Published online Jul 19, 2024. doi: 10.5498/wjp.v14.i7.1080
Revised: May 21, 2024
Accepted: June 5, 2024
Published online: July 19, 2024
Processing time: 94 Days and 18.6 Hours
Intracranial high-density areas (HDAs) have attracted considerable attention for predicting clinical outcomes; however, whether HDAs predict worse neurological function and mental health remains controversial and unclear, which requires further investigation.
To investigate the predictive value of intracranial HDAs for neurological function and mental health after endovascular treatment.
In this prospective study, 96 patients with acute ischemic stroke (AIS) who accepted endovascular mechanical thrombectomy (EMT) were included. The enrolled patients underwent cranial computed tomography (CT) examination within 24 hours after EMT. Clinical data in terms of National Institutes of Health Stroke Scale (NIHSS), the 3-month modified Rankin Scale (mRS), self-rating depression scale (SDS), and self-rating anxiety scale (SAS) scores were collected and compared between patients with HDAs and non-HDAs and between patients with good and poor clinical prognosis.
Compared to patients without HDAs, patients with HDAs presented severe neurological deficits (admission NIHSS score: 18 ± 3 vs 19 ± 4), were more likely to have post-stroke disabilities (mRS < 3: 35% vs 62%), and suffered more severe depression (SDS score: 58 ± 16 vs 64 ± 13) and anxiety disorder (SAS score: 52 ± 8 vs 59 ± 10). Compared to patients with a good prognosis, patients with a poor prognosis presented severe neurological deficits (admission NIHSS score: 17 ± 4 vs 20 ± 3), were more likely to have HDAs on CT images (64% vs 33%), and suffered more severe depression (SDS score: 55 ± 19 vs 65 ± 11) and anxiety (SAS score: 50 ± 8 vs 58 ± 12). Multivariate analysis revealed that HDAs were independent nega
In conclusion, HDAs on CT images predicted poor prognosis and severe depressive and anxiety symptoms in patients with AIS who underwent EMT.
Core Tip: High-density areas (HDAs) have great potential in predicting clinical outcomes in patients with acute ischemic stroke (AIS). This prospective study focused on the association of intracranial HDAs with neurological function and mental health after endovascular mechanical thrombectomy for AIS. Through comprehensive evaluation of National Institutes of Health Stroke Scale, the 3-month modified Rankin Scale, self-rating depression scale, and self-rating anxiety scale scores, our study demonstrated that intracranial HDAs on computed tomography images predict poor prognosis and severe depressive and anxiety symptoms.
- Citation: Lu ZJ, Lai JX, Huang JR, Xie SH, Lai ZH. Predictive value of intracranial high-density areas in neurological function. World J Psychiatry 2024; 14(7): 1080-1086
- URL: https://www.wjgnet.com/2220-3206/full/v14/i7/1080.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i7.1080
Acute ischemic stroke (AIS), with high morbidity, disability, and mortality rates, has become one of the most common causes of death in China in recent years. The lifetime risk of stroke in China is estimated to be 39.3%, ranking first in the world[1]. Moreover, the highest risk is 41.1% in males and 36.7% in females[1]. Intracranial large-artery occlusion in AIS, especially in the anterior circulation, occurs with a high disability rate and severe complications such as stroke-related pneumonia, bedsores, and even death, ultimately leading to a heavy economic burden on households. Over the past three decades, the age-standardized morbidity and mortality rates of stroke have decreased in China, but the stroke burden remains substantial[2].
Early revascularization is key to the treatment of AIS. Endovascular mechanical thrombectomy (EMT) is a safe and effective method for treating AIS resulting from large-artery occlusions[3]. In clinical practice, intracranial high-density areas (HDAs) are observed in patients who undergo initial (within 24 hours) computed tomography (CT) after EMT, with an incidence of 31.2%-60%[4]. Most HDAs are caused by contrast leakage, and a few are caused by intracerebral hemorrhage or a combination of both[4]. Recent studies have suggested that intracranial HDAs after vascular recanalization may indicate a poor prognosis[5-7]. However, one study showed that the occurrence of HDAs was not related to prognosis[4]. The reasons for the association between HDAs and ineffective recanalization are unknown, but the presence of HDAs may indicate blood–brain barrier injury and dysfunction after reperfusion[8].
Although the occluded artery is recanalized, and blood flow is restored after EMT, not all patients with arterial recanalization have good clinical outcomes. This study aimed to investigate the relationship between the occurrence of intra
A total of 96 patients with acute anterior circulation ischemic stroke who underwent EMT at the Ganzhou People’s Hospital, China, between January 2022 and December 2023 were included. The inclusion criteria were as follows: (1) Patients admitted to the intensive care unit for ≥ 3 days; (2) patients with AIS in the anterior circulation within 24 hours of onset who underwent endovascular intervention; and (3) patients who provided informed consent for participation in the study. The exclusion criteria were as follows: (1) Cerebral infarction caused by other causes, such as vasculitis; (2) age < 14 years; (3) pregnant or lactating females; (4) concurrent multi-site cerebral infarction; (5) patients with intracranial space-occupying lesions; (6) patients with incomplete data collection due to various factors; and (7) patients in other clinical trials or studies.
This prospective cohort study included patients with AIS who required endovascular thrombectomy due to occlusion or stenosis of large arteries in the anterior circulation. All patients underwent cranial CT (Philips Healthcare, Best, The Netherlands/Artis zee ceiling, Siemens Healthcare, Erlangen, Germany) examination within 24 hours after EMT and were subsequently divided into HDA and non-HDA groups. Baseline data, including age, sex, risk factors, and routine stroke scales, including the National Institutes of Health Stroke Scale (NIHSS) score and modified Rankin Scale (mRS), were compared between the two groups. The mRS was used to assess clinical prognosis at 3 months, with a good prognosis defined as an mRS score < 3. The self-rating depression scale (SDS) and self-rating anxiety scale (SAS) were used to assess the severity of depressive and anxiety disorders, respectively, and were compared between the non-HDA and HDA groups. According to the mRS score, patients were divided into the good prognosis group (mRS score 0-3) and the poor prognosis group (mRS score 4-6) at 3 months. The HDA, SDS, and SAS scores were compared between the two groups to determine the role of HDAs in clinical outcomes.
This prospective cohort study was a triple-blind study. Continuous variables are presented as mean ± SD. Categorical variables are presented as frequencies and percentages, n (%). Differences in the continuous variables and the categorical variables were examined with student’s t-test and the chi-square or Fisher exact test, respectively. The Wilcoxon rank-sum test was used to compare multiple samples of categorical variables and McNemar’s test was used to compare two paired samples of continuous variables. Multivariate logistic regression analysis was used to determine the correlation between variables and clinical outcomes. Statistical significance was set at P < 0.05. All data were collected using Epidata 3.0, and statistically analyzed using SPSS software (version 13.0).
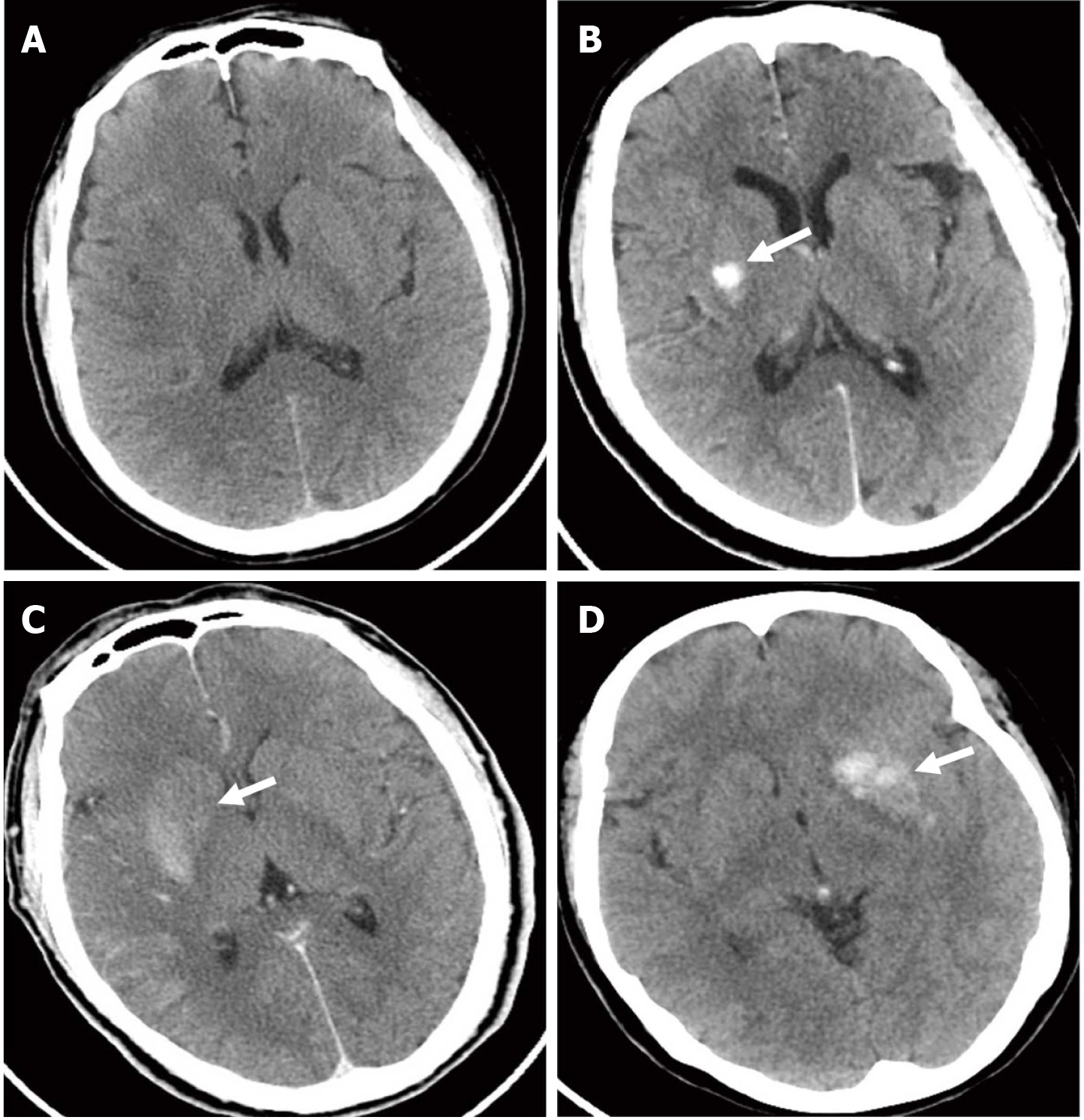
In this study, a triple-blind design was adopted. All patients with acute anterior circulation ischemic stroke underwent cranial CT within 24 hours after EMT. They were divided into non-HDA and HDA groups according to CT image outcomes. Among the 96 patients included in this study, 46 (48%) had non-HDAs (Figure 1A) and 50 (52%) had HDAs on CT images (Figure 1B-D).
The clinical characteristics of patients in the non-HDA and HDA groups are shown in Table 1. The mRS was used to assess patient prognosis at 3 months. No significant differences were observed in the baseline data between the two groups (P > 0.05). Compared to patients with non-HDAs, those with HDAs presented a higher admission NIHSS score (P = 0.047). Among the 46 patients with non-HDAs, 16 had a poor prognosis, while 31 of 50 patients with HDAs had a poor prognosis (mRS < 3: 35% vs 62%, P = 0.001).
| non-HDAs (n = 46) | HDAs (n = 50) | P value | |
| Age (years, mean) | 63 ± 10 | 67 ± 14 | 0.683 |
| Gender (male) | 25 (54) | 26 (52) | 0.869 |
| Hypertension | 20 (43) | 23 (46) | 0.453 |
| Hyperlipidemia | 5 (11) | 7 (14) | 0.879 |
| Diabetes mellitus | 8 (17) | 10 (20) | 0.648 |
| Heart disease | 24 (52) | 27 (54) | 0.872 |
| Admission NIHSS score (mean) | 18 ± 3 | 19 ± 4 | 0.047 |
| mRS score < 3 | 16(35) | 31 (62) | 0.001 |
Based on the clinical outcomes at 3 months, patients were divided into good and poor prognosis groups. The clinical characteristics of the patients in the two groups are shown in Table 2. No significant differences were observed in the baseline data between the two groups (P > 0.05). Poor prognosis was observed in 47 (49%) of the 96 patients in the study. Compared to patients with a good prognosis, those with a poor prognosis presented a higher admission NIHSS score (P = 0.007) and were more likely to have HDAs on CT images (64% vs 33%, P = 0.0.004). Based on the results of multivariate logistic regression analysis, HDAs on CT (OR = 0.326; 95%CI: 0.253-0.323, P = 0.002) were independently associated with a poor prognosis.
| Good prognosis (n = 49) | Poor prognosis (n = 47) | P value | |
| Age (years, mean) | 65 ± 11 | 67 ± 16 | 0.878 |
| Gender (male) | 26 (53) | 25 (53) | 0.889 |
| Hypertension | 22 (45) | 21 (45) | 0.552 |
| Hyperlipidemia | 6 (12) | 6 (13) | 0.879 |
| Diabetes mellitus | 7 (14) | 11 (23) | 0.068 |
| Heart disease | 26 (53) | 25 (53) | 0.872 |
| Admission NIHSS score (mean) | 17 ± 4 | 20 ± 3 | 0.007 |
| HDAs presence | 16 (33) | 30 (64) | 0.004 |
SDS and SAS were used to assess the severity of depressive and anxiety disorders, respectively, in patients with and without HDAs and in patients with good or poor prognosis who accepted EMT. Compared to patients with non-HDAs, patients with HDAs were likely to have more severe depression (P = 0.015) and anxiety (P = 0.032) (Table 3). Moreover, compared to patients with a good prognosis, those with a poor prognosis had more severe depressive (SDS score: 55 ± 19 vs 65 ± 11, P = 0.021) and anxiety (SAS score: 50 ± 8 vs 58 ± 12, P = 0.044) disorders (Table 3).
| non-HDAs (n = 46) | HDAs (n = 50) | P value | |
| SDS score | 58 ± 16 | 64 ± 13 | 0.015 |
| SAS score | 52 ± 8 | 59 ± 10 | 0.032 |
| Good prognosis (n = 49) | Poor prognosis (n = 47) | ||
| SDS score | 55 ± 19 | 65 ± 11 | 0.021 |
| SAS score | 50 ± 8 | 58 ± 12 | 0.044 |
This study aimed to investigate the predictive value of HDAs on CT images for neurological function and mental health after EMT in patients with AIS. These findings suggest that the presence of HDAs predicts severe clinical prognosis and depressive and anxiety symptoms.
AIS is a common cerebrovascular disease with high disability and mortality rates and a low cure rate. To treat AIS, it is essential to restore perfusion to the ischemic brain tissue and protect the function of brain cells. EMT is a safe and effective method for treating AIS with large-artery occlusion. However, numerous studies have demonstrated that although endovascular therapy improves recanalization rates (defined as an mRS score < 3 at 3 months), only approximately 50% of patients have a good prognosis[9-13]. Approximately 50% of patients have poor outcomes, which can be explained by ineffective recanalization after EMT (mRS score > 3 at 3 months), despite recanalization of blood vessels with a blood flow grade after thrombolytic therapy for cerebral infarction > grade 2b[14]. Intracranial HDAs are observed on follow-up CT of patients after EMT with an incidence of 31.2%-60%[4]. Chen et al[7] retrospectively analyzed the clinical treatment of 82 patients with AIS due to anterior circulation large-artery occlusion and showed that the presence of HDAs on C-arm CT scans immediately after EMT resulted in ineffective recanalization, suggesting a poor prognosis. However, An et al[15] reported that the presence or absence of HDAs on dual-energy CT scans within 12-24 hours of EMT was not related to clinical outcomes. Whether HDAs predict poor prognosis remains controversial and more extensive research is needed.
In this study, we explored the association between HDAs and neurological function, as well as mental health in patients with AIS after EMT. Based on the CT images, patients were divided into groups with and without HDAs. There were no significant differences in the baseline data including age, sex, and risk factors, whereas the routine stroke scales including admission NIHSS and 3 month-mRS were significantly different between the two groups. Compared to patients without HDAs, those with HDAs presented a higher admission NIHSS score, suggesting severe neurological deficits. Three months after EMT, patients with HDAs were more likely to have post-stroke disabilities, with an mRS score < 3. The SDS and SAS scores at 3 months were significantly higher in patients with HDAs than in those without HDAs, indicating that the presence of HDAs suggests depression and anxiety severity. Moreover, according to the definition of good prognosis with an mRS score < 3 at 3 months, patients were divided into good and poor prognosis groups. The results indicated that patients with a poor prognosis presented with severe neurological deficits, were more likely to have HDAs on CT images, and had more severe depressive and anxiety disorders than patients with a good prognosis. Multivariate analysis showed that the presence of HDAs was an independent negative prognostic factor in patients with AIS. The potential mechanism of the relationship between HDAs and poor prognosis may be related to failed recanalization. HDAs were observed in approximately 25% of patients with AIS following EMT[5]. Previous studies reported that HDAs are related to an increased risk of symptomatic intracerebral hemorrhage and to unfavorable clinical outcomes[5,6,16-18]. Poor outcomes may be attributed to unsuccessful reperfusion hemorrhage[6,19,20]. To the best of our knowledge, this is the first study on the association between HDAs and the mental health of patients with AIS who received EMT. The underlying mechanism remains unclear and further studies are required to clarify this mecha
Although our findings suggest that the presence of HDAs predict a severe clinical prognosis and depressive and anxiety symptoms, this study had two limitations. First, thrombectomy devices, procedural techniques, timing of postoperative CT re-examination, and definition of HDAs varied across studies, which probably led to different results in different studies. Second, the present study included a small sample size and was performed at a single center. Therefore, the current findings cannot be generalized to all patients with acute anterior circulation ischemic stroke who received intravascular reperfusion therapy, which needs to be confirmed in large-scale and multicenter clinical studies.
In summary, the presence of HDAs on CT images predicts poor prognosis despite successful EMT. Moreover, the presence of HDAs indicates severe depressive and anxiety symptoms. These findings may serve as a reference for clinicians during the treatment of AIS.
| 1. | GBD 2016 Lifetime Risk of Stroke Collaborators; Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, Abajobir AA, Abate KH, Abd-Allah F, Abejie AN, Abyu GY, Ademi Z, Agarwal G, Ahmed MB, Akinyemi RO, Al-Raddadi R, Aminde LN, Amlie-Lefond C, Ansari H, Asayesh H, Asgedom SW, Atey TM, Ayele HT, Banach M, Banerjee A, Barac A, Barker-Collo SL, Bärnighausen T, Barregard L, Basu S, Bedi N, Behzadifar M, Béjot Y, Bennett DA, Bensenor IM, Berhe DF, Boneya DJ, Brainin M, Campos-Nonato IR, Caso V, Castañeda-Orjuela CA, Rivas JC, Catalá-López F, Christensen H, Criqui MH, Damasceno A, Dandona L, Dandona R, Davletov K, de Courten B, deVeber G, Dokova K, Edessa D, Endres M, Faraon EJA, Farvid MS, Fischer F, Foreman K, Forouzanfar MH, Gall SL, Gebrehiwot TT, Geleijnse JM, Gillum RF, Giroud M, Goulart AC, Gupta R, Gupta R, Hachinski V, Hamadeh RR, Hankey GJ, Hareri HA, Havmoeller R, Hay SI, Hegazy MI, Hibstu DT, James SL, Jeemon P, John D, Jonas JB, Jóźwiak J, Kalani R, Kandel A, Kasaeian A, Kengne AP, Khader YS, Khan AR, Khang YH, Khubchandani J, Kim D, Kim YJ, Kivimaki M, Kokubo Y, Kolte D, Kopec JA, Kosen S, Kravchenko M, Krishnamurthi R, Kumar GA, Lafranconi A, Lavados PM, Legesse Y, Li Y, Liang X, Lo WD, Lorkowski S, Lotufo PA, Loy CT, Mackay MT, Abd El Razek HM, Mahdavi M, Majeed A, Malekzadeh R, Malta DC, Mamun AA, Mantovani LG, Martins SCO, Mate KK, Mazidi M, Mehata S, Meier T, Melaku YA, Mendoza W, Mensah GA, Meretoja A, Mezgebe HB, Miazgowski T, Miller TR, Ibrahim NM, Mohammed S, Mokdad AH, Moosazadeh M, Moran AE, Musa KI, Negoi RI, Nguyen M, Nguyen QL, Nguyen TH, Tran TT, Nguyen TT, Anggraini Ningrum DN, Norrving B, Noubiap JJ, O’Donnell MJ, Olagunju AT, Onuma OK, Owolabi MO, Parsaeian M, Patton GC, Piradov M, Pletcher MA, Pourmalek F, Prakash V, Qorbani M, Rahman M, Rahman MA, Rai RK, Ranta A, Rawaf D, Rawaf S, Renzaho AM, Robinson SR, Sahathevan R, Sahebkar A, Salomon JA, Santalucia P, Santos IS, Sartorius B, Schutte AE, Sepanlou SG, Shafieesabet A, Shaikh MA, Shamsizadeh M, Sheth KN, Sisay M, Shin MJ, Shiue I, Silva DAS, Sobngwi E, Soljak M, Sorensen RJD, Sposato LA, Stranges S, Suliankatchi RA, Tabarés-Seisdedos R, Tanne D, Nguyen CT, Thakur JS, Thrift AG, Tirschwell DL, Topor-Madry R, Tran BX, Nguyen LT, Truelsen T, Tsilimparis N, Tyrovolas S, Ukwaja KN, Uthman OA, Varakin Y, Vasankari T, Venketasubramanian N, Vlassov VV, Wang W, Werdecker A, Wolfe CDA, Xu G, Yano Y, Yonemoto N, Yu C, Zaidi Z, El Sayed Zaki M, Zhou M, Ziaeian B, Zipkin B, Vos T, Naghavi M, Murray CJL, Roth GA. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N Engl J Med. 2018;379:2429-2437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1152] [Cited by in RCA: 1012] [Article Influence: 144.6] [Reference Citation Analysis (0)] |
| 2. | Ma Q, Li R, Wang L, Yin P, Wang Y, Yan C, Ren Y, Qian Z, Vaughn MG, McMillin SE, Hay SI, Naghavi M, Cai M, Wang C, Zhang Z, Zhou M, Lin H, Yang Y. Temporal trend and attributable risk factors of stroke burden in China, 1990-2019: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2021;6:e897-e906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 500] [Article Influence: 125.0] [Reference Citation Analysis (0)] |
| 3. | Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López-Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Pérez M, Goyal M, Demchuk AM, von Kummer R, Gallofré M, Dávalos A; REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3402] [Cited by in RCA: 3685] [Article Influence: 368.5] [Reference Citation Analysis (0)] |
| 4. | Parrilla G, García-Villalba B, Espinosa de Rueda M, Zamarro J, Carrión E, Hernández-Fernández F, Martín J, Hernández-Clares R, Morales A, Moreno A. Hemorrhage/contrast staining areas after mechanical intra-arterial thrombectomy in acute ischemic stroke: imaging findings and clinical significance. AJNR Am J Neuroradiol. 2012;33:1791-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Shi ZS, Duckwiler GR, Jahan R, Tateshima S, Szeder V, Saver JL, Kim D, Sharma LK, Vespa PM, Salamon N, Villablanca JP, Viñuela F, Feng L, Loh Y, Liebeskind DS. Early Blood-Brain Barrier Disruption after Mechanical Thrombectomy in Acute Ischemic Stroke. J Neuroimaging. 2018;28:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Rouchaud A, Pistocchi S, Blanc R, Engrand N, Bartolini B, Piotin M. Predictive value of flat-panel CT for haemorrhagic transformations in patients with acute stroke treated with thrombectomy. J Neurointerv Surg. 2014;6:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Chen WH, Yi TY, Wu YM, Zhang MF, Lin DL, Lin XH. Parenchymal hyperdensity on C-arm CT images after endovascular therapy for acute ischaemic stroke predicts a poor prognosis. Clin Radiol. 2019;74:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Renú A, Amaro S, Laredo C, Román LS, Llull L, Lopez A, Urra X, Blasco J, Oleaga L, Chamorro Á. Relevance of blood-brain barrier disruption after endovascular treatment of ischemic stroke: dual-energy computed tomographic study. Stroke. 2015;46:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4460] [Cited by in RCA: 4864] [Article Influence: 486.4] [Reference Citation Analysis (0)] |
| 10. | Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3948] [Cited by in RCA: 4277] [Article Influence: 427.7] [Reference Citation Analysis (0)] |
| 11. | Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4180] [Cited by in RCA: 4541] [Article Influence: 454.1] [Reference Citation Analysis (0)] |
| 12. | Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3555] [Cited by in RCA: 3841] [Article Influence: 384.1] [Reference Citation Analysis (0)] |
| 13. | Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millán M, Davis SM, Roy D, Thornton J, Román LS, Ribó M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4230] [Cited by in RCA: 5415] [Article Influence: 601.7] [Reference Citation Analysis (0)] |
| 14. | Nie X, Pu Y, Zhang Z, Liu X, Duan W, Liu L. Futile Recanalization after Endovascular Therapy in Acute Ischemic Stroke. Biomed Res Int. 2018;2018:5879548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | An H, Zhao W, Wang J, Wright JC, Elmadhoun O, Wu D, Shang S, Wu C, Li C, Wu L, Chen J, Duan J, Zhang H, Song H, Ding Y, Ji X. Contrast Staining may be Associated with Intracerebral Hemorrhage but Not Functional Outcome in Acute Ischemic Stroke Patients Treated with Endovascular Thrombectomy. Aging Dis. 2019;10:784-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Bonatti M, Lombardo F, Zamboni GA, Vittadello F, Currò Dossi R, Bonetti B, Pozzi Mucelli R, Bonatti G. Iodine Extravasation Quantification on Dual-Energy CT of the Brain Performed after Mechanical Thrombectomy for Acute Ischemic Stroke Can Predict Hemorrhagic Complications. AJNR Am J Neuroradiol. 2018;39:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Byrne D, Walsh JP, Schmiedeskamp H, Settecase F, Heran MKS, Niu B, Salmeen AK, Rohr B, Field TS, Murray N, Rohr A. Prediction of Hemorrhage after Successful Recanalization in Patients with Acute Ischemic Stroke: Improved Risk Stratification Using Dual-Energy CT Parenchymal Iodine Concentration Ratio Relative to the Superior Sagittal Sinus. AJNR Am J Neuroradiol. 2020;41:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Payabvash S, Khan AA, Qureshi MH, Saeed O, Suri MF, Qureshi AI. Detection of Intraparenchymal Hemorrhage After Endovascular Therapy in Patients with Acute Ischemic Stroke Using Immediate Postprocedural Flat-Panel Computed Tomography Scan. J Neuroimaging. 2016;26:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Portela de Oliveira E, Chakraborty S, Patel M, Finitsis S, Iancu D. Value of high-density sign on CT images after mechanical thrombectomy for large vessel occlusion in predicting hemorrhage and unfavorable outcome. Neuroradiol J. 2021;34:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Kang Z, Liu G, Fan R, Sun D, Zhou G, Wu X, Nie C, Qiu H, Mei B, Zhang J. Prognosis and Prediction of Asymptomatic Intracranial Hemorrhage After Endovascular Thrombectomy: A Multi-Center Study. J Endovasc Ther. 2023;15266028231219990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |