Published online Jun 19, 2024. doi: 10.5498/wjp.v14.i6.954
Revised: April 22, 2024
Accepted: May 7, 2024
Published online: June 19, 2024
Processing time: 112 Days and 1.9 Hours
Interstitial cystitis/bladder pain syndrome (IC/BPS) is an at least 6-mo noninfectious bladder inflammation of unknown origin characterized by chronic suprapubic, abdominal, and/or pelvic pain. Although the term cystitis suggests an inflammatory or infectious origin, no definite cause has been identified. It occurs in both sexes, but women are twice as much affected.
To systematically review evidence of psychiatric/psychological changes in persons with IC/BPS.
Hypothesizing that particular psychological characteristics could underpin IC/BPS, we investigated in three databases the presence of psychiatric symptoms and/or disorders and/or psychological characteristics in patients with IC/BPS using the following strategy: ("interstitial cystitis" OR "bladder pain syndrome") AND ("mood disorder" OR depressive OR antidepressant OR depression OR depressed OR hyperthymic OR mania OR manic OR rapid cyclasterisk OR dysthymiasterisk OR dysphoriasterisk).
On September 27, 2023, the PubMed search produced 223 articles, CINAHL 62, and the combined PsycLIT/ PsycARTICLES/PsycINFO/Psychology and Behavioral Sciences Collection search 36. Search on ClinicalTrials.gov produced 14 studies, of which none had available data. Eligible were peer-reviewed articles reporting psychiatric/psychological symptoms in patients with IC/BPS, i.e. 63 articles spanning from 2000 to October 2023. These studies identified depression and anxiety problems in the IC/BPS population, along with sleep problems and the tendency to catastrophizing.
Psychotherapies targeting catastrophizing and life stress emotional awareness and expression reduced perceived pain in women with IC/BPS. Such concepts should be considered when implementing treatments aimed at reducing IC/BPS-related pain.
Core Tip: Interstitial cystitis/bladder pain syndrome (IC/BPS) occurs in 2%-18% of the general population, most commonly in women and in people having first-degree relatives affected by the same syndrome. Despite its name suggesting that an inflammation could be involved, no inflammatory aetiology has been found to date. The syndrome causes major sufferance in affected patients and may even affect their psychological status. In spite of efforts to resolve it, no treatment currently exists. Catastrophizing is all too often present in pain syndromes and may be targeted by psychotherapy to reduce the impact of IC/BPS in affected people.
- Citation: Mazza M, Margoni S, Mandracchia G, Donofrio G, Fischetti A, Kotzalidis GD, Marano G, Simonetti A, Janiri D, Moccia L, Marcelli I, Sfratta G, De Berardis D, Ferrara O, Bernardi E, Restaino A, Lisci FM, D'Onofrio AM, Brisi C, Grisoni F, Calderoni C, Ciliberto M, Brugnami A, Rossi S, Spera MC, De Masi V, Marzo EM, Abate F, Boggio G, Anesini MB, Falsini C, Quintano A, Torresi A, Milintenda M, Bartolucci G, Biscosi M, Ruggiero S, Lo Giudice L, Mastroeni G, Benini E, Di Benedetto L, Caso R, Pesaresi F, Traccis F, Onori L, Chisari L, Monacelli L, Acanfora M, Gaetani E, Marturano M, Barbonetti S, Specogna E, Bardi F, De Chiara E, Stella G, Zanzarri A, Tavoletta F, Crupi A, Battisti G, Monti L, Camardese G, Chieffo D, Gasbarrini A, Scambia G, Sani G. This pain drives me crazy: Psychiatric symptoms in women with interstitial cystitis/bladder pain syndrome. World J Psychiatry 2024; 14(6): 954-984
- URL: https://www.wjgnet.com/2220-3206/full/v14/i6/954.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i6.954
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic (lasting 6 mo or more) non-infectious bladder inflammation of unknown origin. The cardinal symptom is chronic suprapubic, abdominal, and/or pelvic pain. At the same time, patients may show pollakiuria, nocturia, urinary urgency, and dyspareunia[1]. Alterations in the sexual, behavioural, cognitive, and emotional domains are not uncommon[2]. Furthermore, after diagnosis, patients with IC/BPS are commonly untreated or treated for more than two-thirds of cases with drugs lacking approval from official agencies[3], thus increasing the occurrence of comorbidities and amplifying its healthcare costs.
The epidemiological data reported in the literature are partly inaccurate due to a high misdiagnosis rate. However, IC/BPS seems to affect 20% of women, while being quite uncommon between men[3].
Since the aetiopathogenesis of IC/BPS is little known, its diagnosis is difficult to make and the therapeutic options are limited. Its pathophysiology is believed to involve pelvic wall defects with increased permeability of the transitional epithelium to urinary toxins and consequent transmural inflammation[1]. The initial trigger may be infectious (Proteus mirabilis, Klebsiella pneumoniae, Citrobacter, Enterobacter, Pseudomonas, Enterococcus faecalis, Staphylococcus saprophyticus, and group B streptococci, but most of all Escherichia coli[4]) or not, and may induce aberrant immune and inflammatory responses[5]. These may result in increased production, activation and degranulation of mast cells and eosinophils, and the subsequent release of histamine and proinflammatory cytokines may ensue in inflammation, consequent vasodilation, sensory nerve stimulation and eventually, tissue damage[5]. Neurogenic inflammation and nerve fibre proliferation, with nerve hyperstimulation and sensory abnormalities[1,6,7] and recurrent urinary infections[1] may also concur. Finally, IC/BPS has been associated with early emotional trauma and hyperactivation of the stress axis[2].
The pelvic diaphragm has an extremely complex innervation that includes several neurological pathways, the function of which has not been elucidated. It is probable that this very intricate innervation is under the control of higher centres; in fact, psychological and emotional factors affect profoundly the function of the pelvic floor. It could be that pelvic organs cross-sensitize in response to earlier threat or traumatic events, prompting some investigators to add a bladder component to the brain-gut axis (including the microbiome) and speak about the bladder-gut-brain axis[8]. This cross-sensitization could result in perceived conjoint bladder- and gut-related emotional distress.
Psychosocial factors, like comorbid anxiety and depression, low quality of life, and trauma-related symptoms accompany and intensify the illness[9,10]. While psychosocial factors influence the development of chronic pain[11], unaddressed psychosocial elements of chronic pain can in turn shape patient perceptions and behaviour, often leading to symptom persistence through central sensitization[12] and are associated with poorer functioning, adjustment, prognosis, and response to treatment[12]. These findings suggest not only a strong association, but also that psychosocial symptoms and bladder-specific symptoms reinforce one another bi-directionally.
In addition, the perception of pain and its chronicity may be modified by emotional and cognitive factors[13]. Increasing evidence suggests that the tendency to magnify the threat value of the pain stimulus, the sense of helplessness in the face of pain, and the inability to inhibit pain-related thoughts, which together constitute the psychic phenomenon known as pain catastrophizing (PC), are associated with the activation of brain regions implicated in processing the affective dimensions of pain but also in cognitive regulation of emotion and cognition, such as the anterior cingulate cortex and ventromedial and dorsolateral prefrontal cortices and can lead to aberrant hypothalamic-pituitary-adrenal axis activity and altered cytokine responses to pain[14], thus resulting in maladaptive plastic changes responsible for the maintenance of chronic pain[13]. Since chronic pain is the cardinal symptom of IC/BPS, these processes could be involved in the pathophysiology of the disorder. Therefore, careful assessment of pain and PC should be included in the clinical workup of IC. In fact, PC was shown to be present in IC/BPS and constitute a core factor[15,16] with a bidirectional relationship[17], with its effect on IC/BPS being mediated by other psychological factors[18].
From this perspective, the poor outcomes in terms of therapeutic efficacy could be traced to inadequate care-taking[19] that underestimates psychological factors and concomitant psychiatric disturbances[20].
The aim of this review was to identify psychological and mental symptoms in IC and chronic bladder pain to underline the urgent need for integration of psychological assessment and management into care plans for IC/CBP.
To identify studies dealing with the presence of psychiatric/psychological symptoms in patients with the IC/BPS, we investigated the PubMed, CINAHL and PsycLIT/PsycARTICLES/PsycINFO/Psychology and Behavioral Sciences Collection databases on November 7, 2023. We also used the ClinicalTrials.gov site to identify ongoing studies, using the following strategy: Condition/disease: Interstitial cystitis; Other terms: Psychological symptoms; Intervention/treatment: blank. To be included, studies had to be original and reporting the proportion of psychiatric disorders or symptoms or psychological symptoms in patients with IC/BPS. Articles ought to be published after a peer reviewing process, to explicitly provide data for psychiatric or psychological symptoms, include patients with IC/BPS and quantify their psychiatric or psychological symptoms as a group, not lumping their data along with data of patients with other conditions (in such case, studies were excluded and labelled as “no IC/BPS” and added to the studies that did not include patients with IC/BPS), nor reporting impressions with no clear data (in such case they were excluded as “no data”). When studies referred to the same sample or to an increased sample including previously analysed patients, only the study with more data (or more complete analyses) was included, with the others excluded as “overlapping” samples. Further exclusion criteria were not reporting psychiatric or psychological symptoms, labelled as “nopsysy”, a design focused on other outcomes (labelled “off-target”, which comprised both studies with an inadequate design as referred to our aims, and unfocused papers with outcomes different from those we investigated), being not related to the subject matter (“unrelated”), surveys of treating physicians or the lay public containing their opinions than data of patients (i.e. the sample was not composed of patients, but consisted of physicians or people interviewed by the survey promoters, labelled as “survey”), other opinion papers without data, such as editorials or letters to the editor or hypotheses and qualitative studies without precise figures as to emerged themes, labelled as “opinion”, animal or in vitro studies (preclinical), labelled as “animal”, protocols of future researches with not even preliminary data, labelled as “protocol”, reviews and meta-analyses, labelled as “review” (but their reference lists were hand-searched to identify possibly eligible studies that eluded our search strategies), and obviously duplicates or corrections of a published paper already existing in a given database; overlapping records between databases were also labelled as “duplicates”.
Our search strategy was ("interstitial cystitis" OR "bladder pain syndrome") AND ("mood disorder" OR depressive OR antidepressant OR depression OR depressed OR hyperthymic OR mania OR manic OR rapid cycl* OR dysthymi OR dysphori) for all databases and was carried out on November 7, 2023.
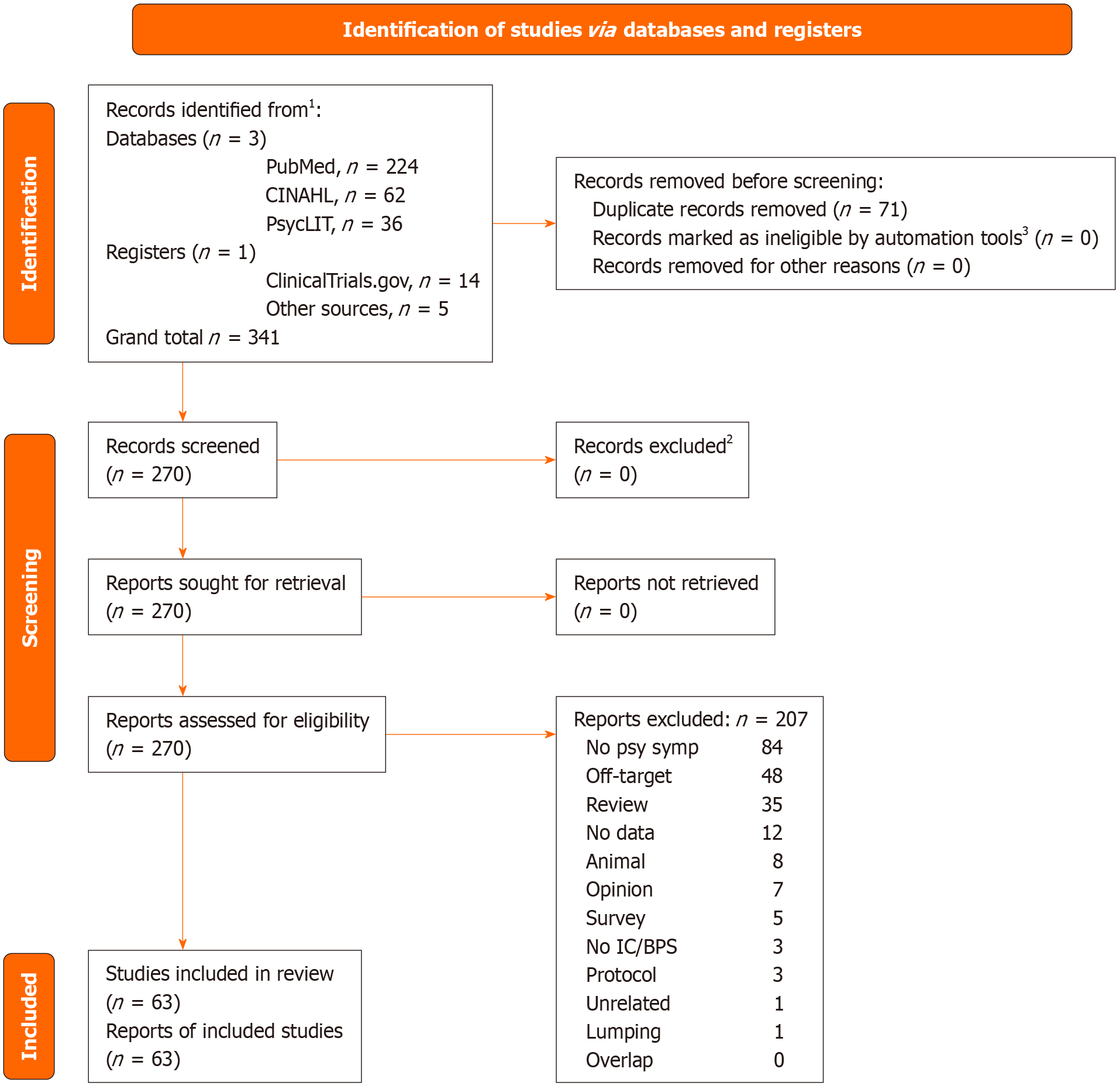
To decide eligibility of a given study, we performed Delphi rounds among all authors until complete consensus was reached. Not more than three were necessary for all articles. We conducted this systematic review adopting the PRISMA statement[21]. Detailed results of databases searching, PRISMA checklist and flowchart are shown in the Supplement. Overall judgments for each rated study and comments are shown in the online Supplement (Supplementary Table 1). We did not register our review on PROSPERO. The articles resulting from our search are shown in the Supplement, along with inclusion/exclusion decisions and the reasons for the latter. The selection process is depicted in Figure 1, which shows the PRISMA flowchart. Risk of Bias (RoB) was assessed through the Cochrane RoB method as described in the Cochrane Handbook[22].
Our search on November 7, 2023 yielded 224 records on PubMed, 62 on CINAHL, and 36 on PsycLIT/PsycARTICLES/PsycINFO/Psychology and Behavioral Sciences Collection, and 1 added from other sources, for a total of 323 records, 67 of which were duplicates and immediately removed. The inclusion process is depicted in Supplementary Table 1. Records identified by the search strategy spanned from March 1988 to October 25, 2023, with eligible records ranging from July 2000 to October 2023. There were 63 records identified as eligible; they are summarised in Table 1[23-85]. These studies were longitudinal (n = 29) or cross-sectional (n = 34). There were some studies conducted with the same sample, but they focused on different outcomes, so we retained them all[35,36,49,54,55,60,70] (Table 1).
| Ref. | Population | Design | Psychiatric symptoms | Conclusions/observations |
| Rabin et al[23], 2000 | 80 females, treated for IC, aged 16-75 yr (mean age = 44.6 ± 12.4 yr) | CS. Questionnaires and scales administered included Demographics; General Questionnaire; Disability; ICES; Pain Scale; Self-Stigmatization Scale; CES-D | 52.6% of IC sample reported dep sym; levels of dep experienced by IC pts are > than general population/other chronic pain populations; regression showed dep to be associated with self-efficacy for male aging pain (P < 0.01), self-stigmatization (P < 0.05), and pain (P < 0.05) | Females with IC reported physical and emotional burden and showed ↑ dep levels |
| Rothrock et al[24], 2002 | 65 female pts 22 to 81 yr (mean age ± SD: 51.0 ± 16.1) with IC + 40 HC 25-82 yr (mean age 52.6 ± 15.8) | CS. Administered scales comprised BDI; MOS SF-36; HAM-D | Pts reported significantly poorer QoL than HC across all MOS domains, including emotional difficulty, and mental health (P < 0.01). Pts reported > dep sym on the BDI than HCs (95%CI: 4.1-7.1 vs 1.5-4.9, P < 0.05,) as well as on the HAM-D (95%CI: 6.1-9.6 vs 0.7-2.3, P < 0.001,). In pts, mean HAM-D score was 7.9-6.8 (range 0-25), indicating mild dep sym. Only 10.2% of pts scored in the moderate-to-severe range of dep sym on the HAM-D | A diagnosis of IC is related to poorer functioning in various life domains. ↑ sym severity related to poorer physical/social functioning and mental health |
| Rothrock et al[25], 2003 | 64 female pts with IC | CS. Scales administered included questionnaires assessing QoL, coping and symptoms; HAM-D | Pts coping with greater catastrophising reported ↑ impairments in dep sym, general mental health, social functioning, vitality, and ↑ pain. Seeking social support was associated with ↓ dep sym | Maladaptive coping strategies are associated with ↑ levels of dep sym and ↓ QoL in pts with this condition. Psychosocial interventions aimed at ↑ adaptive coping may positively impact IC |
| Novi et al[26], 2005 | 46 females with IC+ 46 HC | CS. 46 females with IC and 46 HC were evaluated by PHQ-9 DM (MD defined as a score ≥ 10); RIISQ to find out the diagnosis of IBS | Compared with HC, IC pts were more likely to be diagnosed with IBS (OR 11, 95%CI: 2.7-52, P < 0.001) and dep (OR 3.97, 95%CI: 1.17-14.1, P < 0.05) | The association of IBS and dep appears to be > in females with IC |
| Wu et al[27], 2006 | 749 pts with IC and < 65 yr + HC (646 females and 103 male) | Lo. Costs incurred in the 1st yr after IC diagnosis and co-morbidities were compared between IC pts and HC. A multivariate two-part model was applied to estimate the IC direct medical cost, indirect cost and total cost to adjust for observed pts demographics and comorbidities. Statistical significance was evaluated by the bootstrap method | IC pts had 130% higher direct costs (P < 0.05) and 84% higher indirect costs than HC. IC pts also had a higher diagnostic prevalence of prostatitis (RR = 40.0), endometriosis (RR = 7.4), vulvodynia (RR = 6.9), chronic pelvic pain (RR = 5.8) and urinary tract infections (RR = 5.1; all P < 0.05). IC pts were also more likely to report dep (RR = 2.8) and anx (RR = 4.5) than HCs (all P < 0.05) | IC is a costly disease associated with co-morbidities. More accurate diagnosis and earlier and more appropriate treatment of IC would lead to better management of co-morbidities and ↓ healthcare costs |
| Fan et al[28], 2008 | 47 IC pts (38 females and 9 male) +31 HC | CS. 47 IC pts and a group of 31 age-matched, asymptomatic females received HAM-D and HRSA. IC pts also completed questionnaires relating to IC symptom severity, including urgency and frequency and O'Leary Sant index | Mean dep scores = 16.6. 15 pts (31.9%) with mild dep symptoms, 5 (10.6%) mild-to-moderate and 20 (42.6%) moderate-to-severe dep. mean anx score = 21.0, with 21 (44.7%), 9 (19.1%) and 17 (36.2%) pts displaying mild, mild-to-moderate, and moderate-to-severe anx symptoms, respectively. Pain scale and O'Leary Sant index were significantly correlated to anx and dep score | Most of IC pts feature significant dep and anx (85% of IC pts featured significant affective symptoms). The extent of affective symptoms would appear to correlate well with IC symptom severity |
| Clemens et al[29], 2008 | 239 IC female pts and 717 matched HC (1:3 ratio) | Lo (case-control). A computer search of the administrative database at Kaiser Permanente Northwest, Portland, Oregon was performed for 1 May, 1998 to 30 April, 2003. All females with a medical record diagnosis of IC (ICD-9 code 595.1) were identified. These cases were matched with 3 controls each based on age and duration in the health plan. Assigned ICD-9 diagnoses to these 2 groups were compared using ORs | 239 cases and 717 matched controls were analysed. 23 diagnoses were significantly > in IC pts than in HC (P = 0.005): 7/23 were other urological or gynaecological. Additional specific conditions associated with IC were gastritis (OR 12.2), child abuse (OR 9.3), FM (OR 3.0), anx disorder (OR 2.8), headache (OR 2.5), oesophageal reflux (OR 2.2), unspecified back disorder (OR 2.2) and dep (OR 2.0) | IC was associated with multiple other unexplained physical symptoms and certain psychiatric conditions. The possible biological explanations for these associations remain to be established |
| Clemens et al[30], 2008 | 174 male pts with chronic prostatitis/CPPS (mean age = 52) and 72 male, age-matched HC. 111 female pts with interstitial cystitis/PBS (mean age = 50) and 175 females, age-matched HCs | Lo (case-control). Pts and HC were analysed. Demographic information, current medication use, medical history was collected; NIH-CPSI for male subjects, and the ICSI and ICPI for females; PHQ used to assess mental health | Mental health disorders were identified in 13% of the chronic prostatitis/CPPS cases and 4% of male HC (OR 2.0, P = 0.04), as well as in 23% of IC/PBS cases and 3% of females HCs (OR 8.2, P < 0.0001). Disease status (case vs control) (OR 10.4, P = 0.001) and income > 50000 USD (OR 0.34, P = 0.008) were the only 2 variables independently predictive of the presence of a mental health diagnosis. Medications for anx, dep or stress were taken by 18% of pts with chronic prostatitis/CPPS, 37% of those with IC/PBS, 7% of male HCs and 13% of females HCs | Dep and PA are ↑ in male and females with pelvic pain conditions than in HCs. Furthermore, anx and dep may be more difficult to treat in pts with urological pain syndromes than in HCs |
| Goldstein et al[31], 2008 | 141 females diagnosed with IC (mean age = 45.9 yr) | CS. Prevalence of dep was measured using the BDI-II; Prevalence of abuse was evaluated using the validated DAQ | 98 (70%) pts scored ≥ 14 on the BDI-II. The mean score of the total sample was 14.6 (SD 9.2), representing mild dep. Of all of those that scored in the dep range (≥ 14), the mean score was 22.4 (SD 6.4) representing moderate dep. The prevalence of sexual abuse from validated questionnaires was 36%; the prevalence of childhood sexual abuse was 21%; physical abuse was 31% | Pts with IC had > prevalence of dep and sexual abuse than the general population. Females with IC should be screened for dep and abuse and referred to a mental health expert as necessary for treatment |
| Kim and Heitkemper[32], 2009 | 298 females (mean age = 74.3 ± 6.20) | Lo. To estimate the prevalence of IC/PBS symptoms and describe the relationships among symptoms, general sample characteristics. dep and QOL in older Korean females → ICSI/ICPI, KGDS, HRQOL, KHQ. Statistical analysis → SPSS/WIN 15.0 program; prevalence/urologic characteristics → freq, mean, and severity; correlations among demographic characteristics. ICSI-K and ICPI-K, KGDS, and KHQ → Pearson; group differences in variables by ICSI-K cut-off score 5 → ANOVA | The prevalence of mild to severe IC symptoms using ICSI-K was 54%. The percentage at risk for IC using summed scores of ICSI-K and ICPI-K) was 43.6%. The ICSI-K scores had moderate correlations with KHQ and had mild correlations with KGDS; The ICPI had strong positive correlations with KHQ and had mild correlations with KGDS. KHQ scores had mild positive correlation with KGDS | Almost half of older Korean females in this sample had IC/PBS symptoms using IC/PBS cut-off score of 5. IC symptoms and problems impacted limitation in life highly |
| Tsai et al[33], 2010 | 69 IC pts [mean age = 42.0 ± 16.3 (range: 20–79 yr)], 52 females (mean age = 44.2 ± 16.0 yr), 17 males (mean age = 35.2 ± 15.6 yr); P < 0.05 | CS. PSQI and HADS were used to evaluate quality of sleep and dep level, respectively. Multiple linear regressions were used to identify independent factors of sleep quality | Mean PSQI global score was 9.5 ± 4.2 (range: 1-19); 81.2% of pts had poor sleep quality (PSQI > 5). Regression analysis suggested that IC severity (β coefficient = 0.42, P < 0.001) and level of anx and dep (β coefficient = 0.26, P < 0.05) were significant independent risk factors for poor sleep quality | Poor sleep quality is common in IC pts and severity of urological symptoms and dep levels are important independent risk factors |
| Giannantoni et al[34], 2010 | 14 female pts with IC | Lo. Controlled-trial. 14 pts received 1 BoNT/A inj under cystoscopic guidance. At pre- and 3 mo post- treatment all pts underwent urological assessment, VAS, HAM-A, HAM-D and SF-36 to assess QoL | At pre-treatment all 14 pts had ↑ daytime and nighttime urinary frequency and ↑ VAS scores. 9 pts had pathological HAM-A and HAM-D scores. At the 3-mo fup 10/14 pts reported a subjective improvement in pain. Mean VAS score, mean daytime and nighttime urinary frequency ↓ (P < 0.01, < 0.01 and < 0.01). All SF-36 and HAM-A domains significantly improved (P < 0.01). All HAM-D domains, except weight and sleep disorders, significantly improved, particularly somatoform syms (P < 0.01), cognitive performance (P < 0.01), and circadian variations (P < 0.01) | In pts with refractory PBS with symptoms of anx, dep and poor QoL, BoNT/A intravesical treatment reduced BPS, improved psychological functioning, and well-being |
| Bogart et al[35], 2011 | 1469 females who met criteria for BPS/IC | CS. A telephone screening of 146,231 households and telephone interviews with females with BPS/IC symptoms were conducted. Health-related QoL was measured using the Short-Form 36-item Health Survey physical health scale; The Patient Health Questionnaire-8 items was used to assess dep symptoms; females who had a current partner were asked the number of times they had engaged in vaginal sex in the past year and the extent to which they experienced the 6 BPS/IC-specific sexual dysfunction symptoms and 5 general sexual dysfunction symptoms in the past 4 wk | Of those with a current sexual partner (75%), 88% reported general sexual dysfunction symptom and 90% reported BPS/IC-specific sexual dysfunction symptom in the past 4 wk. In the multivariate models, BPS/IC-specific sexual dysfunction was significantly associated with more severe BPS/IC symptoms, younger age, worse depression symptoms, and worse perceived general health | Females with BPS/IC symptoms experience very high levels of sexual dysfunction and higher level of dep |
| Watkins et al[36], 2011 | 1469 females who met criteria for BPS/IC | CS. A telephone screening of 146,231 households and telephone interviews with females with BPS/IC symptoms. A weighted probability sample of 1469 females who met BPS/IC criteria was identified. Measures of BPS/IC severity, dep symptoms, PA, and treatment utilization were administered. T and χ2 tests used to examine differences between groups | > ⅓ of the sample (n = 536) had a probable diagnosis of dep, and 52% (n = 776) reported recent PA. females with a probable diagnosis of dep or current PA reported worse functioning and ↑ pain and were less likely to work | Rates of probable current dep and PA are high, and there is considerable unmet need for treatment |
| Moskovenko[37], 2011 | 112 female pts with IC | CS. Clinical evaluation | ↑ Neuroticism in 74 (66.1%) cases, moderate or high-reactive anxiety in 98 (87.5%), high personal anxiety in 36 (32.1%); 8.0% pts had depressive disorders > moderate | Pts with IC have higher odds for having psychoemotional disturbances |
| Peters et al[38], 2011 | 639 females: 425 HC(s), 36 with ulcerative IC/PBS (ULC) and 178 non-ulcerative IC/PBS (N-ULC) | CS. females with IC/PBS and HC(s) completed a mailed survey assessing for 21 diagnoses. IC/PBS subtype was determined by hydrodistention reports. Standardized questionnaires assessed IC/PBS symptoms (ICSI-PI) and for undiagnosed fibromyalgia, IBS, and dep (SIS; Rome III Functional Bowel Questionnaire; CES-D). Data were analysed using the Pearson chi-square, Fisher exact, Wilcoxon rank test, or Spearman rank correlation coefficient | ULC IC/PBS pts were older (median 63 yr; P < 0.01) and less employed (P < 0.01), but groups were similar on other demographic characteristics. N-ULC reported more chronic diagnoses (mean 3.5 ± 2.3) than ULC (2.3 ± 2.0) and controls (1.2 ± 1.5; P < 0 .01). When N-ULC and ULC IC/PBS patients were compared, more N-ULC IC/PBS patients had fibromyalgia (P = 0 .03), migraines (P = 0.03), temporomandibular joint disorder (P < 0.01), and higher CES-D (P = 0.02) and SIS scores (P = 0.01). The ULC IC/PBS group voided more frequently during the daytime (P = 0.03) and nighttime (P < 0.01) and had smaller mean bladder capacity than N-ULC (P < 0.01). No significant differences were seen between N-ULC and ULC IC/PBS patients on the ICSI-PI and Rome III | Notable differences in the number of comorbid diagnoses and symptoms were seen between IC/PBS subtypes and controls |
| Panzera et al[39], 2011 | 407 females with IC | Lo. All participants were asked to complete PSQI and ICSI/ICPI | Mean global PSQI score = 13.12 (SD ± 3.61) with all pts reporting a score of 6 or above. Results from the hierarchical multiple regression revealed that after controlling for age, menstrual status, years with IC, and dep, the 4 symptom predictors of IC (pain, urinary frequency, urinary urgency, and nocturia) alone explained 21% of the variance (F(4, 398) = 8.41, P < 0.001) in sleep quality. Only pain, nocturia, and urinary urgency contributed significantly (P < 0.05) | Females with IC have disrupted sleep and poor subjective sleep quality. Predominant symptoms of IC related to poor sleep include nocturia and pain |
| Nickel et al[40], 2011 | 207 IC/BPS female pts and 117 HC matched for age, partner status and education | Lo (case-control). All participants were asked to complete the CTES, the ICSI, the ICPI, the MPQ-SF, the CES-D, the STAI, the FSFI, the MSPSS and the MOS SF-12 | Before 17 yr of age, the IC/BPS cases reported > prevalence of "raped or molested" compared to HCs (24.0% vs 14.7%; P = 0.047). Within the IC/BPS group, cases reporting previous sexual abuse endorsed > sensory pain, dep and poorer physical QoL at the present time compared to IC cases without a sexual abuse history | Childhood traumatic events are reported as more common in IC/BPS pts than HCs |
| Hepner et al[41], 2012 | 1019 females with BPS/IC symptoms | CS. In order to estimate SI prevalence in IC pts, females with and without recent SI were compared based on demographics. dep symptoms, BPS/IC symptoms, functioning, and treatment | 11.0% (95%CI: 8.73-13.25) reported SI in the past 2 wk. Females who endorsed SI reported worse mental health functioning, physical health functioning, and BPS/IC symptoms. Multivariate logistic regression analyses indicated that BPS/IC sym severity did not independently predict likelihood of endorsing SI | BPS/IC severity may not ↑ the likelihood of SI except via severity of dep symptoms |
| Keller et al[42], 2012 | 9269 pts (7584 females and 1685 male) with BPS/IC and 46345 (37920 females and 8425 male) randomly selected comparison ctrl | Lo. Case-control. Conditional logistic regression analyses were performed to calculate the odds ratio for each of the 32 medical comorbidities (included dep disorder, psychoses, alcohol abuse and drug abuse) between pts with and ctrl without BPS/IC | With the exception of metastatic cancer, pts with BPS/IC had a significantly ↑ prevalence of all the medical comorbidities analysed than ctrl without BPS/IC. Compared with ctrl without BPS/IC, pts with BPS/IC had particularly ↑ odds of comorbid mental illnesses | Pts with BPS/IC had > prevalence of multiple comorbidities |
| Clemens et al[43], 2012 | 3397 females with IC/BPS | CS. Pts completed a survey asking if they had comorbidities as IBS, FM, chronic fatigue syndrome, migraines, PA, or dep and the age of symptom onset. All pts were also asked to provide the date of IC/BPS symptom onset | 2185/3397 females reported a diagnosis of at least one of the nonbladder conditions. Dep tended to occur earlier (P < 0.05), whereas FM generally occurred later (P < 0.05). Mean age of onset was lowest for migraine symptoms, dep symptoms, and PA symptoms, and greatest for FM and chronic fatigue syndrome symptoms. Mean age of irritable bowel syndrome and IC/BPS symptom onset was between these other conditions | These findings confirm the common co-occurrence of IC/BPS with chronic nonbladder conditions. In females with IC/BPS symptoms and coexistent nonbladder conditions, bladder symptoms do not uniformly predate the nonbladder symptoms |
| Katz et al[44], 2013 | 196 females IC (recruited from existing IC/BPS pts databases); mean age: 52 yr | CS. Examined mediation through structural equation modelling; MPQ, Pain Disability Index, CES-D; STAI; PCS | Negative affect (P < 0.001) and catastrophising (P < 0.001) significantly explained the relationship between impairments and functional disability, whereas social support did not | Negative affect and catastrophising partially explained disability in IC pts. Due to IC refractoriness, biopsychosocial patient management is essential. ↓ in negative affect and catastrophising will probably lead to improvements in pain-related disability. CS design does not allow for establishing causality |
| Keller et al[45], 2013 | 832 IC/BPS female pts and 4160 HCs (total = 4992) tracked for a 1-yr period; mean age 48.7 ± 16.2 yr | Lo. Cox proportional hazards regressions (stratified by age group and index year) | DD incidence = 4.69 (95%CI: 3.38-6.34) ×100 person- yr in pts with BPS/IC and 0.94 (95%CI: 0.68-1.27) ×100 person-yr in HCs. HR of DD during the 1-yr fup period for BPS/IC pts = 5.06 (95%CI: 3.21-7.96, P < 0.001). Adjusted HR for DD associated with BPS/IC = 10.33 for pts aged 40-49 (95%CI: 3.68-29.04) | ↑ Risk for being diagnosed with DD during 1st yr after receiving diagnosis of IC |
| Nickel et al[46], 2015 | 173 IC females | CS. case control. CES-D to assess dep, STAI for anxiety, PSS for perceived stress, PCS for catastrophising | 157 pts (81%) reported more sensory type pain, poorer physical QoL, and greater somatic dep and sleep disturbance than 36 (19%) pts with pelvic pain only. This last phenotype reported ↑ IBS prevalence and fibromyalgia, and more general fatigue sym and psychiatric conditions | Two distinct pain location phenotypes, pelvic pain only and more than pelvic pain, were identified analysing IC/CPPS pts |
| Kairys et al[47], 2015 | 33 females with IC without comorbidities (mean age 39.5 ± 12 yr; mean symptom duration 9.1 ± 9 yr | CS. Anatomical MRI data were acquired across 5 MAPP discovery sites; high resolution T1 structural images were acquired for each pt; Symptom were measure with the following questionnaires: SYM-Q; FGPI; PROMIS; sleep disturbance scale; SF-MPQ; HADS, Positive and Negative Affect Scale; Gracely Box Scales to measure pain and unpleasantness during the scan | Compared to HC(s), females with IC displayed significantly more GM volume in several regions including the right S1 (P < 0.05, FWE SVC), SPL/precuneus bilaterally (left P < 0.05, FWE SVC; right P < 0.001, uncorrected) and left SMA (P < 0.001, uncorrected, Table 1, Figure 1). GM volume in the right primary somatosensory cortex was associated with greater pain (McGill pain sensory total; r = 0.396, P = 0.025), anxiety (HADS, r = 0.447, P = 0.01) and urological symptoms (r = 0.449, P = 0.01) | Alterations in somatosensory GM may have an important role in pain sensitivity as well as affective and sensory aspects of IC |
| Chuang et al[48], 2015 | 16185 IC/BPS diagnosed during 2002-2010 [11865 (73.3%) females, 4320 (26.69%) male) vs 32370 HCs (23823 (73.60%) females, 8547 (26.40%) male); mean age 46 yr | Lo. Cohort study, based in part on data from NHIRD. Outcome risk assessed with Kaplan-Meier curves; Poisson regression analysis, and Cox proportional hazards models | IR (10000 person-yr) significantly ↑ in IC pts compared to HCs (92.9 vs 38.4 for anxiety; 101.0 vs 42.2 for depression, and 47.5 vs 23.0 for insomnia). IRRs of IC-associated anxiety and dep were ↑ in male compared to females (2.6 vs 2.4 for anxiety; 3.1 vs 2.3 for dep). IC remained a significant predictor with HR and 95%CIs 2.4 (2.2-2.7) for anxiety, 2.4 (2.2-2.6) for dep, and 2.1 (1.8-2.4) for insomnia | IC associated with ↑ risks of anxiety, dep, and insomnia in initially sym-free pts |
| Griffith et al[49], 2016 | 424 pts with UCPPs [233 (55%) females, 191 (45%) males]; mean age 43.4 ± 15.1 yr | CS. MAPP Research Network. Scale GUPI, ICSI, ICPI. Aim of the study was also to examine relationships with symptoms of depression as a comorbidity of UCPPS | Dep was predicted by pain (B ± SE = 0.24 ± 0.04, 95%CI: 0.16–0.32, P < 0.001) In contrast dep was not significantly related to urinary symptoms ( B, mean ± SE = 0.06 ± 0.04, 95%CI: 0.02-0.13, P = 0.127) | The data suggest that pain and dep are closely linked in pts with UCPPS, and that pain and urinary symptoms should be assessed separately |
| Tripp et al[50], 2016 | (Tot 307 females pts) 190 IC mean age 49.20 ± 14.94 yr; 117 HCs mean age 47.83 ± 13.52 yr | CS. MPQ, IC syms, PHQ-9 | 23% IC pts endorsed SI in the past 2 wk vs 6% in HCs. In both IC pts and HCs, ↑ SI associated with ↑ pain and ↑ dep, whereas, for IC pts, ↑ SI was associated further with pain catastrophising | This study indicates that tertiary care pts with IC/BPS have an alarming rate of SI. Dep, catastrophising characterised by helplessness about managing pain, and pain are all significantly associated with ↑ SI. Catastrophising as a predictor of SI in IC/BPS points to its key role as a psychological predictor of negative pain-related outcomes |
| Kanter et al[51], 2017 | 15 females IC in a total of four focus groups. mean age = 52.6 yr, mean IC duration = 6.3 yr | Lo. Qualitative analysis of emerging themes. Session recording and transcription with information deidentified. Transcripts coded and analysed by three independent physicians | 3 concepts identified: IC/PBS is debilitating, pts experience significant isolation, SI found in all groups | Pts with IC preferred organized treatment plans with diverse choices and providers who offered hope in dealing with their condition; focusing on the doctor-pt relationship to overcome isolation and suicidality, physicians may help IC pts |
| Abernethy et al[52], 2017 | 40 females (20 IC; 20 HCs); mean age 34 yr | CS study. Catastrophising Scale, PDI, BDI, BAI. Urinary microbiomes and cytokine levels analysed with standard immunoassay | Pts IC scored ↑ on dep (P = 0.008) and anxiety (P = 0.019) screens compared with HCs | IC pts’ urinary microbiome less likely to contain Lactobacillus species and associated with ↑ levels of proinflammatory cytokines. No correlation between Lactobacillus species and cytokine levels |
| Chen et al[53], 2017 | 1612 IC pts, [1283 (79.6%) females, 309 male (20.4%) mean age 48.4 ± 16.4 yr) vs 3224 HCs (2466 females (76.5%), 758 male (23.5%) mean age 48.9 ± 16.4 yr). 1436 SoDi, 2872 non-SoDi. mean age 48.4 ± 16.4. IC pts 79.6% females HCs 76.5% females | Lo. Case-control and retrospective cohort studies. OdR for SoDi calculated with conditional logistic regression and HR for IC in SoDi pts estimated with Cox regression, cumulative risk with Kaplan-Meier | OdR for SoDi = 2.46. mean time until IC development in HCs = 11.5 ± 1.3 yr (shorter in SoDi pts, 6.3 ± 3.6 yr). HR for developing IC = 2.2. Pts and HCs differed in cumulative survival probability for IC (P < 0 .05) | SoDi can be used as a predictor of IC. While examining pts with IC, it is recommended to investigate past history of SoDi |
| Chiu et al[54], 2017 | 94 females IC/BPS pts. mean age 40.6 ± 10.0 yr | CS. Link between urogenital syms, psychiatric syms, and potentially traumatizing experience CTQ, BVAQ, BDI-II, BAI, TDS | The high-CTQ group had ↑ dep, ↑ anxiety, ↑ dissociation, ↑ alexithymia and ↓initial and follow-up bladder capacities. A combination of higher scores of cognitive alexithymia and lower scores of affective alexithymia was associated with ↑ bladder capacity | In pts with IC/BPS, ↑ anaesthetic bladder capacity was associated with a set of psychological factors that commonly prevail in functional somatic syndrome. This result suggests that a psychological mechanism independent of a bladder- centric defect may underlie the mental and somatic symptoms of a subgroup of pts with IC/BPS and that IC/BPS in a subgroup of pts may represent a functional somatic syndrome |
| Chiu et al[55], 2017 | 94 females IC/BPS pts. mean age 40.6 ± 10.0 yr. 47 females with AC. mean age 43.4 ± 9.9 yr | CS. Childhood trauma and urological sym in pts wit IC/BPS. FUP, OSQ, BBTS, BDI-II, TDS, BAI, SDQ-20 | Pts in the IC/BPS group reported ↑ abusive experiences than did the AC group pts; however, this difference reached significance for physical abuse. Pts in the IC/BPS group reported ↑ childhood trauma by close others | The study hypothesizes that IC/BPS may be a heterogeneous condition that involves a multifactorial aetiology where a psychosocial phenotype of IC/BPS with a unique pathogenetic mechanism may exist; in which, CT may play an important role |
| Hosier et al[56], 2018 | 2007 pts (1523 male mean age 45 ± 13.5, 484 females mean age 45.7 ± 17.4 yr) with UCPPS from a single site | Lo. Retrospective study. Demographics. sym scores, pain scales, described clinical UPOINT scoring between 1998 and 2016 (data from UCPPS clinic) | Male had ↑ prevalence of dep (31% vs 18.4%), and ↑ alcohol use (44.2% vs 10.8%), ↑ IBS, ↑chronic fatigue syndrome, ↑ fibromyalgia, ↑ drug allergies, ↑ diabetes compared to females with UCPPS (all P < 0.001) | Male with UCPPS have ↑ prevalence of systemic disorders/syms and worse urinary symptoms than females with UCPPS. Findings indicate that male and females with UCPPS have distinct and different clinical phenotypes |
| Liang et al[57], 2018 | 30 female pts IC undergoing several intravesical HA instillations with time vs 30 age-matched HCs | Lo. Prospective study. HADS, O'Leary-Sant score, PISQ-12, and a pain visual analogue scale completed before and after treatment; same for the HC | IC pts had a significant ↑ in HADS dep subscale and total scores. After HA treatment, 73% of IC pts showed ↓ in their urological syms, but no significant changes in HADS and PISQ-12 scores | Bladder pain and lower urinary tract syms in pts with IC/BPS may ↓ after a 6-mo intravesical HA treatment. No significant changes in psychological and sexual functional scores |
| Muere et al[58], 2018 | 341 females IC mean age 49.77 ± 14.49 | CS. Demographics. CES-D, PCS. BCPCI | Pts who reported ↑ dep symps and with a ↑ tendency to catastrophize were more likely to engage in illness-focused coping strategies, which contributed to the reporting of ↑ sensory and affective pain | To manage pain in IC/BPS we need evidence-based techniques that ↓ catastrophising, ↓ illness-focused coping, and ↓ dep. These techniques seem to function most in pts with ↑ dep |
| Van Moh et al[59], 2018 | 150 participants, 36% male (11/31) and 25% females (30/119) with HLs. The difference in median age was 17 yr (58 vs 41, P < 0.001) | Lo. Pelvic syms assessed with the following questionnaires: (1) ICSI, ICPI; and (2) PUF. Presence and distribution of non-urologic pain assessed with: (1) Self-reported history of IBS, fibromyalgia, chronic fatigue syndrome, migraine headache, vulvodynia (females only), and (2) using a body map diagram described previously to identify participants who reported “pelvic pain and beyond” and “widespread pain” patterns, and the number of pain sites beyond the pelvis. The intensity of non-urologic pain was assessed using a 0-10 numeric rating scale. BPI was used to assess pain severity and pain interference. Psychosocial health was assessed by: History of depression, history of anxiety attacks, and somatic symptom burden | 27% (n = 41) had HLs (36% of male, 25% of females). Participants with HLs were significantly older (median age 58 vs 41, P < 0.001) and reported less intense urologic pain (5 vs 7, P = 0.024) but more nocturia (ICSI nocturia symptom score: 4 vs 3, P = 0.007). had less frequently a history of IBS (15% vs 36%, P = 0.013) and anxiety attacks (22% vs 44%, P = 0.013) | HLs can be identified in both females and male. The presence of HLs was associated with older age, less bladder pain, more nocturia, and lower probability of IBS and anxiety attacks |
| Rodríguez et al[60], 2019 | 233 females and 191 male UCPPS. Pts with sym duration < 2 vs ≥ 2 yr compared for sym severity, COPC, and mental health comorbidities | CS. HAD, PCS | Male (but not females) with UCPPS sym duration ≥ 2 yr had ↑ severe syms than those with < 2 yr (P = 0.045). Participants with shorter (< 2 yr) and longer (≥ 2 yr) sym duration were as likely to experience COPC | Sym duration did not appear to affect severity of UCPPS pain. male with UCPPS syms ≥ 2 yr experienced more severe urinary syms than male with syms < 2 yr |
| Carty et al[61], 2019 | 37 female pts with CUP+ 25 controls (mean age 45 yr, primarily Caucasian and relatively well educated, and more than half (58.9%) were married or in a committed relationship) | Lo. RCT. Females with CUP received either a single 90-minute life stress interview (n = 37) or no interview (treatment-as-usual control; n = 25). Self-report measures of pain severity (primary outcome), pain interference, pelvic floor symptoms, and psychological symptoms (anx and dep) were completed at BL and 6-wk fup | Pain severity was significantly ↓ at fup in the interview condition than the control condition (F(1, 58) = 4.52, P = 0.038), with a medium effect size. Within the interview condition, there was a ↓ in pain over time (ns), whereas among controls, there was ↑ in pain (ns). Pelvic floor symptoms were significantly ↓ at fup for the interview condition than the control condition (F(1, 58) = 8.01, P = 0.006), with a large effect size. The interview condition had a significant ↓ in pelvic floor symptoms over time (t(36) = 2.95, P = 0.006), but controls did not change (t(24) = 0.09, P = 0.93). Finally, the two conditions did not differ at fup on pain interference (F(1,58) = 1.02, P = 0.62), anx symptoms (F(1, 58) = 0.30, P = 0.59), or dep symptoms (F(1, 59) = 0.20, P = 0.66) | An intensive life stress emotional awareness expression interview improved physical but not psychological symptoms among females with CUP |
| Cepeda et al[62], 2019 | 3973695 eligible non-IC at BL from the general population (2011471 females, 1962224 male) | Lo. Comparative descriptive study using retrospectively recorded data in a US claims database (Optimum). The first outpatient visit was the ID for the general population, and the diagnosis of dep was the ID for pts with dep | 3973695 people from the general population; 2293 (0.06%) developed IC within 2 yr [mean age (yr) 50.87 ± 16.86 vs 47.47 ± 18.30 of non-IC; n = 1995 (87%) females]. Of 249200 individuals with dep, 320 (0.13%) developed IC | ↑ Incidence of IC in pts with dep. Pts who developed IC had ↑ chronic pain conditions, dep, malaise, and inflammatory disorders |
| Thu et al[63], 2019 | 51 OAB [39 females, 12 males; mean age (yr) 53.8 ± 11.9], 27 IC/BPS (all females; mean age (yr) 44.8 ± 16.6), and 30 [17 females, 13 males; mean age (yr) 54.2 ± 12.3] CTRL | Lo. Non-urologic pain was assessed using a whole-body map and BPI. Urologic pain was assessed using the IC Symptom and Problem indexes, Genitourinary Pain Index, and 0-10 pain scale. Urogenital pain was assessed using a genital map, and report of pain related to bladder filling and urination | OAB pts with pelvic pain had worse urinary symptoms (OAB-q SS: 21.7 vs 17.2, P = 0.025; OAB-q HRQOL: 39.7 vs 25.4, P = 0.015; UDI-6: 16.5 vs 10.8, P = 0.004; IIQ-7: 16.2 vs 5.1, P < 0.001), anx (HADS-A, 10.1 vs 6.1, P = 0.003) and dep (HADS-D, 7.6 vs 4.1, P = 0.004) compared to OAB pts without pelvic pain. The P-value for PSS almost reached statistical significance (P = 0.05) | OAB pts has pain inside and/or outside the pelvis. The intensity and distribution of pain in OAB was intermediate between IC/BPS and controls. OAB pts with pelvic pain have worse urinary symptoms and PSS. Systemic processes such as central sensitization should be examined in this population |
| Lai et al[64], 2019 | 211 pts IC/BPS or chronic prostatitis/CPPS (159 females, 52 males; mean age [years] 43.1 ± 15.9) | CS. Clinical variables included in k-means clustering (uro- and non-uro pain severity, urinary urgency, frequency and UPOINT scoring) | The k-means clustering algorithm identified 3 pt clusters: (1) Mild pelvic syms in approximately 30%; (2) severe pelvic syms approximately 40%; and (3) systemic syms approximately 30%. The clusters had an equal likelihood to have HLs in bladder | Pts in the systemic cluster were younger by approximately 5-7 yr and more likely to be females. They had the most severe urinary syms, the most severe pelvic and nonpelvic pain and were more likely to have chronic overlapping pain conditions, psychosocial issues (dep, anxiety and somatic syms) and poorer QoL than pts in the other two pelvic clusters |
| Crawford et al[65], 2019 | 135 females IC recruited from tertiary care clinics, mean age 52.57 yr | Lo. PHQ-9 for dep, PCS for pain, DERS for emotion regulation at BL, 6 mo, and 1 yr. Serial mediation was used to test models of pain, catastrophising, and dep | The significant indirect path was from BL dep to catastrophising at 6 mo to pain at 1 yr (b = 0.10; 95%CI: 0.0049-0.2520). Helplessness was the key factor of catastrophising driving this relationship (b = 0.17; CI: 0.0282-0.3826) | ↓ Feelings of helplessness and ↑ pt feelings of control are important ways to limit the effect of low mood on pt’s pain experience. De-catastrophising interventions should be part of the referral strategy for IC sym management |
| Tu et al[66], 2020 | 212 females with moderate-to-severe dysmenorrhoea [166 with dysmenorrhoea (mean age 24.5 ± 0.5 yr) and 46 dysmenorrhoea with bladder sensitivity (mean age 23.8 ± 0.9 yr)], 44 HCs (mean age 23.8 ± 1.0 yr), and 27 BPS pts (mean age 29.0 ± 1.1 yr) aged 18-45 yr | Lo. Prospective cohort study. Medical/menstrual history and pain history were evaluated with questionnaires. Psychosocial profile and impact were measured with PROMIS and a BSI | Participants with dysmenorrhea plus bladder pain had PROMIS Physical T-scores of 47.7 ± 0.9, lower than in females with dysmenorrhea only (52.3 ± 0.5), and healthy controls 56.1 ± 0.7 (P < 0.001). Similar specific impairments were observed on PROMIS for anxiety, depression, and sleep in participants with dysmenorrhea plus bladder pain vs healthy controls | Females with dysmenorrhea who are unaware they also have bladder sensitivity exhibit broad somatic sensitivity and elevated psychological distress |
| McKernan et al[67], 2020 | 27 females with IC/BPS (mean age = 45 ± 16.30 yr) | CS. 27 females with IC/BPS participated in a focus group and completed validated self-report assessments evaluating urinary symptoms, pain emotional functioning and affective vulnerability using PHQ-9 and PROMIS | Pts voiced pervasive and severe emotional distress related to IC/BPS. They acknowledged the reciprocal nature between emotional states and symptomology, with emotional distress both preceding and following symptoms. Both anxiety and depression symptoms were correlated with overall severity of IC/BPS, rPROMIS = 0.48, P = 0.013; rPHQ-9 = 0.68, P < 0.001 | The physiological and emotional consequences of IC/BPS were reported, highlighting their impact on interpersonal relationships and challenges obtaining appropriate treatment for IC/BPS. Dep symptoms appeared to better capture the role of psychological factors better than anx symptoms since quantitative analysis showed dep levels were significantly associated with worsened IC/BPS symptomology |
| Krsmanovic[68], 2020 | 87 females IC/BPS, mean age = 46.3 ± 14.6 (treatment group = 49; controls = 38) | Lo. Case-control study. 49 pts enrolled in the online self-management treatment program+38 controls. Outcome measures divided into primary (physical and mental QoL → SF-12) and secondary outcomes (IC/BPS syms → ICSI/ICPI, pain → VAS, dep → PHQ-9, pain catastrophising → PCS. social support → MSPSS, disability → PDI). Primary outcome completed at BL, mid-study (week 5), endpoint (1-wk post survey/program completion), and during 3-mo fup assessment. Measures on IC/BPS syms and disability completed at BL and endpoint only, pain, dep, pain catastrophising, and social support assessed at all timepoints | Study pts did not obtain statistically significant improvements in physical and mental QoL, dep, pain catastrophising, or social support following study completion | Given the lack of understanding of pathophysiological mechanisms of this condition, and the inadequacy of medical treatment, it is pertinent to develop treatments that can improve pt outcomes |
| Volpe et al[69], 2020 | 2301 females with IC and 4459 females with CPP and OAB (mean age IC group = 53.1 ± 15.5 yr; mean age OAB/CPP group = 52.5 ± 13.6 yr) | CS. Case-control study. Pts were enrolled using the ICD-9 or ICD-10 diagnosis code for IC/BPS. Using ICD-9 and ICD-10 codes they identified comorbidities common in IC/BPS population including history of dep, history of alcohol abuse, history of PTSD | At BL, females with OAB and CPP were more likely to identify as minority (P < 0.001). Anx (57.3% vs 49.5%), dep (39.0% vs 46.0%), and PTSD (29.7 vs 26.4%) were all more common in the CPP and OAB group than in the IC group | A history of depression (P = 0.030) and IBS (P = 0.021) were statistically more prevalent among females with IC/BPS than HC |
| Clemens et al[70], 2020 | A total of 191 male and 233 females with IC/BPS or CPPS | Lo. Prospective cohort study Pts were followed for 12 mo with bimonthly completion of SF-12 to assess general mental and physical HRQOL and with biweekly assessment of condition-specific HRQOL using GPI | Higher levels of BL problems most connected to the domain seemed to be the best predictors of declining outcome on that domain after controlling for initial HRQOL levels. Mental HRQOL outcomes were impacted by being male, BL UCPPS sym(s), widespread pain, non-urologic medical sym(s), and all measured psychosocial variables. Stress, dep, and being male remained independently associated with poorer HRQOL, Dep Score OR 0.907 (0.840–0.980) P = 0.0130, Perceived Stress OR 0.932 (0.894–0.972) P = 0.0010, being male OR 0.580 (0.380–0.885) P = 0.0115 | These findings primarily highlight the impact of psychosocial factors on the HRQOL of UCPPS pts. Clinicians who treat UCPPS should involve mental health care in the management of pts who exhibit syms of dep, stress, or poor coping |
| Lai et al[71], 2021 | 385 females and 193 males with UCPPS. Among them, 12.5% had HL and 87.5% did not | Lo. COPC were assessed using the CMSI. Anx and dep were assessed using HADS. Stress and pain catastrophising were assessed using PSS and CSQ respectively. Quality of life measures included the SF-12 and GUPI | UCPPS without HL also had higher anx (HADS 7.2 vs 4.1), perceived stress (PSS: 15.9 vs 12.5), and pain catastrophising (CSQ: 11.9 vs 8.3) than those with HL, but there was no difference in dep | UCPPS pts without HL were more likely to have a systemic pain syndrome outside the pelvis compared to those with HL associated with more psychosocial syms |
| Crawford et al[72], 2021 | Females’ pts with IC/BPS (T0) → n 226, mean age = 49.29 ± 15.67; (T2) → n 183, mean age = 51.53 ± 15.47; (T3) → n 151, mean age = 53.22 ± 14.82 | Lo. Pts were asked to complete the same set of questionnaires at T0, 6 mo after the initial urology appointment (T2) and 1-yr post-appointment (T3). Those included: Demographics. SF-MPQ, PCS | SF-MPQ score (mean ± SD): T0 = 16.77 ± 11.19; T2 = 14.27 ± 11.32; T3 = 13.11 ± 10.98; PCS score (mean ± SD): T0 = 23.68 ± 14.39; T1 = 19.88 ± 14.36; T3 = 17.96 ± 3.05; early changes in magnification predicted later changes in pain (P < 0.001); early changes in pain predicted later changes in rumination (P = 0.03); early changes in pain predicted later changes in helplessness (P = 0.03); and early changes in helplessness predicted later changes in pain (P = 0.001) | Pain catastrophising should be considered a prime target in psychological treatment for chronic pain in pts with IC/BPS |
| Laden et al[73], 2021 | 872 IC/BPS pts; mean age = 57.1 ± 15.3 yr [355 (41%) male, 517 (59%) females] and 558 non-IC/BPS pts mean age = 53.9 ± 16.2 yr; [291 (52%) male, 267 (48%) females] | CS. Case-control study Pts were identified from random samples of females and male pts with and without an ICD-9/ICD-10 diagnosis of IC/BPS. Presence of comorbidities and psychosocial factors (alcohol abuse, PTSD, sexual trauma, and history of dep) were determined using ICD-9 and ICD-10 codes | The odds of psychosocial factors was higher in the IC/BPS cohort (OR = 1.9; 95%CI: 1.5-2.4; P < 0.001). Notably, the odds of a PTSD diagnosis were higher among IC/BPS pts than non-IC/BPS pts (OR = 2.0; 95%CI: 1.5-2.5; P < 0.001), like Dep History (OR = 2.0; 95%CI: 1.6-2.6; P < 0.001). Health behaviours including alcohol abuse, smoking history, and diabetes were not significantly different between IC/ BPS and non-IC/BPS pts (P = 0.083, P = 0.067, P = 0.626 respectively). females IC/BPS pts had greater odds of psychosocial factors than male IC/BPS pts (OR = 1.9; 95%CI: 1.3-2.8; P < 0.001). The females IC/BPS pts had a significantly higher prevalence of sexual trauma compared to the females non-IC/BPS pts (13% vs 6%, P < 0.05), while none of the male, IC/BPS reported sexual trauma | This study bolsters the existing literature that psychosocial comorbidities are more common among IC/BPS pts and vary by sex |
| Lee et al[74], 2021 | Male = 1.479 (49.3%); females = 1.521 (50.7%), Age: 40 s = 1.037 (34.6%); 50 s = 982 (32.7%); 60 s = 608 (20.3%); 70 s = 373 (12.4%) | Lo. All participants were surveyed using PUF, Patient Symptom Scale and GDS. The primary outcome was the prevalence of BPS-like symptoms, defined as a total PUF score of ≥ 12 | The prevalence of BPS-like symptoms was 16.4% (483 of 3000 participants). females (21.4%) had a significantly > prevalence of BPS-like symptoms than male (10.7%; P < 0.01). The prevalence by age was significantly > in the 70 s group than in the other age groups (P < 0.01), and ↑ significantly with the ↑ severity of dep on the GDS (P < 0.01) | BPS-like symptoms are widespread among the general population of South Korea and can negatively affect many people's QoL |
| Yang et al[75], 2021 | 1103 IC/BPS pts and 4412 non-IC/BPS pts (5515; 4495 females, 1020 male). 81.5% females and 18.5% male, in both IC/BPS group and HC. Age: 22.57% < 35 yr; 30.28% = 35-50 yr; 25.93% = 50-65 yr; 21.21% > 65 yr | CS. Case-control study. The study investigated in the association between SRDs and a subsequent association of IC/BPS using ICD-9 codes | For all SRDs, the significantly increased risks were obtained in 2 yr before IC/BPS diagnosis, and the higher OR was observed within 3 mo before the diagnosis of IC/BPS. dep (OR = 1.54, 95%CI: 1.24-1.91), sleep disorders (OR = 1.45, 95%CI: 1.19-1.78), within 2 yr had a significant risk of IC/BPS. OR for dep [2.04 (1.52 to 2.75)] and sleep disorder [1.59 (1.18 to 2.15)] is even higher when they appeared in the past 3 mo | The study demonstrates that the health care for SRDs within the previous 2 yr is associated with an ↑ risk of subsequent IC/BPS also the study demonstrates that most SRDs are associated with an ↑ risk of subsequent IC/BPS, especially when peptic ulcer, IBS, dep, sleep disorders, and allergic rhinitis appeared in the past 3 mo |
| Tripp et al[76], 2021 | Females IC/BPS pts (n = 813; range 18–80 yr, mean = 46.60 ± 14.10) | CS. This research reports suicide risk prevalence and its biopsychosocial predictors for a community IC/BPS sample. Pts were assessed with the following scales: SHS, PHQ-9, PAS, SBQ-R | Using the adult general population SBQ-R cutoff created an at-risk group (n = 310, M 9.73, SD 2.65) and a not at-risk group (n = 503, M 3.96, SD 1.11), with 38.1% of the sample meeting the suicide risk threshold. In the suicide risk group he predictors of greater risk included a previously reported exposure to suicide (odds ratio OR 2.71, 95%CI: 1.84-4.01), and the greater presence of psychological factors, such as psychache (i.e. psychological pain) (OR 1.04, 95%CI: 1.02-1.07), greater hopelessness (OR 1.12, 95%CI: 1.06-1.17), and more perceptions that the participant was a burden to others (i.e. perceived burdensomeness; OR 1.07, 95%CI: 1.03-1.11). Pts were also classified for pain group and predictors such as exposure to suicide, psychache, hopelessness, and perceived burdensomeness predicted suicide risk in all groups | The results confirm that suicide risk is a significant concern within the IC/BPS population and work is needed to understand how to address the increased needs of the at-risk females. Suicide risk is more related to psychosocial factors than physical IC/BPS factors. In particular, hopelessness, psychache, perceived burdensomeness, and exposure to previous suicide are important predictor |
| Brünahl et al[77], 2021 | 36 pts included in the intervention group [mean age = 48.6 ± 14.8; n = 19 (52.8%) females; n = 17 (47.2%) males] and 24 in the CTRL group [mean age = 50.6 ± 14.5; n = 14 (58.3%) females; n = 10 (41.7%) males] | Lo. Pts were non-randomly allocated to the intervention group with two consecutive treatment modules (physiotherapy and CBT) with a duration of 9 wk each or to the control group (treatment as usual) + Psychometric assessments (BL and post-treatment): GAD-7, PCS. PDI, PHQ-9, PHQ-15, PSQ, SF-12 PCS; SF-12 MCS; SF-MPQ total, SF-MPQ Sen, SF-MPQ aff., NIH-CPSI total Pain subscale, Urinary subscale, QoL subscale | The intervention group reported significantly ↓ symptom burden as measured by the PDI (P = 0.02, d = −0.73), and the PHQ-9 (P = 0.04, d = −0.62), but no significant changes in the SF-12 and others | The combination of physiotherapy and psychotherapy for pts with CPPS seems to be feasible and potentially promising with regard to effect |
| van Knippenberg et al[78], 2022 | 77 pts (46 females, 31 male), 29 with OBS and 48 with UPS (mean age = 54 yr, range 27-78) | CS. Retrospective observational cohort study. The objective of the study is to investigate the effect of integrated outpatient care by a urologist and a psychiatrist on the symptomatology of pts with functional urological disorders. Pts were screened with HADS, OAB-questionnaire and ICSI | An association was found between pelvic pain and anx (P = 0.032) and panic disorders (P = 0.040). OR were 0.22 (0.06–0.76) for anx disorders and 0.26 (0.08–0.87) for panic disorders. An even stronger association was found between these variables in the group of urological pain syndromes (P = 0.001 in both groups). For anx disorders the OR was 0.02 (0.00–0.18) and for panic disorders 0.03 (0.00–0.24). A psychological trauma in the past was associated with a dep disorder (P = 0.044), with an OR of 2.93 (1.01–8.50). Of the pts with a psychological trauma in the past, 62.3% had urological pain syndromes and 83.3% suffered from pelvic pain. After a multidisciplinary intervention the integrated approach led to the following results: o difference is noticed in both groups (P = 0.219) in the HADS-Anx score before and after the multidisciplinary treatment. However, a significant 2-point reduction in the HADS-dep score is found (P = 0.001). The GAF score ↑to the category 71–80, which indicates no more than slight impairment in social, occupational, or school functioning | The study reveals a pre–post comparison before and after multidisciplinary treatment by urologist and psychiatrist. A significant ↓ in HADS-depression scores was observed, and the GAF shows an ↑in functioning |
| Yu et al[79], 2022 | 60 pts with IC/BPS, 55 females, 5 males (mean age = 53.5 ± 12.6 yr) | Lo. Pts with IC/BPS were randomized to the bladder monotherapy (BT) or combined CBT (CBT) group. The primary endpoint was the self-reported outcome GRA. Secondary endpoints included IC symptoms, BAI, and depression inventory, and objective parameters were also compared. Psychological assessments including DS14 PSS-10 were also performed | Post-treatment anxiety according to BAI showed significant improvement at 8 and 12 wk. Between-group changes also showed significant differences in BAI and GRA at 12 wk. The study showed a significant effect on self-reported treatment outcomes [F(2, 108) = 7.161, P = 0.001] and anx severity [F(2, 108) = 3.519, P = 0.033] within the CBT group | This study reveals that multimodal treatment including CBT combined with suitable bladder treatment was more effective than bladder treatment alone. The CBT intervention significantly improved subjective treatment outcomes and severity of anx in pts with IC/BPS with moderate anxiety refractory to conventional therapy |
| Wuestenberghs et al[80], 2022 | 1453 pts with dyspeptic symptoms, of whom 61% with FD. BPS present in 16% of pts without FD, 22.2% of pts with only FD and 36.4% of pts with overlapping FD and IBS. (mean age = 47.4 ± 15.7 yr, sex ratio male/females, = 0.35); 187 females and 53 males with BPS | CS. Functional dyspepsia and IBS were diagnosed according to Rome III and IV criteria. Pts were assessed with GIOLI to assess QoL, HADS for anxiety and depression, PSQI for sleep quality, and ISI for insomnia | In PTS with BPS overlapping with FD, dyspeptic symptoms severity, anxiety, depression, and insomnia levels were ↑, while quality of life and sleep quality were ↓, (P < 0.05 for all). These results were even more pronounced in case of overlap with IBS, Factors independently associated with overlapping BPS in FD pts were altered QoL and overlap with IBS | BPS is present in 26.9% of FD pts and is associated with higher gastrointestinal sym(s), psychological distresses, sleep symptom burdens, and with reduced quality of life. The presence of overlap with BPS or IBS in FD is associated with younger age, increased female predominance, reduced QoL, ↑ symptoms severity, ↑, anx and dep levels |
| Sutherland et al[81], 2023 | 55 females with IC (mean age = 55.05 ± 14.97 yr) | CS. The study focuses on the hypothesis that greater use of compensatory coping behaviours would be significantly associated with greater psychological distress. Compensatory bladder behaviours assessed with the OABq-QoL, anxiety and depression with the PROMIS | The use of coping strategies related to greater symptoms of depression, but not anxiety. Depressive symptoms positively predicted use of compensatory coping, t(52) = 2.33, P = 0.024; while anxiety was not significantly related to compensatory coping, t(52) = 1.310, P = 0.142 | ↑ Use of compensatory coping behaviours related to ↑ dep syms, even after controlling for level of bladder impairment |
| Şahin et al[82], 2023 | 35 BPS pts, (mean age = 50.2 ± 13.32; 24 females and 11 males | Lo. Pts were administered the KHQ, BAI, BDI, OAB-V8, and VAS at each visit. The same questionnaires were completed and compared with pre-pandemic scores to examine the possible clinical aggregation of the pandemic period on BPS pts | Three (8.6%) of our pts had an asymptomatic COVID-19 infection, but no one had an active disease diagnosis. The mean OAB-V8, BAI, BDI, and VAS scores of the pts at their last visits before the pandemic period were 8.54 ± 4.33, 5.66 ± 7.77, 5.37 ± 5.92, and 4.54 ± 2.03, respectively. All scores of these questionnaires ↑ during the pandemic period, but only the OAB-V8 and VAS scores ↑ statistically significantly (P = 0.02 and 0.02, respectively) | BPS pts have been negatively affected by the emotional effects of the COVID-19 pandemic and their BPS symptoms exacerbated |
| Cardaillac et al[83], 2023 | CPP females with a HSS (High Score of sensitisation) → n = 29; mean age = 37 ± 10; CPP females with a LSS (Low Score of sensitisation) → n = 24; mean age = 40 ± 10 | Lo. females with CPP and a HSS (> 5/10; n = 29) vs LSS (< 5/10; n = 24) according to the Convergences PP criteria underwent a non-invasive bladder sensory test, a rectal barostat test, and a muscular and a vulvar sensory test+ poststimulation pain (minutes), QoL (MOS SF-12/SF-36) and psychological state, comprising anx (STAI), dep (BDI-SF), and catastrophising (PCS), were assessed | Pts mostly suffered from endometriosis (35.8%), IBS (35.8%), BPS (32.1%), and vestibulodynia (28.3%). BL characteristics were similar. CPP females with a high sensitization score had more painful diseases diagnosed (2.7 ± 1.3 vs 1.6 ± 0.8; P = 002) and suffered for longer (11 ± 8 vs 6 ± 5 yr; P = 0.028) than pts with a low score. The bladder maximum capacity was equivalent between pts, however, the pain felt at each cystometric threshold was ↑in females with HSS. A longer period was needed for pts with HSS to obtain a VAS < 3/10 after bladder (4.52 ± 5.26 vs 1.27 ± 2.96 minutes; P = 0.01), rectum (3.75 ± 3.81 vs 1.19 ± 1.23 min; P = 0.009), and muscles (1.46 ± 1.69 vs 0.64 ± 0.40 min; P = 0.002) stimulation. The psychological state was equivalent between groups. No association was found between the sensory thresholds and the psychological state results. The physical component of the QoL score was ↓ in females with HSS (P = 0.0005), with no difference in the mental component | There are objective elements to assess for the presence of central sensitization, independently of psychological factors; high- vs low-sensitisation pts did not differ on catastrophising |
| Panisch et al[84], 2023 | 133 females, diagnosis of CPP, aged 18-65 yr (mean age = 60%) | CS. All pts completed a survey assessing symptoms of somatoform dissociation (SDQ-20), PTSD, pelvic pain severity, history of CPP-related surgeries, and mental and physical HRQOL | 17% had SDQ-20 scores ≥ 35 (cutoff). 60% had experienced at least 1 traumatic event and 57% had PC-PTSD-5 scores ≥ 3 (cutoff). Bivariate correlations revealed significant relationships between somatoform dissociation and PTSD symptoms (r = 0.30, P = 0.12) and mental (r = −0.49, P < 0.001) and physical (r = −0.47, P < 0.001) HRQOL. Inverse relationships were also found between PTSD symptoms and mental (r = −0.49, P < 0.001) and physical (r = −0.37, P < 0.001) HRQOL. Mental HRQOL was also correlated with seeking counselling services (r = −0.34, P < 0.001) and physical HRQOL was associated with pelvic pain severity (r = 0.50, P < 0.001) HRQOL. Multiple regression analysis revealed that mental HRQOL was significantly related to symptoms of both somatoform dissociation and PTSD and that physical HRQOL was significantly associated with pelvic pain severity and symptoms of somatoform dissociation. A post-hoc correlation analysis showed that pts with CPP had high correlations between Mental and Physical QOL measures and sensory alterations, more localized pain and functional difficulties related to the genital region and greater generalized analgesia and numbness in relation to the body as a whole | An integrated approach in care protocols for females with IC or CPP that takes into account assessments of trauma exposure and symptoms of somatoform dissociation should be encouraged |
| Porru et al[85], 2023 | 69 female pts, mean age = 49,4; 42 with BPS/IC + 27 with chronic non neoplastic pain | CS. Administered questionnaires included PHQ-9; ICSI-ICPI, BPI (pain short questionnaire), psychological interview; other psychosocial variables | Mean PHQ-9 scores, 10.3 in pts with IC/BPS and 6.9 in CTRL. The main SD in group 0 had a CI: 8.4-12.19, with 95% of pts having a total value in this range. The CI in the second group was 4.7-9.12 (the difference was statistically significant, P < 0.02) | BPI and CI have an important psychological impact; psychosocial factors are involved in the evolution of the clinical picture |
The search conducted on the ClinicalTrials.gov site provided 14 trials. Of them, 9 were interventional and 5 observational (Table 2). Ten were completed, one was recruiting, one was not yet recruiting, and two had an unknown status. None had data to provide or publications related with their data. However, three of them had received publication by the above date (one in a preprint, not peer-reviewed, Table 2).
| Study title | NCT number | Dates | Status | Conditions | Interventions | Sponsor | Responsible | Results | Study type | Ref. |
| Smartphone-based Self-care Education Program for Women with Interstitial Cystitis: Educational Remote IC Aide | NCT05260112 | February 17, 2022 Last, April 1, 2023 | Completed | Cystitis, Interstitial | Other: ERICA | University of Pennsylvania, Philadelphia, PA, United States | Edward Kim | Submitted March 31, 2023, quality control still not concluded | Interventional | None |
| Interstitial Cystitis: Monitoring of the Psychic State and Counseling Intervention in the COVID-19 Era | NCT05752344 | February 28, 2023 | Completed | Cystitis, Interstitial | Other: Counselling | Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy | Marianna Mazza | Not submitted; study conducted from November 1, 2020 to November 1, 2022 | Observational | None |
| Safety and Efficacy of Aloe Vera in the Management of the Symptoms of Interstitial Cystitis | NCT04734106 | February 1, 2021 Last, January 24, 2024 | Not yet recruiting | Interstitial Cystitis Chronic Interstitial Cystitis Bladder Pain Syndrome | Drug: Desert Harvest Aloe Vera Capsules Other: Placebo Capsules | Wake Forest University Health Sciences, Winston-Salem, N.C., United States | Wake Forest University Health Sciences | Not started | Interventional | None |
| Biopsychosocial and Conventional Approach in Bladder Pain Syndrome | NCT05155384 | December 10, 2021 Last, January 3, 2022 | Unknown status | Interstitial Cystitis/Bladder Pain Syndrome, Randomised Control Trial | Other: Pain Neuroscience Education; Relaxation exercises; Cognition target exercise; Pelvic floor stretching exercises; Transcutaneous electrical nerve stimulation | Hacettepe University, Ankara, Turkey | Ceren Gursen | Some results submitted; quality control still not concluded; completion estimated October 15, 2022; no notice since then | Interventional | None |
| Study of Biomarkers and the Relaxation Response Using Guided Imagery in Women With IC | NCT00420550 | September 1, 2007, Last March 21, 2012 | Completed | Interstitial Cystitis Pelvic Pain | Behavioural: Relaxation Response using Guided Imagery, Phase 2 | William Beaumont Hospitals, Royal Oak, MI, United States | Kenneth Peters | No results posted | Interventional | None |
| Quality of Life Analysis in Bladder Pain Syndrome/Interstitial Cystitis | NCT05630742 | November 18, 2022, Last November 30, 2022 | Completed | Interstitial Cystitis, Bladder Pain Syndrome, Quality of Life | Other: chronic non-neoplastic pain | IRCCS Policlinico S. Matteo, Pavia, Italy | Daniele Porru | Results not posted | Observational | Porru et al[75] |
| Identifying Predictors of Treatment Success in Painful Bladder Syndrome | NCT01410461 | August 4, 2011, Last August 5, 2011 | Unknown status | Painful Bladder Syndrome | Device: quantitative sensory testing; Ultrasound testing | Rambam Health Care Campus, Haifa, Israel | Lior Lowenstein, Dalia Kesner | No results posted | Observational | None |
| Bladder Instillations Versus Onabotulinumtoxin A for Treatment of Interstitial Cystitis/Bladder Pain Syndrome | NCT04401176 | May 19, 2020, Last October 12, 2023 | Completed | Interstitial Cystitis Bladder Pain Syndrome | Drug: Heparin & Alkalinized Lidocaine Bladder Instillation; Onabotulinum Toxin A, Phase 2 | Walter Reed National Military Medical Center, Bethesda, Maryland, United States | Eva Kwong Welch | No results posted | Interventional | None |
| Interstitial Cystitis: Elucidation of the Psychophysiologic and Autonomic Characteristics (ICEPAC) Study | NCT01616992 | June 8, 2012, Last February 4, 2015 | Completed | Interstitial Cystitis/Painful Bladder Syndrome Myofascial Pelvic Pain | Drug: Bupivacaine | Case Western Reserve University, Cleveland, Ohio, United States | Thomas C Chelimsky, Medical College of Wisconsin; Jeffrey Janata, University Hosp. Cleveland | Results not posted | Observational | Williams et al[150] |
| Vestibulodynia: Understanding Pathophysiology and Determining Appropriate Treatments | NCT03844412 | February 15, 2019, Last November 29, 2023 | Recruiting | Vestibulodynia Temporomandibular Disorder Fibromyalgia Syndrome | Drug: 5% lidocaine/5 mg/ml 0.02% oestradiol compound cream; Nortriptyline; Placebo cream | Duke University, Durham, North Carolina, United States | Andrea Nackley – Duke, Andrea Rapkin – UCLA, Erin Carey-Elizabeth Geller – Duke | To be completed on December 1, 2024 (estimated) | Interventional | None |
| Translational Research in Pelvic Pain | NCT04001244 | May 16, 2019, Last April 4, 2023 | Completed | Endometriosis Bladder Pain Syndrome/Chronic Pain | Differentiate between two types of pelvic pain condition (endometriosis-associated pain and bladder pain syndrome) | University of Oxford, United Kingdom – IBMC Porto, Portugal – Boston Children's Hospital - Michigan State University – Bayer Grünenthal GmbH – Esteve – Queen Mary University of London – Aalborg University – Endometriosis.org – International Painful Bladder Foundation – Pelvic Pain Support Network – King's College London – Universität Heidelberg – University of Edinburgh – Universität Jena – Universität Münster | Katy Vincent – University of Oxford | No results posted | Observational | Dimitriou et al[149] |
| Life-Stress Interview for Women With Chronic Urogenital Pain Conditions | NCT02286115 | November 5, 2014, Last December 14, 2016 | Completed | Chronic Urogenital Pain | Behavioural: Life-Stress Interview | William Beaumont Hospitals, Royal Oak, MI, United States | Jennifer Carty, Mark A. Lumley – Wayne State University | No results posted | Interventional | Imamura et al[151]; mentioned in Carty et al[152] and Carty[153] |
| Improving Female Sexual Wellness | NCT04824820 | March 29, 2021, Last April 5, 2023 | Completed | Urinary Incontinence, Sexual Dysfunction, Pelvic Organ Prolapse, Pelvic Floor Disorders, Interstitial Cystitis, Female Sexual Dysfunction, Hypoactive Sexual Desire Disorder, Sexuality Orgasmic Disorder, Sexual Desire Disorder | Behavioural: Vibrator | Cedars-Sinai Medical Center, Los Angeles, CA, United States | Karyn Eilber, principal investigator; contact: Alexandra Dubinskaya | No results posted | Interventional | None |
| Safety and Clinical Outcomes Study: SVF Deployment for Orthopedic, Neurologic, Urologic, and Cardio-pulmonary Conditions | NCT01953523 | September 2, 2013, Last September 25, 2018 | Completed | Neurodegenerative Diseases Osteoarthritis, Erectile Dysfunction, Autoimmune Diseases, Cardiomyopathies, Emphysema | Procedure: Administration of autologous adipose derived SVF | Elliot Lander, Rancho Mirage, CA, United States | Mark H Berman | Completed January 1, 2017; No results posted | Interventional | Wyles et al[154] and Khera et al[155] |
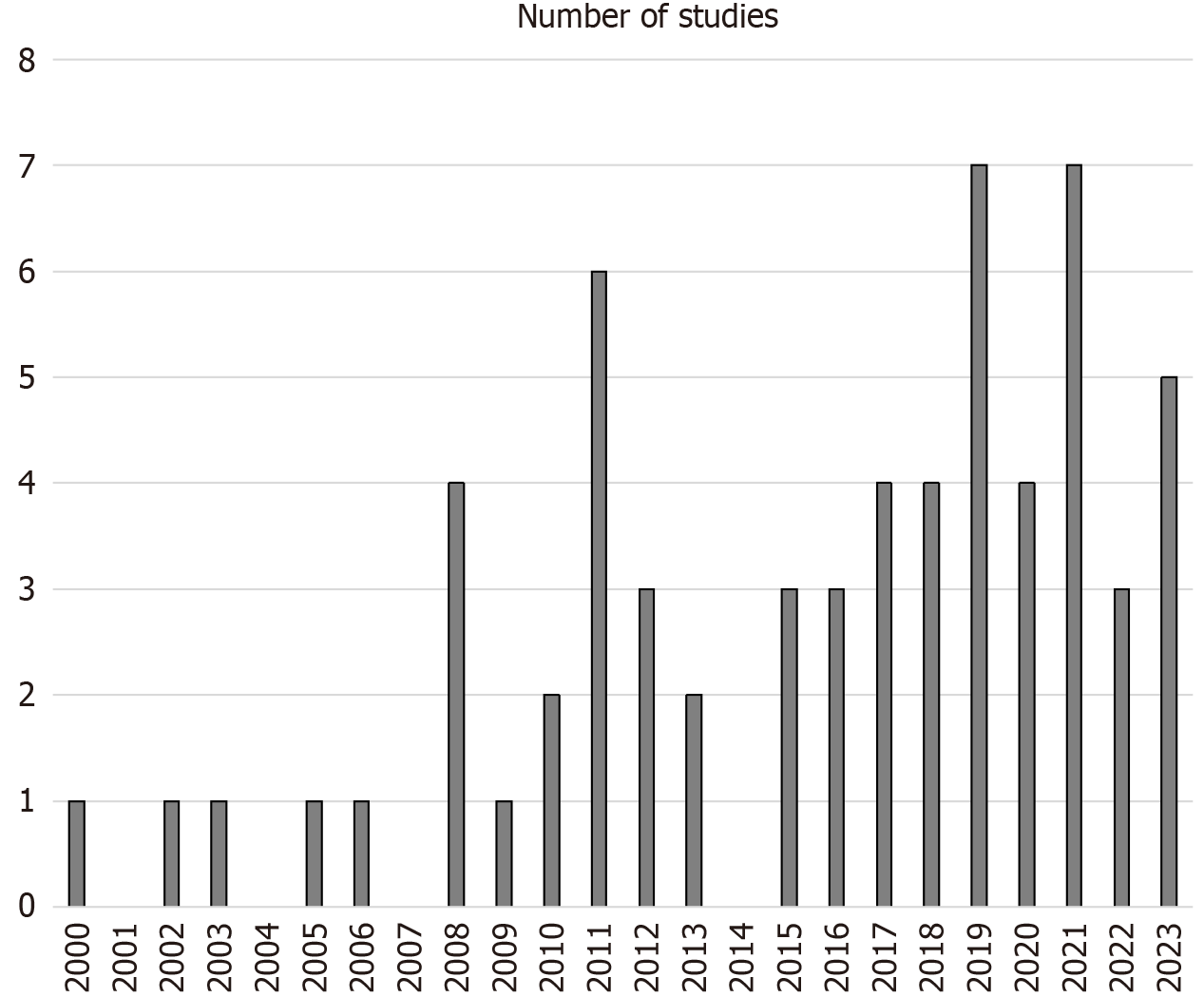
Figure 2 shows the distribution of included studies across time. An increase can be noted from early to recent years, which is not steady, but rather fluctuating. However, the 17-year 2000-2016 period provided 29 studies, less than those of the 7-year 2017-2023 period (n = 34).
Studies were scattered though many sites worldwide, although most of them are concentrated in the United States-North America. Among the 14 ongoing or unpublished studies in the ClinicalTrials.gov database, 9 are in the United States and the United States participates in another multinational study (United Kingdom, Portugal, Germany, and Denmark). The other studies in this database are two Italian, one Turkish, and one Israeli. Among eligible studies, 31 were performed in the United States alone, two were performed conjointly with Canada, three were international studies involving Canada, Denmark, and India, while five were Canadian only studies, eleven Chinese, two French, two South Korean, two Italian, one each German, Turkish, Russian, and Dutch and one was “international” (unspecified where from, but data processed in Philadelphia, PA, United States). Surprisingly, no eligible study was based in Australia-New Zealand, and no study came from Africa, presumably for economic reasons.
Initially, most studies were single-site, prevalently cross-sectional[23-26], and regarded patients seen vis-à-vis; later, from 2008 on, there started international collaborations and multicentre studies[39,40,46,72], and those accessing extensive databases[29,35,36], thus enriching the potential of identifying IC/BPS cases appropriately. Details of the assessment scales used are provided in Table 1.
There were 157 psychological research foci in the 63 eligible studies, i.e. about 2.5 foci per study. These were depression (56 studies), anxiety (31 studies), quality-of-life (QoL, 15 studies), catastrophizing (14 studies), sleep quality (9 studies), alcohol use disorder-related (4 studies, 1 also drug use disorder), perceived stress (4 studies), post-traumatic stress disorder (PTSD, 3 studies), sexual trauma (2 studies), or childhood abuse (2 studies), panic disorder (3 studies), somatoform disorders (3 studies), suicide-related issues (3 studies, one each suicidal ideation, suicidal behaviour, and psychache), general mental health (2 studies), emotional regulation (2 studies), and one study each alexithymia, dissociation, neuroticism, and psychosis.
Of the 56 studies that focused on depression, 22 found definitely increased levels of depression [mostly assessed with the Hospital Anxiety and Depression Scale (HADS)[86]] in patients with IC/BPS, while 5 found associations/correlations between having depressive symptoms and severity of IC/BPS. Longitudinal studies addressing treatment of IC/BPS found either no change in existing depressive symptoms post-treatment[57] or a decrease of depression with treatment[78]. Seven of the studies that investigated anxiety symptoms found a definite increase of these symptoms in the IC/BPS population, while all studies investigating functioning or QoL found them to be both decreased and impaired in this patient population. Of note, all studies addressing catastrophizing but two[46,68], found either high levels to correlate with anxiety and negative affect (depression)[25,44,52] or increased levels in IC/BPS patients compared to control populations[50] and also a beneficial effect of targeted psychotherapies[58,65]. Studies assessing suicidality showed contrasting results. One identified suicidal thinking in all IC/BPS groups[51], another showed that suicidal thinking was prominent in an IC/BPS sample, involving more than 38% of patients (more than the SBQ-R, Suicidal Behaviors Questionnaire-Revised[87] cutoff) and was most often accompanied by perceived burdensomeness, hopelessness and psychache[76], and still another found suicidal ideation to be independent from IC/BPS symptoms or the IC/BPS condition per se, but to be possibly mediated by depression[41]. One study related suicidal ideation to catastrophising in an IC/BPS sample[50]. Similarly, traumatised patients, either with PTSD or sexual or physical abuse, were more prone to develop IC/BPS. Such patients, as expected were found to have emotional distress and/or dysregulation[23,24,37,65,67]. We may speculate that any psychological symptom one wishes to investigate in IC/BPS has a high likelihood to be found altered.
Of the 63 included studies, 36 were carried out on women only, while the other 27 included patients of both sexes. This is a serious bias, since IC/BPS is found in both sexes, although they are more prevalent in women with female-to-male ratios varying from 1.6 in the United States[88] to 4 in China (Taiwan)[89]. Furthermore, it was not possible to calculate the exact number of participants in the various eligible studies, because many of them reported on the same databases through the years with variously overlapping samples (and participating sites). However, adding the figures provided in the studies involving both sexes, we obtained a female-to-male ratio of 1.034 (with total populations of 2041347 and 1974723, respectively), which is not credible, biased toward the male sex and does not match most epidemiological data.
This review showed a high prevalence of IC/BPS in the human female population and a high prevalence of psychological and psychiatric symptoms. There was an imbalance between male and female patients in favour of the latter in the sample taken into consideration in the eligible studies mainly because many studies excluded males. This way it is impossible to generalise on the psychological underpinning of this condition, but we may draw conclusions that may mostly apply to affected women.
IC/BPS is a condition affecting both sexes, although diagnosis is still a problem, and sex-related cultural factors may contribute to underdiagnosis in the male sex[90]. It is estimated that IC/BPS occurs between 2%-18% of the general population[91], with a female-to-male ratio oscillating between 6.5 and 12[92]. Although it is reported that there is an increased incidence of this condition, this may only be an impression[93] and is mainly based on data from some parts of the world where increased interest in searching the term on the internet[92], it is possible that this does not reflect true changes in incidence, but rather changes in diagnostic criteria and awareness (or unawareness)[94-96]. Diagnostic criteria differ among the various countries, so the differences reported on the prevalence of IC/BPS may depend both on how the condition is diagnosed in a specific country and on cultural or ethnicity factors and on when the study was performed. For example, a first study in Finland conducted on 960,000 people identified 95 women and 8 men with IC/BPS in 1975, i.e. a prevalence of 18.1/100000 women of all ages, while for both sexes the prevalence was 10.6/100000[93]. The incidence in this sample was 1.2/100000 women each year[93]. Twenty years later in the Netherlands another study found a prevalence of 8-16/100000 women, thus approximating the figures in the first Finnish study[97]. Meanwhile, in the United States, there were two studies, using different assessment methods and criteria, which found very different prevalence rates; one found a prevalence of 30/100000 in 1987 using mailed surveys of board certified urologists[94], the other reported a prevalence of 510/100000 in 1989 based on participants’ self-reports in 1989[98]. A second Finnish report presented data obtained through self-reports of contacted people in a representative sample and identified a prevalence rate of 450/100000[99], much higher than the preceding study, which was based on medical record examinations[93]. A third Finnish study[100] and an Austrian[101] one administered the O’Leary-Sant IC symptom index and IC problem index[102] in 2005-2007 to women; one found a clinically confirmed probable IC prevalence of 230/100000 and of possible/probable IC of 530/100000[100], while the other found an overall prevalence of 306/100000, which was higher in the middle-aged group (40-49 years of age, who had a prevalence of 464/100000)[101]. Another United States study carried out in 2005 was also based on patient surveys and diagnosed them according to two criteria: pelvic pain for at least 3 months plus urgency or frequency for at least 3 months or the preceding plus pain increasing with bladder filling and/or pain relieved by urination; the prevalence of IC/BPS according to the first criterion was 11.2% in women and 4.6% in men, and according to the second criterion 6.2% in women and 2.3% in men, respecting the “more than twice as many women affected than men” principle with both widened and restricted criteria[103]. A still further United States study conducted in 2007 identified a prevalence of ≥ 197/100000 among women and ≥ 41/100000 among men[104].
Prevalence changes when using relaxed criteria and extend cases to people perceiving pelvic pain. A Boston (Massachusetts) study reported a point prevalence of 2% (1.3% in men and double as much in women) when defining painful bladder syndrome as “pain increasing as the bladder fills and/or pain relieved by urination for at least 3 months”[105]. Using high specificity and high-sensitivity definitions, 2.70% and 6.53% of American women met symptom criteria, respectively[106]. For men, high specificity definition applied to 1.9% of the sample and high sensitivity to 4.2%[107]. In the United States, prevalence of self-reported IC and clinician-rated IC symptoms were 3.7% and 4.4%, respectively, pointing to lack of overreporting of such symptoms among women; however, expanding to women reporting only pelvic pain, the prevalence rose to 17.3%[108]. Studies from Asian countries reported data similar to those of Western countries, i.e. 0.98% in the Fuzhu, Fujian province of China, based on mailed questionnaires[109], and 0.26% in South Korea in 2011, based on a telephone survey with the administration of the O’Leary-Sant IC symptom index and IC problem index[110], but a more recent study in South Korea, conducted 10 years later, based on an online survey and computer-assisted personal interviews and using structured questionnaires, reported an alarmingly high prevalence of 16.4%, with women (21.4%) being affected at a double rate then men (10.7%)[111]. The lack of uniformly adopted criteria and cultural changes may account for the discrepancies observed. It is curious that the annual incidence was always found to be low (or slow if you prefer), but from 1975 to 2008 it went from 1.2/100000[93] to 15/100000 women[112]. How is it possible to have few cases each year and have also high total numbers of a condition? Something must be wrong with epidemiological calculations.
In special settings, as expected, higher proportions of cases meet criteria for IC/BPS. In a United States primary care setting, 13.1% patients scored high enough on the Pelvic Pain and urgency/frequency questionnaire[113] to suggest probable IC; again, women (17.5%) were approximately double than men (8.3%)[114]. In Spain, out of 9312 patients of both sexes attending functional urology and urodynamics units in the first 4 mo of 2014, 5.4% (i.e. n = 503) were diagnosed with IC/BPS, with 90% of them (n = 453) being women[115]. Despite the higher specificity of the Spanish services for capturing cases of IC/BPS, more cases were found in the rather aspecific United States setting. Cultural differences could account for this discrepancy. Furthermore, the male-to-female ratio was smaller in Spain. Summarising, both incidence and prevalence of IC/BPS appear to be rising, but rates are far from being established, since measuring methods have not found consensus.
The question whether IC/BPS triggers psychological problems or it is triggered by already existing ones could be responded to by neuroimaging studies. Some few studies focused on the neuroimaging of IC/BPS. In December 2009, the “Multidisciplinary Approach to the Study of Chronic Pelvic Pain” (MAPP) Research Network was established and focused on various outcomes of female IC/BPS and its male counterpart, chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS)[116]. In 2012 there was a first animal model report[117], and by 2014, the first neuroimaging projects were set[118]. The MAPP network is based on collaboration between various North American university sites and encompasses various research focus areas, including epidemiology/phenotyping, neuroimaging/neurobiology, biomarkers, and urologic chronic pelvic pain syndrome (UCPPS) translational animal models[119,120]. The Network is still validating and fine-tuning its methods[121] and has already identified sources of inter-site variations among its recruiting sites in in voxel-based morphometry (VBM), functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) measures despite using similar apparatuses for neuroimaging[122]. The MAPP Network and other studies have identified neuroimaging correlates of IC/BPS and CP/CPPS, collectively known now as UCPPS, which some are specific to UCPPS and others may extend to other chronic pain syndromes (Table 3)[47,123-140]. These involved both structural and functional neuroimaging, adopting 3 Tesla apparatuses and VBM, DTI, and proton magnetic resonance spectrometry (1H-MRS) and functional connectivity based on fMRI. However, these studies identified only correlates through cross-sectional designs, and longitudinal follow-up studies within the Network did not focus on neuroimaging. There is need that the baseline and follow-up neuroimaging is performed and focused on changes accompanying the variations of the clinical status in order to establish causes and effects.
| Ref. | Population | Technique | Findings |
| Farmer et al[123], 2011 | Male 19 with CP/CPPS vs 16 HCs | fMRI during pain rating task; NMR, VBM; DTI | ↑ FC in right anterior insula correlating with pain intensity; no GM differences between groups, but GM density in anterior insula and ACC correlated with pain intensity and chronicity; the normal correlation between WM FA and neocortex GM volume lost in CP/CPPS Pts |
| Bagarinao et al[124], 2014 | Female 33 with IC/BPS (mean age = 39.51 ± 12.09 yr, 7 with vulvodynia and 8 with TMJD) vs 33 HCs (mean age = 38.95 ± 11.64 yr) | NMR, T1-weighted analysed with SPM8; used SVM algorithm; ROI approach | SVM predicted accurately to which group cases belonged in 73% of cases. SVM identified ↑ GM density areas in bilateral PSC, left preSMA, bilateral hippocampus, left amygdala. Correlation of ↑ cortical GM density with pain, symptom and mood self-ratings |
| Kilpatrick et al[125], 2014 | Female 82 IC/BPS vs 85 HCs | fMRI, RS | Oscillation frequency power at lower frequencies ↑ in IC/BPS vs HCs in postcentral gyrus, anterior paracentral lobule, ventral and medial SMA and ↓ in right posterior insula; ↑ FC in Pts vs HCs between medial SMA and right midbrain, ventral SMA and cerebellum/bilateral midbrain, between anterior paracentral lobule and superior parietal Cx/cerebellum/right midbrain; ↓ FC in Pts vs HCs between right posterior insula and right medial insula |
| Farmer et al[126], 2015 | Female 22 with IC/BPS (mean age = 37.73 ± 12.98 yr; no comorbidity or abnormal pelvis) vs 32 HCs (mean age = 32.84 ± 10.29 yr) | DTI, NMR T1-weighted, voxel-wise analysis | IC/BPS vs HCs ↓ WM density in left cerebellar corticospinal tract, right cerebellar ATR, right IFOF in right frontal orbital Cx, right ILF in lateral occipital Cx, left forceps major adjacent to precuneus, right ATR in right frontal pole, SLF in parietal Cx, and 8) temporal part of left SLF near ACC; ↑ FA in ILF, IFOF at right insula border, forceps major near left ACC, SLF in right postcentral gyrus and left PSC, and corticospinal tract passing through left PSC |
| Kutch et al[127], 2015 | Male 28 with CP/CPPS vs 27 HCs | fMRI, RS | ↓ FC between precentral gyrus/STG/IFG and right posterior insula in CP/CPPS Pts vs HCs |
| Gupta et al[128], 2015 | Female 29 LPVD, 29 HCs, and 29 IBS | fMRI | Intrinsic connectivity LPVD > HCs in SMA, LPVD > IBS in left PMC, left and right SMA, IBS > LPVD in right STC and left and right PSC |
| Martucci et al[129], 2015 | Female 45 UCPPS vs 45 HCs | fMRI, RS | ↓ FC in DMN in PoMeCx (left precuneus and PCC); ↑ FC between PCC and insula, DLPFC, thalamus, pallidum, putamen, amygdala and hippocampus; ↓ FC between left precuneus and OFC, ACC, VMPFC, AG, SPL, IPL |
| Kairys et al[47], 2015 | Female 33 with IC/BPS (mean age = 39.5 ± 12 yr; no comorbidity) vs 33 HCs (mean age = 39 ± 11.6 yr) | VBM, NMR, T1-weighted analysed with SPM8 under MATLAB® 7.6 | ↑ GM volume in IC/BPS vs HCs; SVM identified ↑ GM density areas in bilateral PSC, left preSMA, bilateral hippocampus, left amygdala. Correlation of ↑ cortical GM density with pain, symptom and mood self-ratings |
| Woodworth et al[130], 2015 | 45 UCPPS (19 female IC/BPS, 26 male CP/CPPS; mean age = 40 ± 14 yr) 56 HCs (26 female, 30 male, mean age = 38 ± 13 yr), 39 IBS (23 female, 16 male, mean age = 35 ± 12 yr) | DTI, 3T NMR post-bladder emptying; voxel-wise SPM; Pts were administered McGPQ and PANAS | ↑ MD in UCPPS vs HCs in left/right putamen, left globus pallidus, right posterior coronal radiate, right left forceps minor and GCC, SplCC, right/left sensorimotor integration fibres, right ACC, right external and internal capsules, left posterior SLF, right left ILF, left precentral gyrus association fibres, right prefrontal WM projections; ↑ MD in UCPPS vs IBS in basal ganglia, right periventricular WM, temporal lobe projections, and fibres projecting to right primary motor cortex and association fibres in right hemisphere frontal areas; longer symptom duration correlated with MD and FA in WM clusters differing in MD between UCPPS and HCs |
| Kleinhans et al[131], 2016 | Female 10 twins (5 mono- and 5 dizygotic) discordant for UCPPS (10 UCCPS vs 10 asymptomatic) | fMRI, RS after urination and 50 minutes after drinking 1/2 Lt of water; 3T NMR. ROIs bilateral PAG and amygdala | The symptomatic twins reported more pain at all timepoints and more urgency before scan; ↑ FC in symptomatic twins following water consumption and stable thereafter; in asymptomatic twins, ↑ FC only after bladder distention; ↑ FC in in asymptomatic twins between right PAG and cerebellar/cortical regions involved in sensorimotor planning; ↑ FC between laterobasal amygdala to ACC, insula, somatosensory, premotor Cxs, thalamus and VMPFC |
| Wei et al[132], 2016 | Female 46 PDM vs 49 HCs | fMRI during menstruation and periovulatory phase | PDM, adaptive hyperconnectivity between PAG and SMCx during painful menstruation; maladaptive hypoconnectivity between PAG and DLPFC and DMN during menstruation or periovulatory phase |
| Deutsch et al[133], 2016 | Female 11 with IC/BPS (4 non-comorbid; 5 comorbid with fibromyalgia, 5 vulvodynia, 4 IBS, 3 chronic fatigue) vs 11 HCs | ASL fMRI rCBF at BL, empty bladder and heat pain; 3T NMR | At empty bladder, ↓ rCBF in bilateral insula, middle and PCC in Pts with IC/BPS vs HCs. Bladder distension associated with ↑ rCBF in IC/BPS vs HCs SMA, motor and sensory Cxs, bilateral insula, hippocampus, and middle and PCC in Pts with IC/BPS. During heat pain, ↓ rCBF in IC/BPS Pts vs HCs, who showed ↑ rCBF in bilateral amygdala |
| Huang et al[134], 2016 | 52 UCPPS (23 female, 29 male), 39 IBS (24 female, 15 male), 61 HCs (32 female, 29 male) | 3T NMR, T1-weighted; DTI, FA | ↑ FA in UCPPS in left corticospinal tract, left forceps major, left SLF at precentral gyrus; left superior corona radiata at ACC; forceps major, right IFOF, ILF and cingulum projecting to parahippocampal gyri at precuneous compared to IBS and HCs; ↑ FA in right ATR in IBS compared to HCs; regional changes independent of local grey matter FA and density |
| Kutch et al[135], 2017 | 52 UCPPS (34 female IC/BPS; 18 male CP/CPPS), mean age = 38.8 ± 11.9 yr | fMRI, RS; 3T NMR; clinical reassessment after 3, 6, and 12 mo | Strong BL FC, particularly in the fronto-parietal network, was associated with symptom improvement at 3 mo, but not at 6 mo or 12 mo. FC data predicted accurately the improver/non-improver status at 3 mo for 73.1% of the sample, with 69.2% sensitivity and 75.0% specificity; improver status was not maintained at later timepoints |
| Kutch et al[136], 2017 | 48 female (IC/BPS) 24 male (CP/CPPS) with UCPPS (n = 110) vs 23 female fibromyalgia vs 49 female HCs | fMRI, NMR | Pts with UCPPS and widespread pain show ↑ GM volume in right SMA and cingulate Cx and bilateral SMCx as well as ↑ FC between SMCx and insula with the salience circuit, compared to UCPPS Pts with localised pain and controls |
| Harper et al[137], 2018 | Female 18 UCPPS (mean age = 34.8 ± 11.0 yr) vs 20 HCs (mean age = 34.7 ± 12.3 yr) | 1H-MRS, T1-weighted scans; McGPQ, PANAS, HADS | UCPPS Pts had ↓ GABA and ↑ choline and choline-to-total creatine ratio than HCs in the ACC; the two groups did not differ for other metabolites (glutamate + glutamine; glutamate; N-acetylaspartate; and inositol); ↑ ACC choline level correlated with McGPQ pain, HADS Depression and PANAS negative affect scores |
| Woodworth et al[138], 2018 | 30 UCPPS (13 female IC/BPS; 17 male CP/CPPS); mean age = 39.9 ± 13.5 yr | DTI (FA, ADC), correlations with urinary proteins | ADC in a small WM cluster adjacent to right motor cortex correlated with urinary MMP2; ADC in a WM cluster in DRN and LCC correlated with MMP9 and MMP9/NGAL complex; FA in SMCx-connecting areas correlated with MMP9 as did midbrain areas and left SMCx with MMP9/NGAL complex; large WM clusters’ ADC and FA correlated with NGAL urinary concentrations; no correlations with VEGF |
| Gupta et al[139], 2019 | 85 UCPPS mean age = 39.36 ± 12.80 yr (56 female IC/BPS, mean age = 38.96 ± 12.41 yr; 29 male CP/CPPS; mean age = 40.11 ± 13.71 yr) vs 86 HCs mean age = 37.90 ± 12.23 (59 female mean age = 35.44 ± 10.82 yr; 27 males; mean age = 49.60 ± 13.55 yr) | fMRI, RS; CTES to measure EALEs. Network centrality was measured for each of the main brain networks* (DMN, basal ganglia, sensorimotor, executive control and salience) using graph theory | UCPPS Pts with → ↓ centrality in right anterior insula than HCs (salience network hub). UCPPS male → ↓ centrality in right anterior insula than male HCs. UCPPS female → ↑ centrality in right caudate nucleus and left angular gyrus than female HCs [condition effects]. UCPPS male → ↓ centrality in left PCC (DMN), angular gyrus, middle temporal gyrus, and superior temporal sulcus; UCPPS male → ↑ centrality in precuneus and anterior mid-cingulate Cx than UCPPS female [sex effect]. ↑ reports of EALEs associated with ↑ centrality in left precuneus and left anterior mid-cingulate Cx in UCPPS female. Moderating effect of CTES on condition effect on betweenness centrality of right anterior insula (salience network hub); EALEs moderated centrality in amygdala at high CTES scores |
| Fenske et al[140], 2020 | 100 UCPPS mean age = 39.2 ± 13.3 male/female = 34/66 vs 109 HCs mean age = 36.7 ± 12.2 male/female = 34/75 | fMRI, RS; PAG characterisation as a ROI according to the MNI | ↑ FC in HCs vs Pts in right (pars triangularis) and left IFG (pars opercularis); right PCC, left IFG (pars orbitalis) and left IPL shown when Pts and HCs were compared on the MNI-trace ROI compared to the MNI-sphere ROI |
Another important issue linking chronic pain condition is the chronic pain patient’s tendency to catastrophise it. In our review we found evidence for the importance of pain catastrophising in the psychological symptoms displayed by IC/BPS–CP/CPPS patients[25,44,50,58,65,71,72]. Pain catastrophising, which is the patient’s bleak outlook about the outcome of his/her pain, is also present in other types of pain[141-144], and this has been shown to be related to emotional dysregulation[145]. We found the two constructs to be related also in women with IC/BPS[65]. It would be interesting to understand the neurobiological underpinnings of pain catastrophising and emotional dysregulation and whether altered brain functional connectivity matches the findings of functional connectivity alterations in UCPPS. For example, one hypothesis is that PC and pain perception is associated with reduced or higher neurotrophic factor levels [i.e. lower urinary brain-derived neurotrophic factor (BDNF) and higher nerve growth factor and vascular endothelial growth factor, which in turn would trigger neuroinflammation][16]. It is fascinating to investigate whether psychotherapy focusing on pain catastrophising or life stress, emotional awareness, and emotional expression can reduce perceived pain in IC/BPS patients through BNF-related mechanisms. In fact, some psychotherapies have been reported to increase BDNF levels[146], which probably involves improvement in cognition, even in severely ill patients with psychosis[147]. Further steps would involve looking at which specific psychotherapy or pharmacotherapy obtains desired changes in the neurobiological signature of IC/BPS–CP/CPPS.
Another issue is the effect of integrated, interdisciplinary treatment of IC/BPS compared to single treatments, like pharmacotherapy, psychotherapy or physical therapies. In fact, it was recently shown that combining cognitive behavioural therapy with as-usual bladder treatment was superior to bladder treatment alone[148]. Unfortunately, there is only one such credible study available in the literature at this moment. Further replications of this study will pave the way to appropriate multimodal treatment in IC/BPS–CP/CPPS.
An integrated clinical approach focusing on the patients’ emotional and psychological state and global health and personalized treatments could ensure proper symptom control and promote adequate prevention of comorbidities and future disease-related implications[10]. Thus, it would be possible to cope innovatively with a complex and disabling condition, limiting the dramatic impact on patients' QoL, self-esteem, and social functioning. Future perspectives could involve finding neuroimaging alterations in people with IC/BPS that overlap with those of established alterations in psychiatric conditions which are frequently encountered in IC/BPS. However, to be sure whether alterations precede or follow the development of IC/BPS (the egg or the chicken dilemma), entire populations should be followed with neuroimaging data and look for the clinical evolution of selected people who subsequently develop UCPPSs. Although cross-sectional studies might tell us something about the factors determining IC/BPS, the 0.85 Longitudinal/cross-sectional ratio we found here should surpass the unit in future studies, should we understand the deeper underpinnings of IC/BPS–CP/CPPS. Future research should ascertain the reciprocal causal relationships between psychiatric/psychological factors and IC/BPS–CP/CPPS symptomatology in longitudinal studies, which are the only ones that could enable us to provide a response to the egg or the chicken dilemma. In fact, the simultaneous presence of factors does not legitimize us to establish cause and effect relationships, while factors present at baseline could be related or not related to a future development of a pathological trait.
The authors are grateful to the Scientific Administration of the Bibliographic and Bibliometric Support Service, Fondazione Policlinico A. Gemelli IRCCS, in particular, Dr. Maria Pattuglia, and Ms. Mimma Ariano, Ms. Ales Casciaro, Ms. Teresa Prioreschi, and Ms. Susanna Rospo, Librarians of the Sant’Andrea Hospital, Faculty of Medicine and Psychology, Sapienza University of Rome, for rendering precious bibliographical material accessible.
| 1. | Newsome G. Interstitial cystitis. J Am Acad Nurse Pract. 2003;15:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Osório FL, Carvalho AC, Donadon MF, Moreno AL, Polli-Neto O. Chronic pelvic pain, psychiatric disorders and early emotional traumas: Results of a cross sectional case-control study. World J Psychiatry. 2016;6:339-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Hsieh KL, Chin HY, Lo TS, Long CY, Ho CH, Huang SK, Chuang YC, Wu MP. Interstitial cystitis/bladder pain syndrome patient is associated with subsequent increased risks of outpatient visits and hospitalizations: A population-based study. PLoS One. 2021;16:e0256800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Echols RM, Tosiello RL, Haverstock DC, Tice AD. Demographic, clinical, and treatment parameters influencing the outcome of acute cystitis. Clin Infect Dis. 1999;29:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Grover S, Srivastava A, Lee R, Tewari AK, Te AE. Role of inflammation in bladder function and interstitial cystitis. Ther Adv Urol. 2011;3:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Erickson DR. Interstitial cystitis: update on etiologies and therapeutic options. J Womens Health Gend Based Med. 1999;8:745-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Steers W. Pathogenesis of the overactive bladder and its attendant risk factors. BJU Int. 2000;85 Suppl 3:69; discussion 70-69; discussion 71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Panicker JN, Marcelissen T, von Gontard A, Vrijens D, Abrams P, Wyndaele M. Bladder-bowel interactions: Do we understand pelvic organ cross-sensitization? International Consultation on Incontinence Research Society (ICI-RS) 2018. Neurourol Urodyn. 2019;38 Suppl 5:S25-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | McKernan LC, Walsh CG, Reynolds WS, Crofford LJ, Dmochowski RR, Williams DA. Psychosocial co-morbidities in Interstitial Cystitis/Bladder Pain syndrome (IC/BPS): A systematic review. Neurourol Urodyn. 2018;37:926-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Di Giacomo D, Ranieri J, Nasta L, Moscato S, Guerra F, Passafiume D. Psychological distress in Interstitial Cystitis/Bladder Pain Syndrome: crosssectional study on emotional pattern. Mediterr J Clin Psyc. 2019;7:2. [DOI] [Full Text] |
| 11. | Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. J Pain. 2016;17:T70-T92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 552] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 12. | Kaya S, Hermans L, Willems T, Roussel N, Meeus M. Central sensitization in urogynecological chronic pelvic pain: a systematic literature review. Pain Physician. 2013;16:291-308. [PubMed] |
| 13. | Delgado-Gallén S, Soler MD, Cabello-Toscano M, Abellaneda-Pérez K, Solana-Sánchez J, España-Irla G, Roca-Ventura A, Bartrés-Faz D, Tormos JM, Pascual-Leone A, Cattaneo G. Brain system segregation and pain catastrophizing in chronic pain progression. Front Neurosci. 2023;17:1148176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Quartana PJ, Buenaver LF, Edwards RR, Klick B, Haythornthwaite JA, Smith MT. Pain catastrophizing and salivary cortisol responses to laboratory pain testing in temporomandibular disorder and healthy participants. J Pain. 2010;11:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Chen A, Argoff C, Crosby E, De EJ. Chronic Pelvic Pain Patients Demonstrate Higher Catastrophizing in Association with Pelvic Symptoms and Comorbid Pain Diagnoses. Urology. 2021;150:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Soriano A, Allen A, Malykhina AP, Andy U, Harvie H, Arya L. Relationship of Pain Catastrophizing With Urinary Biomarkers in Women With Bladder Pain Syndrome. Female Pelvic Med Reconstr Surg. 2021;27:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother. 2009;9:745-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1057] [Cited by in RCA: 945] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 18. | Campbell CM, Kronfli T, Buenaver LF, Smith MT, Berna C, Haythornthwaite JA, Edwards RR. Situational versus dispositional measurement of catastrophizing: associations with pain responses in multiple samples. J Pain. 2010;11:443-453.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Lopez SR, Mangır N. Current standard of care in treatment of bladder pain syndrome/interstitial cystitis. Ther Adv Urol. 2021;13:17562872211022478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Xie J, Li X, Lü Y, Huang C, Long X, Liu Y, Lu H, Long J, Chen B, Luo Z, Mo Z. Female chronic posterior urethritis is underestimated in patients with lower urinary tract symptoms. Transl Androl Urol. 2021;10:3456-3464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 21. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 37930] [Article Influence: 9482.5] [Reference Citation Analysis (2)] |
| 22. | Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. London: Cochrane, 2023. |
| 23. | Rabin C, O'Leary A, Neighbors C, Whitmore K. Pain and depression experienced by women with interstitial cystitis. Women Health. 2000;31:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Rothrock NE, Lutgendorf SK, Hoffman A, Kreder KJ. Depressive symptoms and quality of life in patients with interstitial cystitis. J Urol. 2002;167:1763-1767. [PubMed] |
| 25. | Rothrock N, Lutgendorf SK, Kreder KJ. Coping strategies in patients with interstitial cystitis: relationships with quality of life and depression. J Urol. 2003;169:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Novi JM, Jeronis S, Srinivas S, Srinivasan R, Morgan MA, Arya LA. Risk of irritable bowel syndrome and depression in women with interstitial cystitis: a case-control study. J Urol. 2005;174:937-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Wu EQ, Birnbaum H, Mareva M, Parece A, Huang Z, Mallett D, Taitel H. Interstitial Cystitis: Cost, treatment and co-morbidities in an employed population. Pharmacoeconomics. 2006;24:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Fan YH, Lin AT, Wu HM, Hong CJ, Chen KK. Psychological profile of Taiwanese interstitial cystitis patients. Int J Urol. 2008;15:416-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Clemens JQ, Meenan RT, O'Keeffe Rosetti MC, Kimes TA, Calhoun EA. Case-control study of medical comorbidities in women with interstitial cystitis. J Urol. 2008;179:2222-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Clemens JQ, Brown SO, Calhoun EA. Mental health diagnoses in patients with interstitial cystitis/painful bladder syndrome and chronic prostatitis/chronic pelvic pain syndrome: a case/control study. J Urol. 2008;180:1378-1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Goldstein HB, Safaeian P, Garrod K, Finamore PS, Kellogg-Spadt S, Whitmore KE. Depression, abuse and its relationship to interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1683-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Kim JI, Heitkemper MM. Interstitial cystitis/painful bladder syndrome: prevalence estimates, quality of life and depression among older adult Korean women. Korean J Women Health Nurs. 2009;15:381-386. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Tsai CF, Ouyang WC, Tsai SJ, Hong CJ, Lin TL. Risk factors for poor sleep quality among patients with interstitial cystitis in Taiwan. Neurourol Urodyn. 2010;29:568-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Giannantoni A, Cagini R, Del Zingaro M, Proietti S, Quartesan R, Porena M, Piselli M. Botulinum A toxin intravesical injections for painful bladder syndrome: impact upon pain, psychological functioning and Quality of Life. Curr Drug Deliv. 2010;7:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Bogart LM, Suttorp MJ, Elliott MN, Clemens JQ, Berry SH. Prevalence and correlates of sexual dysfunction among women with bladder pain syndrome/interstitial cystitis. Urology. 2011;77:576-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Watkins KE, Eberhart N, Hilton L, Suttorp MJ, Hepner KA, Clemens JQ, Berry SH. Depressive disorders and panic attacks in women with bladder pain syndrome/interstitial cystitis: a population-based sample. Gen Hosp Psychiatry. 2011;33:143-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Peters KM, Killinger KA, Mounayer MH, Boura JA. Are ulcerative and nonulcerative interstitial cystitis/painful bladder syndrome 2 distinct diseases? A study of coexisting conditions. Urology. 2011;78:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Panzera AK, Reishtein J, Shewokis P. Sleep disruption and interstitial cystitis symptoms in women. Urol Nurs. 2011;31:159-165, 172. [PubMed] |
| 40. | Nickel JC, Tripp DA, Pontari M, Moldwin R, Mayer R, Carr LK, Doggweiler R, Yang CC, Mishra N, Nordling J. Childhood sexual trauma in women with interstitial cystitis/bladder pain syndrome: a case control study. Can Urol Assoc J. 2011;5:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Hepner KA, Watkins KE, Elliott MN, Clemens JQ, Hilton LG, Berry SH. Suicidal ideation among patients with bladder pain syndrome/interstitial cystitis. Urology. 2012;80:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Keller JJ, Chen YK, Lin HC. Comorbidities of bladder pain syndrome/interstitial cystitis: a population-based study. BJU Int. 2012;110:E903-E909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Clemens JQ, Elliott MN, Suttorp M, Berry SH. Temporal ordering of interstitial cystitis/bladder pain syndrome and non-bladder conditions. Urology. 2012;80:1227-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Katz L, Tripp DA, Nickel JC, Mayer R, Reimann M, van Ophoven A. Disability in women suffering from interstitial cystitis/bladder pain syndrome. BJU Int. 2013;111:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Keller JJ, Liu SP, Lin HC. Increased risk of depressive disorder following diagnosis with bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. 2013;32:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Nickel JC, Tripp DA; International Interstitial Cystitis Study Group. Clinical and psychological parameters associated with pain pattern phenotypes in women with interstitial cystitis/bladder pain syndrome. J Urol. 2015;193:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Kairys AE, Schmidt-Wilcke T, Puiu T, Ichesco E, Labus JS, Martucci K, Farmer MA, Ness TJ, Deutsch G, Mayer EA, Mackey S, Apkarian AV, Maravilla K, Clauw DJ, Harris RE. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in patients with interstitial cystitis/painful bladder syndrome. J Urol. 2015;193:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 48. | Chuang YC, Weng SF, Hsu YW, Huang CL, Wu MP. Increased risks of healthcare-seeking behaviors of anxiety, depression and insomnia among patients with bladder pain syndrome/interstitial cystitis: a nationwide population-based study. Int Urol Nephrol. 2015;47:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Griffith JW, Stephens-Shields AJ, Hou X, Naliboff BD, Pontari M, Edwards TC, Williams DA, Clemens JQ, Afari N, Tu F, Lloyd RB, Patrick DL, Mullins C, Kusek JW, Sutcliffe S, Hong BA, Lai HH, Krieger JN, Bradley CS, Kim J, Landis JR. Pain and Urinary Symptoms Should Not be Combined into a Single Score: Psychometric Findings from the MAPP Research Network. J Urol. 2016;195:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Tripp DA, Nickel JC, Krsmanovic A, Pontari M, Moldwin R, Mayer R, Carr LK, Yang CC, Nordling J. Depression and catastrophizing predict suicidal ideation in tertiary care patients with interstitial cystitis/bladder pain syndrome. Can Urol Assoc J. 2016;10:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Kanter G, Volpe KA, Dunivan GC, Cichowski SB, Jeppson PC, Rogers RG, Komesu YM. Important role of physicians in addressing psychological aspects of interstitial cystitis/bladder pain syndrome (IC/BPS): a qualitative analysis. Int Urogynecol J. 2017;28:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Abernethy MG, Rosenfeld A, White JR, Mueller MG, Lewicky-Gaupp C, Kenton K. Urinary Microbiome and Cytokine Levels in Women With Interstitial Cystitis. Obstet Gynecol. 2017;129:500-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 53. | Chen IC, Lee MH, Lin HH, Wu SL, Chang KM, Lin HY. Somatoform disorder as a predictor of interstitial cystitis/bladder pain syndrome: Evidence from a nested case-control study and a retrospective cohort study. Medicine (Baltimore). 2017;96:e6304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Chiu CD, Lee MH, Chen WC, Ho HL, Wu HC. Alexithymia and anesthetic bladder capacity in interstitial cystitis/bladder pain syndrome. J Psychosom Res. 2017;100:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Chiu CD, Lee MH, Chen WC, Ho HL, Wu HC. Childhood trauma perpetrated by close others, psychiatric dysfunction, and urological symptoms in patients with interstitial cystitis/bladder pain syndrome. J Psychosom Res. 2017;93:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Hosier GW, Doiron RC, Tolls V, Nickel JC. The X-Y factor: Females and males with urological chronic pelvic pain syndrome present distinct clinical phenotypes. Can Urol Assoc J. 2018;12:E270-E275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Liang CC, Lin YH, Hsieh WC, Huang L. Urinary and psychological outcomes in women with interstitial cystitis/bladder pain syndrome following hyaluronic acid treatment. Taiwan J Obstet Gynecol. 2018;57:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Muere A, Tripp DA, Nickel JC, Kelly KL, Mayer R, Pontari M, Moldwin R, Carr LK, Yang CC, Nordling J. Depression and Coping Behaviors Are Key Factors in Understanding Pain in Interstitial Cystitis/Bladder Pain Syndrome. Pain Manag Nurs. 2018;19:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Van Moh F, Vetter J, Lai HH. Comparison of urologic and non-urologic presentation in interstitial cystitis/bladder pain syndrome patients with and without Hunner lesions. Neurourol Urodyn. 2018;37:2911-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Rodríguez LV, Stephens AJ, Clemens JQ, Buchwald D, Yang C, Lai HH, Krieger JN, Newcomb C, Bradley CS, Naliboff B; MAPP Research Network. Symptom Duration in Patients With Urologic Chronic Pelvic Pain Syndrome is not Associated With Pain Severity, Nonurologic Syndromes and Mental Health Symptoms: A Multidisciplinary Approach to the Study of Chronic Pelvic Pain Network Study. Urology. 2019;124:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Carty JN, Ziadni MS, Holmes HJ, Tomakowsky J, Peters K, Schubiner H, Lumley MA. The Effects of a Life Stress Emotional Awareness and Expression Interview for Women with Chronic Urogenital Pain: A Randomized Controlled Trial. Pain Med. 2019;20:1321-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 62. | Cepeda MS, Reps J, Sena AG, Ochs-Ross R. Risk Factors for Interstitial Cystitis in the General Population and in Individuals With Depression. Int Neurourol J. 2019;23:40-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Thu JHL, Vetter J, Lai HH. The Severity and Distribution of Nonurologic Pain and Urogenital Pain in Overactive Bladder are Intermediate Between Interstitial Cystitis and Controls. Urology. 2019;130:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 64. | Lai HH, Thu JHL, Moh FV, Paradis A, Vetter J. Clustering of Patients with Interstitial Cystitis/Bladder Pain Syndrome and Chronic Prostatitis/Chronic Pelvic Pain Syndrome. J Urol. 2019;202:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Crawford A, Tripp DA, Nickel JC, Carr L, Moldwin R, Katz L, Muere A. Depression and helplessness impact interstitial cystitis/bladder pain syndrome pain over time. Can Urol Assoc J. 2019;13:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Tu FF, Datta A, Atashroo D, Senapati S, Roth G, Clauw DJ, Hellman KM. Clinical profile of comorbid dysmenorrhea and bladder sensitivity: a cross-sectional analysis. Am J Obstet Gynecol. 2020;222:594.e1-594.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | McKernan LC, Bonnet KR, Finn MTM, Williams DA, Bruehl S, Reynolds WS, Clauw D, Dmochowski RR, Schlundt DG, Crofford LJ. Qualitative Analysis of Treatment Needs in Interstitial Cystitis/Bladder Pain Syndrome: Implications for Intervention. Can J Pain. 2020;4:181-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 68. | Krsmanovic A. Online self-management treatment program for women diagnosed with interstitial cystitis/bladder pain syndrome. Ph.D. Thesis, Queen’s University. 2020. Available from: https://qspace.library.queensu.ca/server/api/core/bitstreams/d9df7272-6661-4bb9-9ec6-da557bf98e53/content. |
| 69. | Volpe KA, Cichowski SB, Komesu YK, Rogers RG, Murata A, Murata G. Female Veterans With Diagnoses of Both Chronic Pelvic Pain and Overactive Bladder; How Do They Compare to Women Diagnosed With Interstitial Cystitis? Female Pelvic Med Reconstr Surg. 2020;26:591-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Clemens JQ, Stephens-Shields AJ, Newcomb C, Rodriguez LV, Lai HH, Bradley CS, Naliboff BD, Griffith JW, Taple BJ, Gupta P, Afari N, Harte SE, Strachan E, Guo W, Landis JR. Correlates of 1-Year Change in Quality of Life in Patients with Urologic Chronic Pelvic Pain Syndrome: Findings from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. J Urol. 2020;204:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Lai HH, Newcomb C, Harte S, Appleby D, Ackerman AL, Anger JT, Nickel JC, Gupta P, Rodriguez LV, Landis JR, Clemens JQ; MAPP Research Network. Comparison of deep phenotyping features of UCPPS with and without Hunner lesion: A MAPP-II Research Network Study. Neurourol Urodyn. 2021;40:810-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Crawford A, Muere A, Tripp DA, Nickel JC, Doiron RC, Moldwin R, Katz L. The chicken or the egg: Longitudinal changes in pain and catastrophizing in women with interstitial cystitis/bladder pain syndrome. Can Urol Assoc J. 2021;15:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 73. | Laden BF, Bresee C, De Hoedt A, Dallas KB, Scharfenberg A, Saxena R, Senechal JF, Barbour KE, Kim J, Freedland SJ, Anger JT. Comorbidities in a Nationwide, Heterogenous Population of Veterans with Interstitial Cystitis/Bladder Pain Syndrome. Urology. 2021;156:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 74. | Lee JW, Yoo KH, Choi H. Prevalence of Bladder Pain Syndrome-like Symptoms: a Population-based Study in Korea. J Korean Med Sci. 2021;36:e293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 75. | Yang MH, Huang JY, Chen SL, Wei JC. Association of Interstitial Cystitis/Bladder Pain Syndrome with Stress-Related Diseases: A Nationwide Population-Based Study. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 76. | Tripp DA, Dueck J, Holden RR, Moreau J, Doiron RC, Nickel JC. Biopsychosocial predictors of suicide risk in patients with interstitial cystitis/bladder pain syndrome. Can Urol Assoc J. 2021;15:E630-E636. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 77. | Brünahl CA, Klotz SGR, Dybowski C, Albrecht R, Höink J, Fisch M, Ketels G, Löwe B. Physiotherapy and combined cognitive-behavioural therapy for patients with chronic pelvic pain syndrome: results of a non-randomised controlled feasibility trial. BMJ Open. 2021;11:e053421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 78. | van Knippenberg V, Leue C, Vrijens D, van Koeveringe G. Multidisciplinary treatment for functional urological disorders with psychosomatic comorbidity in a tertiary pelvic care center-A retrospective cohort study. Neurourol Urodyn. 2022;41:1012-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 79. | Yu WR, Jhang JF, Chen BY, Ou SR, Li HM, Kuo HC. Multimodal Treatment with Cognitive Behavioral Therapeutic Intervention Plus Bladder Treatment Is More Effective than Monotherapy for Patients with Interstitial Cystitis/Bladder Pain Syndrome-A Randomized Clinical Trial. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 80. | Wuestenberghs F, Baron M, Melchior C, Desprez C, Cornu JN, Leroi AM, Gourcerol G. Overlaps with bladder pain syndrome and irritable bowel syndrome are associated with higher symptom burden and reduced quality of life in functional dyspepsia. Neurogastroenterol Motil. 2022;34:e14414. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 81. | Sutherland S, Grace Kelly A, Ryden A, Dmochowski RR, Reynolds WS, McKernan LC. Compensatory coping and depression in women with interstitial cystitis/bladder pain syndrome. Neurourol Urodyn. 2023;42:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 82. | Şahin MF, Özcan R, Malak A, Doğan Ç, Yazıcı CM, Özcan M, Akgül M. The effect of the pandemic period on Bladder Pain Syndrome patients under amitriptyline treatment. Neurourol Urodyn. 2023;42:1188-1193. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 83. | Cardaillac C, Levesque A, Riant T, Mortier A, Neunlist M, Perrouin-Verbe MA, Volteau C, Thubert T, Brochard C, Ploteau S. Evaluation of a scoring system for the detection of central sensitization among women with chronic pelvic pain. Am J Obstet Gynecol. 2023;229:530.e1-530.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Panisch LS, Rogers RG, Breen MT, Nutt S, Dahud S, Salazar CA. Dissociation Among Women with Chronic Pelvic Pain: Relation to Surgical Treatment, Pelvic Pain Severity, and Health-Related Quality of Life. J Trauma Dissociation. 2023;24:296-311. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 85. | Porru D, Anselmo D, Magliano A, Scalmati S, DE Silvestri A, Ilardi M, Gardella B, Spinillo A, Politi P, Naspro R. Quality of life analysis in bladder pain syndrome/interstitial cystitis: implications for a multimodal integrated treatment. Minerva Urol Nephrol. 2023;75:634-641. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 86. | Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28548] [Cited by in RCA: 31584] [Article Influence: 752.0] [Reference Citation Analysis (0)] |
| 87. | Osman A, Bagge CL, Gutierrez PM, Konick LC, Kopper BA, Barrios FX. The Suicidal Behaviors Questionnaire-Revised (SBQ-R): validation with clinical and nonclinical samples. Assessment. 2001;8:443-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1176] [Cited by in RCA: 1439] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 88. | Anger JT, Dallas KB, Bresee C, De Hoedt AM, Barbour KE, Hoggatt KJ, Goodman MT, Kim J, Freedland SJ. National prevalence of IC/BPS in women and men utilizing veterans health administration data. Front Pain Res (Lausanne). 2022;3:925834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 89. | Lin HY, Lee MH, Chang KM, Wu HC. Comparison of outpatient reimbursement for interstitial cystitis/bladder pain syndrome and rheumatoid arthritis treatment in Taiwan: A nationwide population-based study. Low Urin Tract Symptoms. 2019;11:O162-O167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 90. | Clemens JQ, Meenan RT, Rosetti MC, Gao SY, Calhoun EA. Prevalence and incidence of interstitial cystitis in a managed care population. J Urol. 2005;173:98-102; discussion 102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 91. | Davis NF, Gnanappiragasam S, Thornhill JA. Interstitial cystitis/painful bladder syndrome: the influence of modern diagnostic criteria on epidemiology and on Internet search activity by the public. Transl Androl Urol. 2015;4:506-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 92. | Davis NF, Brady CM, Creagh T. Interstitial cystitis/painful bladder syndrome: epidemiology, pathophysiology and evidence-based treatment options. Eur J Obstet Gynecol Reprod Biol. 2014;175:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 93. | Oravisto KJ. Epidemiology of interstitial cystitis. Ann Chir Gynaecol Fenn. 1975;64:75-77. [PubMed] |
| 94. | Held PJ, Hanno PM, Wein AJ, Pauly MV, Cahn MA. Epidemiology of Interstitial Cystitis: 2. In: Interstitial Cystitis. Hanno PM, Staskin DR, Krane RJ, Wein AJ, editors. London: Springer. 1990. [DOI] [Full Text] |
| 95. | Ghoniem GM, Khater UM. Interstitial Cystitis-Painful Bladder Syndrome. Pelvic Floor Dysfunction In: Davila GW, Ghoniem GM, Wexner SD, editors. London: Springer. 2008. [DOI] [Full Text] |
| 96. | Cruz F, Pinto R, Mendes PA. Bladder Pain Syndrome: Interstitial Cystitis. Female Genitourinary and Pelvic Floor Reconstruction. In: Martins FE, Holm HV, Sandhu J, McCammon KA, editors. Cham: Springer. 2023. [DOI] [Full Text] |
| 97. | Bade JJ, Rijcken B, Mensink HJ. Interstitial cystitis in The Netherlands: prevalence, diagnostic criteria and therapeutic preferences. J Urol. 1995;154:2035-7; discussion 2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 98. | Jones CA, Nyberg L. Epidemiology of interstitial cystitis. Urology. 1997;49:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 153] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 99. | Leppilahti M, Tammela TL, Huhtala H, Auvinen A. Prevalence of symptoms related to interstitial cystitis in women: a population based study in Finland. J Urol. 2002;168:139-143. [PubMed] |
| 100. | Leppilahti M, Sairanen J, Tammela TL, Aaltomaa S, Lehtoranta K, Auvinen A; Finnish Interstitial Cystitis-Pelvic Pain Syndrome Study Group. Prevalence of clinically confirmed interstitial cystitis in women: a population based study in Finland. J Urol. 2005;174:581-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 101. | Temml C, Wehrberger C, Riedl C, Ponholzer A, Marszalek M, Madersbacher S. Prevalence and correlates for interstitial cystitis symptoms in women participating in a health screening project. Eur Urol. 2007;51:803-8; discussion 809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | O'Leary MP, Sant GR, Fowler FJ Jr, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 506] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 103. | Clemens JQ, Meenan RT, O'Keeffe Rosetti MC, Brown SO, Gao SY, Calhoun EA. Prevalence of interstitial cystitis symptoms in a managed care population. J Urol. 2005;174:576-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 104. | Parsons JK, Kurth K, Sant GR. Epidemiologic issues in interstitial cystitis. Urology. 2007;69:5-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Link CL, Pulliam SJ, Hanno PM, Hall SA, Eggers PW, Kusek JW, McKinlay JB. Prevalence and psychosocial correlates of symptoms suggestive of painful bladder syndrome: results from the Boston area community health survey. J Urol. 2008;180:599-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 106. | Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 401] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 107. | Suskind AM, Berry SH, Ewing BA, Elliott MN, Suttorp MJ, Clemens JQ. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology male study. J Urol. 2013;189:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 108. | Ibrahim IA, Diokno AC, Killinger KA, Carrico DJ, Peters KM. Prevalence of self-reported interstitial cystitis (IC) and interstitial-cystitis-like symptoms among adult women in the community. Int Urol Nephrol. 2007;39:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 109. | Song Y, Zhang W, Xu B, Hao L, Song J. Prevalence and correlates of painful bladder syndrome symptoms in Fuzhou Chinese women. Neurourol Urodyn. 2009;28:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 110. | Choe JH, Son H, Song YS, Kim JC, Lee JZ, Lee KS. Prevalence of painful bladder syndrome/interstitial cystitis-like symptoms in women: a population-based study in Korea. World J Urol. 2011;29:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Lee JW, Han DY, Jeong HJ. Bladder pain syndrome treated with triple therapy with gabapentin, amitriptyline, and a nonsteroidal anti-inflammatory drug. Int Neurourol J. 2010;14:256-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 112. | Patel R, Calhoun EA, Meenan RT, O'Keeffe Rosetti MC, Kimes T, Clemens JQ. Incidence and clinical characteristics of interstitial cystitis in the community. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1093-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 113. | Parsons CL, Dell J, Stanford EJ, Bullen M, Kahn BS, Waxell T, Koziol JA. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 257] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 114. | Rosenberg MT, Page S, Hazzard MA. Prevalence of interstitial cystitis in a primary care setting. Urology. 2007;69:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 115. | Morales-Solchaga G, Zubiaur-Libano C, Peri-Cusí L, Adot-Zurbano JM, Arlandis-Guzmán S, Franco-de Castro A, Castillejo C; Grupo de Investigación de Resultados en Salud en Urología Funcional y Urodinámica (Grupo IFU). Bladder pain syndrome: Prevalence and routine clinical practice in women attending functional urology and urodynamics units in Spain. Actas Urol Esp (Engl Ed). 2019;43:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 116. | University of Pennsylvania. Chronic Pelvic Pain Study of Individuals With Diagnoses or Symptoms of Interstitial Cystitis and/or Chronic Prostatitis (MAPP-EP). [accessed 8 Aug 2010]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/ NCT01098279 ClinicalTrials.gov Identifier: NCT01098279. |
| 117. | Crock LW, Kolber BJ, Morgan CD, Sadler KE, Vogt SK, Bruchas MR, Gereau RW 4th. Central amygdala metabotropic glutamate receptor 5 in the modulation of visceral pain. J Neurosci. 2012;32:14217-14226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 118. | Clemens JQ, Mullins C, Kusek JW, Kirkali Z, Mayer EA, Rodríguez LV, Klumpp DJ, Schaeffer AJ, Kreder KJ, Buchwald D, Andriole GL, Lucia MS, Landis JR, Clauw DJ; MAPP Research Network Study Group. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol. 2014;14:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 119. | Landis JR, Williams DA, Lucia MS, Clauw DJ, Naliboff BD, Robinson NA, van Bokhoven A, Sutcliffe S, Schaeffer AJ, Rodriguez LV, Mayer EA, Lai HH, Krieger JN, Kreder KJ, Afari N, Andriole GL, Bradley CS, Griffith JW, Klumpp DJ, Hong BA, Lutgendorf SK, Buchwald D, Yang CC, Mackey S, Pontari MA, Hanno P, Kusek JW, Mullins C, Clemens JQ; MAPP Research Network Study Group. The MAPP research network: design, patient characterization and operations. BMC Urol. 2014;14:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 120. | Clemens JQ, Kutch JJ, Mayer EA, Naliboff BD, Rodriguez LV, Klumpp DJ, Schaeffer AJ, Kreder KJ, Clauw DJ, Harte SE, Schrepf AD, Williams DA, Andriole GL, Lai HH, Buchwald D, Lucia MS, van Bokhoven A, Mackey S, Moldwin RM, Pontari MA, Stephens-Shields AJ, Mullins C, Landis JR. The Multidisciplinary Approach to The Study of Chronic Pelvic Pain (MAPP) Research Network*: Design and implementation of the Symptom Patterns Study (SPS). Neurourol Urodyn. 2020;39:1803-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 121. | Schrepf AD, Mawla I, Naliboff BD, Gallop B, Moldwin RM, Tu F, Gupta P, Harte S, Krieger JN, Yang C, Bradley C, Rodriguez L, Williams D, Magnotta V, Ichesco E, Harris RE, Clemens Q, Mullins C, Kutch JJ. Neurobiology and long-term impact of bladder-filling pain in humans: a Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) research network study. Pain. 2023;164:2343-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 122. | Alger JR, Ellingson BM, Ashe-McNalley C, Woodworth DC, Labus JS, Farmer M, Huang L, Apkarian AV, Johnson KA, Mackey SC, Ness TJ, Deutsch G, Harris RE, Clauw DJ, Glover GH, Parrish TB, Hollander Jd, Kusek JW, Mullins C, Mayer EA; MAPP Research Network Investigators. Multisite, multimodal neuroimaging of chronic urological pelvic pain: Methodology of the MAPP Research Network. Neuroimage Clin. 2016;12:65-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 123. | Farmer MA, Chanda ML, Parks EL, Baliki MN, Apkarian AV, Schaeffer AJ. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2011;186:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 124. | Bagarinao E, Johnson KA, Martucci KT, Ichesco E, Farmer MA, Labus J, Ness TJ, Harris R, Deutsch G, Apkarian VA, Mayer EA, Clauw DJ, Mackey S. Preliminary structural MRI based brain classification of chronic pelvic pain: A MAPP network study. Pain. 2014;155:2502-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 125. | Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff BD, Labus JS, Jiang Z, Farmer MA, Apkarian AV, Mackey S, Martucci KT, Clauw DJ, Harris RE, Deutsch G, Ness TJ, Yang CC, Maravilla K, Mullins C, Mayer EA. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol. 2014;192:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 126. | Farmer MA, Huang L, Martucci K, Yang CC, Maravilla KR, Harris RE, Clauw DJ, Mackey S, Ellingson BM, Mayer EA, Schaeffer AJ, Apkarian AV; MAPP Research Network. Brain White Matter Abnormalities in Female Interstitial Cystitis/Bladder Pain Syndrome: A MAPP Network Neuroimaging Study. J Urol. 2015;194:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 127. | Kutch JJ, Yani MS, Asavasopon S, Kirages DJ, Rana M, Cosand L, Labus JS, Kilpatrick LA, Ashe-McNalley C, Farmer MA, Johnson KA, Ness TJ, Deutsch G, Harris RE, Apkarian AV, Clauw DJ, Mackey SC, Mullins C, Mayer EA. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP: Research Network Neuroimaging Study. Neuroimage Clin. 2015;8:493-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 128. | Gupta A, Rapkin AJ, Gill Z, Kilpatrick L, Fling C, Stains J, Masghati S, Tillisch K, Mayer EA, Labus JS. Disease-related differences in resting-state networks: a comparison between localized provoked vulvodynia, irritable bowel syndrome, and healthy control subjects. Pain. 2015;156:809-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 129. | Martucci KT, Shirer WR, Bagarinao E, Johnson KA, Farmer MA, Labus JS, Apkarian AV, Deutsch G, Harris RE, Mayer EA, Clauw DJ, Greicius MD, Mackey SC. The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network-a resting-state study from the MAPP Research Network. Pain. 2015;156:1755-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 130. | Woodworth D, Mayer E, Leu K, Ashe-McNalley C, Naliboff BD, Labus JS, Tillisch K, Kutch JJ, Farmer MA, Apkarian AV, Johnson KA, Mackey SC, Ness TJ, Landis JR, Deutsch G, Harris RE, Clauw DJ, Mullins C, Ellingson BM; MAPP Research Network. Unique Microstructural Changes in the Brain Associated with Urological Chronic Pelvic Pain Syndrome (UCPPS) Revealed by Diffusion Tensor MRI, Super-Resolution Track Density Imaging, and Statistical Parameter Mapping: A MAPP Network Neuroimaging Study. PLoS One. 2015;10:e0140250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 131. | Kleinhans NM, Yang CC, Strachan ED, Buchwald DS, Maravilla KR. Alterations in Connectivity on Functional Magnetic Resonance Imaging with Provocation of Lower Urinary Tract Symptoms: A MAPP Research Network Feasibility Study of Urological Chronic Pelvic Pain Syndromes. J Urol. 2016;195:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 132. | Wei SY, Chao HT, Tu CH, Li WC, Low I, Chuang CY, Chen LF, Hsieh JC. Changes in functional connectivity of pain modulatory systems in women with primary dysmenorrhea. Pain. 2016;157:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 133. | Deutsch G, Deshpande H, Frölich MA, Lai HH, Ness TJ. Bladder Distension Increases Blood Flow in Pain Related Brain Structures in Subjects with Interstitial Cystitis. J Urol. 2016;196:902-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 134. | Huang L, Kutch JJ, Ellingson BM, Martucci KT, Harris RE, Clauw DJ, Mackey S, Mayer EA, Schaeffer AJ, Apkarian AV, Farmer MA; MAPP Research Network. Brain white matter changes associated with urological chronic pelvic pain syndrome: multisite neuroimaging from a MAPP case-control study. Pain. 2016;157:2782-2791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 135. | Kutch JJ, Labus JS, Harris RE, Martucci KT, Farmer MA, Fenske S, Fling C, Ichesco E, Peltier S, Petre B, Guo W, Hou X, Stephens AJ, Mullins C, Clauw DJ, Mackey SC, Apkarian AV, Landis JR, Mayer EA; MAPP Research Network. Resting-state functional connectivity predicts longitudinal pain symptom change in urologic chronic pelvic pain syndrome: a MAPP network study. Pain. 2017;158:1069-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 136. | Kutch JJ, Ichesco E, Hampson JP, Labus JS, Farmer MA, Martucci KT, Ness TJ, Deutsch G, Apkarian AV, Mackey SC, Klumpp DJ, Schaeffer AJ, Rodriguez LV, Kreder KJ, Buchwald D, Andriole GL, Lai HH, Mullins C, Kusek JW, Landis JR, Mayer EA, Clemens JQ, Clauw DJ, Harris RE; MAPP Research Network. Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain. 2017;158:1979-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 137. | Harper DE, Ichesco E, Schrepf A, Halvorson M, Puiu T, Clauw DJ, Harris RE, Harte SE; MAPP Research Network. Relationships between brain metabolite levels, functional connectivity, and negative mood in urologic chronic pelvic pain syndrome patients compared to controls: A MAPP research network study. Neuroimage Clin. 2018;17:570-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 138. | Woodworth DC, Dagher A, Curatolo A, Sachdev M, Ashe-McNalley C, Naliboff BD, Labus JS, Landis JR, Kutch JJ, Mayer EA, Lee RS, Moses MA, Ellingson BM; MAPP Research Network. Changes in brain white matter structure are associated with urine proteins in urologic chronic pelvic pain syndrome (UCPPS): A MAPP Network study. PLoS One. 2018;13:e0206807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 139. | Gupta A, Bhatt RR, Naliboff BD, Kutch JJ, Labus JS, Vora PP, Alaverdyan M, Schrepf A, Lutgendorf S, Mayer EA; MAPP Research Network. Impact of early adverse life events and sex on functional brain networks in patients with urological chronic pelvic pain syndrome (UCPPS): A MAPP Research Network study. PLoS One. 2019;14:e0217610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 140. | Fenske SJ, Bierer D, Chelimsky G, Conant L, Ustine C, Yan K, Chelimsky T, Kutch JJ. Sensitivity of functional connectivity to periaqueductal gray localization, with implications for identifying disease-related changes in chronic visceral pain: A MAPP Research Network neuroimaging study. Neuroimage Clin. 2020;28:102443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 141. | Nasir M, Scott EJ, Westermann RC. Pain Catastrophizing, Kinesiophobia, Stress, Depression, and Poor Resiliency Are Associated With Pain and Dysfunction in the Hip Preservation Population. Iowa Orthop J. 2023;43:125-132. [PubMed] |
| 142. | Stragapede E, Huber JD, Corsini-Munt S. My Catastrophizing and Your Catastrophizing: Dyadic Associations of Pain Catastrophizing and the Physical, Psychological, and Relational Well-being of Persons With Endometriosis and Their Partners. Clin J Pain. 2024;40:221-229. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 143. | Whitaker MM, Odell D, Deboeck PR, Stefanucci JK, Okifuji A. Increased Pain Variability in Patients With Chronic Pain: A Role for Pain Catastrophizing. J Pain. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 144. | Fang Y, Liu M, Wu M, Liu M, Niu T, Zhang X. Pain intensity and self-perceived burden mediate the relationship between family functioning and pain catastrophizing in patients with neuropathic pain. Nurs Health Sci. 2024;26:e13097. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 145. | Yuan Y, Schreiber K, Flowers KM, Edwards R, Azizoddin D, Ashcraft L, Newhill CE, Hruschak V. The relationship among emotion regulation and pain catastrophizing in chronic pain patients. Pain Med. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 146. | Piotrkowicz M, Janoska-Jazdzik M, Koweszko T, Szulc A. The Influence of Psychotherapy on Peripheral Brain-Derived Neurotrophic Factor Concentration Levels and Gene Methylation Status: A Systematic Review. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 147. | Farcas A, Knopik L, Piccolotto C, Iftene F. BDNF changes as a result of non-pharmacological interventions in schizophrenia – A scoping review. Psychiatry Res Commun. 2023;3:100127. [DOI] [Full Text] |
| 148. | Burgio KL, Kraus SR, Johnson TM 2nd, Markland AD, Vaughan CP, Li P, Redden DT, Goode PS. Effectiveness of Combined Behavioral and Drug Therapy for Overactive Bladder Symptoms in Men: A Randomized Clinical Trial. JAMA Intern Med. 2020;180:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 149. | Demetriou P, Krassowski M, Mendes PA, Garbutt K, Vitonis AF, Wilkins E, Coxon L, Arendt-Nielsen L, Aziz Q, Birch J, Horne AW, Hoffman A, Hummelshoj L, Lunde CE, Meijlink J, Perro D, Rahmioglu N, Terry KL, Pogatzki-Zahn E, Sieberg CB, Treede RD, Becker CM, Cruz F, Missmer SA, Zondervan KT, Nagel J, Vincent K. Clinical profiling of specific diagnostic subgroups of women with chronic pelvic pain. 2022 Preprint . [DOI] [Full Text] |
| 150. | Williams DP, Chelimsky G, McCabe NP, Koenig J, Singh P, Janata J, Thayer JF, Buffington CA, Chelimsky T. Effects of Chronic Pelvic Pain on Heart Rate Variability in Women. J Urol. 2015;194:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 151. | Imamura M, Scott NW, Wallace SA, Ogah JA, Ford AA, Dubos YA, Brazzelli M. Interventions for treating people with symptoms of bladder pain syndrome: a network meta-analysis. Cochrane Database Syst Rev. 2020;7:CD013325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 152. | Carty J, Ziadni M, Lumley M, Holmes H, Tomakowsky J, Schubiner H, Dove-Medows E, Peters Kenneth M. The effects of a stress and emotion interview for women with urogenital pain: A randomized trial. Society of Urodynamics, Female Pelvic Medicine& Urogenital Reconstruction2017 Winter Meeting February 28–March 4, 2017; Hyatt Regency Scottsdale Resort & Spa at Gainey Ranch Scottsdale, Arizona. Neurourol Urodyn. 2017;36 Suppl 1:S52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 153. | Carty J. The Effects of a Life-Stress Interview For Women With Chronic Urogenital Pain: A Randomized Trial. D.Sc. Thesis, Wayne State University Dissertations. 2016. Available from: https://digitalcommons.wayne.edu/cgi/viewcontent.cgi?article=2520&context=oa_dissertations. |
| 154. | Wyles CC, Houdek MT, Behfar A, Sierra RJ. Mesenchymal stem cell therapy for osteoarthritis: current perspectives. Stem Cells Cloning. 2015;8:117-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 155. | Khera M, Albersen M, Mulhall JP. Mesenchymal stem cell therapy for the treatment of erectile dysfunction. J Sex Med. 2015;12:1105-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |