Published online May 19, 2024. doi: 10.5498/wjp.v14.i5.661
Revised: March 26, 2024
Accepted: April 11, 2024
Published online: May 19, 2024
Processing time: 125 Days and 4.2 Hours
Although the specific pathogenesis of preterm birth (PTB) has not been thoroughly clarified, it is known to be related to various factors, such as preg
To analyze the risk factors for PTB to establish a PTB risk prediction model and to assess postpartum anxiety and depression in mothers.
A retrospective analysis of 648 consecutive parturients who delivered at Shenzhen Bao’an District Songgang People’s Hospital between January 2019 and January 2022 was performed. According to the diagnostic criteria for premature infants, the parturients were divided into a PTB group (n = 60) and a full-term (FT) group (n = 588). Puerperae were assessed by the Self-rating Anxiety Scale (SAS) and Self-rating Depression Scale (SDS), based on which the mothers with anxiety and depression symptoms were screened for further analysis. The factors affecting PTB were analyzed by univariate analysis, and the related risk factors were identified by logistic regression.
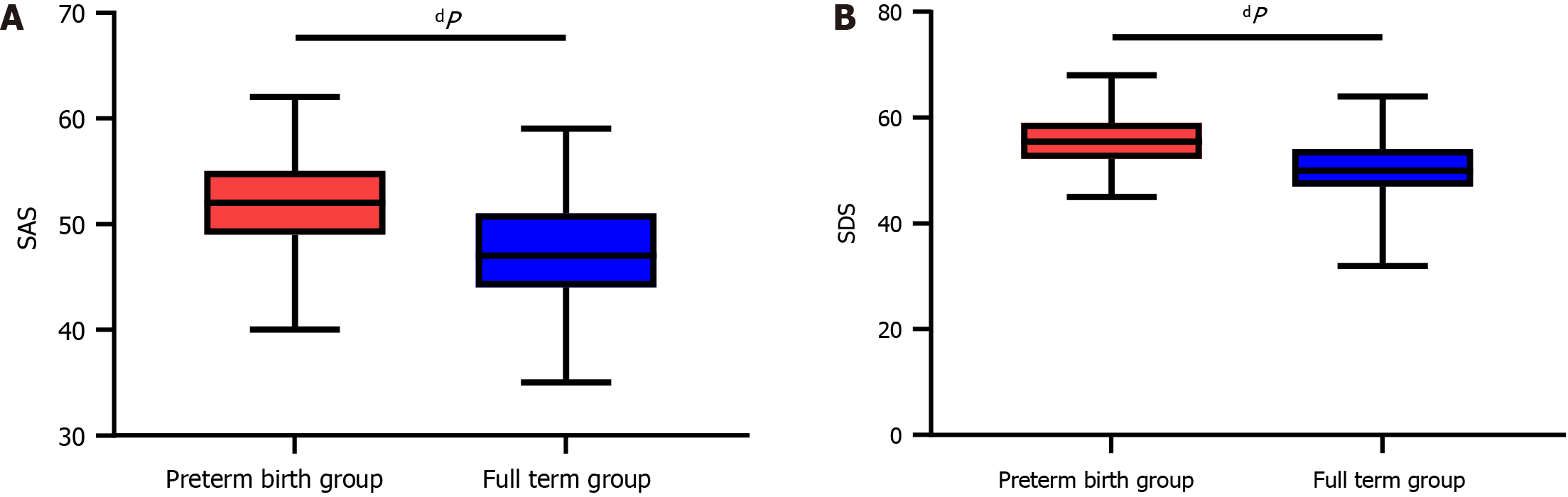
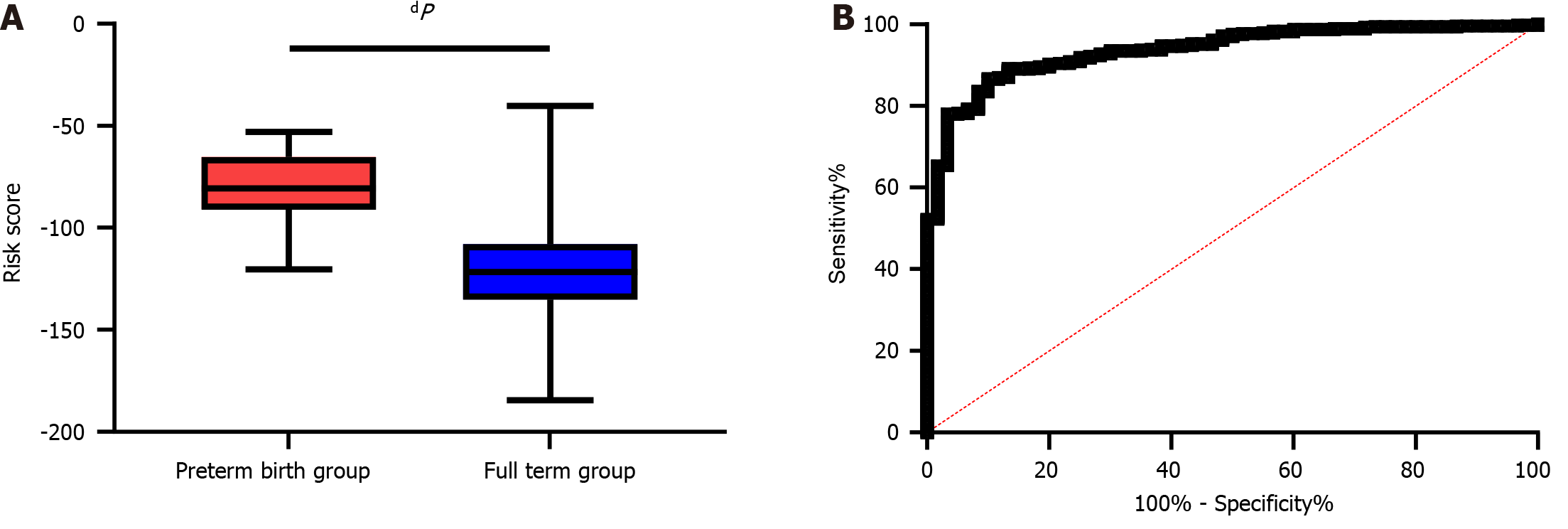
According to univariate analysis, the PTB group was older than the FT group, with a smaller weight change and greater proportions of women who underwent artificial insemination and had gestational diabetes mellitus (P < 0.05). In addition, greater proportions of women with reproductive tract infections and greater white blood cell (WBC) counts (P < 0.05), shorter cervical lengths in the second trimester and lower neutrophil percentages (P < 0.001) were detected in the PTB group than in the FT group. The PTB group exhibited higher postpartum SAS and SDS scores than did the FT group (P < 0.0001), with a higher number of mothers experiencing anxiety and depression (P < 0.001). Multivariate logistic regression analysis revealed that a greater maternal weight change, the presence of gestational diabetes mellitus, a shorter cervical length in the second trimester, a greater WBC count, and the presence of maternal anxiety and depression were risk factors for PTB (P < 0.01). Moreover, the risk score of the FT group was lower than that of the PTB group, and the area under the curve of the risk score for predicting PTB was greater than 0.9.
This study highlights the complex interplay between postpartum anxiety and PTB, where maternal anxiety may be a potential risk factor for PTB, with PTB potentially increasing the incidence of postpartum anxiety in mothers. In addition, a greater maternal weight change, the presence of gestational diabetes mellitus, a shorter cervical length, a greater WBC count, and postpartum anxiety and depression were identified as risk factors for PTB.
Core Tip: This study identified several important risk factors for preterm birth (PTB), including a greater maternal weight change, the presence of gestational diabetes mellitus, a shorter cervical length in the second trimester, a greater white blood cell count, and postpartum anxiety and depression. Based on these factors, a PTB risk prediction model was constructed by our research team, which demonstrated excellent prediction efficiency. In addition, in view of the high prevalence of negative emotions such as anxiety and depression in mothers with PTB, timely psychological intervention is necessary. These findings are helpful for promoting early intervention, reducing the adverse consequences of PTB and providing a new perspective for the management of pregnant women.
- Citation: Chen JJ, Chen XJ, She QM, Li JX, Luo QH. Clinical risk factors for preterm birth and evaluating maternal psychology in the postpartum period. World J Psychiatry 2024; 14(5): 661-669
- URL: https://www.wjgnet.com/2220-3206/full/v14/i5/661.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i5.661
Preterm birth (PTB) definitions vary internationally and are generally set before 37 wk of gestation[1]. China adopts the World Health Organization’s 1976 definition, considering the birth of a fetus weighing over 1000 g between 28 wk and less than 37 wk gestation as PTB[2,3]. Factors such as advancements in reproductive technologies, changes in birth policies to allow couples to have more children, and enhanced living standards have contributed to increased pregnancy complications and PTB rates, which range from 7%-15% in China, slightly higher than the rates of 6%-11% observed in developed countries[4]. PTB is the leading cause of perinatal mortality and morbidity, contributing to one-third of perinatal deaths and three-quarters of perinatal illnesses[5]. Research indicates that PTB survivors often face significant health challenges, including a high likelihood (80%) of experiencing cognitive impairments or neurological sequelae[6]. Additionally, PTB is linked to long-term complications across various bodily systems, including respiratory, digestive, and immune disorders, as well as central nervous system diseases[7]. Long-term observations further revealed that preterm infants (PTI) are at risk for sensory, motor, cognitive, and developmental issues[8], underscoring the critical need for targeted interventions and support.
The risk factors for PTB are very complex, and one of the major categories significantly related to PTB is various complications that occur during pregnancy, including intrahepatic cholestasis of pregnancy, gestational diabetes mellitus (GDM), premature rupture of membranes (PROM), placenta previa, placental abruption, fetal distress, and multiple pregnancy[9,10]. In addition to the abovementioned pregnancy complications, PTB is also related to many other factors, such as socioeconomic factors, prepregnancy physical conditions, nutritional status during pregnancy, lifestyle habits during pregnancy, environmental and psychological factors, and reproductive history[2]. The existing frameworks for predicting PTB involve significant challenges due to the intricate web of risk factors, including genetic, environmental, and socioeconomic factors[11]. These complexities hinder accurate predictions, leading to a management approach that is often reactive rather than preventive. Consequently, there is an urgent need for the development of more sophisticated and nuanced prediction models. Such models would not only improve the accuracy of PTB predictions but also shift the paradigm from emergency care to targeted, preemptive interventions. By enhancing our predictive capabilities, we can create opportunities for developing personalized treatment plans, tailored interventions, and comprehensive support strategies, thereby significantly mitigating the health risks associated with PTB for both mothers and infants[5].
PTIs often require care in the neonatal intensive care unit, while their mothers experience the stress of physical and mental discomfort and separation from their infants[12]. This situation can increase the risk of postpartum anxiety and depression for mothers, who worry about their PTIs’ health, survival, and future development[13]. This study aimed to analyze the clinical risk factors for PTB and assess postpartum anxiety and depression in mothers, providing a theoretical basis for PTB treatment and prevention. Future research should explore innovative interventions to support both PTIs and their mothers, enhancing care strategies and psychological support to mitigate the impact of PTB on families.
In this retrospective study, 1041 parturients who delivered at Shenzhen Bao’an District Songgang People’s Hospital between January 2019 and January 2022 were selected as study participants, and their clinical data were collected for regression analysis.
The inclusion criteria for patients were as follows: All patients who underwent standardized and regular prenatal examinations at our hospital during pregnancy and delivered at our hospital and had complete clinical data.
The exclusion criteria were as follows: Patients with medical PTB, such as PTB due to placenta accreta, threatened uterine rupture, chorioamnionitis, and pregnancy-induced hypertension; patients with early termination of pregnancy due to placental abruption, fetal distress, fetal growth and development delay, etc.; patients who were not advised to continue pregnancy due to clinical safety considerations; patients who underwent cervical cerclage during or before pregnancy due to “cervical insufficiency”; and patients with a prior history of conization of the cervix.
In this study, a total of 648 eligible parturients were screened according to the inclusion and exclusion criteria. According to the diagnostic criteria for PTIs (28 wk ≤ gestational age < 37 wk and fetal weight ≥ 1000 g), the parturients were divided into a PTB group (n = 60) and a full-term (FT) group (n = 588).
Clinical data and laboratory-related indicators were collected from electronic medical records and outpatient records. The clinical data collected included age, prepregnancy body mass index (BMI), prepregnancy disease history (history of malignant tumors, hyperthyroidism, hypothyroidism, diabetes, and heart disease), weight change during pregnancy, ethnicity, parity, mode of conception (spontaneous conception or conception by assisted reproductive technology), history of tobacco and alcohol exposure during pregnancy, whether nutritional supplements were used during pregnancy, and whether regular physical activity was performed during pregnancy (30 min of low-intensity exercise daily). Laboratory indicators included the last routine blood and leucorrhea examination before delivery, the last ultrasound examination before delivery, and the cervical length measured by transvaginal ultrasound in the second trimester. The women were assessed for negative emotions in the postpartum period using the Self-rating Anxiety Scale (SAS) and Self-rating Depression Scale (SDS)[14]. Both scales consist of 20 items answered using a 4-point scale. The standard score was the integer obtained by multiplying the total score of the 20 items by 1.25. Mothers with a standard SAS score ≥ 50 points were considered to have anxiety, and those with a standard SDS score ≥ 53 points were considered to have depression.
The primary outcome measures were as follows: Univariate analysis was performed to identify factors influencing PTB, and the risk factors for PTB were further determined using logistic regression.
The secondary outcome measures were as follows: The postpartum SAS and SDS scores were recorded to screen for anxiety and depression symptoms.
In this study, R language 4.1.1 software (R Foundation for Statistical Computing, Vienna, Australia) was used for data sorting and analysis, and a prediction model was established. Logistic regression was used to screen the influencing factors, and their clinical value was verified by receiver operating characteristic (ROC) curve analysis. Data analysis and visualization were performed with Graph Pad Prism 8.0. Normally distributed data are statistically described as the mean ± SD; comparisons between two groups were performed with a t test, whereas intergroup comparisons were performed with an independent sample t test, and intragroup comparisons were performed with a paired t test. The χ2 test was used to compare count data. In all tests, a significance level of 5% (P < 0.05) was adopted.
The clinical data of the two groups were compared. The results showed that the PTB group was older than the FT group, with a smaller pregnancy weight change and greater proportions of women who underwent artificial insemination and had GDM (all P < 0.05, Table 1); no significant differences were identified in other clinical data (P > 0.05, Table 1).
| Categories | Preterm birth group (n = 60) | Full term group (n = 588) | P value | |
| Age | 0.003b | |||
| ≥ 35 years old | 47 | 341 | ||
| < 35 years old | 13 | 247 | ||
| Prenatal BMI | 0.355 | |||
| ≥ 23 kg/m2 | 42 | 376 | ||
| < 23 kg/m2 | 18 | 212 | ||
| History of pre-pregnancy diseases | 0.540 | |||
| With | 5 | 35 | ||
| Without | 55 | 553 | ||
| Maternal weight change (kg) | 8.8 ± 4.7 | 12.1 ± 3.9 | < 0.001c | |
| Parity | 0.608 | |||
| Primipara | 47 | 441 | ||
| Multipara | 13 | 147 | ||
| Mode of conception | 0.005 | |||
| Spontaneous conception | 52 | 559 | ||
| Artificial insemination | 8 | 29 | ||
| History of alcohol and tobacco exposure during pregnancy | 0.361 | |||
| With | 5 | 35 | ||
| Without | 55 | 553 | ||
| Nutritional supplements during pregnancy | ||||
| Folic acid supplementation in early pregnancy | 47 | 417 | 0.252 | |
| Multivitamin supplements in the second trimester | 49 | 441 | 0.303 | |
| No nutritional supplements during pregnancy | 4 | 41 | 0.771 | |
| Regular physical activity | 0.562 | |||
| Yes | 9 | 106 | ||
| No | 51 | 482 | ||
| Gestational diabetes mellitus | 0.001b | |||
| Yes | 11 | 35 | ||
| No | 49 | 553 |
Compared with the FT group, the PTB group had a significantly greater proportion of women with reproductive tract infections, greater white blood cell (WBC) counts, shorter cervical lengths in the second trimester, and lower neutrophil counts (P < 0.001, Table 2).
We compared postpartum anxiety and depression scores between the two groups. The results revealed lower postpartum SAS and SDS scores in the FT group than in the PTB group (P < 0.0001, Figure 1). Moreover, more mothers in the PTB group than in the FT group experienced symptoms of anxiety and depression, as indicated by the chi-square test (P < 0.001, Table 3).
We assigned values to the abovementioned factors with statistical significance (Table 4) and then used the backward LR method to identify risk factors for PTB. A greater maternal weight change, the presence of GDM, a shorter cervical length in the second trimester, a greater WBC count, and the presence of maternal anxiety and depression were confirmed to be risk factors for PTB (Table 5, P < 0.01).
| Factors | Assignment |
| Age | ≥ 35 years old = 1, < 35 years old = 0 |
| Maternal weight change (kg) | ≥ 10.45 = 1, < 10.45 = 0 |
| Mode of conception | Artificial insemination = 1, spontaneous conception = 0 |
| Gestational diabetes mellitus | With = 1, without = 0 |
| Reproductive tract infection | With = 1, without = 0 |
| Cervical length in the second trimester (mm) | ≥ 29.05 = 1, < 29.05 = 0 |
| White blood cell count (× 109/L) | ≥ 9.35 = 1, < 9.35 = 0 |
| Neutrophil percentage | ≥ 0.85 = 1, < 0.85 = 0 |
| Maternal anxiety and depression | With = 1, without = 0 |
| Delivery status | Premature = 1, full-term = 0 |
| Factors | β | Standard error | χ2 | P value | OR | 95%CI | |
| Lower bound | Upper bound | ||||||
| Age | 0.650 | 0.428 | 2.315 | 0.128 | 1.916 | 0.829 | 4.429 |
| Maternal weight change | -1.453 | 0.396 | 13.456 | < 0.001c | 0.234 | 0.108 | 0.508 |
| Mode of conception | 0.967 | 0.674 | 2.057 | 0.151 | 2.630 | 0.702 | 9.855 |
| Gestational diabetes mellitus | 1.577 | 0.586 | 7.244 | 0.007b | 4.839 | 1.535 | 15.254 |
| Reproductive tract infection | 0.811 | 0.500 | 2.629 | 0.105 | 2.250 | 0.844 | 5.997 |
| Cervical length in the second trimester | -3.784 | 0.521 | 52.804 | < 0.001c | 0.023 | 0.008 | 0.063 |
| White blood cell count | 2.255 | 0.512 | 19.420 | < 0.001c | 9.537 | 3.498 | 26.004 |
| Maternal anxiety and depression | 1.802 | 0.391 | 21.301 | < 0.001c | 6.064 | 2.821 | 13.036 |
A risk prediction model based on logistic regression coefficients was built. Since anxiety and depression are postpartum factors, we excluded them as predictors of PTB. We then constructed a risk formula with a risk score calculated as follows: -1.453 × maternal weight change + 1.577 × GDM + -3.784 × cervical length in the second trimester + 2.255 × WBC count. Then, we compared the risk scores between the FT and PTB groups, and a significantly lower risk score was found in the FT group than in the PTB group (P < 0.0001, Figure 2A). In addition, the area under the ROC curve (AUC) of the risk score for predicting PTB in pregnant women was 0.937, with a specificity of 90.00% and a sensitivity of 86.73% (Figure 2B).
Though it remains to be further elucidated, the pathogenesis of PTB, according to the available findings, is associated with multiple factors, such as pregnancy complications, maternal socioeconomic factors, living habits (smoking, drinking, and eating habits, etc.), reproductive history (history of abortion, history of premature birth, the use of assisted reproductive technology, etc.), environmental and psychological factors, antenatal care, and nutritional status[15]. PTB is known to be extremely harmful to preterm infants’ families and leads to a high mortality rate of PTIs, and those who survive may experience a variety of sequelae and complications[16], imposing a substantial burden on the families of PTIs and society. In this context, it would be beneficial to be able to predict PTB in advance, which would provide more opportunities for early intervention to reduce the negative emotions caused by PTB and the consequences of PTB.
In this study, we analyzed the clinical risk factors for PTB. According to related research[17], PTB and its associated complications are the major factors leading to neonatal death, with approximately 15% of premature babies dying in the neonatal stage. According to World Health Organization statistics[18], there are more than 13 million PTIs worldwide, with a PTB rate of nearly 10%, while the rate in Asia is approximately 9%. The PTB rate in this study was 9.3%, which is consistent with the existing records. In our study, a greater maternal weight change, the presence of GDM, a shorter cervical length in the second trimester, a greater WBC count, and the presence of maternal anxiety and depression were risk factors for PTB.
Overweight and obesity increase the risk for high birth weight and PTB in offspring[19]. On the other hand, an underweight BMI prior to conception is associated with a reduced risk of PTB. In all initial weight categories, insufficient weight change in pregnant women is linked to an increased incidence of spontaneous PTB and PROM-related PTB (PROM-PTB)[20]. Generally, an increase in weight in pregnant women predicts an increase in the probability of all types of PTB[21]. Therefore, maintaining normal weight gain is one of the keys to reducing PTB. GDM, a type of diabetes that pregnant women may experience, occurs during pregnancy and usually disappears after delivery[22]. This may lead to high blood sugar levels in pregnant women, exerting some negative effects on the mother and fetus. Research has shown that GDM may cause a fetus to be too large because too much glucose may be absorbed and converted into fat[23]. An oversized fetus can induce PTB because early labor may be triggered, or doctors may choose early delivery because the fetus is too large. In addition, GDM may increase the risk of pregnancy-induced hypertension and preeclampsia, further increasing the risk of PROM and leading to PTB. Furthermore, GDM can cause maternal blood sugar levels to be difficult to control, which can affect fetal development and increase the risk of PTB. Previously, Pigatti Silva et al[21] reported that pregnancy complications were risk factors for PTB. However, in their research, pregnancy complications included pregnancy-induced hypertension, GDM, intrahepatic cholestasis of pregnancy, prenatal and postpartum hemorrhage, placental abnormalities, etc., which cannot demonstrate the role of GDM as an independent risk factor for PTB. In contrast, Dekker et al[24] reported that GDM was an independent risk factor for PTB, suggesting that maternal blood glucose changes should be monitored in a timely manner and that early detection and treatment should be carried out to improve pregnancy outcomes.
In normal adult nonpregnant women, the cervix is usually 25 mm-30 mm in length. Ultrasonic measurement of cervical length requires a rigorous sagittal section to show the morphology of the internal and external cervical os and the length of the cervical canal[25]. Under normal circumstances, the internal cervical os is closed and has a certain tension that prevents the fetus and its appendages (such as the fetal membrane and placenta) from moving toward the cervical canal. The cervix gradually matures as gestational age increases. However, premature maturation of the cervix can cause the cervix to shorten significantly, causing the cervix to be squeezed as the fetus moves down[26]. This further shortens the length of the cervical canal and even leads to the expansion of the external cervix and the discharge of the mucus plug, ultimately leading to an increased risk of PTB. Inflammation is the physiological response of the body to infection, tissue injury or other stimuli and can lead to the release of inflammatory mediators and cytokines[27]. The WBC count is an index that directly reflects the inflammatory reaction in patients. Infection is one of the most common causes of inflammation-related PTB, and bacterial, viral or other microbial infections can increase the amount of WBCs in the body[28]. The release of massive amounts of inflammatory mediators following an inflammatory response may lead to uterine contraction and cervical relaxation, resulting in premature labor. Postpartum anxiety and PTB affect and interact with each other[29]. Postpartum anxiety may increase the risk of PTB, and PTB itself may also increase the incidence of postpartum anxiety[30]. Preterm mothers face multiple forms of distress and anxiety, including separation anxiety and concerns about the health and development of their baby[31]. Therefore, early psychological support and intervention are crucial for preterm mothers. Emotional support, education and counseling can help mothers cope with anxiety and enhance their coping skills and emotional regulation, thereby reducing the occurrence and impact of postpartum anxiety and positively impacting the health and development of PTIs. Therefore, attention should be given to mental health in the care of preterm mothers, and comprehensive support and care should be provided to promote the overall well-being of PTIs and their mothers. At the end of the study, we built a risk model based on the identified risk factors. It was found that the AUC of the risk prediction model was greater than 0.9, demonstrating that this model is an excellent potential predictive tool.
However, there are still some limitations in this study. First, the participants were all from our hospital, which is a single center, leading to a small sample size. Second, although the model was established, the influencing factors were not systematically reviewed from an evidence-based perspective, and the included indicators were not comprehensive. Furthermore, there may be statistical biases in the data collection and analysis process. Therefore, whether the prediction model can be widely used in clinical practice still needs further verification and discussion.
This study highlights the complex interaction between postpartum anxiety and PTB, i.e., that maternal anxiety may be a potential risk factor for PTB, and PTB may increase the incidence of postpartum anxiety in mothers. In addition, the study identified a greater maternal weight change, the presence of GDM, a shorter cervical length, a greater WBC count, and the presence of postpartum anxiety and depression as risk factors for PTB.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade C
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Gibson G, United States S-Editor: Zhang L L-Editor: A P-Editor: Zhao S
| 1. | Walani SR. Global burden of preterm birth. Int J Gynaecol Obstet. 2020;150:31-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 503] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 2. | Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 567] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 3. | Bonuccelli GA, Negrini R, da Silva Ferreira RD. Premature Birth in Women with Endometriosis: a Systematic Review and Meta-analysis. Reprod Sci. 2022;29:250-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reprod Biol Endocrinol. 2017;15:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 5. | Cnattingius S, Johansson S, Razaz N. Apgar Score and Risk of Neonatal Death among Preterm Infants. N Engl J Med. 2020;383:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 6. | Lorthe E. [Epidemiology, risk factors and child prognosis: CNGOF Preterm Premature Rupture of Membranes Guidelines]. Gynecol Obstet Fertil Senol. 2018;46:1004-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Sykes L, Bennett PR. Efficacy of progesterone for prevention of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Song J, Wang Y, Xu F, Sun H, Zhang X, Xia L, Zhang S, Li K, Peng X, Li B, Zhang Y, Kang W, Wang X, Zhu C. Erythropoietin Improves Poor Outcomes in Preterm Infants with Intraventricular Hemorrhage. CNS Drugs. 2021;35:681-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Lee WL, Chang WH, Wang PH. Risk factors associated with preterm premature rupture of membranes (PPROM). Taiwan J Obstet Gynecol. 2021;60:805-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Jiang M, Mishu MM, Lu D, Yin X. A case control study of risk factors and neonatal outcomes of preterm birth. Taiwan J Obstet Gynecol. 2018;57:814-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Shen ZZ, Wang YW, Ma S, Zhan YL, Wu SS, Feng YH, Cai SY, Ma LK, Jiang Y; Chinese Pregnant Women Cohort Study-Peking Union Medical College Collaborative Group. [Risk factors for preterm birth, low birth weight and small for gestational age: a prospective cohort study]. Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40:1125-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 12. | Ribeiro MKA, Alcântara-Silva TRM, Oliveira JCM, Paula TC, Dutra JBR, Pedrino GR, Simões K, Sousa RB, Rebelo ACS. Music therapy intervention in cardiac autonomic modulation, anxiety, and depression in mothers of preterms: randomized controlled trial. BMC Psychol. 2018;6:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Lebel C, MacKinnon A, Bagshawe M, Tomfohr-Madsen L, Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J Affect Disord. 2020;277:5-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 576] [Article Influence: 115.2] [Reference Citation Analysis (0)] |
| 14. | Yue T, Li Q, Wang R, Liu Z, Guo M, Bai F, Zhang Z, Wang W, Cheng Y, Wang H. Comparison of Hospital Anxiety and Depression Scale (HADS) and Zung Self-Rating Anxiety/Depression Scale (SAS/SDS) in Evaluating Anxiety and Depression in Patients with Psoriatic Arthritis. Dermatology. 2020;236:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Brown K, Langston-Cox A, Unger HW. A better start to life: Risk factors for, and prevention of, preterm birth in Australian First Nations women - A narrative review. Int J Gynaecol Obstet. 2021;155:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Abdelwahab M, Petrich M, Wang H, Walker E, Cleary EM, Rood KM. Risk factors for preterm birth among gravid individuals receiving buprenorphine for opioid use disorder. Am J Obstet Gynecol MFM. 2022;4:100582. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Fettweis JM, Serrano MG, Brooks JP, Edwards DJ, Girerd PH, Parikh HI, Huang B, Arodz TJ, Edupuganti L, Glascock AL, Xu J, Jimenez NR, Vivadelli SC, Fong SS, Sheth NU, Jean S, Lee V, Bokhari YA, Lara AM, Mistry SD, Duckworth RA 3rd, Bradley SP, Koparde VN, Orenda XV, Milton SH, Rozycki SK, Matveyev AV, Wright ML, Huzurbazar SV, Jackson EM, Smirnova E, Korlach J, Tsai YC, Dickinson MR, Brooks JL, Drake JI, Chaffin DO, Sexton AL, Gravett MG, Rubens CE, Wijesooriya NR, Hendricks-Muñoz KD, Jefferson KK, Strauss JF 3rd, Buck GA. The vaginal microbiome and preterm birth. Nat Med. 2019;25:1012-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 585] [Reference Citation Analysis (0)] |
| 18. | Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, Lewis C, Rattanakanokchai S, Teng DN, Thinkhamrop J, Watananirun K, Zhang J, Zhou W, Gülmezoglu AM. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7:e37-e46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1752] [Cited by in RCA: 1859] [Article Influence: 309.8] [Reference Citation Analysis (0)] |
| 19. | Kong L, Nilsson IAK, Gissler M, Lavebratt C. Associations of Maternal Diabetes and Body Mass Index With Offspring Birth Weight and Prematurity. JAMA Pediatr. 2019;173:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 20. | Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, Li N, Hu G, Corrado F, Rode L, Kim YJ, Haugen M, Song WO, Kim MH, Bogaerts A, Devlieger R, Chung JH, Teede HJ. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA. 2017;317:2207-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 1135] [Article Influence: 141.9] [Reference Citation Analysis (0)] |
| 21. | Pigatti Silva F, Souza RT, Cecatti JG, Passini R Jr, Tedesco RP, Lajos GJ, Nomura ML, Rehder PM, Dias TZ, Oliveira PF, Silva CM; Brazilian Multicenter Study on Preterm Birth (EMIP) study group. Role of Body Mass Index and gestational weight gain on preterm birth and adverse perinatal outcomes. Sci Rep. 2019;9:13093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Kattini R, Hummelen R, Kelly L. Early Gestational Diabetes Mellitus Screening With Glycated Hemoglobin: A Systematic Review. J Obstet Gynaecol Can. 2020;42:1379-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Malaza N, Masete M, Adam S, Dias S, Nyawo T, Pheiffer C. A Systematic Review to Compare Adverse Pregnancy Outcomes in Women with Pregestational Diabetes and Gestational Diabetes. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 24. | Dekker GA, Lee SY, North RA, McCowan LM, Simpson NA, Roberts CT. Risk factors for preterm birth in an international prospective cohort of nulliparous women. PLoS One. 2012;7:e39154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Dude A, Miller ES. Change in Cervical Length across Pregnancies and Preterm Delivery. Am J Perinatol. 2020;37:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Romero R, Conde-Agudelo A, Da Fonseca E, O'Brien JM, Cetingoz E, Creasy GW, Hassan SS, Nicolaides KH. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 320] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 27. | Gomez-Lopez N, Galaz J, Miller D, Farias-Jofre M, Liu Z, Arenas-Hernandez M, Garcia-Flores V, Shaffer Z, Greenberg JM, Theis KR, Romero R. The immunobiology of preterm labor and birth: intra-amniotic inflammation or breakdown of maternal-fetal homeostasis. Reproduction. 2022;164:R11-R45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 28. | Menon R. Fetal inflammatory response at the fetomaternal interface: A requirement for labor at term and preterm. Immunol Rev. 2022;308:149-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Jung E, Romero R, Yeo L, Diaz-Primera R, Marin-Concha J, Para R, Lopez AM, Pacora P, Gomez-Lopez N, Yoon BH, Kim CJ, Berry SM, Hsu CD. The fetal inflammatory response syndrome: the origins of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin Fetal Neonatal Med. 2020;25:101146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 30. | van der Zee-van den Berg AI, Boere-Boonekamp MM, Groothuis-Oudshoorn CGM, Reijneveld SA. Postpartum depression and anxiety: a community-based study on risk factors before, during and after pregnancy. J Affect Disord. 2021;286:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 31. | Staneva A, Bogossian F, Pritchard M, Wittkowski A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: A systematic review. Women Birth. 2015;28:179-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 441] [Article Influence: 44.1] [Reference Citation Analysis (0)] |