Published online Aug 19, 2024. doi: 10.5498/wjp.v14.i8.1208
Revised: July 3, 2024
Accepted: July 11, 2024
Published online: August 19, 2024
Processing time: 82 Days and 18.8 Hours
Tourette syndrome (TS) is a neurodevelopmental disorder characterized by the presence of motor and vocal tics, typically beginning in childhood. Despite signifi
To investigate the association between DII and the severity, recurrence, and inflammatory levels of TS in children.
A total of 207 children diagnosed with TS in the pediatric department of Qingdao Chengyang People’s Hospital from January 2022 to January 2023 were selected. They were divided into stable and unstable groups based on follow-up condi
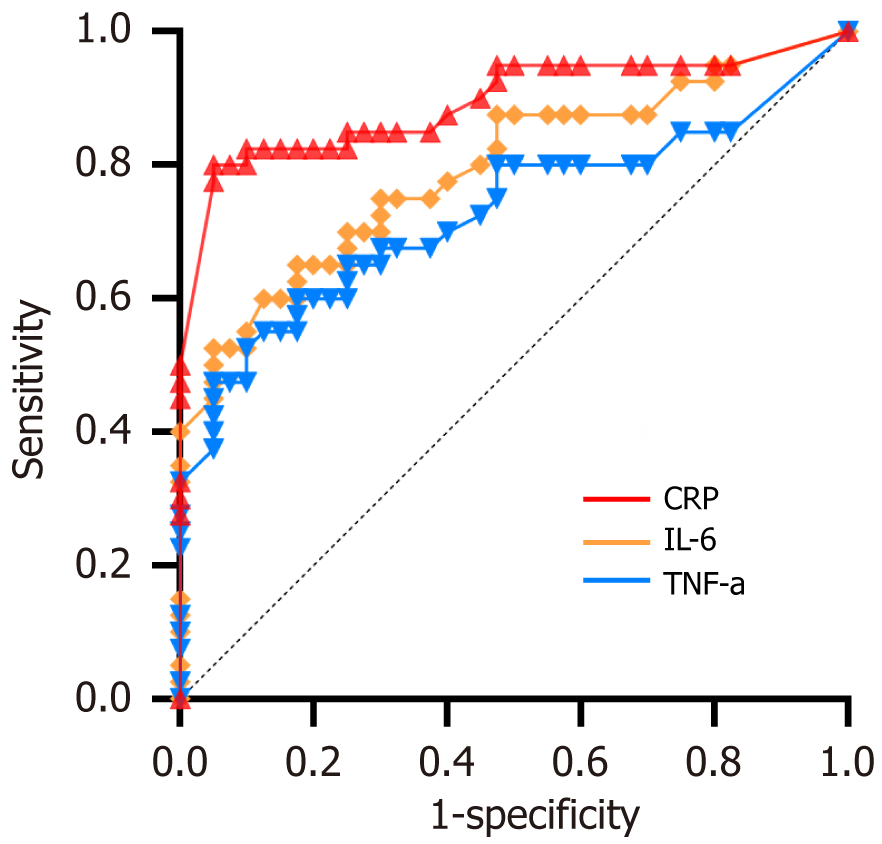
Follow-up surveys were completed by 207 children and their guardians. Among them, 117 children were in the stable group, and 90 were in the recurrent group. We found no statistically significant differences in age, gender, comorbidities, BMI, and disease duration between the two groups (P > 0.05). However, academic stress, electronic device usage, medication, guardian’s education level, and DII scores showed statistically significant differences between the groups (P < 0.05). Multifactorial regression analysis revealed that guardian’s anxiety level, DII score, medication, academic stress, and family history were statistically significant factors (P < 0.05) affecting the recurrence of TS in children. Therefore, anxiety level, DII score, medication status, electronic device usage, and academic stress were identified as factors influencing the recurrence of TS in children. Among them, DII score, academic stress, and family history had odds ratios (OR) greater than 1, indicating risk factors, whereas medication status and guardian’s education level had OR values less than 1, indicating protective factors. According to the YGTSS scores, children were categorized into mild, moderate, and severe groups. Comparative analysis of DII and inflammatory levels in children with different degrees of tic disorders revealed that the severe group had the highest DII and inflammatory levels, followed by the moderate group, and the mild group had the lowest levels. The trend of TS progression was consistent with the DII results. Receiver operating characteristic curves were plotted to predict disease progression in patients with TS via inflammatory markers. The areas under the curve for IL-6, CRP, and TNF-α were 0.894 (95%CI: 0.817-0.969), 0.793 (95%CI: 0.694-0.893), and 0.728 (95%CI: 0.614-0.843) respectively, with statistically significant differences (P < 0.05). According to the Youden index, the optimal cutoff values were IL-6 = 3.775 ng/L (sensitivity 68.1% and specificity 68.4%), CRP = 6.650 mg/L (sensitivity 60.6% and specificity 68.4%), and TNF-α = 0.666 (sensitivity 60.6% and specificity 71.1%).
We found a certain correlation between DII and the severity, recurrence, and inflammatory levels of TS in children. Reasonable reduction in the intake of pro-inflammatory foods may be beneficial in reducing the risk of disease progression in children with TS.
Core Tip: The aim of this study is to investigate the association between dietary inflammatory index (DII) and the severity, recurrence, and inflammatory levels of Tourette syndrome (TS) in children. Conclusion: There is a certain correlation between DII and the severity, recurrence, and inflammatory levels of TS in children.
- Citation: Wu XP, Fang RR, Ji TT. Dietary inflammatory index and its impact on severity and recurrence of Tourette syndrome in children. World J Psychiatry 2024; 14(8): 1208-1215
- URL: https://www.wjgnet.com/2220-3206/full/v14/i8/1208.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i8.1208
Tourette syndrome (TS) are a group of neurological and psychiatric disorders characterized by involuntary, sudden, rapid movements or vocalizations, which can be simple or complex, involving any part of the body[1]. The incidence and prevalence of TS vary among different regions and populations. In general, TS mainly occur in children and adolescents, with a higher proportion of male patients than female patients[2]. Typically, they first appear in late childhood to early adolescence, with an average onset age between 3 and 9 years old, often recurring clinically. Clinical treatment aims to alleviate symptoms, improve quality of life, and help children adapt to social and learning environments.
The dietary inflammatory index (DII) is an indicator used to assess the impact of food and nutrients on the inflammatory levels in the body. Research has shown that inflammation plays a central role in the development of many chronic diseases, and dietary factors indirectly influence the development and progression of these diseases by affecting the body’s inflammatory response[3]. In recent years, studies have begun to explore the role of inflammation in neurological and psychiatric disorders, especially in developmental brain disorders such as TS, where neuroinflammation is considered a key mechanism in the pathogenesis of TS[4].
By quantifying the pro-inflammatory or anti-inflammatory effects of food components on the inflammatory response, DII provides a method to measure the overall inflammatory potential of the diet. A high DII score suggests that the diet may increase the level of inflammation in the body, whereas a low DII score indicates potential anti-inflammatory effects of the diet[5]. At present, the research on the specific association between DII and TS is still in its infancy, and more clinical and epidemiological studies are needed to verify the strength and specific mechanism of this association. This study aims to explore the correlation between DII and the severity and recurrence of TS in children through a retrospective study, so as to provide effective evidence for clinical diagnosis and treatment.
This study employed a retrospective research design to investigate patients who sought treatment for pediatric TS at Qingdao Chengyang People’s Hospital’s pediatric department from January 2022 to January 2023. All participants volunteered to take part and signed informed consent forms (signed by guardians for children). All cases were follow-up cases. On the basis of whether there was a recurrence of symptoms within 1 year of follow-up, patients were diagnosed into the stable and recurrent groups.
Inclusion criteria were as follows: (1) Meeting the diagnostic criteria for TS outlined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)[6]: Onset age < 18 years; presence of one or more motor and/or vocal tics not attributable to substances (e.g., cocaine) or other conditions (e.g., Huntington’s disease and post-encephalitic conditions); and (2) Age 4-12 years old, Yale Global Tic Severity Scale (YGTSS) total score ≥ 25 points, no relevant medication received in the past 2 weeks, and informed consent signed by the child and legal guardian.
Exclusion criteria were as follows: Exclusion of refractory TS, tics caused by other conditions or secondary TS, comorbidities including attention deficit hyperactivity disorder, obsessive compulsive disorder, learning difficulties, sleep disorders, mood disorders, self-injurious behaviors, and individuals exhibiting sexually inappropriate behavior, those with comorbid primary diseases such as cardiovascular diseases, hepatic or renal diseases, metabolic diseases, and blood system diseases, as well as individuals allergic to test drugs or their components.
Dropout criteria were as follows: (1) Occurrence of severe adverse reactions or need for adjustment of treatment plan due to changes in symptoms; (2) Poor compliance, failure to take medication as required, or lack of cooperation with laboratory tests; and (3) Loss to follow-up or withdrawal midway through the study.
Data collection and follow-up: Information including age, gender, body mass index (BMI), guardian’s education level, DII, medical history (e.g., recurrent respiratory infections and gastrointestinal disorders), family history, academic stress, electronic device usage (hours per day), medication (regular/irregular), and disease progression (reported by guardians, stable/recurrent) of children with TS was collected through the hospital’s electronic medical records system and outpatient/telephone follow-up.
DII: The DII was calculated using a classic algorithm developed by Professor Shivappa from the University of South Carolina, United States. First, dietary intake data of individual patients were collected through dietary questionnaire surveys. On the basis of dietary data from 11 countries, Z-scores were calculated as follows: Z-score = (individual daily intake of dietary component - global average intake of dietary component)/standard deviation of global intake of dietary component. The values ranged from -1 to + 1, and the Z-values were converted to centered percentile values. Individual DII for each dietary component was calculated as percentile value multiplied by the inflammation score of the respective dietary component. Finally, the DII scores of all dietary components were summed up to obtain the overall DII score[7]. In this study, we calculated the DII for the observation group and the control group separately. Using the tertile method (≤ 0.06, -0.06 to 1.11, > 1.11), dietary DII scores were sorted from low to high and grouped into low pro-inflammatory, intermediate, and high pro-inflammatory categories, with low pro-inflammatory category as the reference group.
Serum inflammatory level detection: Fasting venous blood samples (3 mL) were collected from patients in the observation group and the control group on the day of follow-up visit, and enzyme-linked immunosorbent assay was used to detect interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) levels. C-reactive protein (CRP) levels were measured using the immunoturbidimetric assay method.
YGTSS: YGTSS is one of the most commonly used tools for assessing the severity of tic symptoms, which include aspects such as tic frequency, intensity, complexity, interference, and overall impairment on daily life. The severity of TS is classified into three groups according to YGTSS scores (motor tic score + vocal tic score + overall impairment score): < 25 points for mild, 25-50 points for moderate, and > 50 points for severe[8]. This questionnaire was used to assess the condition of TS in children.
Experimental data of the measurement data with normal distribution to said, comparing the two groups using independent sample t test, multiple comparison using F test, the count data to the number of cases or rate, according to comparing the two groups by χ2 test, multiple sets of hierarchical data comparing the Kruskal Wallis rank and inspection, The diagnostic value was evaluated by receiver operating characteristic (ROC) curve. In all analyses, a significance level of P < 0.05 was considered statistically significant.
A total of 207 children and their guardians completed the follow-up survey, with 117 children in the stable group and 90 cases in the unstable group. We found no statistically significant differences in age, gender, comorbidities, BMI, or disease duration between the two groups (P > 0.05). However, comparisons between the two groups showed statistically significant differences in academic stress, electronic device usage, medication, guardian’s education level, and DII score (P < 0.05), as shown in Table 1.
| Item | Stable group (117 cases) | Unstable group (90 cases) | Statistical value | P value |
| Age (year, mean ± SD) | 7.3 ± 2.22 | 8.0 ± 2.4 | 0.585 | 0.561 |
| Gender, n (%) | χ2 = 0.03 | 0.863 | ||
| Male | 69 (58.97) | 52 (57.78) | ||
| Female | 48 941.02) | 38 (42.23) | ||
| Guardian’s educational level | Fisher accurate probability test | 0.037 | ||
| Associate degree and below | 10 | 36 | ||
| Bachelor’s degree | 98 | 23 | ||
| Bachelor’s degree and above | 2 | 1 | ||
| Family history | χ2 = 4.276 | 0.039 | ||
| Yes | 74 (63.24) | 44 (44.44) | ||
| No | 43 (36.75) | 46 (51.11) | ||
| Coexisting chronic diseases | Fisher accurate probability test | 0.064 | ||
| Recurrent respiratory inflammation | 67 (57.26) | 75 (83.33) | ||
| Digestive dysfunction | 23 (19.65) | 15 (16.66) | ||
| Other chronic diseases | 12 (10.25) | 0 | ||
| None | 15 (12.82) | 10 (11.11) | ||
| BMI (kg/m², mean ± SD) | 24.23 ± 9.29 | 23.91 ± 10.22 | t = 0.868 | 0.386 |
| Medication adherence | χ2 = 5.227 | < 0.001 | ||
| Regular | 33 (35.76) | 7 (7.00) | ||
| Irregular | 62 (65.26) | 83 (93.00) | ||
| Electronic device usage (h/day) | 3.45 ± 1.24 | 4.34 ± 1.12 | t = 1.025 | 0.028 |
| Academic pressure | ||||
| Yes | 66 (56.41) | 62(68.88) | χ2 = 6.305 | 0.012 |
| No | 51 (43.58) | 28 (31.11) | ||
| DII score [points, M (P25-P75)] | 0.56 (-0.9-1.59) | 0.17 (-1.34 to 1.42) | 6.723 | 0.000 |
| Duration of disease | χ2 = 1.372 | 0.561 | ||
| Within 6 months | 69 (58.97) | 50 (55.55) | ||
| Over 6 months | 48 (41.02) | 40 (44.44) | ||
Using the significant variables identified in the univariate analysis, with recurrence of TS (0 = no, + 1 = yes) as the dependent variable and the statistically significant indicators from the univariate analysis as independent variables, categorical data were coded (guardian’s education level, academic stress, electronic device usage, medication status, and family history), whereas continuous data (DII score) were entered as raw values. Details are shown in Table 2.
| Variable | Assignment explanation |
| Guardian’s education level | Associate degree and below = 1, bachelor’s = 2, above bachelor’s = 3 |
| DII index | Original value entered |
| Academic pressure | None = 0; Yes = 1 |
| Electronic device usage | < 4 hours = 1, ≥ 4 hours = 0 |
| Medication adherence | Irregular = 0, Regular = 1 |
| Family history | No = 0, Yes = 1 |
Multifactorial regression analysis revealed that differences in guardian’s anxiety level, DII score, medication status, academic stress, and family history were statistically significant (P < 0.05). Therefore, guardian’s anxiety level, DII score, medication status, electronic device usage, and academic stress were identified as factors influencing the recurrence of TS in children. Among these factors, DII score, academic stress, and family history had odds ratios (OR) greater than 1, indicating that they were risk factors, whereas medication status and guardian’s education level had OR values less than 1, indicating that they were protective factors. Details are shown in Table 3.
| Variable | β | SE | Wald χ2 | P value | OR (95%CI) |
| Guardian’s education level | -0.416 | 0.163 | 6.281 | 0.0122 | 0.666 (0.485-0.915) |
| DII index | 1.033 | 0.334 | 9.566 | 0.0021 | 2.809 (1.459-5.407) |
| Academic pressure | 0.830 | 0.287 | 7.827 | 0.0062 | 2.125 (1.242-3.673) |
| Electronic device usage | 0.754 | 0.274 | 7.573 | 0.0052 | 1.351 (0.499-3.657) |
| Family history | 0.383 | 0.1566 | 6.027 | 0.0142 | 1.467 (1.080-1.991) |
| Medication adherence | -0.372 | 0.138 | 7.267 | 0.0071 | 0.689 (0.526-0.903) |
Children were categorized into mild, moderate, and severe groups based on their scores on the YGTSS during follow-up. The stable group comprised 75 cases of mild, 36 cases of moderate, and 6 cases of severe TS. Among the unstable patients, there were 32 cases of mild, 22 cases of moderate, and 36 cases of severe TS.
The severe group had the highest average DII and inflammatory levels, followed by the moderate group, whereas the mild group had the lowest. The trend of TS progression was consistent with the DII index results (Tables 4 and 5).
| Group | Number of cases | DII | IL-6 (pg/mL) | CRP (mg/L) | TNF-α (pg/mL) |
| Mild | 75 | 0.45 ± 0.17 | 40.09 ± 7.01 | 6.18 ± 1.92 | 6.03 ± 1.52 |
| Moderate | 36 | 0.61 ± 0.07 | 52.11 ± 6.72 | 8.15 ± 3.23 | 8.42 ± 2.08 |
| Severe | 6 | 0.58 ± 0.12 | 68.47 ± 8.27 | 9.23 ± 2.74 | 12.35 ± 3.17 |
| F value | 66.632 | 60.455 | 4.785 | 32.863 | |
| P value | < 0.001 | < 0.001 | 0.008 | < 0.001 |
| Group | Number of cases | DII | IL-6 (pg/mL) | CRP (mg/L) | TNF-α (pg/mL) |
| Mild | 32 | 0.35 ± 0.02 | 42.51 ± 7.14 | 6.57 ± 1.61 | 6.24 ± 1.22 |
| Moderate | 22 | 0.67 ± 0.05 | 51.01 ± 7.09 | 8.43 ± 2.48 | 8.27 ± 2.14 |
| Severe | 36 | 1.52 ± 0.21 | 68.81 ± 8.18 | 10.97 ± 2.51 | 12.91 ± 3.08 |
| F value | 389.120 | 89.433 | 123.715 | 92.813 | |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
ROC curves were plotted to assess the predictive ability of IL-6, CRP, and TNF-α for disease progression in patients with TS. The AUCs for IL-6, CRP, and TNF-α were 0.894 (95%CI: 0.817-0.969), 0.793 (95%CI: 0.694-0.893), and 0.728 (95%CI: 0.614-0.843), respectively, with statistically significant differences (P < 0.05). According to the maximum Youden index, the optimal cutoff values were determined as follows: IL-6 = 3.775 ng/L (sensitivity 68.1% and specificity 68.4%), CRP = 6.650 mg/L (sensitivity 60.6% and specificity 68.4%), and TNF-α = 0.666 (sensitivity 60.6% and specificity 71.1%), as shown in Figure 1.
This study explored the relationship between DII and the severity and recurrence of TS in pediatric patients through retrospective analysis. Results indicated a significant positive correlation between high DII values and the severity and recurrence of TS. Thus, a diet high in inflammation may exacerbate the severity of TS and contribute to disease recurrence by increasing systemic inflammation. Inflammation has been widely recognized as a key factor in many neuropsychiatric disorders, including TS[9]. In this study, high DII values were associated with elevated levels of IL-6, CRP, and TNF-α, further confirming the link between dietary inflammation and systemic inflammation. The elevation of these inflammatory factors may directly impact the neurotransmitter system in the brain, thereby influencing the progression of TS in patients. Elevated levels of inflammatory factors such as IL-6, TNF-α, and CRP not only reflect the inflammatory status in the body but have also been shown to cross the blood-brain barrier, directly affecting brain function. These inflammatory factors can influence the synthesis and release of neurotransmitters such as serotonin, dopamine, and norepinephrine, which play crucial roles in regulating mood, attention, and motor control. Therefore, the inflammatory status may indirectly affect the progression of TS through these mechanisms[10]. Long-term inflammation not only affects the neurotransmitter system but may also lead to changes in brain structure. For example, inflammation may promote neuronal death, affect neuronal connections, and impact brain functional areas, particularly those related to motor control and behavior regulation. Thus, the brains of patients with TS are highly sensitive to these inflammatory changes, leading to fluctuations in the disease.
The pathophysiology of TS is multifaceted, involving genetic, environmental, and neurobiological factors. The elevation of inflammatory factors may serve as a bridge connecting these factors. For instance, specific genetic backgrounds may render individuals to be highly sensitive to inflammatory responses, whereas environmental factors (such as diet) may trigger or exacerbate this inflammatory state[11]. Therefore, inflammatory factors may play a crucial role in the occurrence and development of TS. This finding provides a new perspective for the research and treatment of TS. Future studies can further explore the exact relationship between inflammatory factors and TS, especially how they affect the neurotransmitter system and brain structure. In terms of treatment, in addition to traditional medication and behavioral interventions, managing the patient’s inflammatory status may need to be considered, such as by improving dietary habits or using anti-inflammatory drugs. By analyzing the mechanisms of action of inflammatory factors, we can not only thoroughly understand the pathophysiological basis of TS but also provide comprehensive and personalized diagnostic and treatment strategies for patients[12].
Recent studies have begun to reveal the association between dietary habits and neuropsychiatric disorders such as depression, anxiety, and cognitive impairments. In particular, some studies have indicated a correlation between a high-inflammatory diet and increased incidence of these disorders. For example, research has found that a Western diet (high in sugar, fat, and processed foods) is associated with high levels of inflammation and an increased risk of mental health problems. Compared with these studies, the present work incorporated the association between inflammatory diets and TS, expanding the field of research on the relationship between dietary inflammation and neuropsychiatric disorders. DII, as a tool for assessing the inflammatory potential of diets, has been widely used in studying chronic diseases, especially cardiovascular diseases and certain cancers[13,14]. By quantifying the promotion or inhibition of inflammatory responses by foods, DII provides an objective standard for evaluating the health effects of diets. The innovation of this study lies in the application of DII in the field of neuropsychiatric disorders, especially TS, which not only enriches the application scope of DII but also provides new scientific evidence for exploring the relationship among diet, inflammation, and TS[15]. Although medication and behavioral interventions remain the mainstay of TS treatment, the results of this study suggested that reducing inflammation through dietary adjustments may become one of the adjunctive treatment measures. This conclusion was consistent with the viewpoint proposed in some literature that lifestyle changes, including diet, exercise, and sleep, should be considered in the treatment of neuropsychiatric disorders, which may positively impact the overall health of patients[16].
Although this study provides preliminary evidence for the association between dietary inflammation and TS, further research is needed to explore the specific mechanisms and intervention measures. Future studies should consider using prospective designs, large sample sizes, and precise dietary assessment tools to deepen understanding of how dietary inflammation affects the development and progression of TS. This study was only conducted in one pediatric department of Qingdao People's Hospital, which may limit the generalizability of the study results to the wider population. Further replication of the study in different Settings and populations will strengthen its external validity. Additionally, randomized controlled trials can be used to evaluate the effects of specific dietary interventions on patients with TS, providing direct guidance for clinical treatment.
In summary, this study not only revealed the association between DII and the severity and recurrence of TS but also emphasized the potential value of anti-inflammatory diets in TS management. This work provides new ideas for the comprehensive treatment of TS, especially in the application of dietary interventions. With a deep understanding of the pathophysiological mechanisms of TS and related research, we hope that highly personalized and effective diagnostic and treatment strategies can be provided for patients with TS in the future.
| 1. | Vermilion J, Mink JW. Tic Disorders. Pediatr Rev. 2023;44:294-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Roessner V, Eichele H, Stern JS, Skov L, Rizzo R, Debes NM, Nagy P, Cavanna AE, Termine C, Ganos C, Münchau A, Szejko N, Cath D, Müller-Vahl KR, Verdellen C, Hartmann A, Rothenberger A, Hoekstra PJ, Plessen KJ. European clinical guidelines for Tourette syndrome and other tic disorders-version 2.0. Part III: pharmacological treatment. Eur Child Adolesc Psychiatry. 2022;31:425-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 3. | Garcia-Delgar B, Servera M, Coffey BJ, Lázaro L, Openneer T, Benaroya-Milshtein N, Steinberg T, Hoekstra PJ, Dietrich A, Morer A; EMTICS collaborative group. Tic disorders in children and adolescents: does the clinical presentation differ in males and females? A report by the EMTICS group. Eur Child Adolesc Psychiatry. 2022;31:1539-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Pringsheim T, Okun MS, Müller-Vahl K, Martino D, Jankovic J, Cavanna AE, Woods DW, Robinson M, Jarvie E, Roessner V, Oskoui M, Holler-Managan Y, Piacentini J. Practice guideline recommendations summary: Treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. 2019;92:896-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 249] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 5. | Alesi S, Villani A, Mantzioris E, Takele WW, Cowan S, Moran LJ, Mousa A. Anti-Inflammatory Diets in Fertility: An Evidence Review. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Ueda K, Black KJ. A Comprehensive Review of Tic Disorders in Children. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Shakya PR, Melaku YA, Shivappa N, Hébert JR, Adams RJ, Page AJ, Gill TK. Dietary inflammatory index (DII®) and the risk of depression symptoms in adults. Clin Nutr. 2021;40:3631-3642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Szejko N, Robinson S, Hartmann A, Ganos C, Debes NM, Skov L, Haas M, Rizzo R, Stern J, Münchau A, Czernecki V, Dietrich A, Murphy TL, Martino D, Tarnok Z, Hedderly T, Müller-Vahl KR, Cath DC. European clinical guidelines for Tourette syndrome and other tic disorders-version 2.0. Part I: assessment. Eur Child Adolesc Psychiatry. 2022;31:383-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 9. | Stino AM, Naddaf E, Dyck PJ, Dyck PJB. Chronic inflammatory demyelinating polyradiculoneuropathy-Diagnostic pitfalls and treatment approach. Muscle Nerve. 2021;63:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 10. | Erhan E, Salcan I, Bayram R, Suleyman B, Dilber M, Yazici GN, Coban TA, Altuner D, Suleyman H. Protective effect of lutein against acrolein-induced ototoxicity in rats. Biomed Pharmacother. 2021;137:111281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Pape K, Tamouza R, Leboyer M, Zipp F. Immunoneuropsychiatry - novel perspectives on brain disorders. Nat Rev Neurol. 2019;15:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 319] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 12. | Giovannoni F, Quintana FJ. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020;41:805-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 403] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 13. | Marazziti D, Palermo S, Arone A, Massa L, Parra E, Simoncini M, Martucci L, Beatino MF, Pozza A. Obsessive-Compulsive Disorder, PANDAS, and Tourette Syndrome: Immuno-inflammatory Disorders. Adv Exp Med Biol. 2023;1411:275-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 14. | Stiede JT, Woods DW. Pediatric Prevention: Tic Disorders. Pediatr Clin North Am. 2020;67:547-557. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 15. | Kammermeier J, Lamb CA, Jones KDJ, Anderson CA, Baple EL, Bolton C, Braggins H, Coulter TI, Gilmour KC, Gregory V, Hambleton S, Hartley D, Hawthorne AB, Hearn S, Laurence A, Parkes M, Russell RK, Speight RA, Travis S, Wilson DC, Uhlig HH. Genomic diagnosis and care co-ordination for monogenic inflammatory bowel disease in children and adults: consensus guideline on behalf of the British Society of Gastroenterology and British Society of Paediatric Gastroenterology, Hepatology and Nutrition. Lancet Gastroenterol Hepatol. 2023;8:271-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Gilbert DL. Inflammation in Tic Disorders and Obsessive-Compulsive Disorder: Are PANS and PANDAS a Path Forward? J Child Neurol. 2019;34:598-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |