Published online Jun 19, 2024. doi: 10.5498/wjp.v14.i6.767
Revised: May 5, 2024
Accepted: May 23, 2024
Published online: June 19, 2024
Processing time: 165 Days and 11.9 Hours
Although significant advances have been made in understanding the patho-physiology of psychiatric disorders (PDs), therapeutic advances have not been very convincing. While psychotropic medications can reduce classical symptoms in patients with PDs, their long-term use has been reported to induce or exagge
Core Tip: Psychotropic medications are the first line of treatment for psychiatric disorders; however, their long-term use has been shown to induce various metabolic abnormalities including diabetes, obesity, and fatty liver disease. Although mechanism(s) underlying these metabolic abnormalities is not clear, lipid/fatty acid accumulation caused by enhanced de novo lipogenesis (DNL) could be the primary mediator. In this regard, various anti-inflammatory drugs that are used in combination therapy, have been shown to reduce DNL and the aforementioned metabolic abnormalities in laboratory animals. This suggests that DNL could be a potential pathological and therapeutic target, and a new avenue for translational research in psychiatric disorders.
- Citation: Khan MM, Khan ZA, Khan MA. Metabolic complications of psychotropic medications in psychiatric disorders: Emerging role of de novo lipogenesis and therapeutic consideration. World J Psychiatry 2024; 14(6): 767-783
- URL: https://www.wjgnet.com/2220-3206/full/v14/i6/767.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i6.767
Although a great success has been made in understanding the pathophysiology of psychiatric disorders (PDs), therapeutic advances have not been very convincing[1-4]. While psychotropic medications including antipsychotic drugs (APs) and antidepressants (ADs) can reduce classical symptoms in patients with PDs, their long-term use has been reported to induce the development or exacerbate various pre-existing metabolic abnormalities including insulin resistance, adiposity/obesity and non-alcoholic fatty liver disease (NAFLD)[5-8]. Evidence suggests that oxidative stress and inflammation could be the major risk factors associated with various metabolic abnormalities in PDs; however, the underlying mechanisms remain(s) unclear[8-12]. Finding the underlying mechanism(s) could play a crucial role in developing effective therapies/drugs for minimizing the development of various metabolic abnormalities and improving treatment outcome and the quality of life in patients with PDs.
Over the years several mechanisms have been sown to induce oxidative stress and inflammation and associated metabolic abnormalities, they are triggered initially by the accumulation of intracellular fatty acids synthesized via de novo pathway/de novo lipogenesis (DNL)[12-19]. Although DNL produces both saturated fatty acids (SAFs) and monounsaturated fatty acids (MUFAs), evidence suggest that effect of SAFs on metabolic abnormalities could be detrimental as they increase oxidative stress and inflammation by disrupting calcium homeostasis, endoplasmic reticulum (ER) and mitochondrial function, whereas, MUFAs can induce metabolic abnormalities, specially, insulin resistance even without increasing oxidative stress and inflammation[17-21]. Since both APs and ADs have been shown to induce/deteriorate insulin resistance and other metabolic abnormalities, their effects could be most likely mediated via enhanced DNL.
In this review, several emerging evidence are discussed, which suggest that lipid/fatty acid accumulation caused by enhanced DNL could be the primary mechanism associated with the development of obesity, diabetes, and NAFLD during long term treatment with psychotropic medication in patients with PDs. In support of this, outcome of preliminary clinical trial studies and prospects of various adjunctive drugs/anti-inflammatory agents in reducing the development of the aforementioned metabolic abnormalities in patients with schizophrenia and depression are discussed. References cited in this review article were searched using PubMed, Scopus and Google. Only indexed articles published in English within the last five years were included. Articles published in French or German were considered only when necessary. Older articles were considered only when deemed necessary.
Psychotropic medications are synthetics drugs/agents used in treating a wide variety of PDs including schizophrenia psychosis, depression, bipolar disorder, mood disorder, anxiety, attention deficit hyperactivity disorder and others[22]. The most common psychotropic medications are APs, ADs, mood stabilizers, and anxiolytics or anti-anxiety drugs. However, in this review we have focused mainly on APs and ADs, their receptor binding profiles and mechanism of action are shown in Table 1.
| Antipsychotic drugs | Main mechanism of action[23-25] | Main mechanism of action[28,29] | Weight gain[8,26,27] | Weight gain[31,32] |
| Typical APs | ||||
| Chloropromazine | Blocks post-synaptic dopamine D2 receptors in the brain | +++ | ||
| Haloperidol | Blocks post-synaptic dopamine D2 receptors in the brain | + | ||
| Thiothixene | Blocks post-synaptic dopamine D1, D2, D3, D4 receptors in the brain | +++ | ||
| Fluphenazine | Blocks post-synaptic dopamine D1 and D2 receptors in the brain | + | ||
| Atypical APs | ||||
| Clozapine | Blocks dopamine D2 and 5HT serotonin receptors in the brain | +++ | ||
| Olanzapine | Blocks dopamine D1, D2, D3, D4 receptors, and serotonin 5HT2A, 5HT2C, 5HT3 and 5HT6, the alpha-1 adrenergic receptor | +++ | ||
| Quetiapine | Blocks dopamine D2 and serotonin 5HT2A receptors | +++ | ||
| Ziprasidone | Blocks dopamine D2 and serotonin 5HT2A receptors | -/+ | ||
| Risperidone | Blocks dopamine D2 and serotonin 5HT2A receptors | ++ | ||
| Aripiprazole | Partially agonizes dopamine D2, 5-HT1A receptors, blocks serotonin 5HT2A receptors | + | ||
| Paliperidone | Blocks dopamine D2 and serotonin 5HT2A receptors | + | ||
| Zotepine | Blocks dopamine D1, D2 and serotonin 5HT2A, 5HT2C, 5HT6 receptors | +++ | ||
| Sertindole | Blocks dopamine D2 and serotonin 5HT2A, 5HT2C alpha-1 adrenergic receptor | + | ||
| Amisulpride | Blocks dopamine D2 and D3 receptors | + | ||
| Antidepressants | ||||
| SSRIs | ||||
| Sertraline | Increase serotonin 5HT level by blocking reuptake at presynaptic terminals | ++ | ||
| Fluoxetine | ||||
| Excitalopram | ||||
| Trazodone | ||||
| Citalopram | ||||
| Paroxetine | ||||
| SNRIs | ||||
| Duloxetine | Block serotonin and norepinephrine reuptake in the synapse, increase postsynaptic receptors’ stimulation | ++ | ||
| Venlafaxine | ||||
| Levomilnacipran | ||||
| Atypical ADs | ||||
| Bupropion | Inhibits reuptake of dopamine and norepinephrine at the presynaptic cleft by binding to norepinephrine transporter and dopamine transporter | + | ||
| Mirtazapine | Increases release of norepinephrine into the synapse by blocking alpha-2 adrenergic receptors. Also antagonizes 5-HT receptor, increasing norepinephrine and dopamine | ++ | ||
| Viladozone | Enhances the release of serotonin across the brain’s serotonergic pathways specifically by inhibiting the serotonin transporter | |||
| Tricyclic ADs | ||||
| Imipramine | Increase norepinephrine and serotonin concentration by inhibiting reuptake at the presynaptic neuronal membrane | +++ | ||
| Nortriptyline | ||||
| Amitriptyline | ||||
| Doxepin | ||||
| MAOIs | ||||
| Phenelzine | Increase the levels of norepinephrine, epinephrine, serotonin, and dopamine by blocking reuptake of catecholamines and serotonin at the presynaptic neuronal membrane | ++ | ||
| Isocarboxazie | ||||
| Tranylcypromine |
APs are the first line of treatment for schizophrenia and related psychiatric conditions. They are broadly classified into two categories; first generation or typical APs and second generation or atypical Aps[23,24]. Regarding the mechanism of action, first-generation APs are designed to block dopaminergic neurotransmission, and it has been suggested that their effectiveness is optimum when they block about 72%-75% of the dopamine-2 (D2) receptors in the brain. In addition to D2 receptor blocking, first generation APs have been found to also block noradrenergic, cholinergic, and histamine receptors. On the other hand, second-generation APs work by blocking D2 receptors as well as serotonin (5-hydroxytryptamine) receptor. Among the various serotonin receptors, 5-HT2A subtype of serotonin receptor is most commonly involved in the action of second-generation APs[23-25].
Although APs effectively reduce psychotic symptoms but, when used for extended duration, they can induce various adverse effects including sedation or dry mouth, constipation, akathisia, sexual dysfunction, acute dystonia, tardive dyskinesia, myocarditis, agranulocytosis and weight gain. Some adverse effects of APs such as hyperprolactinemia and dyslipidemia may involve long-term risk of medical complications. Although compared to the typical APs, atypical APs have been found to have the lowest propensity to cause extrapyramidal symptoms but they have highest propensity for causing weight gain and metabolic syndrome[8,26,27].
ADs are used for treating depression and major depressive disorders[22]. Over the years numerous ADs have been developed and approved by Food and Drug Administration for treating children, adults and geriatric patients with depression/major depression and various related conditions[28,29]. ADs are classified into the following groups: Selective serotonin re-uptake inhibitors (SSRIs), selective serotonin and norepinephrine re-uptake inhibitors (SNRIs), tricyclic ADs (TCAs), monoamine oxidase inhibitors and atypical ADs[28,29]. Evidence suggests that overall outcome and tolerance profile is better with the more recent ADs (SSRIs, SNRIs) than with the older agents (TCAs). Receptor binding profiles and mechanisms of action of various ADs are shown in Table 1.
Although ADs can effectively reduce symptoms of depression, their long-term use, like APs, has been shown to induce various side effects including sexual dysfunction, gastrointestinal problems, sleep disturbance, apathy, fatigue/drowsiness, insomnia, tremor, apathy and weight gain[30]. A recent meta-analysis has reported that weight gain was more prevalent in patients who received long-term treatment with TCAs[31,32]. We have discussed later the role of DNL and the mechanism associated with weight gain and other metabolic abnormalities induced by long-term treatment with both APs and ADs.
Lipogenesis is a term used for lipid synthesis from fatty acids obtained either from the diet or synthesized de novo from glucose inside the cells. One the other hand, the term DNL is used for lipid synthesis from fatty acids, which are synthesized exclusively by de novo pathway from glucose. Excess glucose obtained from the diet or synthesized from intermediary metabolites including citrate, lactate, pyruvate, glutamate, glutamine, and glycerol can be converted into glucose and used in DNL[33-35].
In energy sufficient states or fed state, glucose is converted to pyruvate through glycolysis. Pyruvate then enters mitochondria to metabolize through Krebs cycle (tricarboxylic acid cycle) and produce citrate, which is transported back into the cytosol where it is converted to acetyl-CoA. DNL starts with ATP-dependent carboxylation of acetyl-CoA leading to the production of malonyl-CoA. In the next step, alonyl-CoA and acetyl-CoA are converted into palmitic acid (a C16 SFA) by a multi-subunit enzyme called fatty acid synthase. Palmitic acid is the predominant fatty acid synthesized during DNL. Palmitic acid can be further elongated to yield stearic acid (a C18 SFA) and also undergoes desaturation process by the enzyme stearoyl-CoA desaturase-1 (SCD-1) to produce palmitoleic acid (C16:1 MUFA). Evidence suggest that SCD-1 can convert stearoyl-CoA to oleoyl-CoA, which is a major source for triacylglycerol (TG) synthesis. Palmitic acid and stearic acid can be further elongated and desaturated to give higher MUFAs including nervonic acid as the terminal product[33-35]. Although under normal physiological conditions DNL is a tightly regulated process, enhanced DNL has been associated with various metabolic diseases[35], which could be a likely scenario in patients with PDs treated with psychotropic medications[12].
Over the years several studies have shown that membrane lipid/fatty acid abnormalities are strongly associated with cognitive and classical symptoms in patients with PDs[36-39]. Although most of these studies have focused mainly on polyunsaturated fatty acids (PUFAs), little or no attention is given to the role of SAFs and MUFAs, which are supplied mainly by DNL. Evidence suggests that DNL is essential for brain and peripheral tissue development and metabolic homeostasis[40-43]. However; enhanced DNL has been associated with inflammation and various metabolic abnormalities including insulin resistance/diabetes, obesity, and NAFLD[12,13,34,35,44-48]. Since psychotropic medi-cations have been shown to induce or exaggerate these metabolic abnormalities, enhanced DNL could be a major mediator.
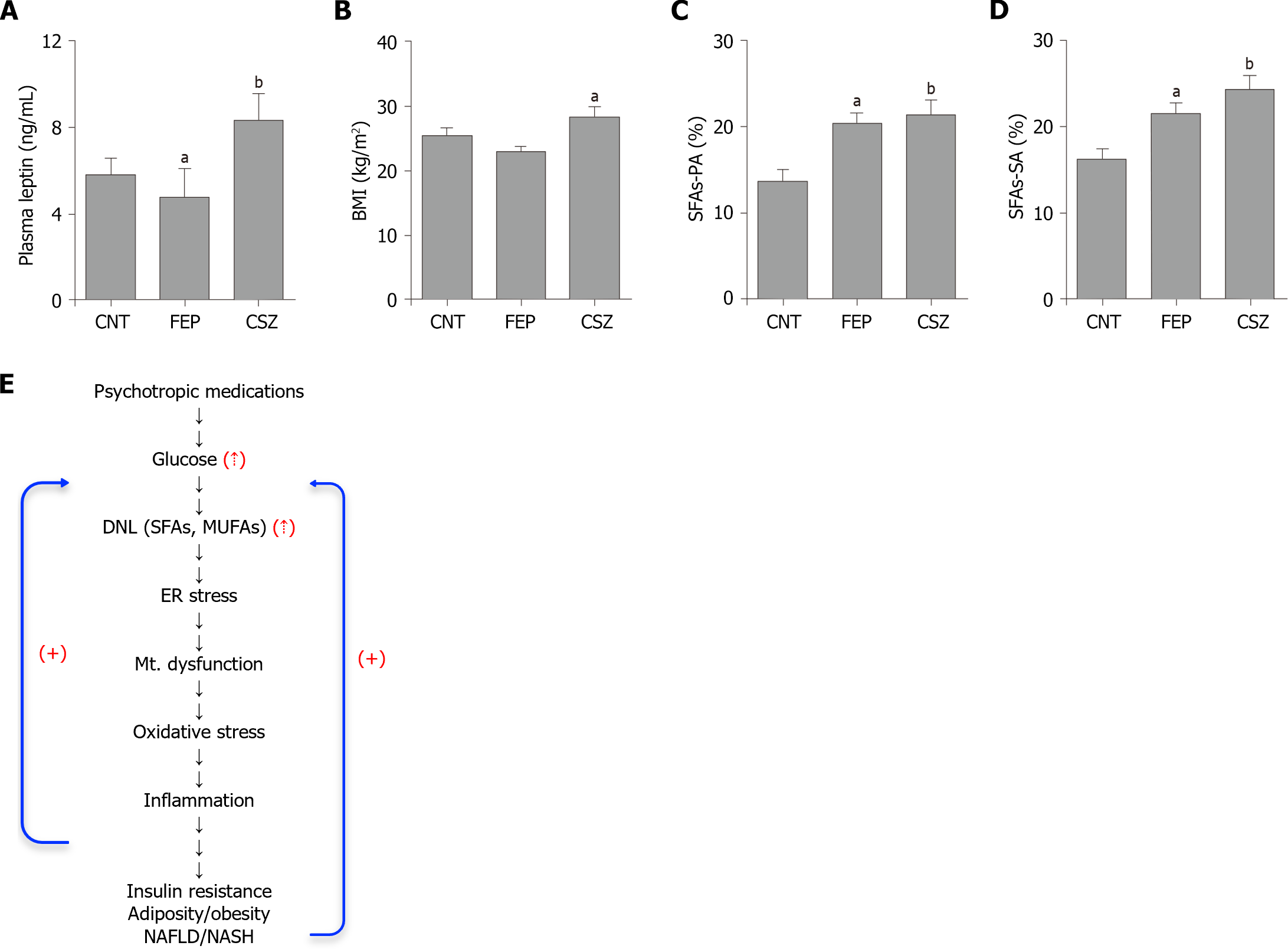
Red blood cells (RBCs) membrane fatty acids (SAFs and MUFAs) have been used to measure the extent of DNL in health and disease including PDs[46,49-51]. In schizophrenia, we reported long back that the levels of RBC’s SFAs, MUFAs, and PUFAs were significantly elevated in patients with psychosis treated with APs compared to the untreated patients and control subjects (Figure 1 and Table 2)[36]. A number of other studies including those conducted in recent years have also reported similar changes in the levels of SFAs, MUFAs, and PUFAs in the RBC membrane from patients with psychosis after treatment with APs[37-39,42,52,53]. In addition to RBCs fatty acids, plasma free fatty acids and TG levels have also been found to be significantly increased after treatment with APs[54-56]. Changes in membrane fatty acids and TGs seem to be the result of enhanced DNL, and not due to binge eating or other confounders because; they showed strong association with cognitive and clinical symptom scores[37-39,57].
| Parameters | Antipsychotic drugs | Antidepressants |
| De novo lipogenesis (markers) | ||
| SFAs | Increased[36,39] | Increased[58,61] |
| MUFAs | Increased[36,39] | Increased[58,61] |
| PUFAs1 | Increased[36,39] | Increased[58,61] |
| Gluconeogenesis (precursors) | ||
| Lactate | Increased[125,126] | Decreased[128] |
| Citrate | Increased[129] | ? |
| Pyruvate | Increased[129] | Increased[128] |
| Glutamate | Increased[129,130] | Increased[128] |
| Metabolic abnormalities | ||
| Blood glucose | Increased[72,121] | Increased[122,131] |
| IR/insulin level2 | Increased[55,133] | Increased[65,74,75] |
| Triglycerides | Increased[8,55,56,72] | Increased[122] |
| Obesity (BMI) | Increased[8,55,72] | Increased[31,32,122] |
| Leptin | Increased[57,87] | Increased[132] |
| Adiponectin | Increased[55,89] | No change[65,134] |
| Resistin | Increased[55,89] | Reduced[134] |
| Diabetes | Increased[8,55,72] | Increased[5,65,74,135] |
| NAFLD | Increased[7,97,123] | Increase[95,96] |
In depression, several studies have reported increase in the RBC’s fatty acid contents after treatment with various ADs[58-61]. Evidence suggests that treatment with ADs can also increase plasma as well as hepatic TGs most likely by increasing DNL[62]. Further, changes in various fatty acids and TG levels were strongly associated with clinical symptoms scores in patients with depression[59-62]. Altogether, the above evidence suggests that treatment with both APs and ADs can increase the levels of both SFAs and MUFAs via increasing DNL in patients with PDs. This could be a potential risk factor associated with various metabolic abnormalities including insulin resistance/diabetes, obesity and NAFLD induced by long-term treatment with psychotropic medications.
Evidence suggests that under normal physiological condition, insulin regulate both gluconeogenesis and DNL, whereas, insulin resistance stimulates gluconeogenesis and DNL[47,63]. It has been reported in humans that the level of SAFs of DNL in adipose tissue is negatively associated with insulin sensitivity[64]. Thus, elevated SFAs along with MUFAs synthesized via DNL could be the major players involved in insulin resistance in patients with PDs.
Although evidence suggest that insulin resistance could be developed from the early stage of the illness in patients with PDs, treatment with psychotropic medications may further deteriorate insulin resistance[65-68]. In drug-naïve patients with early psychosis, Steiner et al[69] assessed homeostatic model assessment of insulin resistance (HOMA-IR) and stress hormone levels, and found that insulin resistance and disrupted glucose homeostasis could be illness related and not due to pharmacotherapy, adiposity, or hormonal stress axis activation; although, levels of serum stress hormone may be increased. In another study, Chouinard et al[68] studied insulin resistance in patients with first-episode psychosis and suggested that abnormal glucose metabolism could be related to risk for psychosis, independent of disease expression and treatment effects. Pillinger et al[70] performed a meta-analysis and noticed elevated HOMA-IR in drug-naive patients with first-episode compared with controls. Thus, while the above evidence suggest that insulin resistance may develop from the early stage of the illness in patients with PDs, recent studies have reported that treatment with APs further deteriorate insulin resistance, which could be aligned with the increase in body weight[71,72].
Likewise, in depression several studies have shown that insulin resistance could be present in a significantly high proportion of patients before the diagnosis of classical symptoms, and it may either remain unchanged or deteriorate further leading to the development of diabetes and obesity after long-term treatment with ADs[65,67,73,74]. Although, there may be some controversies, a recent meta-analysis has reported that risk of insulin resistance is also increased even in children and adolescence after treatment with ADs[75]. Altogether, the above evidence suggests that insulin resistance could be an intrinsic risk factor, which may deteriorate further triggering the development of obesity and NAFLD following treatment with psychotropic medications.
Although patients with PDs may have elevated risk for adipose tissue dysfunction from the early stage of illness, obesity usually develops or become more severe after treatment with psychotropic medication[72,73,76,77]. Adipose tissue is one of the two major sites for DNL under normal conditions, evidence suggests that adipose tissue DNL could be enhanced in patients with PDs[12,55,57,60,61,67]. Although adipocytes can synthesize and store excess lipids/fats without being inflammatory, insulin resistance has been associated with adipocyte hypertrophy and secretion of pro-inflammatory cytokines[78-80]. In addition, hypersensitized adipocytes can released SAFs and MUFAs into circulation, which can lead to the activation and transformation of circulating monocytes into macrophages[81,82]. Intriguingly, several evidence suggest that monocytes could be activated and associated with increased macrophage activation and inflammation in patients with PDs[83,84]. Activated macrophages, in turn, can accumulate SAFs via enhanced DNL and secrete various pro-inflammatory cytokines in adipose tissue; some of these cytokines such as tumor necrosis factor (TNF)-α, can activate nearby adipocytes leading to the formation of a paracrine inflammatory loop between macrophages and adipocytes[14,15,81,82]. Evidence suggests that formation of inflammatory loop between adipocytes and macrophages can result in hypersensitization of adipose tissue leading to irreversible increase in body weight and insulin resistance[81,82].
Adipocyte-macrophage inflammatory cascade, involving activated monocytes, could be the primary mediator of adipose tissue abnormalities induced by long-term treatment with psychotropic medications in patients with PDs[55,77,84,85]. In support of this, several studies including our own, have shown that membrane SFAs, fasting glucose, C-reactive protein, and adipokines including adiponectin and resistin are increased but leptin is decreased in patients with recent onset PDs[28,39,57,86-89]. Evidence suggests that while all fatty acids can inhibit adipokine/leptin production, effect of SAFs could be detrimental[12,57,89,90]. In addition, elevated SAFs in adipocytes and intercalated macrophages can stimulate de novo biosynthesis of ceramides, which can further potentiate inflammatory effect of SFAs in adipose tissue by disrupting adipokine secretion and signaling in patients with PDs[21,86,91,92]. Moreover, adipose tissue abnormalities are directly associated with cardio-vascular dysfunctions in obese individuals; therefore, cardio-vascular dysfunction in patients with PDs could be influenced by both impaired membrane fluidity of vascular endothelial cells as well as adipose tissue abnormalities most likely induced by elevated SFAs synthesized via DNL[53,55,93].
Over the years several authors have investigated the prevalence of liver disease before and after treatment with psychotropic medications in patients with PDs. The available data suggests that a great majority of patients with PDs possess pre-existing risk of developing NAFLD/non-alcoholic steatohepatitis (NASH) within 1-3 years following treatment with psychotropic medications[7,94-96]. Epidemiological studies have shown that extent of NAFLD/NASH prevalence may vary from 27% in United States to as high as 50% in China in patients with PDs compared to the general population. And evidence suggest that the onset of NAFLD/NASH could be positively associated with circulating triglycerides, body mass index, combination and dosage of psychotropic medications, and clinical symptoms in PDs[94-97].
Liver inflammation/NAFLD, irrespective of the cause, is triggered by the dysregulation of DNL leading to lipid/fatty acid accumulation within the hepatocytes[45]. In PDs, whether increased prevalence of NAFLD/NASH is associated with enhanced DNL remains to be validated. However, recent studies have shown that increased plasma and liver free fatty acids and TGs in normal population with NAFLD/NASH are primarily a result of enhanced DNL in liver. Since treatment with both APs and ADs increases plasma free fatty acid, TGs, and the risk of NAFLD/NASH; therefore, enhanced DNL could be a major risk factor associated with the development of NAFLD/NASH in patients with PDs[7,45,95,96,98,99].
As discussed before, several lines of evidence suggest that insulin resistance could be a potential risk factor for developing PDs[66]. Insulin resistance also strongly stimulates hepatic DNL leading to lipid/fatty acid accumulation and development of NAFLD/NASH[37,68,100]. Since, insulin resistance may develop from the early childhood age in patients with PDs, as a consequence, DNL could also be enhanced coinciding with the development of insulin resistance[66]. Although increased SFAs, synthesized via DNL, can activate several pro-inflammatory pathways associated with insulin resistance, they can also be incorporated into membrane phospholipids resulting into reduced membrane fluidity, which can further potentiate inflammatory response and hepatic insulin resistance and progression to NAFLD/NASH, and this could be a likely scenario after treatment with psychotropic medications in patients with PDs. Further, evidence suggest that excess SFAs and TGs produced by liver DNL could be released in circulation, and can activate adipocytes and blood immune cells, specially, monocytes leading to further potentiation of pro-inflammatory cues in patients with PDs[45,98,100,101].
Regarding the mechanism(s) associated with the development of various metabolic abnormalities by psychotropic medications, elevated fatty acids/lipids (SFAs, MUFAs, TG) and leptin together can activated/alter multiple signaling pathways involved in oxidative stress, inflammation and development of various metabolic abnormalities in PDs[12,18,19,59]. Although it is not clear how psychotropic medications increase fatty acid/lipid and leptin synthesis, disruption of calcium homeostasis/signaling could be the major causative factors because; both APs and ADs have been shown to block/inhibit various voltage-gated and non-voltage gated calcium channels, and calcium supplementation has been shown to significantly reverse the early weaning-induced metabolic abnormalities including hyperleptinemia in adult animals[102-106]. Also, several studies have shown that calcium supplementation reduces plasma leptin production (increased leptin production beyond physiological limit is positively associated with obesity), and development of obesity and NAFLD in obese individuals and laboratory animals[106-109]. Thus, while the above findings suggest that psychotropic medications may increase leptin synthesis, body weight and the extent of NAFLD in patients with PDs, it could be a result of perturbed calcium signaling/availability.
An overwhelming body of evidence suggests that elevated SFAs can disrupt insulin signaling and energy homeostasis by altering ER and mitochondrial function (Figure 1). Cell culture studies have shown that treatment with SFAs causes abrupt release of Ca2+ from ER thereby depleting ER Ca2+ store. This leads to a sharp increase in cytosolic and mitochon
It has been shown that SFAs-induced activated adipocytes as well as macrophages, mainly, inflammatory type (M1 type) play a major role in inflammation by producing several pro-inflammatory cytokines including interleukin (IL)-1b, IL-6, IL-8, and TNF-α[19]. Since SAFs have been shown to increase these pro-inflammatory markers, and SFAs are further increased after treatment with psychotropic medications, which therefore could be a major contributing factor in the development of pro-inflammatory response and metabolic abnormalities during long-term treatment with psychotropic medication in patients with PDs[36,58-61].
Several lines of evidence suggest that elevated leptin can induce adiposity/fat mass accumulation. It has been shown to potentiate inflammatory, lipogenic, and adipogenic response in cellular and animal models[110-112]. Leptin treatment of adipocytes has been shown to increase the synthesis of various inflammatory cytokines including TNF-α, IL-10, and IL-6[110]. Evidence suggest that together with TNF-α, leptin can activate macrophages leading to increased secretion of inflammatory cytokines, which may further amplify inflammatory response[113-115]. Also, leptin either alone or in association with TNF-α can induce inflammation of the pancreas disrupting β-cell function and insulin secretion[110,116,117], a scenario typically seen in patients with PDs after long-term treatment with psychotropic medications.
Adipogenic effect of leptin could be enhanced further by increased DNL and adiposity/obesity[110]. It has been shown that leptin can increase the production of PLIN1, CAV-1, PPARγ, SREBP1C, and/or adiponectin[110]. These proteins together increase transcription of various genes involved in adipocyte differentiation. Regarding the signaling pathways involved in lipogenic effect, evidence suggest that leptin can increase lipid accumulation in adipocytes via mechanistic target of rapamycin-dependent pathway[110], which may occur even without insulin action that is crucial for pre-adipocyte differentiation. These findings suggest that leptin may stimulate adipocyte differentiation and DNL even in the absence of insulin signaling. In support of this, it has been shown recently that removing circulating plasma leptin can reduce body weight and hyperglycemia in obese rats[112]. This is an interesting outcome, which may lead to designing leptin-based treatment for reducing obesity and diabetes develop during long-term treatment with psychotropic medications.
Regarding the role of leptin in the development of NAFLD, elevated leptin has been associated with the increased risk of NAFLD. In one study, analysis of 4571 patients with NAFLD, leptin level progressively increased with the increase in the severity of NAFLD[118]. Although, some report suggests that higher leptin level may be protective against NAFLD, result of recent meta-analyses suggest that elevated leptin could be a potential risk factor for developing NAFLD[119,120]. Moreover, since leptin elevation is strongly associated with obesity, and obesity is positive associated with NAFLD; therefore, it can be hypothesized that hyperleptinemia in obese individuals may accelerate the development of NAFLD, a scenario that most likely develops during long-term treatment with psychotropic medication in patients with PDs.
Although psychotropic medications are the first line of treatment for PDs, as discussed above that their long-term use can induce or exacerbate various metabolic abnormalities including insulin resistance/diabetes, obesity, and NAFLD[7,8,72-75,95,96,121-123]. Even early intervention with psychotropic medications has been shown to trigger the development of various metabolic abnormalities in children and adolescents with PDs[124,125]. The mechanism(s) underlying these metabolic abnormalities remains to be documented; however, as discussed before that DNL dysregulation leading to fatty acid accumulation could be the likely mechanisms involved[7,12,57]. In support of this, several studies have shown that the levels of RBC’s SFAs and MUFAs are increased in patients with PDs after treatment with psychotropic medications compared to the untreated patients or control subjects[36,39,58,61]. Since RBC’s fatty acid (SAFs and MUFAs) compo
| Agents/drugs | Psychosis1 | Depression2 | Insulin resistance3 | NAFLD4 |
| Aspirin | Reduced[136,137] | Reduced[142] | Reduced[151] | Reduced[160] |
| N-acetylcysteine | Reduced[136,137] | Reduced[143] | Reduced[152] | Reduced[161] |
| Minocycline | Reduced[136,137] | No change[144] | Reduced[153] | Increased[162] |
| Pregnenolone | Reduced[137] | Reduced[145] | ? | Reduced[163] |
| Estrogens | Reduced[136,137] | Reduced[146] | Reduced[154] | Reduced[164] |
| Raloxifene | Reduced[137] | ? | May reduce[155] | Reduced[165] |
| Curcumin | Reduced[138] | Reduced[147] | Reduced[156] | Reduced[166] |
| Pioglitazone | Reduced[139] | Reduced[148] | Reduced[157] | Reduced[167] |
| Celecoxib | Reduced[140] | Reduced[149] | Reduced[158] | Reduced[168] |
| w3-PUFAs | Reduced[141] | Reduced[150] | Reduced[159] | Reduced[169] |
In the last two decades, several combination therapy trials have been conducted with adjunctive drugs including anti-inflammatory agents and anti-oxidants in PDs[136-139]. Addition of these adjunctive drugs to the clinically approved doses of APs or ADs have been shown to reduce symptoms of psychosis and depression (Table 3). While these agents also reduce insulin resistance, evidence suggests that this effect could be a result of reduced DNL as evident by decrease in NAFLD/NAD (Table 3). Among these agents, aspirin, minocycline, N-acetylcysteine, pregnanolone, estrogen, raloxifene (estrogen receptor modulators), and curcumin have been found to reduce NAFLD/DNL in various experimental studies (Table 3). Development of NAFLD can affect multiple systems and is associated with various metabolic abnormalities including dyslipidemia, insulin resistance, obesity, and cardiovascular diseases and is triggered primarily by dysregulated DNL[50,170,171].
As shown in Table 3, that most of the adjunctive drugs, mentioned above, have been shown to reduce NAFLD in various experimental studies. These findings, together with the favorable effects of these drugs on symptoms of depression and psychosis suggest that enhanced DNL could be an intrinsic risk factor associated with the etiopathology of PDs. Therefore, large randomized clinical trials with therapeutic agents that inhibit/regulate DNL are warranted. In this context, excellent recent reviews by Batchuluun et al[34], and Jeon et al[35] which have presented a detailed account of functional and clinical significance of various DNL inhibitors, can be considered.
Since the evidence discussed earlier suggests that enhanced DNL could be the primary mediator of insulin resistance, which may develop from the early childhood age in patients with PDs; therefore, early intervention with appropriate therapeutic agents that regulate/inhibit DNL may reverse/normalize cellular signaling(s) that leads to the development of brain and peripheral tissue inflammation, and various metabolic abnormalities in patients with PDs. For early intervention, some adjunctive drugs, namely, N-acetylcysteine, pioglitazone or curcumin can be given preference over others[12]. N-acetylcysteine has been shown to reduce most of the psychotic symptoms, inflammation, insulin resistance and NAFLD, while having positive effect on cognition and neurogenesis (Table 3). Although, estrogen has been found effective in reducing psychosis, possible induction of feminization effect limits its extensive use in men. One the other hand, raloxifene, a synthetic selective estrogen receptor modulator that does not carry the risk of feminization, and therefore, could be effective both in young men and women with schizophrenia. However, its effectiveness in patients with depression remains to be documented (Table 3). In addition to these agents, curcumin has been shown to reduce inflammation, insulin resistance, and NAFLD while significantly reducing symptoms of depression and psychosis in patients with PDs[138,147,156,166]. These findings together with profound influence of curcumin on neurogenesis and cognition in young and aged rats suggest that it could be worthy of further large-scale clinical trials in patients with PDs[172,173].
The evidence discussed above suggests that insulin resistance may develop from the early childhood age in patients with PDs. Since insulin resistance is positively associated with DNL; therefore, DNL could also be enhanced from the early childhood age in patients with PDs. Although elevated intracellular fatty acids (SAFs and MUFAs) synthesized via DNL could be the primary mediators of insulin resistance, both insulin resistance and DNL are further deteriorated after treatment with psychotropic medication leading to the development of obesity and NAFLD. This suggests that DNL could be a potential pathological factor associated with various metabolic abnormalities and, targeting DNL could be an effective strategy for reducing the deterioration or development of these metabolic abnormalities and improving global outcome in patients with PDs after treatment with psychotropic medications.
While clinical trial(s) with specific DNL inhibitor(s) have not been performed, various adjunctive drugs used in combination with psychotropic medications in treating patients with PDs have been shown to reduce the development of insulin resistance and NAFLD in laboratory animals (Table 3). Some of these adjunctive drugs, namely, N-acetylcysteine, pioglitazone and curcumin have satisfactory safety profiles and are therefore worthy of early intervention and long-term use in PDs. Regarding the early intervention, since insulin resistance is potential a risk factor for developing PDs and could be diagnosed during childhood stage or before the onset of classical symptoms in patients with PDs; therefore, early intervention with an appropriate adjunctive drugs or other therapeutic agents that reduced/regulate DNL and insulin resistance may normalize cellular signaling/mechanism, which leads to the development of various metabolic abnormalities in patients with PDs.
We sincerely acknowledge the facilities provided by the Department of Biotechnology, Era’s Lucknow Medical College and Hospital, and Faculty of Science, Era University, Lucknow, India.
| 1. | Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience. 2016;321:138-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 402] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 2. | McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-An Overview. JAMA Psychiatry. 2020;77:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 723] [Article Influence: 144.6] [Reference Citation Analysis (0)] |
| 3. | Jones PB, Barnes TR, Davies L, Dunn G, Lloyd H, Hayhurst KP, Murray RM, Markwick A, Lewis SW. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 699] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 4. | Ioannidis JP. Effectiveness of antidepressants: an evidence myth constructed from a thousand randomized trials? Philos Ethics Humanit Med. 2008;3:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Andersohn F, Schade R, Suissa S, Garbe E. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry. 2009;166:591-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | El Asmar K, Fève B, Colle R, Trabado S, Verstuyft C, Gressier F, Vievard A, Haffen E, Polosan M, Ferreri F, Falissard B, Chanson P, Becquemont L, Corruble E. Early weight gain predicts later metabolic syndrome in depressed patients treated with antidepressants: Findings from the METADAP cohort. J Psychiatr Res. 2018;107:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Galiano Rus S, Ortiz García de la Foz V, Arias-Loste MT, Iruzubieta P, Gómez-Revuelta M, Juncal-Ruiz M, Crespo J, Crespo-Facorro B, Vázquez-Bourgon J. Elevated risk of liver steatosis in first-episode psychosis patients: Results from a 3-year prospective study. Schizophr Res. 2022;246:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Burschinski A, Schneider-Thoma J, Chiocchia V, Schestag K, Wang D, Siafis S, Bighelli I, Wu H, Hansen WP, Priller J, Davis JM, Salanti G, Leucht S. Metabolic side effects in persons with schizophrenia during mid- to long-term treatment with antipsychotics: a network meta-analysis of randomized controlled trials. World Psychiatry. 2023;22:116-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 9. | Ndisang JF, Vannacci A, Rastogi S. Oxidative stress and inflammation in obesity, diabetes, hypertension, and related cardiometabolic complications. Oxid Med Cell Longev. 2014;2014:506948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, Reus VI, Verhoeven JE, Epel ES, Mahan L, Rosser R, Wolkowitz OM, Mellon SH. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology. 2017;76:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 346] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 11. | Fraguas D, Díaz-Caneja CM, Ayora M, Hernández-Álvarez F, Rodríguez-Quiroga A, Recio S, Leza JC, Arango C. Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-analysis. Schizophr Bull. 2019;45:742-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 12. | Khan MM. Role of de novo lipogenesis in insulin resistance in first-episode psychosis and therapeutic options. Neurosci Biobehav Rev. 2022;143:104919. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 937] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 14. | Ecker J, Liebisch G, Englmaier M, Grandl M, Robenek H, Schmitz G. Induction of fatty acid synthesis is a key requirement for phagocytic differentiation of human monocytes. Proc Natl Acad Sci U S A. 2010;107:7817-7822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Wei X, Song H, Yin L, Rizzo MG, Sidhu R, Covey DF, Ory DS, Semenkovich CF. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature. 2016;539:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 16. | Zhou H, Urso CJ, Jadeja V. Saturated Fatty Acids in Obesity-Associated Inflammation. J Inflamm Res. 2020;13:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 17. | Button EB, Mitchell AS, Domingos MM, Chung JH, Bradley RM, Hashemi A, Marvyn PM, Patterson AC, Stark KD, Quadrilatero J, Duncan RE. Microglial cell activation increases saturated and decreases monounsaturated fatty acid content, but both lipid species are proinflammatory. Lipids. 2014;49:305-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Kim JI, Huh JY, Sohn JH, Choe SS, Lee YS, Lim CY, Jo A, Park SB, Han W, Kim JB. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol Cell Biol. 2015;35:1686-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 19. | Ly LD, Xu S, Choi SK, Ha CM, Thoudam T, Cha SK, Wiederkehr A, Wollheim CB, Lee IK, Park KS. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp Mol Med. 2017;49:e291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 20. | Garcia Corrales AV, Haidar M, Bogie JFJ, Hendriks JJA. Fatty Acid Synthesis in Glial Cells of the CNS. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Gaggini M, Ndreu R, Michelucci E, Rocchiccioli S, Vassalle C. Ceramides as Mediators of Oxidative Stress and Inflammation in Cardiometabolic Disease. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 22. | DeBattista C, Schatzberg AF. The Black Book of Psychotropic Dosing and Monitoring. Psychopharmacol Bull. 2021;51:8-58. [PubMed] |
| 23. | Chokhawala K, Stevens L. Antipsychotic Medications. 2023 Feb 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 24. | Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: A new hypothesis. Am J Psychiatry. 2001;158:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 721] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 25. | Lieberman JA, Bymaster FP, Meltzer HY, Deutch AY, Duncan GE, Marx CE, Aprille JR, Dwyer DS, Li XM, Mahadik SP, Duman RS, Porter JH, Modica-Napolitano JS, Newton SS, Csernansky JG. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60:358-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 26. | Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17:341-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 320] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 27. | Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, Arndt T, Bäckers L, Rothe P, Cipriani A, Davis J, Salanti G, Leucht S. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394:939-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 774] [Cited by in RCA: 971] [Article Influence: 161.8] [Reference Citation Analysis (0)] |
| 28. | Sheffler ZM, Patel P, Abdijadid S. Antidepressants. 2023 May 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 29. | de Vries YA, Roest AM, Burgerhof JGM, de Jonge P. Initial severity and antidepressant efficacy for anxiety disorders, obsessive-compulsive disorder, and posttraumatic stress disorder: An individual patient data meta-analysis. Depress Anxiety. 2018;35:515-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Kelly K, Posternak M, Alpert JE. Toward achieving optimal response: understanding and managing antidepressant side effects. Dialogues Clin Neurosci. 2008;10:409-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Chokka P, Tancer M, Yeragani VK. Metabolic syndrome: relevance to antidepressant treatment. J Psychiatry Neurosci. 2006;31:414. [PubMed] |
| 32. | Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years' follow-up: population based cohort study. BMJ. 2018;361:k1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 33. | Williams NC, O'Neill LAJ. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front Immunol. 2018;9:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 408] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 34. | Batchuluun B, Pinkosky SL, Steinberg GR. Lipogenesis inhibitors: therapeutic opportunities and challenges. Nat Rev Drug Discov. 2022;21:283-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 207] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 35. | Jeon YG, Kim YY, Lee G, Kim JB. Physiological and pathological roles of lipogenesis. Nat Metab. 2023;5:735-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 93] [Reference Citation Analysis (0)] |
| 36. | Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr Res. 2002;58:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 37. | Kim SW, Jhon M, Kim JM, Smesny S, Rice S, Berk M, Klier CM, McGorry PD, Schäfer MR, Amminger GP. Relationship between Erythrocyte Fatty Acid Composition and Psychopathology in the Vienna Omega-3 Study. PLoS One. 2016;11:e0151417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Dickens AM, Sen P, Kempton MJ, Barrantes-Vidal N, Iyegbe C, Nordentoft M, Pollak T, Riecher-Rössler A, Ruhrmann S, Sachs G, Bressan R, Krebs MO, Amminger GP, de Haan L, van der Gaag M, Valmaggia L, Hyötyläinen T; EU-GEI High Risk Study Group, Orešič M, McGuire P. Dysregulated Lipid Metabolism Precedes Onset of Psychosis. Biol Psychiatry. 2021;89:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 39. | Li N, Yang P, Tang M, Liu Y, Guo W, Lang B, Wang J, Wu H, Tang H, Yu Y, Wu X, Zeng C, Cao T, Cai H. Reduced erythrocyte membrane polyunsaturated fatty acid levels indicate diminished treatment response in patients with multi- vs first-episode schizophrenia. Schizophrenia (Heidelb). 2022;8:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Chirala SS, Chang H, Matzuk M, Abu-Elheiga L, Mao J, Mahon K, Finegold M, Wakil SJ. Fatty acid synthesis is essential in embryonic development: fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc Natl Acad Sci U S A. 2003;100:6358-6363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Knobloch M, Braun SM, Zurkirchen L, von Schoultz C, Zamboni N, Araúzo-Bravo MJ, Kovacs WJ, Karalay O, Suter U, Machado RA, Roccio M, Lutolf MP, Semenkovich CF, Jessberger S. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2013;493:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 42. | Solinas G, Borén J, Dulloo AG. De novo lipogenesis in metabolic homeostasis: More friend than foe? Mol Metab. 2015;4:367-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 43. | Gonzalez-Bohorquez D, Gallego López IM, Jaeger BN, Pfammatter S, Bowers M, Semenkovich CF, Jessberger S. FASN-dependent de novo lipogenesis is required for brain development. Proc Natl Acad Sci U S A. 2022;119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 44. | Yee JK, Lee WN, Han G, Ross MG, Desai M. Organ-specific alterations in fatty acid de novo synthesis and desaturation in a rat model of programmed obesity. Lipids Health Dis. 2011;10:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 792] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 46. | Imamura F, Fretts AM, Marklund M, Ardisson Korat AV, Yang WS, Lankinen M, Qureshi W, Helmer C, Chen TA, Virtanen JK, Wong K, Bassett JK, Murphy R, Tintle N, Yu CI, Brouwer IA, Chien KL, Chen YY, Wood AC, Del Gobbo LC, Djousse L, Geleijnse JM, Giles GG, de Goede J, Gudnason V, Harris WS, Hodge A, Hu F; InterAct Consortium, Koulman A, Laakso M, Lind L, Lin HJ, McKnight B, Rajaobelina K, Riserus U, Robinson JG, Samieri C, Senn M, Siscovick DS, Soedamah-Muthu SS, Sotoodehnia N, Sun Q, Tsai MY, Tuomainen TP, Uusitupa M, Wagenknecht LE, Wareham NJ, Wu JHY, Micha R, Lemaitre RN, Mozaffarian D, Forouhi NG. Fatty acids in the de novo lipogenesis pathway and incidence of type 2 diabetes: A pooled analysis of prospective cohort studies. PLoS Med. 2020;17:e1003102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 47. | Smith GI, Shankaran M, Yoshino M, Schweitzer GG, Chondronikola M, Beals JW, Okunade AL, Patterson BW, Nyangau E, Field T, Sirlin CB, Talukdar S, Hellerstein MK, Klein S. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. 2020;130:1453-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 510] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 48. | Yang S, Qin C, Hu ZW, Zhou LQ, Yu HH, Chen M, Bosco DB, Wang W, Wu LJ, Tian DS. Microglia reprogram metabolic profiles for phenotype and function changes in central nervous system. Neurobiol Dis. 2021;152:105290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 49. | Mu L, Mukamal KJ, Naqvi AZ. Erythrocyte saturated fatty acids and systemic inflammation in adults. Nutrition. 2014;30:1404-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Qureshi W, Santaren ID, Hanley AJ, Watkins SM, Lorenzo C, Wagenknecht LE. Risk of diabetes associated with fatty acids in the de novo lipogenesis pathway is independent of insulin sensitivity and response: the Insulin Resistance Atherosclerosis Study (IRAS). BMJ Open Diabetes Res Care. 2019;7:e000691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Lee Y, Lai HTM, de Oliveira Otto MC, Lemaitre RN, McKnight B, King IB, Song X, Huggins GS, Vest AR, Siscovick DS, Mozaffarian D. Serial Biomarkers of De Novo Lipogenesis Fatty Acids and Incident Heart Failure in Older Adults: The Cardiovascular Health Study. J Am Heart Assoc. 2020;9:e014119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Yang X, Sun L, Zhao A, Hu X, Qing Y, Jiang J, Yang C, Xu T, Wang P, Liu J, Zhang J, He L, Jia W, Wan C. Serum fatty acid patterns in patients with schizophrenia: a targeted metabonomics study. Transl Psychiatry. 2017;7:e1176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 53. | Alqarni A, Mitchell TW, McGorry PD, Nelson B, Markulev C, Yuen HP, Schäfer MR, Berger M, Mossaheb N, Schlögelhofer M, Smesny S, Hickie IB, Berger GE, Chen EYH, de Haan L, Nieman DH, Nordentoft M, Riecher-Rössler A, Verma S, Thompson A, Yung AR, Amminger GP, Meyer BJ. Comparison of erythrocyte omega-3 index, fatty acids and molecular phospholipid species in people at ultra-high risk of developing psychosis and healthy people. Schizophr Res. 2020;226:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Pillinger T, Beck K, Stubbs B, Howes OD. Cholesterol and triglyceride levels in first-episode psychosis: systematic review and meta-analysis. Br J Psychiatry. 2017;211:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 55. | Osimo EF, Sweeney M, de Marvao A, Berry A, Statton B, Perry BI, Pillinger T, Whitehurst T, Cook SA, O'Regan DP, Thomas EL, Howes OD. Adipose tissue dysfunction, inflammation, and insulin resistance: alternative pathways to cardiac remodelling in schizophrenia. A multimodal, case-control study. Transl Psychiatry. 2021;11:614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Li J, Wang F, Xue R, Si S, Tang F, Xue F. Effects of antipsychotics on triglyceride trajectories and its implications in CVD: A longitudinal cohort study. EBioMedicine. 2022;81:104123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 57. | Khan MM. Disrupted leptin-fatty acid biosynthesis is an early manifestation of metabolic abnormalities in schizophrenia. World J Psychiatry. 2022;12:827-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (2)] |
| 58. | Assies J, Pouwer F, Lok A, Mocking RJ, Bockting CL, Visser I, Abeling NG, Duran M, Schene AH. Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PLoS One. 2010;5:e10635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 59. | Meyer BJ, Grenyer BF, Crowe T, Owen AJ, Grigonis-Deane EM, Howe PR. Improvement of major depression is associated with increased erythrocyte DHA. Lipids. 2013;48:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Mocking RJ, Verburg HF, Westerink AM, Assies J, Vaz FM, Koeter MW, Ruhé HG, Schene AH. Fatty acid metabolism and its longitudinal relationship with the hypothalamic-pituitary-adrenal axis in major depression: Associations with prospective antidepressant response. Psychoneuroendocrinology. 2015;59:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Liu T, Wang L, Guo J, Zhao T, Tang H, Dong F, Wang C, Chen J, Tang M. Erythrocyte Membrane Fatty Acid Composition as a Potential Biomarker for Depression. Int J Neuropsychopharmacol. 2023;26:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 62. | Pan SJ, Tan YL, Yao SW, Xin Y, Yang X, Liu J, Xiong J. Fluoxetine induces lipid metabolism abnormalities by acting on the liver in patients and mice with depression. Acta Pharmacol Sin. 2018;39:1463-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 63. | Onyango AN. Excessive gluconeogenesis causes the hepatic insulin resistance paradox and its sequelae. Heliyon. 2022;8:e12294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 64. | Roberts R, Hodson L, Dennis AL, Neville MJ, Humphreys SM, Harnden KE, Micklem KJ, Frayn KN. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52:882-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 65. | Chen YC, Lin WW, Chen YJ, Mao WC, Hung YJ. Antidepressant effects on insulin sensitivity and proinflammatory cytokines in the depressed males. Mediators Inflamm. 2010;2010:573594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Perry BI, Stochl J, Upthegrove R, Zammit S, Wareham N, Langenberg C, Winpenny E, Dunger D, Jones PB, Khandaker GM. Longitudinal Trends in Childhood Insulin Levels and Body Mass Index and Associations With Risks of Psychosis and Depression in Young Adults. JAMA Psychiatry. 2021;78:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 67. | Fernandes BS, Salagre E, Enduru N, Grande I, Vieta E, Zhao Z. Insulin resistance in depression: A large meta-analysis of metabolic parameters and variation. Neurosci Biobehav Rev. 2022;139:104758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 68. | Chouinard VA, Henderson DC, Dalla Man C, Valeri L, Gray BE, Ryan KP, Cypess AM, Cobelli C, Cohen BM, Öngür D. Impaired insulin signaling in unaffected siblings and patients with first-episode psychosis. Mol Psychiatry. 2019;24:1513-1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 69. | Steiner J, Berger M, Guest PC, Dobrowolny H, Westphal S, Schiltz K, Sarnyai Z. Assessment of Insulin Resistance Among Drug-Naive Patients With First-Episode Schizophrenia in the Context of Hormonal Stress Axis Activation. JAMA Psychiatry. 2017;74:968-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 70. | Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired Glucose Homeostasis in First-Episode Schizophrenia: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2017;74:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 71. | Wu RR, Zhao JP, Liu ZN, Zhai JG, Guo XF, Guo WB, Tang JS. Effects of typical and atypical antipsychotics on glucose-insulin homeostasis and lipid metabolism in first-episode schizophrenia. Psychopharmacology (Berl). 2006;186:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 72. | Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, Beck K, Natesan S, Efthimiou O, Cipriani A, Howes OD. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:64-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 559] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 73. | Vancampfort D, Correll CU, Wampers M, Sienaert P, Mitchell AJ, De Herdt A, Probst M, Scheewe TW, De Hert M. Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: a meta-analysis of prevalences and moderating variables. Psychol Med. 2014;44:2017-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 74. | Rashidian H, Subramaniapillai M, Park C, Lipsitz O, Zuckerman H, Cao B, Lee Y, Gill H, Rodrigues RN, Di Vincenzo JD, Iacobucci M, Jaberi S, Rosenblat JD, McIntyre RS, Mansur RB. Changes in insulin resistance following antidepressant treatment mediate response in major depressive disorder. J Psychopharmacol. 2023;37:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 75. | Sun JW, Hernández-Díaz S, Haneuse S, Bourgeois FT, Vine SM, Olfson M, Bateman BT, Huybrechts KF. Association of Selective Serotonin Reuptake Inhibitors With the Risk of Type 2 Diabetes in Children and Adolescents. JAMA Psychiatry. 2021;78:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 76. | Lee SH, Paz-Filho G, Mastronardi C, Licinio J, Wong ML. Is increased antidepressant exposure a contributory factor to the obesity pandemic? Transl Psychiatry. 2016;6:e759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 77. | Smith E, Singh R, Lee J, Colucci L, Graff-Guerrero A, Remington G, Hahn M, Agarwal SM. Adiposity in schizophrenia: A systematic review and meta-analysis. Acta Psychiatr Scand. 2021;144:524-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 78. | Verboven K, Wouters K, Gaens K, Hansen D, Bijnen M, Wetzels S, Stehouwer CD, Goossens GH, Schalkwijk CG, Blaak EE, Jocken JW. Abdominal subcutaneous and visceral adipocyte size, lipolysis and inflammation relate to insulin resistance in male obese humans. Sci Rep. 2018;8:4677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 79. | Klöting N, Blüher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014;15:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 353] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 80. | Shimobayashi M, Albert V, Woelnerhanssen B, Frei IC, Weissenberger D, Meyer-Gerspach AC, Clement N, Moes S, Colombi M, Meier JA, Swierczynska MM, Jenö P, Beglinger C, Peterli R, Hall MN. Insulin resistance causes inflammation in adipose tissue. J Clin Invest. 2018;128:1538-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 322] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 81. | Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25:2062-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 790] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 82. | Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, Kamei Y, Ogawa Y. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 618] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 83. | Lisi L, Camardese G, Treglia M, Tringali G, Carrozza C, Janiri L, Dello Russo C, Navarra P. Monocytes from depressed patients display an altered pattern of response to endotoxin challenge. PLoS One. 2013;8:e52585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Weber NS, Gressitt KL, Cowan DN, Niebuhr DW, Yolken RH, Severance EG. Monocyte activation detected prior to a diagnosis of schizophrenia in the US Military New Onset Psychosis Project (MNOPP). Schizophr Res. 2018;197:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Zhao S, Lin Q, Xiong W, Li L, Straub L, Zhang D, Zapata R, Zhu Q, Sun XN, Zhang Z, Funcke JB, Li C, Chen S, Zhu Y, Jiang N, Li G, Xu Z, Wyler SC, Wang MY, Bai J, Han X, Kusminski CM, Zhang N, An Z, Elmquist JK, Osborn O, Liu C, Scherer PE. Hyperleptinemia contributes to antipsychotic drug-associated obesity and metabolic disorders. Sci Transl Med. 2023;15:eade8460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 86. | Hamada Y, Nagasaki H, Fujiya A, Seino Y, Shang QL, Suzuki T, Hashimoto H, Oiso Y. Involvement of de novo ceramide synthesis in pro-inflammatory adipokine secretion and adipocyte-macrophage interaction. J Nutr Biochem. 2014;25:1309-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Misiak B, Bartoli F, Stramecki F, Samochowiec J, Lis M, Kasznia J, Jarosz K, Stańczykiewicz B. Appetite regulating hormones in first-episode psychosis: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2019;102:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 88. | Steiner J, Frodl T, Schiltz K, Dobrowolny H, Jacobs R, Fernandes BS, Guest PC, Meyer-Lotz G, Borucki K, Bahn S, Bogerts B, Falkai P, Bernstein HG. Innate Immune Cells and C-Reactive Protein in Acute First-Episode Psychosis and Schizophrenia: Relationship to Psychopathology and Treatment. Schizophr Bull. 2020;46:363-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 89. | Veru-Lesmes F, Guay S, Shah JL, Schmitz N, Giguère CÉ, Joober R, Iyer SN, Malla AK. Adipose tissue dysregulation at the onset of psychosis: Adipokines and social determinants of health. Psychoneuroendocrinology. 2021;123:104915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Cammisotto PG, Gélinas Y, Deshaies Y, Bukowiecki LJ. Regulation of leptin secretion from white adipocytes by free fatty acids. Am J Physiol Endocrinol Metab. 2003;285:E521-E526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Schwarz E, Prabakaran S, Whitfield P, Major H, Leweke FM, Koethe D, McKenna P, Bahn S. High throughput lipidomic profiling of schizophrenia and bipolar disorder brain tissue reveals alterations of free fatty acids, phosphatidylcholines, and ceramides. J Proteome Res. 2008;7:4266-4277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 92. | Martínez L, Torres S, Baulies A, Alarcón-Vila C, Elena M, Fabriàs G, Casas J, Caballeria J, Fernandez-Checa JC, García-Ruiz C. Myristic acid potentiates palmitic acid-induced lipotoxicity and steatohepatitis associated with lipodystrophy by sustaning de novo ceramide synthesis. Oncotarget. 2015;6:41479-41496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 93. | Chait A, den Hartigh LJ. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med. 2020;7:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 755] [Article Influence: 151.0] [Reference Citation Analysis (0)] |
| 94. | Soto-Angona Ó, Anmella G, Valdés-Florido MJ, De Uribe-Viloria N, Carvalho AF, Penninx BWJH, Berk M. Non-alcoholic fatty liver disease (NAFLD) as a neglected metabolic companion of psychiatric disorders: common pathways and future approaches. BMC Med. 2020;18:261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 95. | Todorović Vukotić N, Đorđević J, Pejić S, Đorđević N, Pajović SB. Antidepressants- and antipsychotics-induced hepatotoxicity. Arch Toxicol. 2021;95:767-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 96. | Voican CS, Corruble E, Naveau S, Perlemuter G. Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry. 2014;171:404-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 97. | Morlán-Coarasa MJ, Arias-Loste MT, Ortiz-García de la Foz V, Martínez-García O, Alonso-Martín C, Crespo J, Romero-Gómez M, Fábrega E, Crespo-Facorro B. Incidence of non-alcoholic fatty liver disease and metabolic dysfunction in first episode schizophrenia and related psychotic disorders: a 3-year prospective randomized interventional study. Psychopharmacology (Berl). 2016;233:3947-3952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Lawitz EJ, Li KW, Nyangau E, Field TJ, Chuang JC, Billin A, Wang L, Wang Y, Huss RS, Chung C, Subramanian GM, Myers RP, Hellerstein MK. Elevated de novo lipogenesis, slow liver triglyceride turnover, and clinical correlations in nonalcoholic steatohepatitis patients. J Lipid Res. 2022;63:100250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 99. | Smith ZR, Horng M, Rech MA. Medication-Induced Hyperlactatemia and Lactic Acidosis: A Systematic Review of the Literature. Pharmacotherapy. 2019;39:946-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Roumans KHM, Lindeboom L, Veeraiah P, Remie CME, Phielix E, Havekes B, Bruls YMH, Brouwers MCGJ, Ståhlman M, Alssema M, Peters HPF, de Mutsert R, Staels B, Taskinen MR, Borén J, Schrauwen P, Schrauwen-Hinderling VB. Hepatic saturated fatty acid fraction is associated with de novo lipogenesis and hepatic insulin resistance. Nat Commun. 2020;11:1891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 101. | Castoldi A, Monteiro LB, van Teijlingen Bakker N, Sanin DE, Rana N, Corrado M, Cameron AM, Hässler F, Matsushita M, Caputa G, Klein Geltink RI, Büscher J, Edwards-Hicks J, Pearce EL, Pearce EJ. Triacylglycerol synthesis enhances macrophage inflammatory function. Nat Commun. 2020;11:4107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 102. | Choi KH, Rhim H. Inhibition of recombinant Ca(v)3.1 (alpha(1G)) T-type calcium channels by the antipsychotic drug clozapine. Eur J Pharmacol. 2010;626:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 103. | Horishita T, Yanagihara N, Ueno S, Okura D, Horishita R, Minami T, Ogata Y, Sudo Y, Uezono Y, Sata T, Kawasaki T. Antidepressants inhibit Na(v)1.3, Na(v)1.7, and Na(v)1.8 neuronal voltage-gated sodium channels more potently than Na(v)1.2 and Na(v)1.6 channels expressed in Xenopus oocytes. Naunyn Schmiedebergs Arch Pharmacol. 2017;390:1255-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 104. | Ito K, Nakazawa K, Koizumi S, Liu M, Takeuchi K, Hashimoto T, Ohno Y, Inoue K. Inhibition by antipsychotic drugs of L-type Ca2+ channel current in PC12 cells. Eur J Pharmacol. 1996;314:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 105. | Santi CM, Cayabyab FS, Sutton KG, McRory JE, Mezeyova J, Hamming KS, Parker D, Stea A, Snutch TP. Differential inhibition of T-type calcium channels by neuroleptics. J Neurosci. 2002;22:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 106. | Nobre JL, Lisboa PC, Lima Nda S, Franco JG, Nogueira Neto JF, de Moura EG, de Oliveira E. Calcium supplementation prevents obesity, hyperleptinaemia and hyperglycaemia in adult rats programmed by early weaning. Br J Nutr. 2012;107:979-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 107. | Li P, Fan C, Lu Y, Qi K. Effects of calcium supplementation on body weight: a meta-analysis. Am J Clin Nutr. 2016;104:1263-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 108. | Zhang Z, Liu S, Qi Y, Aluo Z, Zhang L, Yu L, Li Q, Luo Z, Sun Z, Zhou L, Li Y. Calcium supplementation relieves high-fat diet-induced liver steatosis by reducing energy metabolism and promoting lipolysis. J Nutr Biochem. 2021;94:108645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Cammisotto PG, Bukowiecki LJ. Role of calcium in the secretion of leptin from white adipocytes. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1380-R1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 110. | Palhinha L, Liechocki S, Hottz ED, Pereira JADS, de Almeida CJ, Moraes-Vieira PMM, Bozza PT, Maya-Monteiro CM. Leptin Induces Proadipogenic and Proinflammatory Signaling in Adipocytes. Front Endocrinol (Lausanne). 2019;10:841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 111. | Panariello F, Polsinelli G, Borlido C, Monda M, De Luca V. The role of leptin in antipsychotic-induced weight gain: genetic and non-genetic factors. J Obes. 2012;2012:572848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 112. | Zhao S, Zhu Y, Schultz RD, Li N, He Z, Zhang Z, Caron A, Zhu Q, Sun K, Xiong W, Deng H, Sun J, Deng Y, Kim M, Lee CE, Gordillo R, Liu T, Odle AK, Childs GV, Zhang N, Kusminski CM, Elmquist JK, Williams KW, An Z, Scherer PE. Partial Leptin Reduction as an Insulin Sensitization and Weight Loss Strategy. Cell Metab. 2019;30:706-719.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 113. | Tsiotra PC, Tsigos C, Raptis SA. TNFalpha and leptin inhibit basal and glucose-stimulated insulin secretion and gene transcription in the HIT-T15 pancreatic cells. Int J Obes Relat Metab Disord. 2001;25:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 114. | Monteiro L, Pereira JADS, Palhinha L, Moraes-Vieira PMM. Leptin in the regulation of the immunometabolism of adipose tissue-macrophages. J Leukoc Biol. 2019;106:703-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 115. | Kuno R, Wang J, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Autocrine activation of microglia by tumor necrosis factor-alpha. J Neuroimmunol. 2005;162:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 116. | Cases JA, Gabriely I, Ma XH, Yang XM, Michaeli T, Fleischer N, Rossetti L, Barzilai N. Physiological increase in plasma leptin markedly inhibits insulin secretion in vivo. Diabetes. 2001;50:348-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 117. | Seufert J, Kieffer TJ, Leech CA, Holz GG, Moritz W, Ricordi C, Habener JF. Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus. J Clin Endocrinol Metab. 1999;84:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 118. | Rotundo L, Persaud A, Feurdean M, Ahlawat S, Kim HS. The Association of leptin with severity of non-alcoholic fatty liver disease: A population-based study. Clin Mol Hepatol. 2018;24:392-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 119. | Polyzos SA, Aronis KN, Kountouras J, Raptis DD, Vasiloglou MF, Mantzoros CS. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia. 2016;59:30-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 120. | Guo Z, Du H, Guo Y, Jin Q, Liu R, Yun Z, Zhang J, Li X, Ye Y. Association between leptin and NAFLD: a two-sample Mendelian randomization study. Eur J Med Res. 2023;28:215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 121. | Zhang Y, Liu Y, Su Y, You Y, Ma Y, Yang G, Song Y, Liu X, Wang M, Zhang L, Kou C. The metabolic side effects of 12 antipsychotic drugs used for the treatment of schizophrenia on glucose: a network meta-analysis. BMC Psychiatry. 2017;17:373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 122. | Beyazyüz M, Albayrak Y, Eğilmez OB, Albayrak N, Beyazyüz E. Relationship between SSRIs and Metabolic Syndrome Abnormalities in Patients with Generalized Anxiety Disorder: A Prospective Study. Psychiatry Investig. 2013;10:148-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 123. | Koreki A, Mori H, Nozaki S, Koizumi T, Suzuki H, Onaya M. Risk of Nonalcoholic Fatty Liver Disease in Patients With Schizophrenia Treated With Antipsychotic Drugs: A Cross-sectional Study. J Clin Psychopharmacol. 2021;41:474-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 124. | Cockerill RG, Biggs BK, Oesterle TS, Croarkin PE. Antidepressant use and body mass index change in overweight adolescents: a historical cohort study. Innov Clin Neurosci. 2014;11:14-21. [PubMed] |
| 125. | Libowitz MR, Nurmi EL. The Burden of Antipsychotic-Induced Weight Gain and Metabolic Syndrome in Children. Front Psychiatry. 2021;12:623681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 126. | Elmorsy E, Shahda M, Mahmoud el-HM, Rakha SA, Shoaib M. Blood lactate levels as a biomarker of antipsychotic side effects in patients with schizophrenia. J Psychopharmacol. 2016;30:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 127. | Glavina T, Mrass D, Dodig T, Glavina G, Pranić S, Uglešić B. Blood lactate levels in patients receiving first- or second- generation antipsychotics. Croat Med J. 2011;52:41-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 128. | Pu J, Liu Y, Gui S, Tian L, Yu Y, Wang D, Zhong X, Chen W, Chen X, Chen Y, Chen X, Gong X, Liu L, Li W, Wang H, Xie P. Effects of pharmacological treatment on metabolomic alterations in animal models of depression. Transl Psychiatry. 2022;12:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 129. | Yang J, Chen T, Sun L, Zhao Z, Qi X, Zhou K, Cao Y, Wang X, Qiu Y, Su M, Zhao A, Wang P, Yang P, Wu J, Feng G, He L, Jia W, Wan C. Potential metabolite markers of schizophrenia. Mol Psychiatry. 2013;18:67-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 130. | De Luca V, Viggiano E, Messina G, Viggiano A, Borlido C, Viggiano A, Monda M. Peripheral amino Acid levels in schizophrenia and antipsychotic treatment. Psychiatry Investig. 2008;5:203-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 131. | Miidera H, Enomoto M, Kitamura S, Tachimori H, Mishima K. Association Between the Use of Antidepressants and the Risk of Type 2 Diabetes: A Large, Population-Based Cohort Study in Japan. Diabetes Care. 2020;43:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 132. | Schilling C, Gilles M, Blum WF, Daseking E, Colla M, Weber-Hamann B, Lederbogen F, Krumm B, Heuser I, Wudy SA, Kopf D, Deuschle M. Leptin plasma concentrations increase during antidepressant treatment with amitriptyline and mirtazapine, but not paroxetine and venlafaxine: leptin resistance mediated by antihistaminergic activity? J Clin Psychopharmacol. 2013;33:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 133. | Teff KL, Rickels MR, Grudziak J, Fuller C, Nguyen HL, Rickels K. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes. 2013;62:3232-3240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 134. | Weber-Hamann B, Kratzsch J, Kopf D, Lederbogen F, Gilles M, Heuser I, Deuschle M. Resistin and adiponectin in major depression: the association with free cortisol and effects of antidepressant treatment. J Psychiatr Res. 2007;41:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 135. | Barnard K, Peveler RC, Holt RI. Antidepressant medication as a risk factor for type 2 diabetes and impaired glucose regulation: systematic review. Diabetes Care. 2013;36:3337-3345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 136. | Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol Med. 2019;49:2307-2319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 137. | Cho M, Lee TY, Kwak YB, Yoon YB, Kim M, Kwon JS. Adjunctive use of anti-inflammatory drugs for schizophrenia: A meta-analytic investigation of randomized controlled trials. Aust N Z J Psychiatry. 2019;53:742-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 138. | Dinakaran D, Sreeraj VS, Venkatasubramanian G. Role of Curcumin in the Management of Schizophrenia: A Narrative Review. Indian J Psychol Med. 2022;44:107-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 139. | Smith RC, Jin H, Li C, Bark N, Shekhar A, Dwivedi S, Mortiere C, Lohr J, Hu Q, Davis JM. Effects of pioglitazone on metabolic abnormalities, psychopathology, and cognitive function in schizophrenic patients treated with antipsychotic medication: a randomized double-blind study. Schizophr Res. 2013;143:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 140. | Marini S, De Berardis D, Vellante F, Santacroce R, Orsolini L, Valchera A, Girinelli G, Carano A, Fornaro M, Gambi F, Martinotti G, Di Giannantonio M. Celecoxib Adjunctive Treatment to Antipsychotics in Schizophrenia: A Review of Randomized Clinical Add-On Trials. Mediators Inflamm. 2016;2016:3476240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 141. | Goh KK, Chen CY, Chen CH, Lu ML. Effects of omega-3 polyunsaturated fatty acids supplements on psychopathology and metabolic parameters in schizophrenia: A meta-analysis of randomized controlled trials. J Psychopharmacol. 2021;35:221-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 142. | Berk M, Agustini B, Woods RL, Nelson MR, Shah RC, Reid CM, Storey E, Fitzgerald SM, Lockery JE, Wolfe R, Mohebbi M, Dodd S, Murray AM, Stocks N, Fitzgerald PB, Mazza C, McNeil JJ. Effects of aspirin on the long-term management of depression in older people: a double-blind randomised placebo-controlled trial. Mol Psychiatry. 2021;26:5161-5170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 143. | Russell SE, Skvarc DR, Mohebbi M, Camfield D, Byrne LK, Turner A, Ashton MM, Berk M, Dodd S, Malhi GS, Cotton SM, Bush AI, Dean OM. The Impact of N-acetylcysteine on Major Depression: Qualitative Observation and Mixed Methods Analysis of Participant Change during a 12-week Randomised Controlled Trial. Clin Psychopharmacol Neurosci. 2023;21:320-331. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 144. | Calich VL, Burger E, Kashino SS, Fazioli RA, Singer-Vermes LM. Resistance to Paracoccidioides brasiliensis in mice is controlled by a single dominant autosomal gene. Infect Immun. 1987;55:1919-1923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 145. | Brown ES, Park J, Marx CE, Hynan LS, Gardner C, Davila D, Nakamura A, Sunderajan P, Lo A, Holmes T. A randomized, double-blind, placebo-controlled trial of pregnenolone for bipolar depression. Neuropsychopharmacology. 2014;39:2867-2873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 146. | Wium-Andersen MK, Jørgensen TSH, Halvorsen AH, Hartsteen BH, Jørgensen MB, Osler M. Association of Hormone Therapy With Depression During Menopause in a Cohort of Danish Women. JAMA Netw Open. 2022;5:e2239491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 147. | Fusar-Poli L, Vozza L, Gabbiadini A, Vanella A, Concas I, Tinacci S, Petralia A, Signorelli MS, Aguglia E. Curcumin for depression: a meta-analysis. Crit Rev Food Sci Nutr. 2020;60:2643-2653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 148. | Lin KW, Wroolie TE, Robakis T, Rasgon NL. Adjuvant pioglitazone for unremitted depression: Clinical correlates of treatment response. Psychiatry Res. 2015;230:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 149. | Wang Z, Wu Q, Wang Q. Effect of celecoxib on improving depression: A systematic review and meta-analysis. World J Clin Cases. 2022;10:7872-7882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 150. | Liao Y, Xie B, Zhang H, He Q, Guo L, Subramanieapillai M, Fan B, Lu C, McIntyre RS. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl Psychiatry. 2019;9:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 251] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 151. | Pradhan AD, Cook NR, Manson JE, Ridker PM, Buring JE. A randomized trial of low-dose aspirin in the prevention of clinical type 2 diabetes in women. Diabetes Care. 2009;32:3-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 152. | Pereira S, Shah A, George Fantus I, Joseph JW, Giacca A. Effect of N-acetyl-l-cysteine on insulin resistance caused by prolonged free fatty acid elevation. J Endocrinol. 2015;225:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 153. | Mattei D, Djodari-Irani A, Hadar R, Pelz A, de Cossío LF, Goetz T, Matyash M, Kettenmann H, Winter C, Wolf SA. Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun. 2014;38:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 154. | Pereira RI, Casey BA, Swibas TA, Erickson CB, Wolfe P, Van Pelt RE. Timing of Estradiol Treatment After Menopause May Determine Benefit or Harm to Insulin Action. J Clin Endocrinol Metab. 2015;100:4456-4462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 155. | Alvarez Sánchez JA, Aldana Figueroa A. [Various hemodynamic characteristics of the venous circulation of the lower limbs in the patient with recurrent varices]. Angiologia. 1992;44:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 156. | Tian J, Feng B, Tian Z. The Effect of Curcumin on Lipid Profile and Glycemic Status of Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2022;2022:8278744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 157. | Qian X, Wang H, Yang G, Gao Z, Luo Y, Dong A, Zhang F, Xu M, Liu S, Yang X, Chen Y, Li G. Pioglitazone Improved Insulin Sensitivity and First Phase Insulin Secretion Among Obese and Lean People with Diabetes: A Multicenter Clamp Study. Diabetes Ther. 2018;9:815-826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 158. | González-Ortiz M, Pascoe-González S, Esperanzamartínez-Abundis, Kam-Ramos AM, Hernández-Salazar E. Effect of celecoxib, a cyclooxygenase-2-specific inhibitor, on insulin sensitivity, C-reactive protein, homocysteine, and metabolic profile in overweight or obese subjects. Metab Syndr Relat Disord. 2005;3:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 159. | Khalili L, Valdes-Ramos R, Harbige LS. Effect of n-3 (Omega-3) Polyunsaturated Fatty Acid Supplementation on Metabolic and Inflammatory Biomarkers and Body Weight in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of RCTs. Metabolites. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 160. | Han YM, Lee YJ, Jang YN, Kim HM, Seo HS, Jung TW, Jeong JH. Aspirin Improves Nonalcoholic Fatty Liver Disease and Atherosclerosis through Regulation of the PPARδ-AMPK-PGC-1α Pathway in Dyslipidemic Conditions. Biomed Res Int. 2020;2020:7806860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 161. | Hang W, Shu H, Wen Z, Liu J, Jin Z, Shi Z, Chen C, Wang DW. N-Acetyl Cysteine Ameliorates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease and Intracellular Triglyceride Accumulation by Preserving Mitochondrial Function. Front Pharmacol. 2021;12:636204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 162. | Minocycline. 2019 Jan 23. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012– . [PubMed] |
| 163. | Ma Y, Liu D. Activation of pregnane X receptor by pregnenolone 16 α-carbonitrile prevents high-fat diet-induced obesity in AKR/J mice. PLoS One. 2012;7:e38734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 164. | Zhu L, Brown WC, Cai Q, Krust A, Chambon P, McGuinness OP, Stafford JM. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes. 2013;62:424-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 245] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 165. | Luo F, Ishigami M, Achiwa K, Ishizu Y, Kuzuya T, Honda T, Hayashi K, Ishikawa T, Katano Y, Goto H. Raloxifene Ameliorates Liver Fibrosis of Nonalcoholic Steatohepatitis Induced by Choline-Deficient High-Fat Diet in Ovariectomized Mice. Dig Dis Sci. 2015;60:2730-2739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 166. | Yan C, Zhang Y, Zhang X, Aa J, Wang G, Xie Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed Pharmacother. 2018;105:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 167. | Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 729] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 168. | Zhang C, Lu Y, Song Y, Chen L, Hu J, Meng Y, Chen X, Li S, Zheng G, Qiu Z. Celecoxib attenuates hepatosteatosis by impairing de novo lipogenesis via Akt-dependent lipogenic pathway. J Cell Mol Med. 2022;26:3995-4006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 169. | Vell MS, Creasy KT, Scorletti E, Seeling KS, Hehl L, Rendel MD, Schneider KM, Schneider CV. Omega-3 intake is associated with liver disease protection. Front Public Health. 2023;11:1192099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 170. | Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol. 2021;6:578-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 325] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 171. | Fotbolcu H, Zorlu E. Nonalcoholic fatty liver disease as a multi-systemic disease. World J Gastroenterol. 2016;22:4079-4090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 172. | Dong S, Zeng Q, Mitchell ES, Xiu J, Duan Y, Li C, Tiwari JK, Hu Y, Cao X, Zhao Z. Curcumin enhances neurogenesis and cognition in aged rats: implications for transcriptional interactions related to growth and synaptic plasticity. PLoS One. 2012;7:e31211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 173. | Lee Y, Park HR, Lee JY, Kim J, Yang S, Lee C, Kim K, Kim HS, Chang SC, Lee J. Low-dose curcumin enhances hippocampal neurogenesis and memory retention in young mice. Arch Pharm Res. 2023;46:423-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |