INTRODUCTION
Autism spectrum disorder (ASD) is a chronic developmental disability with social dysfunction and repetitive behaviors[1]. According to data from the Centers for Disease Control and Prevention, 1/36 children under the age of 8 years (approximately 4% of boys and 1% of girls) is estimated to have ASD[2]. Beyond the core symptoms of ASD, many of ASD patients experience other symptoms, including gastrointestinal (GI) dysfunction as well as unusual eating and sleeping habits[3]. GI problems commonly occur in patients with ASD, with up to 70% of patients being affected[4]. Generally, GI problems in ASD mainly include diarrhea, constipation, and abdominal pain[5]. Moreover, GI problems in ASD children with ASD are associated with the severity of behavioral ASD symptoms[6]. GI problems directly affect the quality of life of children with ASD and increase the psychological and economic burden of their families.
Although GI symptoms are common in children with ASD, their etiology remains unknown. It was inferred that the pathogenesis may be related to changes in gut microbiota, increased intestinal permeability, and immune abnormality. The gut microbiota constitutes a special intestinal environment that affects brain development by acting on the nervous, endocrine, and immune systems[7,8]. Intestinal villi deformation and inflammatory cell infiltration were observed in rats with gut dysbiosis[9]. Both clinical and animal studies have shown that alterations in gut microbiota and gut infection are related to ASD. Furthermore, the gut microbiome usually coexists with inflammation and neurotransmitter abnormalities[10-13]. The gut microbiota is also involved in the maturation of the immune system.
Interestingly, evidence has suggested that maternal intestinal problems may be a risk factor for the development of ASD[14-16]. Sadik et al[17] found that there was a potential link between maternal inflammatory bowel disease (IBD) and ASD. In animal ASD models, BTBR and SHANK3 mutant mice developed gut microbiome dysbiosis[18,19]. Furthermore, changes in the maternal gut microbiota may promote maternal immune activation-associated ASD model phenotypes[20]. The maternal gut microbiota is vertically transmitted to the offspring, which is important for offspring to establish their metabolic and developmental pathways. Moeller et al[21] showed that vertical transmission is not only related to the mode of delivery, but also to the composition of the maternal gut microbiota.
Intestinal homeostasis is maintained by the interaction of the intestinal mucosa, microbiota, nutrients, and metabolites. Gut dysbiosis leads to intestinal disorders[22] and affects the progression of IBD[23]. Furthermore, nonoptimal maternal nutrition during the embryonic period epigenetically affects the fetus, which may induce susceptibility to the development of colitis[24]. These results suggest that an abnormal maternal gut microbiota not only induces maternal gut inflammation but also adversely affects the offspring.
Although evidence has shown that genes that increase the risk of ASD may be associated with maternal intestinal inflammation and microbial dysbiosis, the effects of adverse environmental factors during pregnancy on the maternal and offspring GI tracts remain unclear. To identify the environmental factors that affect the maternal intestinal condition, we created a valproic acid (VPA)-induced ASD rat model to detect changes in maternal intestinal microbiota and inflammation. Additionally, we wanted to determine whether changes in the maternal GI system are associated with GI problems in children with ASD.
MATERIALS AND METHODS
Animal husbandry and care
We obtained Wistar rats (male and female rats, 270-350 g) from the Beijing Vital River Laboratory Animal Technology Co., Ltd. The animals were housed individually in cages with a 12-12 h light-dark cycle. Food and water were provided ad libitum from the cage lid. Humidity and temperature were maintained at 50% ± 10% and 23 ± 2 °C, respectively. The study protocol was approved by the Institute of Acupuncture and Moxibustion Animal Care and Use Committee (approval No. Y2023-03-14-02). The United States National Institutes of Health Guide for the Care and Use of Laboratory Animals was followed in this study.
VPA rat model
Female and male rats were mated overnight in the same cage. The day when vaginal plugging occurred was considered as embryonic day 0.5 (E0.5). Pregnant rats were randomized into VPA and control [normal saline (NS)] groups. In the VPA group, pregnant rats were intraperitoneally injected with 450 mg/kg of VPA (Sigma: P4543) on embryonic day 12.5 (E12.5). The control group received the same concentration of NS. On postnatal day 21 (PND 21), same-sex offspring were housed separately (2-6 per cage). Male offspring were used in this study. The timeline of the experiments is shown in Figure 1A.
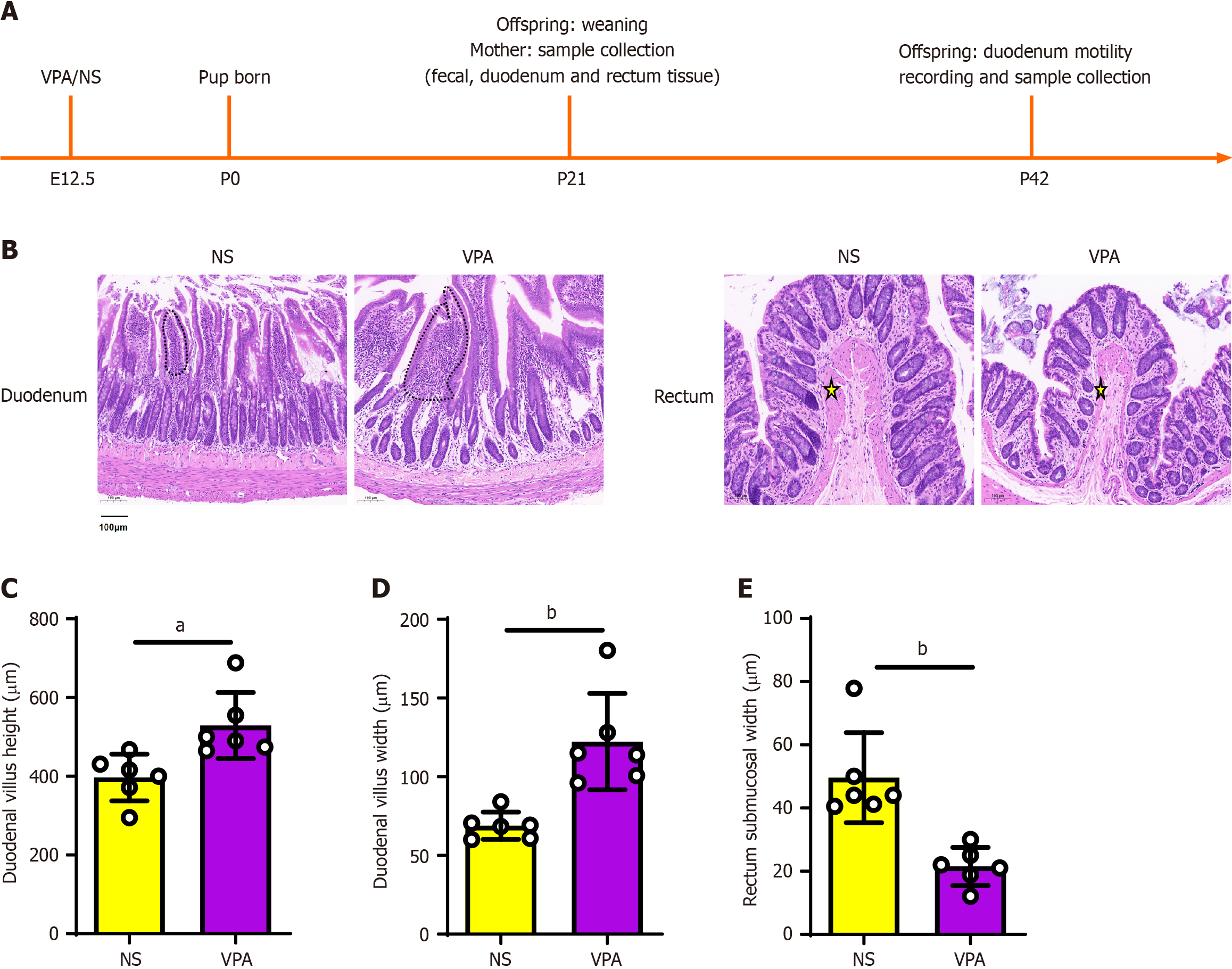
Figure 1 Histological evaluation of intestinal tissue of the normal saline and valproic acid groups (hematoxylin & eosin staining; scale bar = 100 μm; 10 ×).
A: Timeline of experimental design; B: Hematoxylin & eosin staining of the duodenum (above) and rectum (down). The duodenal villi are marked by dotted lines. The submucosa is marked by a yellow star; C and D: Histological statistics of duodenal villi height and width; E: Histological statistics of rectal submucosal width. Data are presented as the mean ± SD (normal saline group n = 6, valproic acid group n = 6). Unpaired t test, aP < 0.05, bP < 0.01. NA: Normal saline; VPA: Valproic acid.
Tissue preparation
Rats were anesthetized by intraperitoneally injecting 10% chloral hydrate and transcardially perfused with NS. Duodenal and rectal tissues were quickly removed and rinsed with 1X phosphate buffered saline (PBS). After sonicating 100 mg of tissue for 1 min in radioimmunoprecipitation assay lysis buffer (RIPA; Beyotime, China) containing 1:100 protease inhibitor, centrifugation was performed at 12000 g for 15 min. Then, the supernatant was removed and stored at -80 °C. We used a BCA Protein Assay kit (Beyotime, China) to measure the total protein concentration of each sample, and the results were interpreted on a BIO-RAD iMark™ micro-plate reader.
Enzyme-linked immunosorbent assay
Before enzyme-linked immunosorbent assay (ELISA), duodenal and rectal tissues were diluted 1:20 and 1:10 with RIPA, respectively. Inflammatory factors [tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-6] and neurotransmitters [acetylcholine (ACh) and nitric oxide synthase (NOS)] in duodenal and rectal tissues were detected by ELISA. The standards or samples were pipetted into the wells of microtiter plates containing monoclonal antibodies against the following proteins: TNF-α (CSB-E11987r, CUSABIO), IL-1β (CSB-E08055r, CUSABIO), IL-6 (CSB-E04640r, CUSABIO), ACh (CSB-E08044r, CUSABIO), and NOS (CSB-E14034r, CUSABIO). Substrate solution was added to the wells after washing to remove unbound antibody-enzyme reagent. The enzymatic reactions resulted in a blue product, which turned yellow when phosphoric acid stop solution was added. The concentrations of the factors of interest in the samples were calculated using standard curves as the intensity of the color was directly proportional to the amount of total target protein bound in the first step. The results were calculated as the target protein concentration vs total protein.
Hematoxylin & eosin staining
Duodenal and rectal tissues were immersed in 4% paraformaldehyde for 4 h and then transferred to containers containing 70% ethanol. Individual lobes were placed in processing cassettes, dehydrated through a series of alcohol gradients, and embedded in paraffin blocks. Tissue sections measuring 5 μm were deparaffinized in xylene, rehydrated through a series of decreasing concentrations of ethanol, and washed in PBS. They were then stained with hematoxylin & eosin (H&E). Images were taken with a Nikon microscope (ECLIPSE Ni-E, Japan).
Fecal 16S rRNA sequencing and microbial analysis
After the rats were anesthetized, we obtained fecal samples from the rectum. Following fecal collection, total DNA was extracted from fecal samples using the CTAB/SDS method. DNA concentration and purity were monitored by 1% agarose gel electrophoresis. DNA was diluted with sterile water to 1 ng/μL. 16S rRNA genes were amplified with specific primers and barcodes. The cycling conditions included an initial denaturation step at 98 °C (1 min), then 30 cycles of 98 °C (30 s), 50 °C (30 s), and 72 °C (30 s), and a final extension at 72 °C (5 min). The polymerase chain reaction products (containing SYB green) were mixed with loading buffer and electrophoresed on a 2% agarose gel. We used Qiagen Gel Extraction Kit (Qiagen, Germany) to purify the mixed polymerase chain reaction products.
The library was sequenced on an Illumina NovaSeq platform at Novogene Bioinformatics Technology Co., Ltd. (Tianjin, China). For high-quality clean tags, the quality filtering on the raw tags were performed with fastp (version 0.20.0) software. To assess the complexity and differences among samples, we used beta diversity, which was based on weighted and unweighted unifrac distances in QIIME2. Nonmetric multidimensional scaling (NMDS) was performed with QIIME modules and visualized using the R package (version 3.5.2). To investigate the differences in community structure between groups, we used the Adonis and Anosim functions in QIIME2 software. To determine the different species at each taxonomic level, we performed MetaStat and t-test analyses with R software (version 3.5.3).
Duodenal motility recording
Duodenal motility recording was performed by PND35-42. To record duodenal motility, we used a rubber condom to create a latex balloon, which was then attached to one tip of a PE-50 tubing. The other end of the tubing was connected to a syringe and a pressure sensor through a tee pipe. The rats were placed in supine position, and a 2-cm incision was made at the ventral median line 1 cm below the xiphoid process. The abdominal skin, muscle layer, and peritoneum were incised. Another incision was made in the duodenum (1 cm from the pylorus), and the latex balloon was placed. We then sutured the duodenal incision, muscle layer, and skin. Double distilled H2O was injected into the latex balloon, and duodenal pressure changes were recorded with Spike2V8.02 software.
Statistical analysis
We used IBM SPSS Statistics 19 (SPSS Inc., Chicago, IL, United States) and GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, United States) for statistical analyses and graph generation. All data sets were normality-tested using the Shapiro-Wilk normality test before choosing the statistical test. Statistical significance was assessed by the unpaired Student’s t-test and Mann-Whitney U test. Results are expressed as the mean ± standard deviation of the mean (SD), and P < 0.05 (two-tailed) was considered statistically significant.
RESULTS
Pathological changes in the duodenum and colon in VPA-induced ASD rats
First, we extracted the duodenal and colonic tissues of mother rats in the VPA and NS groups for H&E staining. H&E staining showed that in the VPA group, the duodenal villi height and width were increased (P < 0.05; Figure 1B-D), while the rectal submucosal width was reduced (P < 0.05; Figure 1E). These results suggest that VPA exposure during pregnancy affects the maternal intestinal structure.
Effects of VPA exposure during pregnancy on maternal intestinal microbiota
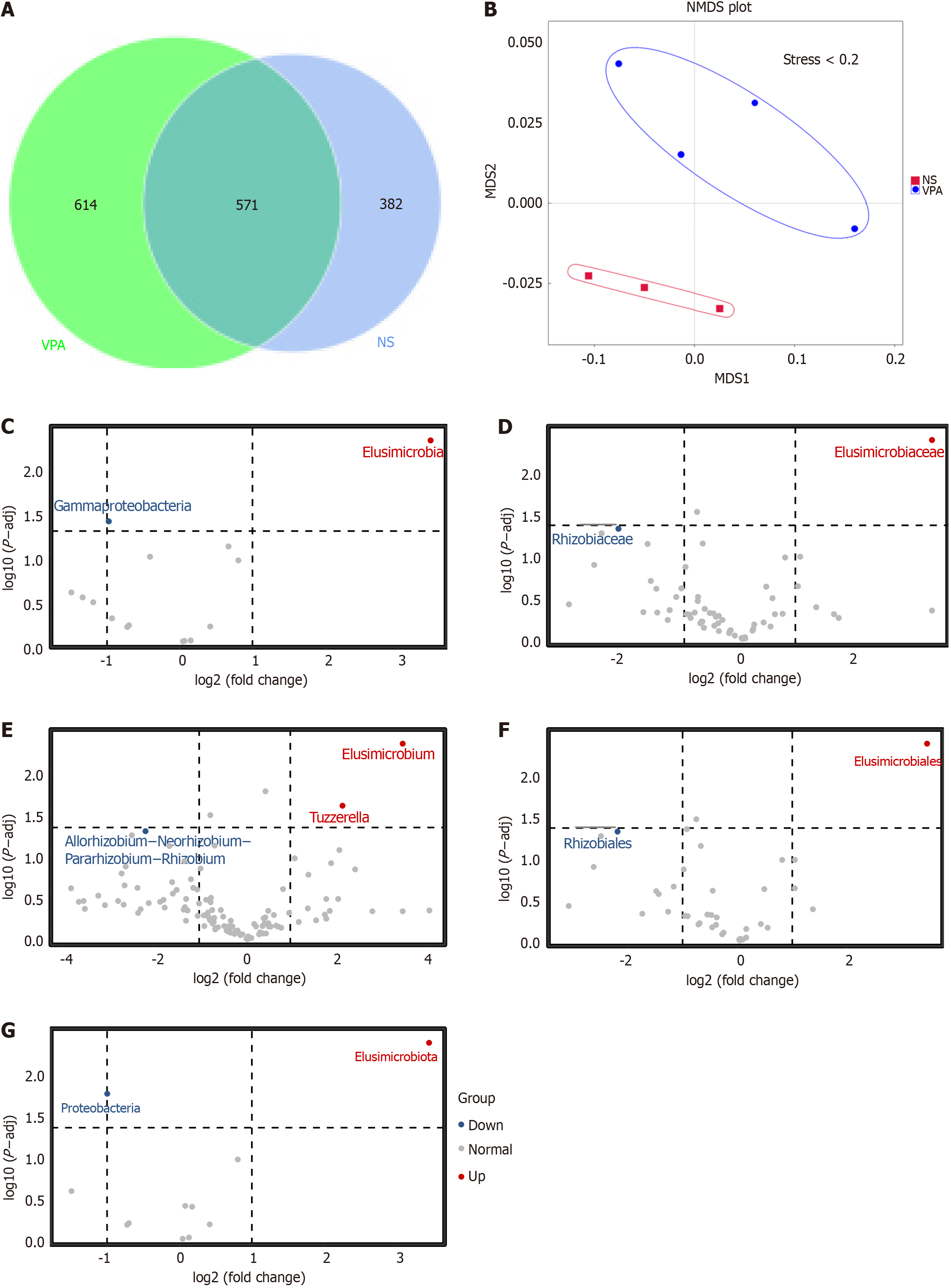
We then explored the differences in gut microbiota between the NS and VPA groups. The Venn diagram displayed 614 unique operational taxonomic unit (OTUs) in the VPA group and 382 in the NS group. Meanwhile, 571 OTUs were shared by the two groups (Figure 2A). NMDS was then conducted to investigate the differences between groups of samples (Figure 2B). When stress was < 0.2, it meant that NMDS accurately reflected the degree of difference between samples. Moreover, the VPA and NS groups displayed different microbial profiles at different levels. Compared with the NS group, populations of gamma-proteobacteria, Rhizobiaceaea, and Proteobacteria were decreased in the VPA group, while some bacterial strains, such as Elusimicrobia and Tuzzerella, were higher in the VPA group (Figure 2C-G). These results further suggest that VPA exposure during pregnancy may alter the composition of the maternal gut microbiota.
Figure 2 Alteration of gut microbiota in normal saline and valproic acid groups according to 16S rRNA data.
A: Venn diagram of observed operational taxonomic units in normal saline and valproic acid (VPA) groups; B: Beta diversity of gut microbiota based on nonmetric multidimensional scaling; C-G: Significantly different species at each taxonomic level (class, family, genus, order, and phylum) based on t-test analysis. Normal saline group n = 3, valproic acid group n = 4. P < 0.05. NA: Normal saline; VPA: Valproic acid.
VPA exposure during pregnancy increases levels of maternal intestinal inflammatory factors
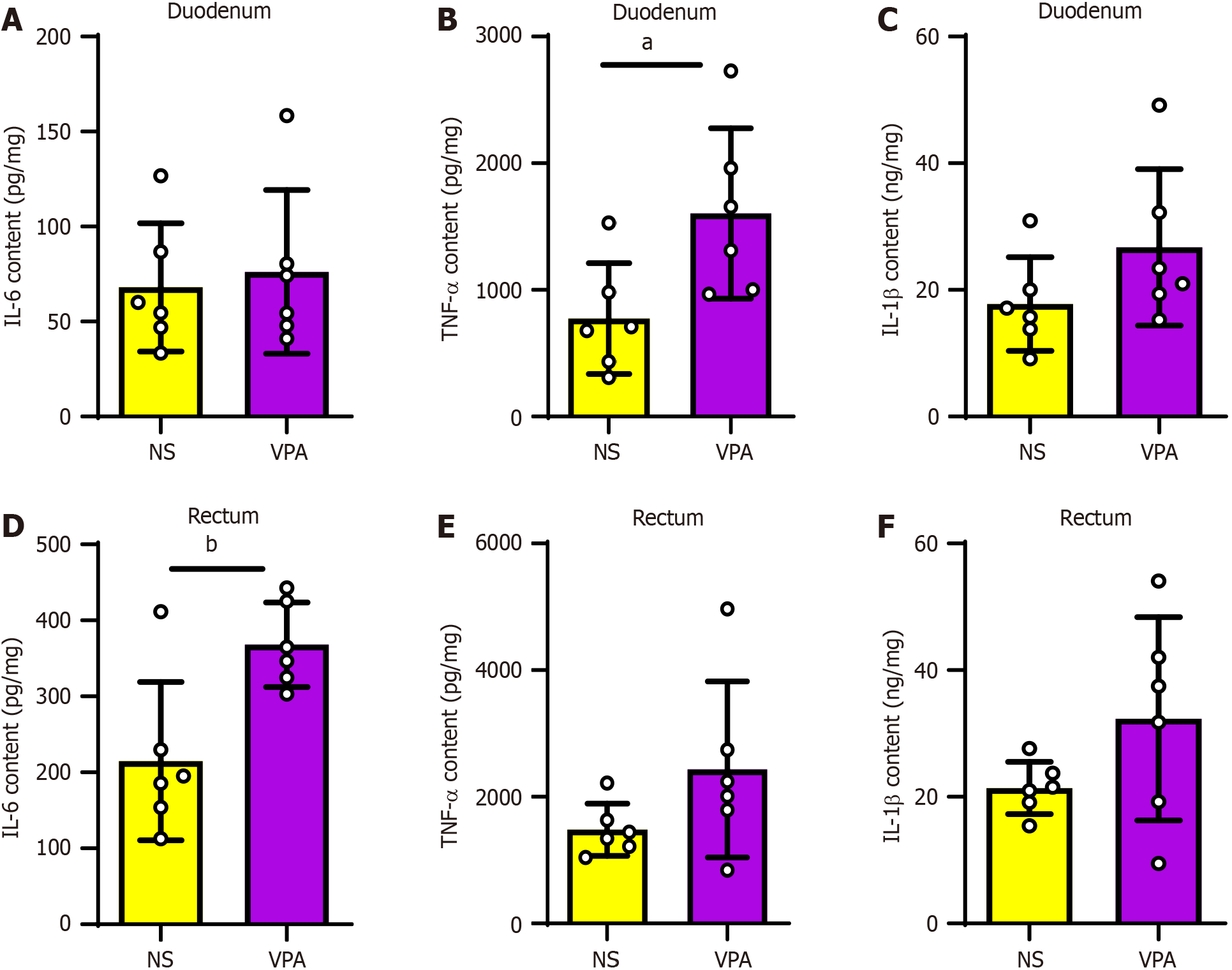
Next, we used ELISA to evaluate the levels of maternal intestinal inflammatory factors in the VPA and NS groups to determine whether VPA exposure induces maternal intestinal inflammation. The results showed that compared with the NS group, the levels of TNF-α were higher in the duodenum, whereas those of IL-6 and IL-1β were not significantly different, in the VPA group (P < 0.05; Figure 3A-C). In rectal tissues of the VPA group, IL-6 levels were higher, whereas there was no significant difference in TNF-α or IL-1β levels (P < 0.01; Figure 3D-F). These results indicate that prenatal VPA exposure increases the levels of maternal intestinal inflammatory factors.
Figure 3 Levels of intestinal inflammatory factors are increased in valproic acid group.
A-C: Interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), and IL-1β levels in the duodenum in normal saline (NS) and valproic acid (VPA) groups; D-F: IL-6, TNF–α, and IL-1β levels in the rectum in NS and VPA groups. Data are presented as the mean ± SD (normal saline group n = 6, valproic acid group n = 6). Unpaired t test, aP < 0.05, bP < 0.01. TNF-α: Tumor necrosis factor-alpha; IL: Interleukin; NA: Normal saline; VPA: Valproic acid.
Intestinal motility is impaired in offspring in the VPA group
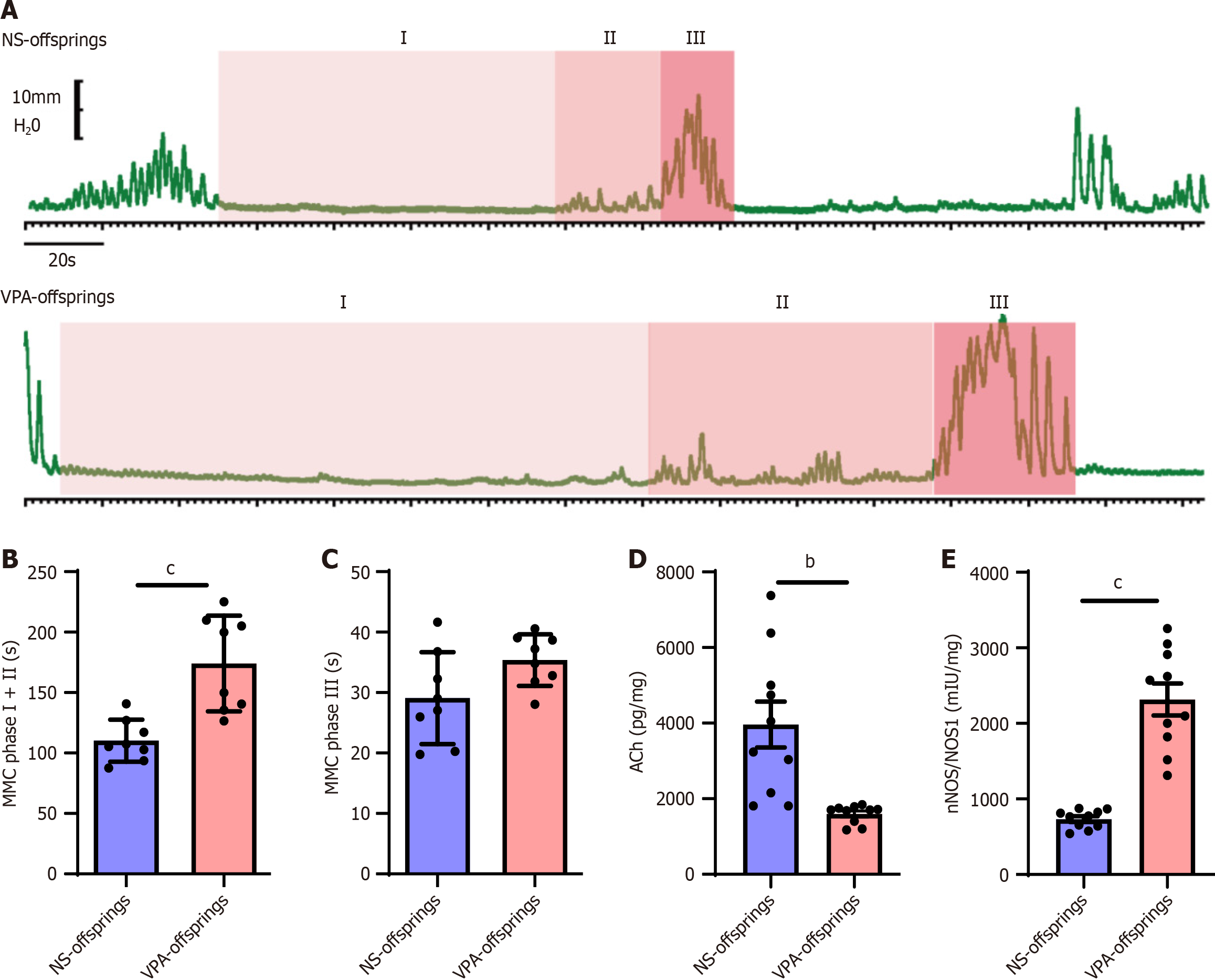
After assessing changes in the maternal gut microbiota and intestinal inflammation, we evaluated intestinal function in offspring in the VPA group. The migrating motor complex (MMC) is a cyclic motility pattern that occurs in the stomach and small bowel during the interdigestive state[25]. MMC can be divided into four phases: Phase I is the quiescent phase with no contractions; phase II is characterized by random contractions; phase III has a sudden onset and ends with a burst of contractions with maximal amplitude and duration; and phase IV is characterized by the rapid decrease of contractions[26]. Phase IV represents a short transition period back to the quiescence of phase I; in this study, we focused on phases I, II, and III. As the duodenum is connected to the pylorus and is responsible for food digestion and absorption, we chose the duodenum to assess intestinal motility during puberty. As shown in Figure 4A-C, the duration of MMC I and II in the VPA offspring group was longer than that of the NS offspring group (P < 0.001).
Figure 4 Duodenal motility and neurotransmitters are impaired after prenatal valproic acid exposure.
A: Duodenal motility in normal saline (NS) and valproic acid (VPA) offspring at different phases of migrating motor complex (MMC); B and C: Duration of MMC phases I + II (B) and III (C) in NS and VPA offspring groups (NS offspring group n = 8, VPA offspring group n = 8). Unpaired t test, aP < 0.05, bP < 0.01; D and E: Levels of acetylcholine (D) and nitric oxide synthase (E) in NS and VPA offspring groups in the duodenum. Data are presented as the mean ± SD (normal saline offspring group n = 10, valproic acid offspring group n = 10). Unpaired t test, bP < 0.01, cP < 0.001. NA: Normal saline; VPA: Valproic acid; MMC: Migrating motor complex; ACh: Acetylcholine; NOS: Nitric oxide synthase.
Meanwhile, there was no significant difference between the VPA offspring group and the NS offspring group in the duration of MMC III. Consistent with the duodenal motility results, ACh levels were lower and NOS levels were higher in the VPA offspring group than in the NS offspring group (P < 0.01; Figure 4D-E). These results suggest that offspring in the VPA group would develop disorders in duodenal motor function, which might be the reason for GI problems in ASD.
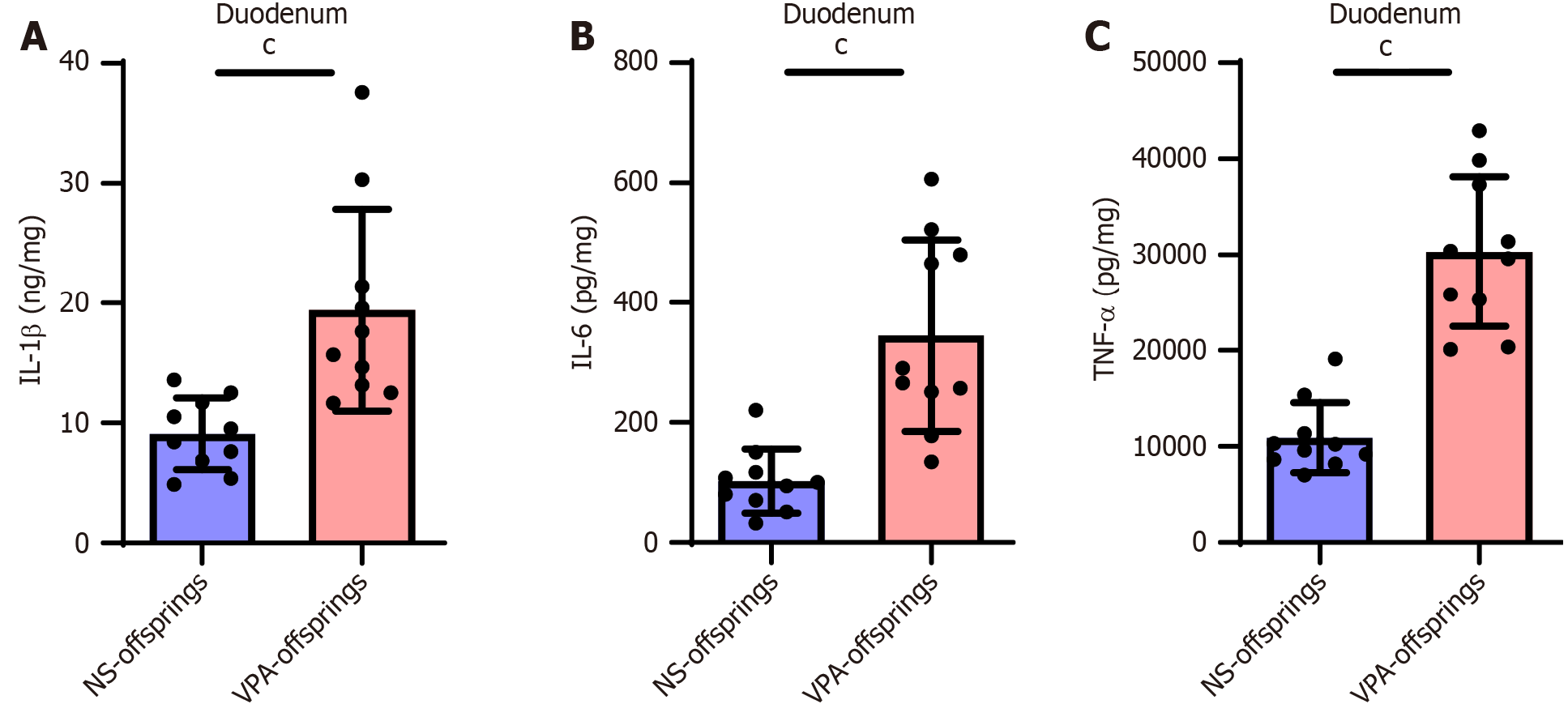
Levels of intestinal inflammatory factors are increased in offspring in the VPA group
Finally, we evaluated intestinal inflammation in offspring in the NS and VPA groups. As expected, the VPA offspring group showed higher levels of intestinal inflammation; the levels of intestinal IL-1β, IL-6, and TNF-α were increased in the duodenum (Figure 5). This suggests that GI problems in patients with ASD may be related to intestinal inflammation.
Figure 5 Levels of duodenal inflammatory factors are increased in valproic acid group.
A: Interleukin (IL)-1β levels in the duodenum in normal saline (NS) and valproic acid (VPA) groups; B: IL-6 levels in the duodenum in NS and VPA groups; C: Tumor necrosis factor-alpha levels in the duodenum in NS and VPA groups. Data are presented as the mean ± SD (normal saline offspring group n = 8, valproic acid offspring group n = 8). Unpaired t test, cP < 0.001. TNF-α: Tumor necrosis factor-alpha; IL: Interleukin; NA: Normal saline; VPA: Valproic acid.
DISCUSSION
Our findings show that VPA exposure during pregnancy can alter the maternal gut microbiota and increase the levels of inflammatory factors. Furthermore, in offspring of VPA-induced ASD rat models, intestinal motility decreased along with changes in intestinal neurotransmitters, and that levels of intestinal inflammatory factors increased. These results suggest that the maternal intestinal condition is involved in the pathogenesis of ASD. Vertical transmission of the maternal microbiota from mother to infant in ASD is worthy of discussion.
VPA is a drug used to treat epilepsy and mood disorders. Epidemiology has demonstrated that VPA exposure during pregnancy is an important risk factor for the pathogenesis of ASD[27-29]. The mechanism for this may be due to the passage of VPA into the fetus through the placenta. However, there is a lack of evidence on the effects of VPA on changes in the maternal GI system. Through daily feeding, we observed the development of diarrhea in rats after VPA injection. H&E staining also showed that VPA causes changes in the maternal intestinal villi and muscle layers. Kim et al[30] also found that the thickness of the GI mucosa and its muscle layers was reduced in offspring of VPA rats. According to our results, exposure to VPA during pregnancy induced intestinal inflammation. Coincidentally, in 2022, a previous study[17] found that there was a potential link between parental, particularly maternal, IBD and ASD in children, and that its results may reflect the influence of the maternal intestinal condition on the prenatal environment. Hence, we hypothesized that the severity of maternal intestinal inflammation might be an important factor in the development of ASD.
Our results demonstrated that the maternal gut microbiota was altered after VPA exposure, which is in line with previous findings. A study found that some symptoms of ASD were associated with specific gut microbiota shared by children and their mothers[17]. Kimura et al[31] also showed that the maternal microbiota shaped the metabolic system of offspring in mice. Our findings provide additional evidence for the vertical transmission of maternal gut microbiota and ASD development.
Compared with the NS group, we did not find differences in the overall structure, diversity, or abundance of maternal gut microbiota in the VPA group. However, t-test analysis revealed that Elusimicrobia and Tuzzerella populations were higher in the VPA group than in the NS group. Elusimicrobia is a gut-associated bacterial phylum that has a relatively small genome. An earlier study reported that Elusimicrobia populations were increased in the intestines of Göttingen minipigs and rhesus macaques after irradiation[32]. Furthermore, Tuzzerella has been demonstrated to grow in different mouse models such as IBD and depression[33,34]. Meanwhile, Proteobacteria populations were decreased in the VPA group. According to a previous review[35], Proteobacteria is a marker for an unstable microbial community. Therefore, from the results of the maternal microbiota analysis in this study, we could infer that VPA exposure during pregnancy alters the maternal gut microbiota and induces inflammation.
Gut microbiota imbalance destroys the intestinal mucosal barrier function, resulting in the entry of bacterial endotoxins and metabolites into the intestinal mucosa and triggering inflammatory responses[36]. The maternal gut microbiota and its inflammatory factors may then be passed to infants through mother-to-child vertical transmission. In line with previous studies[37,38], our results demonstrate that offspring in the VPA group developed GI problems. Meanwhile, in prior studies, the researchers tended to use intestinal permeability to explain GI problems in ASD[39-41]. In this research, we provided new evidence regarding the mechanisms of GI problems in ASD. Similarly, it was reported that Foxp1+/- mice developed GI transit dysfunction[42]. Interestingly, SHANK3 mutant zebrafish also showed GI motility disruption[43]. Hence, GI motility disorders have been observed in multiple animal models of ASD. These results suggest that GI motility should also be considered when treating ASD, and that improving GI motility may be beneficial for improving the core symptoms of ASD.
A widely accepted hypothesis on the development of ASD is excitatory-inhibitory (E-I) ratio imbalance. Most of the evidence for E-I imbalance was obtained from brain regions such as the neocortex, hippocampus, amygdala, and cerebellum[44-46]. For example, an increased E-I ratio in the prefrontal cortex may result in behavioral and social impairments[47]. Meanwhile, the enteric nervous system (ENS) is rich in excitatory and inhibitory neurotransmitters, which can directly act on GI smooth muscle cells. Hence, the ENS is also called the second brain. In this study, our results indicate that ACh levels in the ENS of offspring in the VPA group were decreased, but NOS levels were increased. This phenomenon might partly explain the disorder in intestinal motility and also provide a new perspective on the E-I ratio imbalance in ASD.
Gut dysbiosis and immune alterations are common in children with ASD[48,49]. Gut dysbiosis is related to inflammation and immune activation[50]. Furthermore, the gut microbiota may play an important role in intestinal transit[51]. Thus, our study provides additional evidence on the adverse maternal outcomes of drug exposure and the effects of these adverse outcomes on their offspring. Modulation of the gut microbiota seems to be a promising strategy to ameliorate GI manifestations in ASD, but further studies are warranted.
Currently, studies have been focusing on the gut microbiota to treat GI problems in ASD[52,53]. However, the treatment of GI motility in ASD is rarely reported. As an early therapeutic method, acupuncture has significant benefits in treating GI motility disorders. It was shown that acupuncture can promote GI movement through parasympathetic nerve stimulation[54]. Moreover, acupuncture is also being considered as a potential treatment for ASD[55], with neural plasticity and the brain-gut axis being the postulated mechanisms involved[56,57]. Future studies should pay more attention to the therapeutic effects and mechanisms of acupuncture on GI problems in ASD.
In this study, we only discussed the effects of VPA exposure during pregnancy on the GI system of the mothers and their offspring. A possible limitation of this study is the mechanism of vertical transmission of inflammation and maternal gut microbiota, which should be explored further. Besides, the sample size of this study was limited due to the lack of animal experimental environment. In the future, we will expand the sample size, and hope to discover a therapeutic approach to solve this problem.