Published online Sep 19, 2024. doi: 10.5498/wjp.v14.i9.1354
- This article has been retracted.
- Retraction in: World J Psychiatry. Feb 19, 2025; 15(2): 103118 See also: Errata, Retraction, Duplicate Publication and Comment Policy
Revised: July 4, 2024
Accepted: August 5, 2024
Published online: September 19, 2024
Processing time: 91 Days and 2.5 Hours
To assess the effectiveness of Shugan Jieyu capsules on peripheral blood miR-124, miR-132, and brain-derived neurotrophic factor (BDNF) levels in patients with mild to moderate depression following coronary artery intervention [percuta
To evaluate the therapeutic efficacy of Shugan Jieyu capsules and their effects on the peripheral blood levels of miR-124, miR-132, and BDNF in patients with mild to moderate depression following PCI for coronary heart disease.
Patients with mild-to-moderate depression of the liver-qi stagnation type after PCI for coronary heart disease at the 305th Hospital of the People’s Liberation Army were enrolled from June 2022 to November 2023 and randomly assigned to two groups: Experimental (treated with Shugan Jieyu capsules) and control (tr
No significant difference was observed in any index between the two groups before treatment (P > 0.05). After treatment, the total efficacy rates were 93.33% and 90.00% in the experimental and control groups, respectively. Experimental group had significantly lower scores for the main and secondary syndromes compared to the control group (P < 0.05). No significant difference was observed in the metabolic equivalents between the two groups be
Shugan Jieyu capsules have good efficacy in patients with mild-to-moderate depression after PCI, and its me
Core Tip: The study examines the effects of Shugan Jieyu capsules on patients with mild to moderate depression following percutaneous coronary intervention for coronary heart disease. The results indicate significant improvements in depressive symptoms, biochemical markers such as miR-124, miR-132, and brain-derived neurotrophic factor, as well as immune function. Shugan Jieyu capsules outperformed escitalopram oxalate tablets in reducing depression severity and adverse reactions, suggesting their potential as a safer and more effective treatment alternative for post-percutaneous coronary intervention depression.
- Citation: Zhang X, Liu Y, Tang HF, Jiang F, Chen CL, Wang TT, Gu HZ, Zhao Q, Ma R. Shugan Jieyu capsule effects on peripheral blood micro-124, micro-132, and brain-derived neurotrophic factor in patients with mild to moderate depression. World J Psychiatry 2024; 14(9): 1354-1363
- URL: https://www.wjgnet.com/2220-3206/full/v14/i9/1354.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i9.1354
Percutaneous coronary intervention (PCI) is a widely used medical procedure for treating coronary artery disease[1]. This treatment method is primarily employed for managing coronary heart disease, especially in cases in which coronary artery narrowing or blockage leads to insufficient blood supply to the heart. During PCI, a physician inserts a long thin catheter into the patient’s blood vessels, typically through the leg or arm arteries. The catheter was then guided into the coronary arteries of the heart. Once in place, the physician uses specialized tools, such as balloons, to dilate narrow sections of the blood vessels[2]. In many cases, a small metal mesh structure called a stent is also placed to help keep the blood vessels open. PCI primarily improves the blood supply to the myocardium, alleviates chest pain (angina), enhances the patient’s quality of life, and reduces the risk of heart attacks. Compared with traditional open-heart surgery, PCI is a less invasive option with shorter recovery times and relatively low risks[3].
However, recent research indicates that a significant portion of patients undergoing PCI treatment (approximately 40%) experience postoperative mental health issues such as anxiety and depression[4]. These psychological problems may be attributed to factors such as the stress of the surgery, concerns regarding health status, and lifestyle changes[5]. This psychological stress not only affect the patient’s mental well-being but may also have a negative impact on their physical recovery. Therefore, psychological interventions and support have become crucial components in the overall mana
Research has shown that compared to Western medicine, traditional Chinese medicine (TCM) is more effective in treating patients with post-PCI depression. TCM believes that smooth circulation of blood vessels in the body is essential for the overall wellbeing of patients, and disturbances in emotions can lead to the stagnation of liver qi and blood stasis, causing blockages in the blood vessels. The “Nei Jing” (Yellow Emperor’s Inner Canon), an ancient Chinese medical text, mentions that the heart governs blood vessels and spirits[7]. Dysfunction in these functions can lead to “dual heart diseases”, where heart and emotional disorders mutually influence each other. Shugan Jieyu capsules are widely used in clinical practice because of their calming and liver qi soothing effects. With the rapid development of second-generation sequencing, microRNAs (miRNAs) have gradually become a popular topic in clinical research. Numerous studies have confirmed the abnormal expression of miR-124 and miR-132 in patients with mental disorders and their involvement in regulating vascular neogenesis[8,9]. Therefore, this study used Shugan Jieyu capsules to treat patients with mild to moderate depression following coronary intervention and analyzed their clinical efficacy as well as their impact on the levels of miR-124, miR-132, and brain-derived neurotrophic factor (BDNF), a factor influencing the occurrence of de
This study was approved by the Ethics Committee of Hospital of Beijing Armed Police Forces the initiator of the multi-center joint study. Written informed consent was obtained from the patients and/or their guardians. This randomized controlled trial enrolled patients with PCI depression of liver qi stagnation and blood stasis treated at the 305th Hospital of the People’s Liberation Army from June 2022 to November 2023. Liver qi stagnation and blood stasis were diagnosed based on TCM syndrome differentiation criteria, including symptoms such as chest and hypochondriac fullness, distending pain, depression, and restlessness. The patients were randomly divided into the experimental and control groups. The sampling method employed was consecutive sampling. Random allocation was performed using a com
Inclusion criteria included: (1) Age between 40 and 75 years; (2) Meeting the diagnosis criteria for coronary heart disease[8], coronary angiography showing coronary artery stenosis or occlusion > 50%, undergoing PCI treatment; (3) Meeting the International Classification of Diseases-10[9] diagnosis criteria for mild to moderate depression[10], with a course of at least 4 weeks; (4) Meeting the TCM syndrome differentiation diagnosis criteria for mild to moderate depression, with symptoms lasting for at least 2 weeks: Main symptoms: Chest and hypochondriac fullness, distending pain, mental depression and restlessness; (5) Secondary symptoms: Wandering pain, loss of appetite, restless sleep, thin or greasy tongue coating, and wiry and fine pulse; (6) No other drugs that could affect the trial results were used within 2 weeks before treatment; (7) Patients had clear thinking and were able to cooperate with treatment; and (8) Patients and their family members signed informed consent forms. The exclusion criteria were as follows: (1) Patients with acute myocardial infarction accompanied by hemodynamic instability; (2) Patients with severe depression; (3) Patients with severe liver or kidney dysfunction and/or other malignant primary diseases; (4) Patients with severe infections; (5) Pregnant and lactating women; (6) Patients with suspected or confirmed allergies to the relevant drugs in this study; and (7) Self-administration of other TCM treatments. The exclusion criteria were severe adverse reactions, deterioration of condition, occurrence of other life-threatening conditions requiring emergency measures, poor compliance, voluntary withdrawal from the trial, and loss to follow-up. This study was approved by the hospital Ethics Committee.
Before and after 6 weeks of treatment, the 17-item Hamilton Rating Scale for Depression (HAMD-17) was used to assess the efficacy of depression treatment in patients. The scale consists of 17 items with a total score of 54, with higher scores indicating more severe illness. The efficacy was assessed using the HAMD-17 score reduction rate, calculated as (pre-treatment score - post-treatment score)/pre-treatment score × 100%. Clinical control: HAMD score reduction rate ≥ 70%, significant efficacy: 50% ≤ HAMD score reduction rate < 70%, effective: 20% ≤ HAMD score reduction rate ≤ 50%, ineffective: HAMD score reduction rate ≤ 20%. Additionally, before and after 6 weeks of treatment, TCM syndrome scoring assessments were conducted for depressive symptoms in both groups of patients. The main symptoms were chest and hypochondriac fullness, distending pain, mental depression, and restlessness, while secondary symptoms included wandering pain, loss of appetite, and restless sleep. Symptom severity was divided into four levels: None, mild, mode
Before and after 6 weeks of treatment, morning fasting venous blood was drawn from the patients and centrifuged to obtain the serum, and the BDNF level was determined using an enzyme-linked immunosorbent assay. Low-density lipoprotein cholesterol (LDL-C) levels were determined using an automatic biochemical analyzer, and serum hyper
| Gene | PCR primer sequences |
| miR-124 | Forward: 5’-GCGGTGAATGCCAAAAA-3’ |
| Reverse: 5’-CGCAAGGATGACACGCAAATTCGT-3’ | |
| miR-132 | Forward: 5’-ACCGTGGCTTTCGATTGTTA-3’ |
| Reverse: 5’-GGCGACCATGGCTGTAGACT-3’ | |
| U6 | Forward: 5’-TTGTGGAAAGGACGAAACAC-3 |
| Reverse: 5'-GGCCATGCTAATCTTCTG- 3' |
The experimental data were analyzed using Statistical Package for Social Science software (version 21.0; IBM, Armonk, NY, United States). Continuous variables that met the criteria for a normal distribution were presented as the mean ± SD, and differences between groups were compared using the independent two-sample t-test. Non-normally distributed data are presented as [M(P25, P75)], and intergroup differences were assessed using the non-parametric Mann-Whitney U test. Categorical data were expressed as percentages (%), and intergroup differences were compared using the χ2 or Fisher’s exact test. Statistical significance was set at P < 0.05.
Sixty patients with mild-to-moderate depression of liver qi stagnation and blood stasis following PCI were enrolled between June 2022 and November 2023 at the 305th Hospital of the People’s Liberation Army. Patients were randomly divided into experimental and control groups, with each group comprising 30 patients. As shown in Table 2, no sig
| Characteristic | Experimental group (n = 30) | Control group (n = 30) | P value |
| Age (year) | 60.07 ± 4.89 | 61.79 ± 4.53 | 0.232 |
| BMI (kg/m2) | 23.92 ± 2.14 | 24.08 ± 2.67 | 0.768 |
| Males (%) | 46.7 | 56.7 | 0.436 |
| < 2 stents (%) | 63.3 | 53.3 | 0.432 |
| ≥ 2 stents (%) | 36.7 | 46.7 | 0.432 |
In the comparison of depression treatment efficacy between the two groups, as shown in Table 3, there was no significant difference in the efficacy of treatment for depression between the two groups (P > 0.05). As shown in Table 4, the paired t-test analysis of the HAMD-17 scores within each group before and after treatment showed significant im
| Group | Experimental | Control | χ2 | P value |
| Number of cases | 30 | 30 | ||
| Clinical control, n (%) | 9 (30.00) | 6 (20.00) | ||
| Significant effect, n (%) | 11 (36.67) | 14 (46.67) | ||
| Effective, n (%) | 8 (26.67) | 7 (23.33) | ||
| Ineffective, n (%) | 2 (6.67) | 3 (10.00) | ||
| Total, effectiveness rate n (%) | 28 (93.33) | 27 (90.00) | 0.218 | 0.640 |
| Characteristic | Experimental group (n = 30) | Control group (n = 30) | P value |
| Age (year) | 60.07 ± 4.89 | 61.79 ± 4.53 | 0.232 |
| BMI (kg/m2) | 23.92 ± 2.14 | 24.08 ± 2.67 | 0.768 |
| Males (%) | 46.7 | 56.7 | 0.436 |
| < 2 stents (%) | 63.3 | 53.3 | 0.432 |
| ≥ 2 stents (%) | 36.7 | 46.7 | 0.432 |
In the comparison of TCM syndrome scores between the two groups of patients (Table 5), there was no statistically significant difference in baseline TCM syndrome scores between the two groups (P > 0.05). However, after treatment, the syndrome scores for the main and secondary symptoms in the experimental group were significantly lower than those in the control group (P < 0.05).
In the comparison of exercise endurance between the two groups, there was no significant difference in METs values between the experimental and control groups before treatment [(2.69 ± 0.47) vs (2.85 ± 0.68), P > 0.05]. The METs values between the two groups did not show significant difference between the two groups after treatment [(5.71 ± 1.16) vs (5.23 ± 1.23), P > 0.05].
In the comparison of biochemical indicators between the two groups (Table 6), there were no significant differences in the levels of LDL-C, hs-CRP, BDNF, miR-124, and miR-132 between the two groups before treatment (P > 0.05). After treatment, the miR-132, LDL-C, and hs-CRP levels in the experimental group were significantly lower than those in the control group (P < 0.05), whereas the miR-124 and BDNF levels were significantly higher than those in the control group (P < 0.05).
| Group | Experimental | Control | t | P value |
| LDL-C (mmol/L) | ||||
| Before treatment | 3.21 ± 0.87 | 3.35 ± 0.68 | 0.694 | 0.490 |
| After treatment | 1.61 ± 0.66a | 1.97 ± 0.63a | 2.161 | 0.035 |
| hs-CRP (mg/L) | ||||
| Before treatment | 8.47 ± 1.23 | 8.89 ± 1.56 | 1.157 | 0.252 |
| After treatment | 5.67 ± 0.59a | 6.25 ± 0.67a | 3.558 | 0.001 |
| BDNF (ng/mL) | ||||
| Before treatment | 4.29 ± 0.97 | 4.58 ± 0.88 | 1.213 | 0.230 |
| After treatment | 13.71 ± 3.16a | 10.43 ± 3.93a | 3.563 | 0.001 |
| miR-124 | ||||
| Before treatment | 0.89 ± 0.25 | 0.93 ± 0.26 | 0.607 | 0.546 |
| After treatment | 1.22 ± 0.30a | 1.01 ± 0.27a | 2.850 | 0.006 |
| miR-132 | ||||
| Before treatment | 3.39 ± 0.78 | 3.22 ± 0.74 | 0.866 | 0.390 |
| After treatment | 1.41 ± 0.36a | 1.73 ± 0.49a | 2.883 | 0.006 |
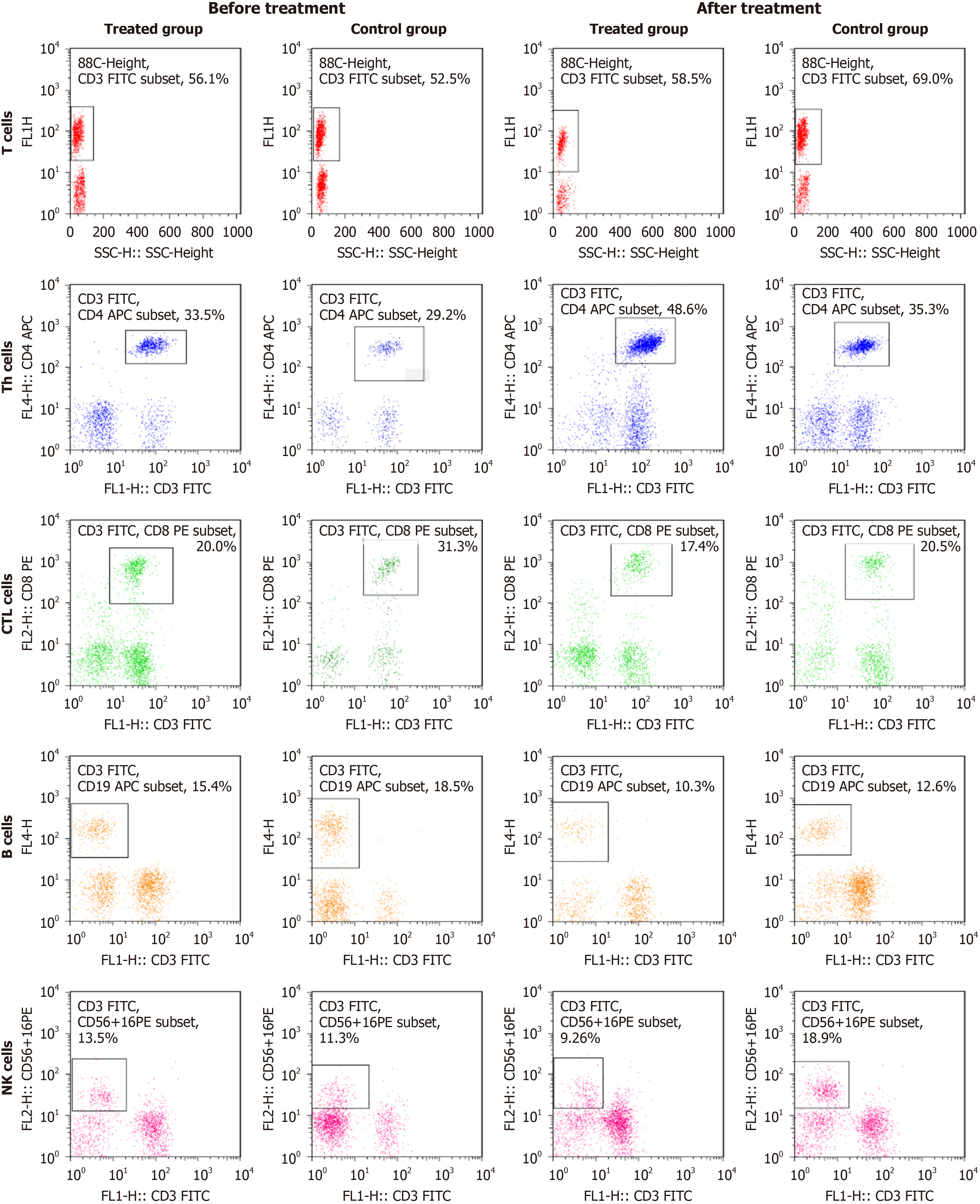
As shown in Figure 1 and Table 7, before treatment, there were no significant differences in the proportions of peripheral blood lymphocyte subsets (CD3+T lymphocytes, CD3+CD4+Th lymphocytes, CD3+CD8+CTL lymphocytes, CD3-CD19+B lymphocytes, CD3-CD56+16+NK cells) and the immune index (CD3+CD4+/CD3+CD8+ ratio) between the two groups (P > 0.05). However, after treatment, the proportions of CD3+T lymphocytes, CD3+CD4+Th lymphocytes, and CD3-CD56+16+NK cells, and the immune index (CD3+CD4+/CD3+CD8+ ratio) were significantly higher in the experimental group than in the control group (P < 0.05). As shown in Table 8, the incidence of adverse reactions in the experimental group was significantly lower than that in the control group (P < 0.05).
| Group | Experimental | Control | t | P value |
| CD3+T cells | ||||
| Before treatment | 53.45 ± 4.56 | 52.13 ± 5.11 | 0.924 | 0.568 |
| After treatment | 69.89 ± 5.03a,b | 58.32 ± 5.25a | 4.857 | 0.012 |
| CD3+CD4+Th cells | ||||
| Before treatment | 29.23 ± 3.28 | 28.65 ± 3.42 | 0.874 | 0.646 |
| After treatment | 40.05 ± 3.96a,b | 32.35 ± 4.17a | 5.231 | 0.009 |
| CD3+CD8+CTL cells | ||||
| Before treatment | 25.34 ± 2.92 | 26.76 ± 2.87 | 0.922 | 0.0324 |
| After treatment | 20.19 ± 3.18 | 24.24 ± 3.62 | 0.845 | 0.425 |
| CD3-CD19+B cells | ||||
| Before treatment | 14.85 ± 2.33 | 12.56 ± 2.12 | 0.984 | 0.266 |
| After treatment | 10.63 ± 2.96 | 15.32 ± 3.33 | 1.011 | 0.123 |
| CD3-CD56+16+NK cells | ||||
| Before treatment | 11.45 ± 2.91 | 12.77 ± 3.04 | 0.651 | 0.758 |
| After treatment | 15.66 ± 3.05a | 13.46 ± 3.12 | 3.864 | 0.011 |
| Group | Experimental | Control | Fisher P value |
| Number of cases | 30 | 30 | |
| Abdominal pain, n (%) | 0 (0.00) | 2 (6.67) | |
| Nausea, n (%) | 0 (0.00) | 2 (6.67) | |
| Vomiting, n (%) | 0 (0.00) | 1 (3.33) | |
| Total incidence, n (%) | 0 (0.00) | 5 (16.67) | 0.020 |
Recently, the number of patients undergoing PCI has increased with the incidence of coronary heart disease[10]. Concurrently, the number of patients with comorbid anxiety and depression has also increased. Research has shown that cardiovascular diseases and patients’ psychological well-being can mutually influence each other[7]. Abnormal changes in emotions can affect the hypothalamic-pituitary-adrenal axis, leading to hormonal imbalances, which may increase the likelihood of coronary heart disease[11]. Therefore, the field of “dual cardiology and psychology” has gained attention from clinical researchers.
Patients with cardiovascular diseases are often treated with selective serotonin reuptake inhibitors, norepinephrine reuptake inhibitors, and other psychotropic drugs to manage depression and anxiety. However, these drugs often have significant adverse effects such as bitterness in the mouth, dry mouth, dizziness, and decreased appetite, which can lead to poor medication adherence and suboptimal treatment outcomes[12]. Studies have shown that TCM can effectively improve the psychological state of patients with coronary heart disease who undergo PCI, reduce the incidence of ad
This study applied Shugan Jieyu capsules in the clinical treatment and found that, compared to escitalopram oxalate tablets, Shugan Jieyu capsules were more effective in alleviating symptoms of depression in patients with PCI and comorbid mild-to-moderate depression[14]. They also demonstrated a more significant improvement in patients’ exercise tolerance, quality of life, and biochemical indicators, with a significant reduction in the incidence of adverse reactions. Although there was no significant difference in the METs values between the two groups, other indicators suggested a positive trend in exercise tolerance, contributing to an overall improvement in the quality of life.
The “Expert Consensus on Integrated Traditional Chinese and Western Medicine Diagnosis and Treatment of Dual Cardiac Diseases” summarizes the causes of dual cardiac diseases into three aspects: Emotional discomfort leading to meridian blockage, dietary irregularities affecting the spleen and stomach, and prolonged cardiovascular disease leading to loss of confidence and heart blood depletion[15]. Shugan Jieyu capsules, mainly composed of Guan Ye Jin Si Tao and Ci Wu Jia, are used to treat liver qi stagnation in the cases of deficiency and weakness, demonstrating effects such as soothing the liver, relieving depression, clearing heat, and promoting dampness.
The results of this study suggest that the antidepressant effects of Shugan Jieyu capsules are comparable to those of escitalopram oxalate tablets. Capsules primarily focus on supplementing deficiencies and purging excesses while regulating the patient’s spirit, resulting in fewer adverse reactions and better relief from clinical symptoms. Multiple studies have also indicated that Shugan Jieyu capsules can alleviate clinical symptoms in patients with coronary heart disease and mild-to-moderate comorbid depression, improve TCM syndrome scores, and align with the results of this study[16].
Furthermore, studies have shown a correlation between the serotonin transporter gene and the occurrence of coronary heart disease combined with depression[17,18]. Patients with coronary heart disease and comorbid mood disorders often have elevated levels of inflammatory factors, such as CRP and abnormal blood lipids, which can lead to vascular endothelial dysfunction. The research by Guo et al[19] found that after treating patients with coronary heart disease with PCI, who also had comorbid anxiety and depression, with Shugan Lishpi capsules, there was a significant decrease in the levels of hs-CRP and a significant increase in BDNF levels, with greater changes than those in the group treated with fluoxetine maleate. The results of this study also showed that after treatment, miR-132 levels in the experimental group significantly decreased, whereas miR-124 and BDNF levels significantly increased compared to those in the control group. This suggests that Shugan Jieyu capsules may regulate oxidative stress, cytokines, and energy metabolism to reduce inflammatory responses and regulate lipid metabolism, while improving depressive symptoms.
In summary, Shugan Jieyu capsules demonstrated good efficacy in patients with mild-to-moderate depression following PCI. They improve the patients’ physical fitness, immune capabilities, and quality of life, thereby significantly reducing the incidence of adverse reactions. This effect may be related to the downregulation of miR-132 and upregulation of miR-124 and BDNF levels.
The findings of this study demonstrate that Shugan Jieyu capsules have a significant therapeutic effect in patients with mild-to-moderate depression following PCI for coronary heart disease. This treatment effectively reduced depressive symptoms, improved biochemical indicators, and enhanced immune function. Compared with escitalopram oxalate tablets, Shugan Jieyu capsules showed a lower incidence of adverse reactions, making them safer alternatives for treating post-PCI depression. Therapeutic mechanisms may involve the regulation of miR-124, miR-132, and BDNF levels, as well as modulation of the immune system. These results suggest that Shugan Jieyu capsules could be a valuable addition to the treatment regimens for patients with depression after PCI.
| 1. | Saini RK, Chaudhury S, Singh N, Chadha DS, Kapoor R. Depression, anxiety, and quality of life after percuataneous coronary interventions. Ind Psychiatry J. 2022;31:6-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Dubovsky SL, Ghosh BM, Serotte JC, Cranwell V. Psychotic Depression: Diagnosis, Differential Diagnosis, and Treatment. Psychother Psychosom. 2021;90:160-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 3. | Mujtaba SF, Sial JA, Karim M. Depression and Anxiety in patients undergoing Percutaneous Coronary Intervention for Acute Coronary Syndrome. Pak J Med Sci. 2020;36:1100-1105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Ashour A, Al-Rawashdeh S, Tanash M, Al-Smadi A, Alshraifeen A, Shajrawi A. Changes in the Anxiety Levels of Patients Undergoing Percutaneous Coronary Intervention. Dimens Crit Care Nurs. 2023;42:15-21. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Song R, Sun N, Song X. The Efficacy of Psychological Capital Intervention (PCI) for Depression From the Perspective of Positive Psychology: A Pilot Study. Front Psychol. 2019;10:1816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Wang S, Li H, Chen X, Yan N, Wen D. The mediating role of psychological capital in the association between life satisfaction and depressive and anxiety symptoms among Chinese medical students during the COVID-19 pandemic: a cross-sectional study. BMC Psychiatry. 2023;23:398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Gu G, Zhou Y, Zhang Y, Cui W. Increased prevalence of anxiety and depression symptoms in patients with coronary artery disease before and after percutaneous coronary intervention treatment. BMC Psychiatry. 2016;16:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | van Dijk MR, Utens EM, Dulfer K, Al-Qezweny MN, van Geuns RJ, Daemen J, van Domburg RT. Depression and anxiety symptoms as predictors of mortality in PCI patients at 10 years of follow-up. Eur J Prev Cardiol. 2016;23:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Patel N, Kellezi B, Williams AC. Psychological, social and welfare interventions for psychological health and well-being of torture survivors. Cochrane Database Syst Rev. 2014;2014:CD009317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Han X, Zhang S, Chen Z, Adhikari BK, Zhang Y, Zhang J, Sun J, Wang Y. Cardiac biomarkers of heart failure in chronic kidney disease. Clin Chim Acta. 2020;510:298-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Askin L, Uzel KE, Tanrıverdi O, Kavalcı V, Yavcin O, Turkmen S. The relationship between coronary artery disease and depression and anxiety scores. North Clin Istanb. 2020;7:523-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Liang B, Gu N. Traditional Chinese Medicine for Coronary Artery Disease Treatment: Clinical Evidence From Randomized Controlled Trials. Front Cardiovasc Med. 2021;8:702110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Min H, Xu YI, Shaowei Y, Xueli WU, Shuoshi W, Diancheng HE. Gehua Jiejiu Dizhi decoction ameliorates alcoholic fatty liver in mice by regulating lipid and bile acid metabolism and with exertion of antioxidant stress based on 4DLabel-free quantitative proteomic study. J Tradit Chin Med. 2024;44:277-288. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Wu T, Yue T, Yang P, Jia Y. Notable efficacy of Shugan Jieyu capsule in treating adult with post-stroke depression: A PRISMA-compliant meta-analysis of randomized controlled trials. J Ethnopharmacol. 2022;294:115367. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Lue HC, Su YC, Lin SJ, Huang YC, Chang YH, Lin IH, Yang SP. Taipei consensus on integrative traditional Chinese and Western Medicine. J Formos Med Assoc. 2021;120:34-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Liu Z, Gu C, Lei J. Meta-analysis of Shugan Jieyu Capsule for depression in patients with coronary heart disease. Medicine (Baltimore). 2023;102:e34685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Bucciarelli V, Caterino AL, Bianco F, Caputi CG, Salerni S, Sciomer S, Maffei S, Gallina S. Depression and cardiovascular disease: The deep blue sea of women's heart. Trends Cardiovasc Med. 2020;30:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 18. | Bozzini S, Gambelli P, Boiocchi C, Schirinzi S, Falcone R, Buzzi P, Storti C, Falcone C. Coronary artery disease and depression: possible role of brain-derived neurotrophic factor and serotonin transporter gene polymorphisms. Int J Mol Med. 2009;24:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Guo M, Zi MJ, Xi RX, Yang QN, Bai RN, Zhang YS, Wang YH, Wang PL, Shi DZ. Effect of Xinyue capsules on patients with coronary heart disease after percutaneous coronary intervention: study protocol for a randomized controlled trial. Trials. 2016;17:412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |