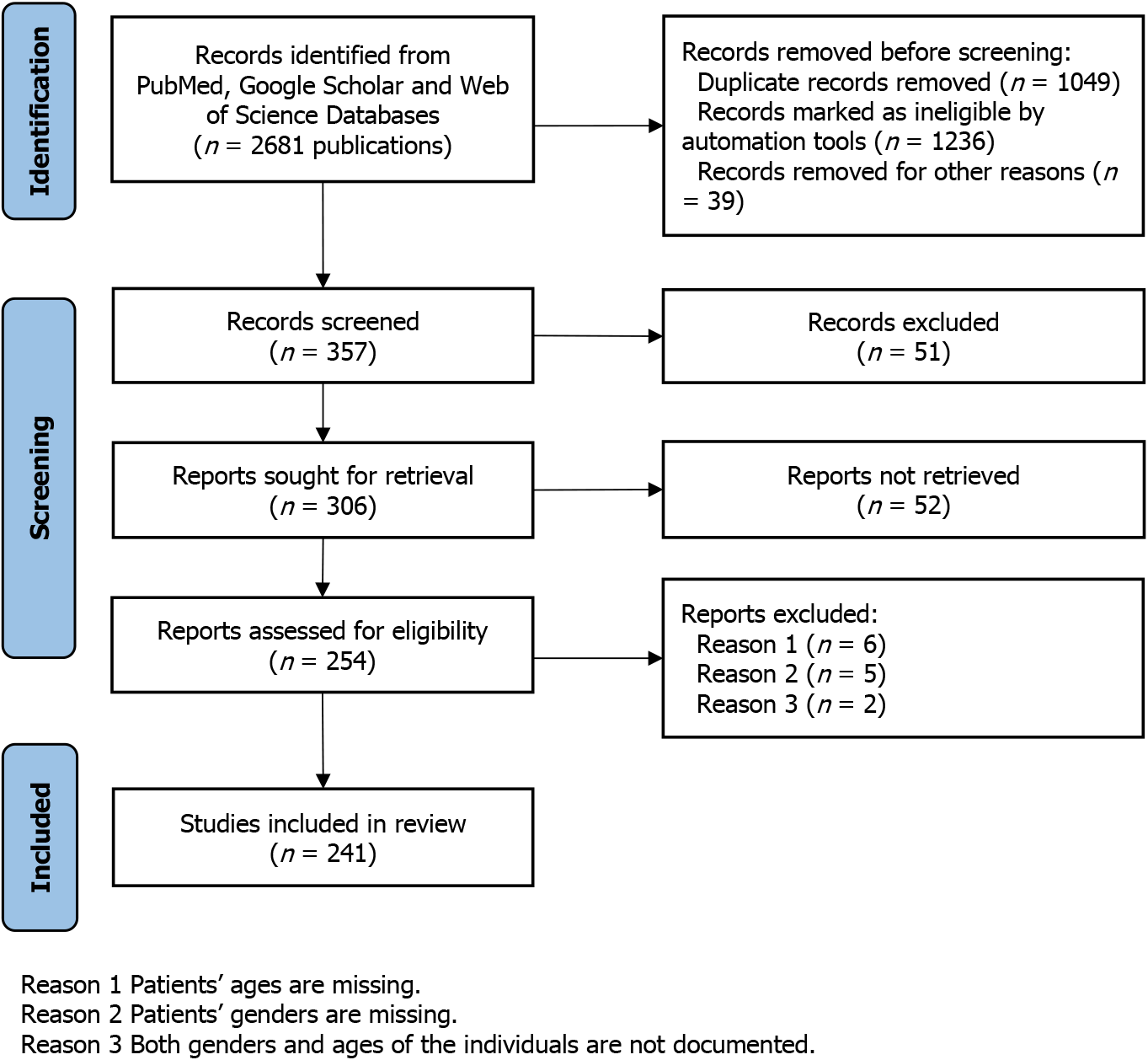
A comprehensive search was conducted in the NCBI PubMed database, Google Scholar, and Web of Science to identify all available articles. The following search terms were employed: “music hallucination,” “music hallucination,” “musical hallucination and mental illness,” “music hallucination and gender difference,” and “music hallucination and psychiatric disease.” No search restrictions were imposed. The search results were initially screened to exclude articles not written in English, those lacking abstracts or full texts, or those unrelated to MH. The selected articles were further scrutinized, and MH subjects were assembled to form the data pool for this study (Figure 1).
Demographic data, including age, sex, treatment, comorbidity, and etiology, were collected for each MH subject. Subjects were categorized based on their etiologies. In cases where a patient could have multiple potential etiologies for MH, the most likely etiology or the one clinically most associated with the onset of MH symptoms was chosen, resulting in each case being classified once. The demographic variables of MH subjects were compared and analyzed among different etiological groups.
To compare the average age of MH populations among different etiological groups, the t-test and ANOVA were employed (a significance level of P < 0.05 was considered statistically significant). The implications of the statistical findings were discussed.
Neuroimaging studies were reviewed to identify the neural networks implicated in MH. The neurobiological processes associated with psychiatric disorders comorbid with MH were summarized and described.
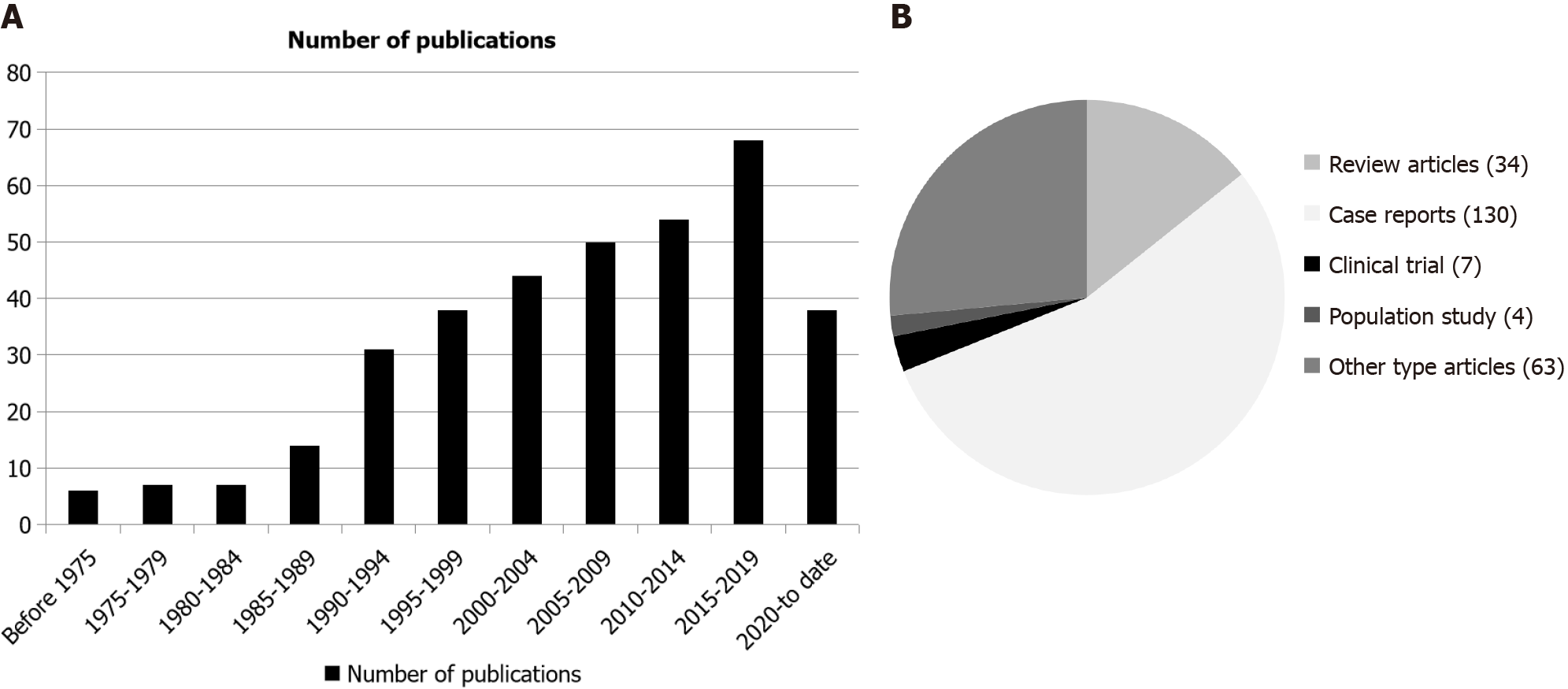
The earliest article pertaining to MH was published in 1958 by Arieff and Brooks[17]. Only 34 articles were identified in connection to MH between 1958 and 1989. As interest in MH grew, more research emerged. Between 1990 and 1995, there were 31 publications on MH, and this number doubled during the 5-year period from 2015 to 2020 (Figure 2A).
From the reviewed publications, those unrelated to MH or lacking relevant information were excluded. This process yielded 238 articles, which formed the MH study database. Within this database, there were 34 review articles, 130 case reports, 7 clinical trials, and 4 population studies. The remaining 63 articles covered topics like basic research, surgical procedures, and auditory studies (Figure 2B). Notably, while some articles mentioned trends in age and gender diffe
A total of 294 MH subjects were gathered by examining the articles in the MH study database. Demographic variables, including age, gender, and comorbidity, were investigated.
Among the 294 MH subjects, the ages of 226 subjects were reported, ranging from 20 to 101 years, with a mean age of 67.9 years (SD = 16.2 years). Among these subjects, there were 151 (66.8%) females and 75 (33.2%) males, resulting in a female-to-male ratio (F:M) of approximately 2:1 (Table 1). For the remaining 67 cases, their ages and genders were not documented.
| Group number | Etiology1 | Case number2 | Gender | Age in years, mean ± SD (range)3 | Nationalities of the cases | Comorbidities |
| 1 | Hearing impairment | 54 (77) | Male = 19, female = 35 | 69.0 ± 12.5 (27-89) | Australia, Belgium, Brazil, Espinal, Germany, India, Israel, Japan, Omaha, Romania, Netherlands, The United Kingdom, Türkiye, United States | Tinnitus, hearing loss, presbycusis, pre-lingual deafness, cochlear implants, pontine lacunar lesions, otosclerosis |
| 2 | Psychiatric disorder | 64 (85) | Male = 20, female = 44 | 65.2 ± 16.4 (20-89) | Finland, Greece, Israel, Italy, Japan, Netherlands, Portugal, The Netherlands, The United Kingdom, United States | OCD, schizophrenia, Alzheimer, anxiety, ADHD, MDD, dementia, personality disorder |
| 3 | Brain lesion | 16 (22) | Male = 5, female = 11 | 69.1 ± 12.2 (38-80) | Japan, Poland, United States | Stroke, traumatic brain injury, whiplash injury, left temporal lobectomy, brainstem lesion |
| 4 | Neuropathological syndromes | 12 (14) | Male = 2, female = 10 | 77.1 ± 15.9 (45-101) | Germany, Japan, Spain, United States | Epilepsy, Parkinson disease, neurodegenerative disorder, Lewy body disorders, Charles Bonnet Syndrome etc. |
| 5 | Substance intoxication | 18 (20) | Male = 8, female = 10 | 59.7 ± 19.3 (23-91) | Japan, United Kingdom, United States | Mirtazapine, tricyclic, ketamine, pentoxifylline, dipyridamole, quetiapine, triazolam, oxycodone, Transient amantadine |
| 6 | Unknown etiology | 42 (53) | Male = 12, female = 30 | 72.9 ± 13.2 (29-88) | France, or Italy, United States | No comorbidities |
| 7 | Surgery | 8 (9) | Male = 1, female = 7 | 61.3 ± 20.5 (27-79) | Brazil, Canada, United States | Childbirth in a female patient on hemodialysis |
| 8 | Infection/immune disorder | 12 (14) | Male = 8, female = 4 | 65.3 ± 15.6 (27-82) | Canada, United States | Listeria rhombencephalitis, anti-IgLON5 disease, listeria rhombencephalitis |
| Total cases | 226 (294) | Male = 75, female = 151 | 67.9 ± 16.2 (20-101) |
In this study, the 294 MH subjects were categorized into 8 distinct groups based on their underlying pathological etiologies (Table 1):
Group 1 (n = 77): MH with Hearing Impairment. This group consisted of MH subjects with varying degrees of hearing loss. Among the 54 subjects with documented demographic information, their ages ranged from 27 to 89 (mean of 69.0 ± 12.5 years). There were 35 females (64.8%) and 19 males (35.2%), resulting in a F:M of 1.8:1, with female predominance. The ages and genders of the remaining 23 subjects were unknown.
Group 2 (n = 85): MH with psychiatric disorders. This was the largest of the 8 etiological groups. The MH subjects in this group presented a wide range of psychiatric disorders, including psychotic disorder, mood disorder, personality disorder, or developmental-related disorder. Among the 64 subjects with reported ages, their ages ranged from 20 to 89 years (mean of 65.2 ± 16.4 years). Of these, 44 were females, and 20 were males, resulting in a F:M ratio of 2.2 (68.7% vs 31.3%). The demographic information for the other 21 subjects was unknown.
Group 3 (n = 22): MH with Brain Lesions. This group included subjects with organic brain lesions, encompassing conditions such as brain injuries, brain tumors, or strokes. Of the 22 subjects in this group, 11 were females and 5 were males, with a mean age of 69.1 ± 12.2 years. Personal information of the other 6 subjects was not provided.
Group 4 (n = 14): MH with Neuropathological Syndromes [epilepsy/Charles Bonnet syndrome (CBS)]. CBS is a neuropathological condition frequently seen in elderly individuals. CBS and epilepsy are the neurological disorders most commonly associated with MH. In this group, 9 patients were diagnosed with CBS, and 5 were reported to have epilepsy disorders. This group showed a relatively later onset age (occurring after 45 years) and had the highest mean age of 77.1 ± 15.2 years. A significant gender difference was noted, with 83% being female and 17% male. Personal information was available for 12 of the 14 subjects, while 2 subjects lacked age and gender data in the original articles.
Group 5 (n = 20): MH with substance intoxications. This group included subjects who developed MH due to substance use or adverse side effects of prescribed medications. It consisted of 8 males, 10 females, and 2 subjects with unknown gender. This group was the youngest, with ages ranging from 23 to 91 and a mean age of 59.7 ± 19.3 years.
Group 6 (n = 53): MH with Unknown Etiology. This group comprised 53 subjects diagnosed with primary MH, with no other psychiatric or medical conditions identified. The etiology for this group remained unknown. Among the 42 subjects with age data, ages ranged from 29 to 88 (average 72.9 ± 13.2), with an F:M ratio of 2.5:1. Demographic information for 11 individuals was unavailable.
Group 7 (n = 9): Surgery-related MH. This group encompassed subjects who experienced MH following surgical procedures other than neurosurgeries, including procedures like pharyngolaryngoesophagectomy, liver transplantation, childbirth, and hemodialysis. Among these subjects, the gender of one was unknown, and the remaining 8 were female and 1 male (F:M = 7:1). The age range was 27 to 79, with an average age of 61.3 ± 20.5 years.
Group 8 (n = 14): Infection/immune disorder group. This group included 14 MH subjects with various concomitant infectious or immune diseases, including conditions like Lyme Disease, Hashimoto’s Encephalopathy, and Listeria Rhombencephalitis. Ages in this group ranged from 27 to 82 (mean of 65.3 ± 15.6) years. Notably, this group had male predominance, with 66.7% males and 33.3% females, resulting in an M:F ratio of 2:1. The demographic information for two additional subjects in this group was not reported.
ANOVA was conducted to assess whether there were statistically significant differences in mean ages among the eight etiological groups. The results indicated significant age differences (P < 0.033).
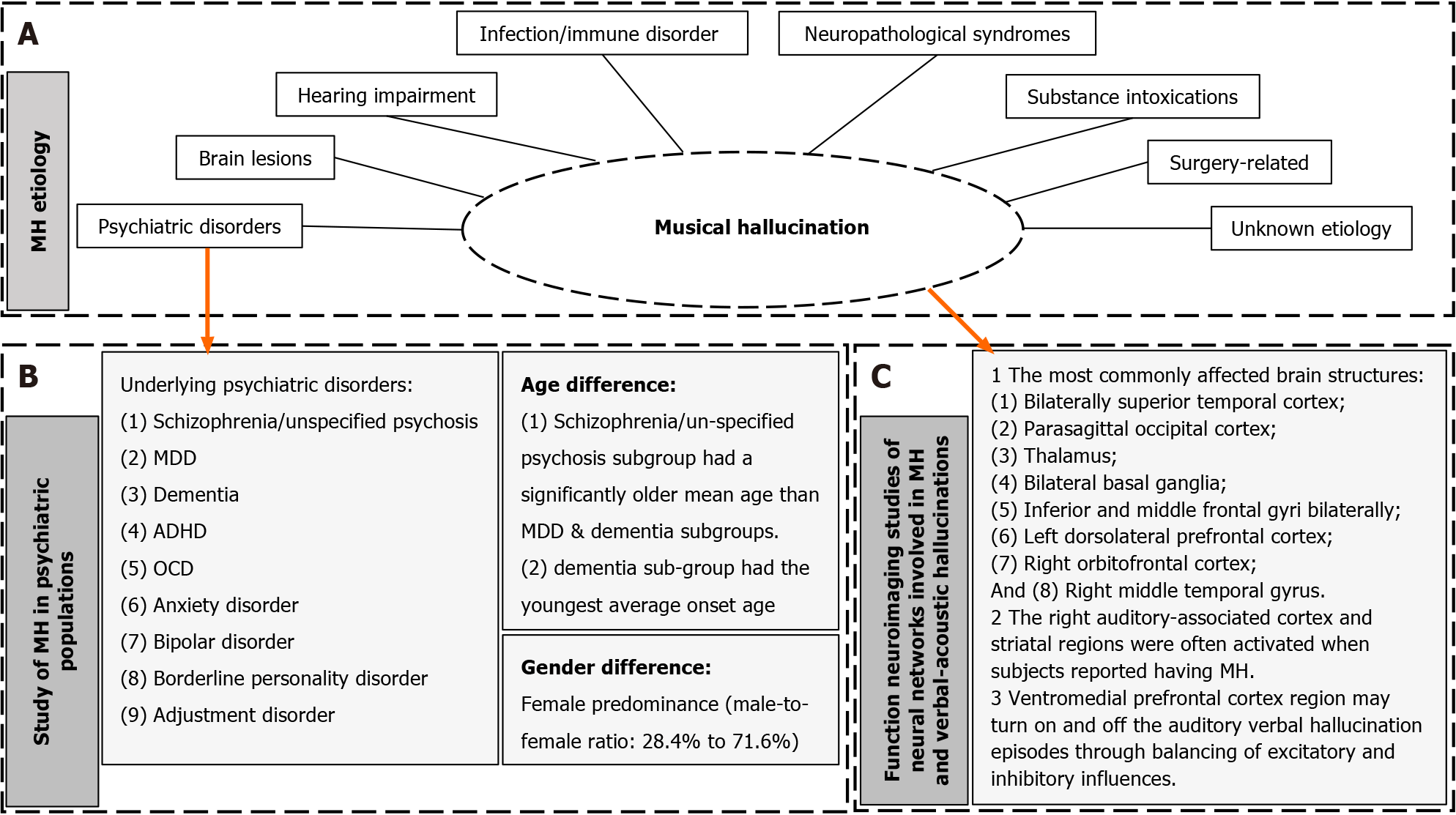
The investigation of the relationship between MH and psychiatric disorders is of particular interest in this study. Out of the 294 subjects, 85 (28.9%) had underlying psychiatric disorders. The prevalent psychiatric disorders comorbid with MH included: Schizophrenia/unspecified psychosis (40 cases), MDD (26 cases), dementia (10 cases), ADHD (2 cases), OCD (2 cases), anxiety disorder (2 cases), bipolar disorder (1 case), borderline personality disorder (1 case), and adjustment disorder (1 case) (Table 2).
| Psychiatric diagnosis1 | Case number2 | Sex | Age in years, mean ± SD3 (range) |
| Psychosis NOS/schizophrenia | 40 | Male = 6, female = 18, unknown = 16 | 71.7 ± 19.7 (20-89) |
| MDD | 26 | Male = 8, female = 17, unknown = 1 | 66.9 ± 11.5 (35-88) |
| Dementia | 10 | Male = 2, female = 8, unknown = 0 | 57.2 ± 18.7 (24-82) |
| ADHD | 2 | Male = 2, female = 0, unknown = 0 | 23, 40 |
| Anxiety | 2 | Male = 0, female = 2, unknown = 0 | 78, 85 |
| OCD | 2 | Male = 0, female = 1, unknown = 1 | 75 |
| Adjustment disorder | 1 | Male = 0, female = 1, unknown = 0 | 57 |
| Bipolar | 1 | Male = 0, female = 1, unknown = 0 | 66 |
| Borderline personality | 1 | Male = 1; female = 0, unknown = 0 | Unknown |
| Total number | 85 | Male = 19, female = 48, unknown = 18 | 67.1 ± 16.5 (20-89) |
In the overall population of the psychiatric etiological group, a female predominance was observed, with a male-to-female ratio of 28.4% males to 71.6% females, approximately 2.5:1 (Table 2). The female predominance trend was also observed in the ideological subgroups, including MDD (32% male to 68% female), dementia (20% male to 80% female), and psychosis (25% male to 75% female).
Various psychiatric disorders were associated with MH, with the three most common diagnoses being psychosis NOS/schizophrenia, MDD, and dementia (Table 2). The mean ages of these three groups were as follows: Psychosis subgroup 71.7 ± 19.7 (range 20-89), MDD subgroup 66.9 ± 11.5 (range 35-88), and dementia subgroup 57.2 ± 18.7 (range 24-82). The psychosis subgroup had a significantly older mean age than the other two subgroups. Interestingly, the MH-associated dementia subgroup had the youngest average onset age (average 57.2 ± 18.7), despite dementia symptoms typically manifesting at a later stage of life in the general population. MH was also sporadically found in individuals with ADHD, adjustment disorder, anxiety, bipolar disorder, OCD, and borderline personality disorder. The one-way ANOVA analysis test did not reveal significant age differences among the subgroups of MDD, dementia, and psychosis (P = 0.059).
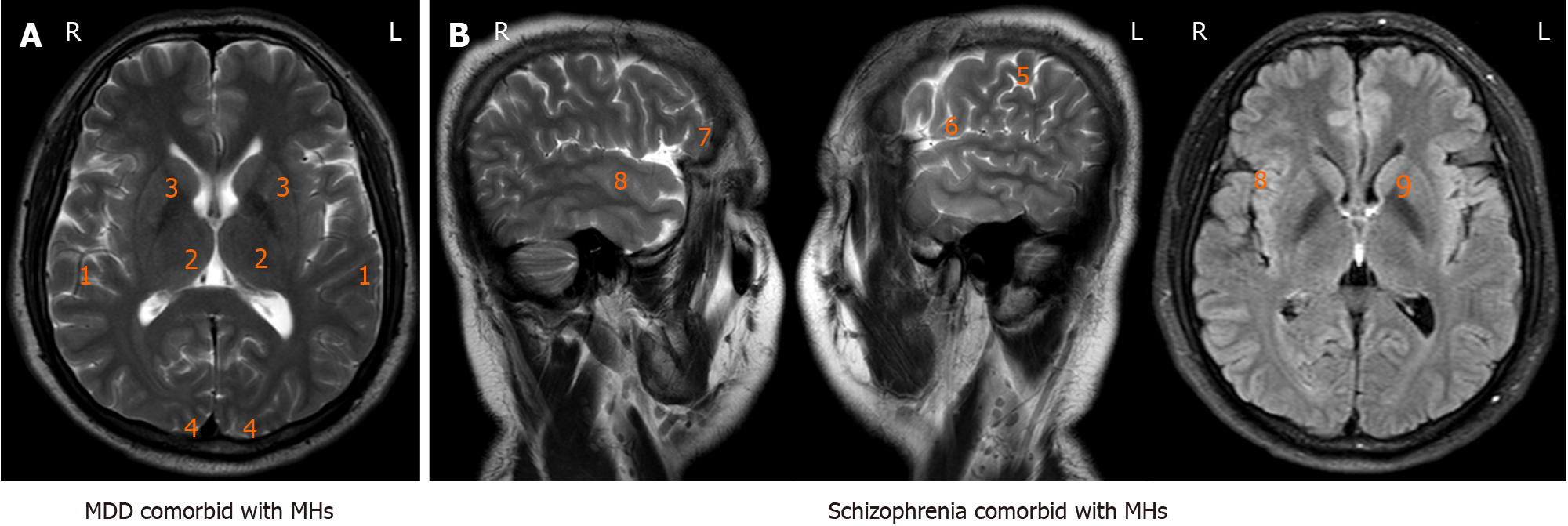
Figure 3 summarizes the increased metabolic activities observed in various cortex and diencephalon structures in neuroimaging studies of mentally ill patients experiencing MH. The most commonly affected brain structures included the bilaterally superior temporal cortex, parasagittal occipital cortex, thalamus, bilateral basal ganglia, inferior and middle frontal gyri bilaterally, left dorsolateral prefrontal cortex, right orbitofrontal cortex, and right middle temporal gyrus. The right auditory-associated cortex and striatal regions were often activated when subjects reported having MH.
MH is a phenomenon characterized by diverse clinical and pathophysiological backgrounds. Previous analyses of its pathophysiological mechanisms identified five etiological causes[6]. The notable increase in MH publications in recent decades reflects a growing interest and recognition of this significant psychoneurological phenomenon. Therefore, a more comprehensive analysis of an updated MH study is essential, particularly from a systematic perspective regarding the psychiatric conditions linked and/or associated with MH. In this study, we expand the pathomechanisms of MH from the previous five major categories (hypoacusis, psychiatric disorders, focal brain lesions, epilepsy, and intoxication) to eight categories, which now include hearing impairment, psychiatric disorders, brain lesions, neuropathological syndromes, substance intoxication, surgical procedures, infection/immune disorders, and unknown etiology (Figure 4).
Most previous studies have reported a higher prevalence of MH in females. Similarly, our data also showed an overall F:M ratio of approximately 2:1, in line with Evers and Ellger’s study[6]. An examination of gender differences among the eight MH groups revealed that in seven out of the eight groups, females dominated. This trend was most pronounced in the CBS/Epilepsy group (F:M = 5:1) and the Surgery group (F:M = 7:1), while it was less prominent in the Substance Intoxication group (F:M = 1.25). Only one group displayed a male preponderance (F:M = 1:2), and this group consisted of subjects with Lyme disease, Hashimoto’s encephalopathy, lung cancer, living-donor liver transplants, and Listeria rhombencephalitis.
Analyzing gender differences is crucial for understanding the underlying pathophysiological mechanisms of clinical phenomena. Some gender-specific risk factors affect women differently than men. For example, females who have been widowed after 30 to 40 years of marriage have a higher incidence of experiencing perceptual changes, such as hearing and/or seeing their deceased spouse, compared to males who have also lost a spouse after a similar period of time. This gender difference might serve as a coping mechanism and source of comfort for women but may not manifest similarly in men. Biologically, sexual hormones and neurotransmitters in the hypothalamic-pituitary-adrenal (HPA) axis are reported to contribute to gender differences in psychiatric symptoms[18]. The “estrogen protection theory” suggests that estrogen may mask developmental insults until menopause[19], and sex differences in the dysregulation of the HPA axis and cortisol response to stress have been consistently observed. Social factors also play a role, but research indicates that they may influence the prevalence of mental illness, though this effect is not independent of genetically determined vulnerability. Drawing a parallel to explain the female preponderance of MH, we might infer that women are more vulnerable to physical and emotional stress and are more likely to develop MH.
Population studies have shown that 10%-15% of the general population occasionally experiences some form of auditory hallucination at some point in their life, which is typically benign and unrelated to any psychiatric condition[20]. MH, as a form of auditory hallucination, is less debilitating to individuals than other verbal hallucinations, which are also more prevalent in non-psychotic populations. In the current study, we found that the majority of MH subjects (71.1%) did not have any underlying mental illness. This discovery holds clinical significance as individuals with MH are often erroneously diagnosed as psychotic due to cultural and social perceptions, even by medical professionals. MH subjects are frequently wrongly treated with antipsychotic medications that can have serious side effects. Furthermore, improper diagnosis and treatment can lead to unnecessary stigmatization and discrimination. A key reason for this is that psychiatry is a field that primarily relies on clinical evaluation for diagnosis. There are often no physical symptoms or definitive biological tests for psychosis. Diagnosing mental illnesses is complex and prone to misdiagnosis. Given the complexity and limited knowledge of MH, these misdiagnoses are not so much errors as inevitable outcomes resulting from cognitive differences and unclear diagnostic procedures.
Therefore, it is crucial to classify MH as either psychotic or non-psychotic to make accurate diagnoses of patients. The choice of the appropriate treatment plan should be based on the patient’s diagnosis and the extent to which their social functioning is impaired, rather than just the presence of the term “hallucination.” This approach will help avoid unnecessary health issues and mental problems resulting from the overuse of antipsychotics.
Using more than the recommended dosage of most medications will not necessarily enhance the intended effect and can lead to severe health problems.
In the case of treating patients, psychotic MH may exacerbate an underlying psychiatric condition that necessitates treatment. In contrast, non-psychotic MH typically has a minimal impact on a person’s life and does not require intervention.
Previous studies have found that individuals with isolated non-psychotic perception disorders may develop schizotypal personality and psychosis later in life. In our study, 53 subjects reported isolated MH symptoms, which calls for further research to examine if this population is at an increased risk of developing a psychotic diagnosis later in life. Thus far, no increased risk of suicidality, homicidality, or significant violent behaviors has been reported in association with MH, whether with or without underlying psychotic conditions.
Neurobiological studies have shown that MH involves specific neuronal populations and various neural networks, leading to abnormal communication between different parts of the brain due to disruptions. Neuroscientists from the Massachusetts Institute of Technology have identified a neural population in the primary auditory cortex that selectively responds to sounds typically categorized as music, but not to speech or other environmental sounds[21]. This suggests that the disruption of the neural population associated with music in the primary auditory cortex and its connected neural circuits is the fundamental mechanism behind MH. Cumulative data from neuroimaging studies indicate that the primary auditory cortex on the superior temporal sulcus, along with its associated neural circuitry, is the most common site of activation in MH patients.
At the biological and molecular level, there are three primary mechanisms responsible for these disruptions in neurons:
Physical injuries: These disruptions can result from physical injuries such as strokes, traumatic brain injuries, surgical brain damage, and tumors. For example, in this study, a 38-year-old patient who suffered a brain injury in a motor vehicle accident and an 80-year-old man who survived an ischemic stroke both developed MH due to their respective injuries.
Chemical transmitter miscommunication: This can occur due to a range of substances, including benzodiazepines, voriconazole, propranolol, tramadol hydrochloride, tricyclic medications, salicylate-containing drugs, pentoxifylline, butane gas, toluene-based solvents, alcohol, opioids, and marijuana. In certain instances, these substances have been reported to cause diffuse brain injuries that lead to MH[22-29]. As substance abuse is more prevalent in younger populations, it is not surprising that the subjects in this group with MH were significantly younger than those in other etiological categories (with a mean age of 59.7 ± 19.3).
Neurodegeneration: Abnormal neuroactivity in MH can be caused by neurodegenerative diseases. An extensive body of structural and imaging literature has reported on studies of mental illnesses associated with MH. These studies indicate that the most commonly affected brain structures are the bilaterally superior temporal cortex, prefrontal cortex, and right middle temporal gyrus, including the right auditory associated cortex and striatal regions. Theoretically, neurodegenerative diseases affecting these neural structures significantly increase the risk of developing MH. This hypothesis is supported by the high prevalence of MH in populations with early-onset dementia (with an average age of 57.2 ± 18.7), mainly presenting with frontotemporal dementia, and in populations with age-related hearing loss.
There is no consensus on the treatment or prognosis of MH. In individual case reports, some authors have demon
MH is an intriguing and complex phenomenon that can manifest in various circumstances. It may occur in isolation, be associated with an underlying psychiatric condition, or stem from physical trauma (most commonly brain injury) and surgical procedures. MH can vary in its impact, from severely debilitating to mild with no harmful consequences. While data suggests a female predominance, it also affects males and can be found across all age groups. There are numerous research avenues yet to be explored in understanding the physiological aspects of MH and how to improve its treatment.
This research, however, is subject to several limitations. For instance, hundreds of MH cases were collected through database searching and many of them do not include full information of the MH patients, such as age, gender or comorbidity conditions. These cases must be eliminated from the study pool which decreases the sample sizes. MH as a condition with low incidence, insufficient sample size for statistical measurements and lack of previous statistic studies on the topic might impact in drawing valid conclusions on MH. In addition, MHs often comorbid multiple medical or medical conditions, these conditions may or may not interact with each other, the true association and/or influence its interpretation need to be carefully considered.
The notable increasing number of publications in MH recently reflects a growing interest and recognition of this significant psychoneurological phenomenon. A more comprehensive review of an updated MH study and extended classification of MH by its pathological etiologies is necessary. A systematic perspective and statistical point of view regarding the psychiatric conditions comorbid with MH were performed in the research. Recent biological and molecular studies suggest possible three mechanisms causing neuron disruptions in MH. The neurobiological processes associated with psychiatric disorders comorbid with MH were summarized.
We express our gratitude to Mr. Eddie Elkin for his invaluable editing and proofreading assistance.
| 2. | Cole MG, Dowson L, Dendukuri N, Belzile E. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry. 2002;17:444-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Teunisse RJ, Olde Rikkert MG. Prevalence of musical hallucinations in patients referred for audiometric testing. Am J Geriatr Psychiatry. 2012;20:1075-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Fukunishi I, Kitaoka T, Shirai T, Watanabe S. Musical hallucinations after childbirth in a female patient on hemodialysis. Nephron. 1998;79:105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Hermesh H, Konas S, Shiloh R, Dar R, Marom S, Weizman A, Gross-Isseroff R. Musical hallucinations: prevalence in psychotic and nonpsychotic outpatients. J Clin Psychiatry. 2004;65:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Evers S, Ellger T. The clinical spectrum of musical hallucinations. J Neurol Sci. 2004;227:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Liikkanen LA. Musical activities predispose to involuntary musical imagery. Psychol Music. 2012;40:236-256. [DOI] [Full Text] |
| 8. | Aizenberg D, Schwartz B, Modai I. Musical hallucinations, acquired deafness, and depression. J Nerv Ment Dis. 1986;174:309-311. [PubMed] [DOI] [Full Text] |
| 9. | Bhatt YM, de Carpentier JP. Musical hallucination following whiplash injury: case report and literature review. J Laryngol Otol. 2012;126:615-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Calabrò RS, Baglieri A, Ferlazzo E, Passari S, Marino S, Bramanti P. Neurofunctional assessment in a stroke patient with musical hallucinations. Neurocase. 2012;18:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | E Fischer C, Marchie A, Norris M. Musical and auditory hallucinations: A spectrum. Psychiatry Clin Neurosci. 2004;58:96-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Janakiraman R, Wildgoose K, Seelam K. ECT associated musical hallucinations in an elderly patient: a case report. Ann Gen Psychiatry. 2006;5:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Ergün U, Bozbaş A, Akin U, Inan L. Musical hallucinations and Parkinson disease. Neurologist. 2009;15:150-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Stricker RB, Winger EE. Musical hallucinations in patients with Lyme disease. South Med J. 2003;96:711-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Fontenelle LF, Cocchi L, Harrison BJ, Shavitt RG, do Rosário MC, Ferrão YA, de Mathis MA, Cordioli AV, Yücel M, Pantelis C, Mari Jde J, Miguel EC, Torres AR. Towards a post-traumatic subtype of obsessive-compulsive disorder. J Anxiety Disord. 2012;26:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Islam L, Scarone S, Gambini O. Obsessive-compulsive disorder presenting with musical obsessions in otosclerosis: a case report. J Med Case Rep. 2014;8:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Arieff AJ, Brooks JM. Musical hallucinations as a psychical seizure: focal epileptic disorder. Q Bull Northwest Univ Med Sch. 1958;32:201-203. [PubMed] |
| 18. | Riecher-Rössler A, Butler S, Kulkarni J. Sex and gender differences in schizophrenic psychoses-a critical review. Arch Womens Ment Health. 2018;21:627-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 19. | Baldwin CH, Srivastava LK. Can the neurodevelopmental theory account for sex differences in schizophrenia across the life span? J Psychiatry Neurosci. 2015;40:75-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res. 2000;97:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 204] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Norman-Haignere SV, Feather J, Boebinger D, Brunner P, Ritaccio A, McDermott JH, Schalk G, Kanwisher N. A neural population selective for song in human auditory cortex. Curr Biol. 2022;32:1470-1484.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Tohhara S, Tani N, Nakajima T, Tsuda E. [Clinical study of butane gas abuse: in comparison with toluene-based solvent and marihuana]. Arukoru Kenkyuto Yakubutsu Ison. 1989;24:504-510. [PubMed] |
| 23. | Gilbert GJ. Pentoxifylline-induced musical hallucinations. Neurology. 1993;43:1621-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Allen JR. Salicylate-induced musical perceptions. N Engl J Med. 1985;313:642-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Gordon AG. Tricyclic-induced musical hallucinations. Biol Psychiatry. 1996;40:309-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Fernandez A, Crowther TR, Vieweg WV. Musical hallucinations induced by propranolol. J Nerv Ment Dis. 1998;186:192-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Keeley PW, Foster G, Whitelaw L. Hear my song: auditory hallucinations with tramadol hydrochloride. BMJ. 2000;321:1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Curtin F, Redmund C. Musical hallucinations during a treatment with benzodiazepine. Can J Psychiatry. 2002;47:789-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Prommer E. Musical hallucinations and opioids: a word of caution. J Pain Symptom Manage. 2005;30:305-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Blumberger DM, Christensen BK, Zipursky RB, Moller B, Chen R, Fitzgerald PB, Daskalakis ZJ. MRI-targeted repetitive transcranial magnetic stimulation of Heschl's gyrus for refractory auditory hallucinations. Brain Stimul. 2012;5:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Terao T, Tani Y. Carbamazepine treatment in a case of musical hallucinations with temporal lobe abnormalities. Aust N Z J Psychiatry. 1998;32:454-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Sosland MD, Edelsohn GA. Hallucinations in children and adolescents. Curr Psychiatry Rep. 2005;7:180-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Zilles D, Zerr I, Wedekind D. Successful treatment of musical hallucinations with the acetylcholinesterase inhibitor donepezil. J Clin Psychopharmacol. 2012;32:422-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | David RR, Fernandez HH. Quetiapine for hypnogogic musical release hallucinations. J Geriatr Psychiatry Neurol. 2000;13:210-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Hugdahl K, Craven AR, Johnsen E, Ersland L, Stoyanov D, Kandilarova S, Brunvoll Sandøy L, Kroken RA, Løberg EM, Sommer IEC. Neural Activation in the Ventromedial Prefrontal Cortex Precedes Conscious Experience of Being in or out of a Transient Hallucinatory State. Schizophr Bull. 2023;49:S58-S67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Goldberg D. Vulnerability factors for common mental illnesses. Br J Psychiatry Suppl. 2001;40:s69-s71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Johnston O, Gallagher AG, McMahon PJ, King DJ. The efficacy of using a personal stereo to treat auditory hallucinations. Preliminary findings. Behav Modif. 2002;26:537-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |