Published online May 19, 2024. doi: 10.5498/wjp.v14.i5.624
Revised: April 20, 2024
Accepted: April 26, 2024
Published online: May 19, 2024
Processing time: 102 Days and 4.7 Hours
Dystonia characterizes a group of neurological movement disorders characterized by abnormal muscle movements, often with repetitive or sustained contraction resulting in abnormal posturing. Different types of dystonia present based on the affected body regions and play a prominent role in determining the potential efficacy of a given intervention. For most patients afflicted with these disorders, an exact cause is rarely identified, so treatment mainly focuses on symptomatic alleviation. Pharmacological agents, such as oral anticholinergic administration and botulinum toxin injection, play a major role in the initial treatment of patients. In more severe and/or refractory cases, focal areas for neurosurgical intervention are identified and targeted to improve quality of life. Deep brain stimulation (DBS) targets these anatomical locations to minimize dystonia symptoms. Surgical ablation procedures and peripheral denervation surgeries also offer potential treatment to patients who do not respond to DBS. These management options grant providers and patients the ability to weigh the benefits and risks for each individual patient profile. This review article explores these pharmacological and neurosurgical management modalities for dystonia, providing a comprehensive assessment of each of their benefits and shortcomings.
Core Tip: Dystonia is a neurological movement disorder affecting different regions of the body with variable responses to current interventions. Pharmacological agents, such as oral anticholinergic and botulinum toxin injection, play a major role in the initial treatment of patients. However severe and/or refractory cases require the identification and targeting of focal areas for neurosurgical intervention. Deep brain stimulation (DBS) targets these anatomical locations to manage symptoms. Surgical ablation procedures and peripheral denervation surgeries also offer potential treatment to patients who do not respond to DBS.
- Citation: Mohamed AA, Faragalla S, Khan A, Flynn G, Rainone G, Johansen PM, Lucke-Wold B. Neurosurgical and pharmacological management of dystonia. World J Psychiatry 2024; 14(5): 624-634
- URL: https://www.wjgnet.com/2220-3206/full/v14/i5/624.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i5.624
Dystonia is the third most common movement related disorder, behind Parkinson’s and essential tremor[1]. Dystonia is defined by numerous characteristics including age of onset, region of body where symptoms occur, and pattern of occurrence[2]. Clinical manifestations of dystonia vary widely but are often characterized by contracted and slowed movements with associated jerky or rapid movements intermittently occurring[3]. It is estimated that 16.7% of patients will also have an observable tremor[4]. They can occur anywhere in the body and once they occur, they usually do not regress[3].
The incidence of dystonia is commonly debated but has been estimated to affect 16.43 per 100000[5]. The incidence rates rise as the subjects age, with a study showing that there were 743 cases per 100000 in patients over the age of 50[6,7]. Pairing this rate with the additional information that only 33% of the subjects being previously diagnosed suggests that the true prevalence of dystonia is higher than officially reported[6,8]. Dystonia is more commonly reported in patients that regularly partake in skillful, precise movements such as painting or playing golf[9]. Dystonia is also more likely to occur in females[10]. Dystonia is highly linked with sensory dysfunction as well and can present as decreased sensation to light touch and proprioception[11,12]. In certain instances of focal hand dystonia, vibration of the affected arm can actually induce dystonia.
Despite the symptoms being well described, the origin of dystonia disorders is not[13,14]. With that being said, there is mounting evidence that suggests there are specific genes that could potentially play a role in the development of dystonia, which vary with the location and subclassification of dystonia[13,15]. The most common cause of early onset dystonia is a deletion at position 302/303 of TorsinA, although the function of the gene itself is still largely unknown[16]. Additional non-genetic factors, including cigarette smoking and head/neck trauma, play a role in the pathogenesis [17,18]. Thirty percent of Parkinson’s patients have dystonia, suggesting there is genetic overlap with the two conditions which are often mistaken for each other[19].
With variable presentation and treatment response based on the affected region of the body, understanding management approaches and their efficacy is crucial in providing patients with dystonia the most suitable treatment options. This review will discuss pharmacological and neurosurgical management options for dystonia, highlighting the benefits and drawbacks of each management approach.
Pharmacological management of dystonia traditionally consists of the use of anticholinergic drugs, baclofen, clonazepam, and other dopamine-related medications[20,21]. This review will also discuss the use of antipsychotics and botulinum toxin (BT) injections.
The most common anticholinergic drug used to treat dystonia is trihexyphenidyl[22]. It is a selective muscarinic acetylcholine receptor agonist that blocks cholinergic activity. It can also increase the availability of dopamine which plays a role in the initiation and control of muscle movements. The onset of action is about an hour after when administered orally, with a peak after 2-3 h, while the duration of action is between 6 h to 12 h[23]. Dosage begins at 1 mg per day and is raised until either a dose of 30 mg is achieved or adverse drug responses present[22]. A study on Dyt1 knocking mice (mice with the ΔE-TorsinA mutation which causes DYT1 dystonia) found that trihexyphenidyl can correct dopamine release in the mice indirectly through the use of a nicotinic receptor-dependent pathway[24]. Another study found that 20% of patients reported a favorable response when treated with the drug[25]. Despite the common use of trihexyphenidyl, there is literature that has shown that it may be ineffective in treating certain types of dystonia like cerebral palsy, although it did show promising results in the treatment of others like Costello syndrome[23,26]. Other anticholinergics have also been used such as benztropine, ethopropazine, procyclidine, and biperiden but did not show the same effectiveness as trihexyphenidyl[27].
Baclofen is a GABA-B agonist that binds to the pre-synaptic GABA-B receptors, leading to the hyperpolarization of the motor horn cells and reduction in the reflexes that lead to muscle spasms. Due to its inability to cross the blood-brain barrier, high doses of oral baclofen are required to reach therapeutic effects resulting in a greater possibility of adverse drug reactions such as muscle weakness, nausea, and dizziness. In contrast, intrathecal baclofen can bypass the blood-brain barrier, allowing for its administration at a lower dose. However, it is reserved for patients who experience intolerable adverse effects or fail to respond to the maximum recommended dose of oral baclofen[28]. It was found that the same proportion of patients reported a similar favorable response to baclofen as trihexyphenidyl[25]. It is admi
Clonazepam is the most commonly used benzodiazepine in the treatment of dystonia[22]. Clonazepam directly impacts the benzodiazepine receptors by impacting the GABAergic transmission in the brain. Administration begins at 0.5 mg and is increased to 1.0 mg to 4.0 mg, divided three times a day. Forty percent of patients reported a favorable response to Clonazepam[25]. Adverse effects include confusion, impaired coordination, depression, and dependence[22].
Some dystonia, such as dopamine-responsive dystonia, can be responsive to treatment with dopamine-related medi
There have also been several different studies that have utilized clozapine to treat dystonia and associated disorders[33-36]. Clozapine is an antipsychotic that will occupy dopamine D2 receptors and will be displaced after a rise in synaptic dopamine[37]. In one study, Clozapine was administered at 12.5 mg per day and was increased by 25.0 mg per day up to a total of 900.0 mg per day unless adverse drug reactions such as persistent symptomatic orthostatic hypotension or tachycardia presented. Of the five participants in the study, all had reached significant improvement in dystonia presentation by the third week, but only two continued to use the medication following the completion of the study[34]. The use of clozapine is limited due to its side effects and need for additional monitoring.
BT is commonly used for blepharospasm, adult spasticity, headache, and cervical dystonia treatment[38,39]. It is the treatment of choice for those with focal dystonia[40,41]. BT inhibits acetylcholine release in the α-motor neuron[42]. It is injected into muscles and produces a localized peripheral paresis[43]. Therapeutic effects usually manifest within 2 wk and will last for about 3 to 4 months[22]. Adverse effects include dry mouth, neck weakness, dysphagia, and voice changes/hoarseness. Most adverse effects were considered to be mild[41].
Certain types of dystonia had a more favorable response to BT injections. For instance, BT injection benefits lasted significantly longer in patients who used treatment for focal handdystonia[44]. There is also some evidence that BT type A could be affected by changes in the cerebral cortex[45]. In order to administer the toxin, ultrasound can be used. This allows the injector to clearly identify and inject the correct muscle rather than relying on blind injections. It also allows for the accurate administration of the drug to deeper muscles[46]. Unfortunately, there is a possibility of developing antibodies for BT, resulting in the need for more frequent injections[47].
Neurosurgical management of dystonia is considered in cases that impact quality of life and activities of daily living, typically following a lack of response to pharmacological interventions[48]. Deep brain stimulation (DBS) is the mainstay of treatment, replacing previously common ablative procedures[49-52]. Along with DBS, other surgical procedures include pallidotomy, thalamotomy, and peripheral denervation[48].
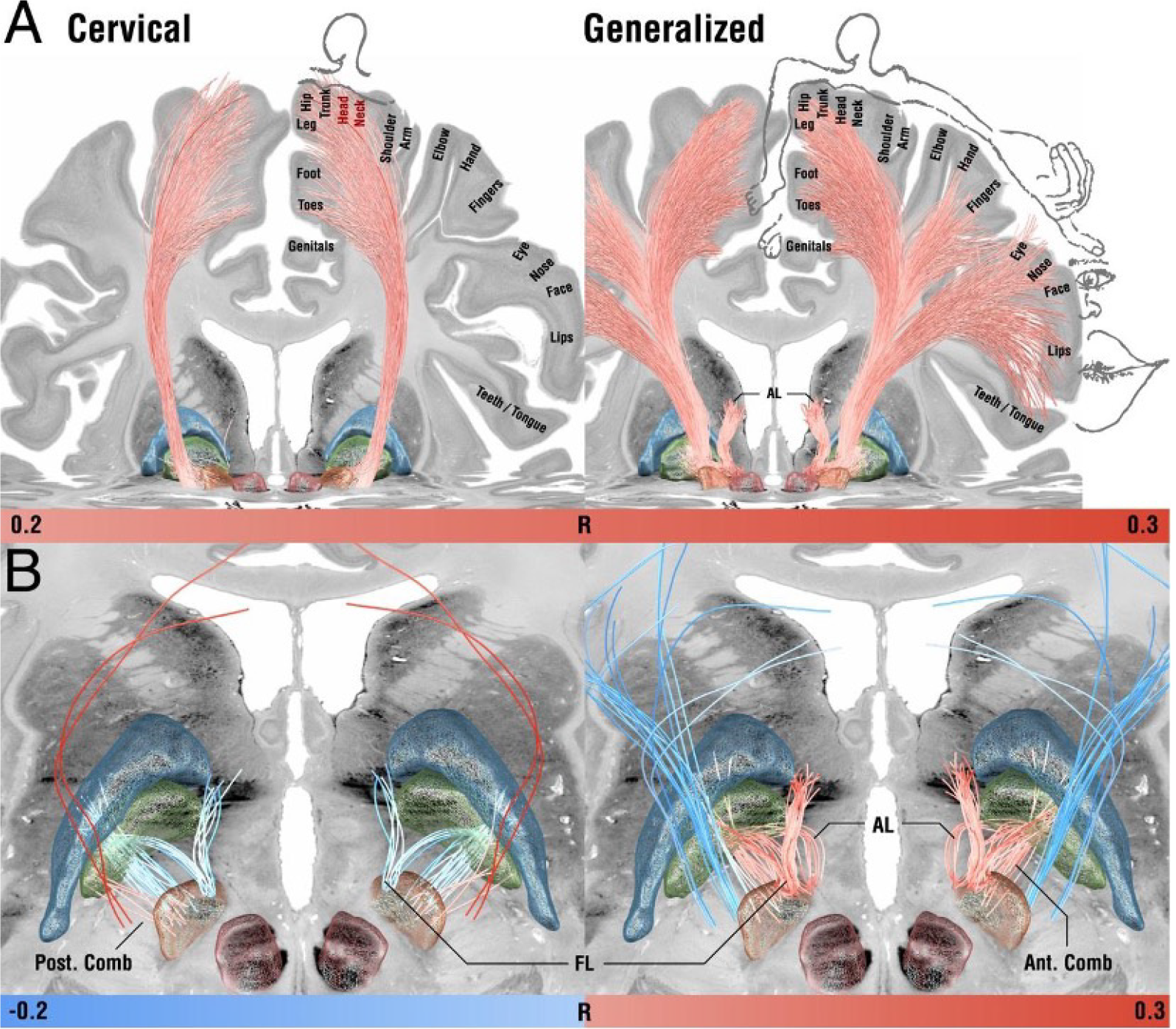
The use of DBS for treatment of dystonia has been validated by several cohort studies, demonstrating significant improvements of symptoms from 3 months to up to 7 years[50,51,53,54]. Several target sites have been investigated for DBS treatment of dystonia, including the ventral intermediate nucleus of the thalamus, the subthalamic nucleus, and the globus pallidus pars interna (Figure 1)[55-59], with the primary target site being the globus pallidus pars interna[60-63]. Electrodes are implanted at sites relevant to muscle contractility such as the subthalamic nucleus and globus pallidus pars interna, exhibiting stimulatory effects on axon terminals, in turn, inducing neurotransmitter release[64]. This external source of activation stimulates the malfunctioning regions to produce their normal functions. The frequency of stimulation has the potential to alter both the rate and pattern of firing of the neurons in the area surrounding the electrode[65]. Adverse effects of treatment may include incoordination, postural instability, rigidity, dysphonia, dysarthria, paresthesia, perioral tingling, and micrographia. These effects are mediated by way of inadvertent targeting of neighboring structures around the globus pallidus pars interna, including the optic tract ventrally, internal capsule medially, and hypothalamus superiorly[66,67]. Other hardware related adverse events include infection of the skin or implant, malfunction related to internal pulse generator erosion of failure, and electrode or extension wire damage[68]. Battery life is an additional challenge of DBS, presenting with the most difficulty in pediatric cases where many battery changes are required over the course of their life and thus a higher risk of surgical complications is apparent[69,70]. The advent of rechargeable devices has somewhat alleviated this problem in both pediatric and adult populations, demonstrating lower complication rates and higher patient satisfaction[71,72]. DBS of the subthalamic nucleus and globus pallidus pars interna has also demonstrated favorable outcomes in patients who previously underwent pallidotomy but continued to present with progressive symptoms[73,74].
In pediatric patients, benefits following DBS have been reported 5 or more years following implantation[69]. The anatomical changes depending on the age of the child also pose difficulties as the anatomical structure is growing rapidly and altering the surgical target site. Intraoperative visualization of brain anatomy and site targeting require significantly increased precision to successfully localize DBS electrodes[75,76]. Furthermore, children may lack the appropriate endurance and communication abilities needed to determine the therapeutic window during the postoperative sti
Electrode types for DBS are platinum-iridium wires and connectors made of nickel alloy encased in a sheath of polyurethane[83]. Electrode configurations vary based on contact number, shape, and spacing. Precise stimulation control is achieved with small contact spacing whereas a greater range of neural targets can be achieved with increased contact spacing. Stimulation types include unipolar, bipolar, interleaving, multiple level, and directional. Unipolar stimulation describes current movement directed either from the battery to the contact or from the contact to the battery. Bipolar stimulation describes current movement between at least one cathode and one anode contact. Interleaving stimulation describes a system of interchanging settings and multiple level stimulation allows for stimulation of several neuronal targets along the electrodes trajectory. Directional stimulation allows for current shaping. Directional stimulation has specifically demonstrated treatment efficacy and reduced adverse effects[84]. Because of the use of radially segmented contacts, directional stimulation enables horizontal plane movement of the stimulation field. In the context of directional stimulation, increasing contact numbers and current amplitudes must be weighed against their impact on treatment feasibility in the context of programming and hindrances on stimulation field shaping.
Ablation approaches include radiofrequency ablation, stereotactic radiosurgery, and magnetic resonance imaging (MRI)-guided focused ultrasound (MRgFUS)[48,85-88]. The main concern for these procedures is the irreversible nature and potential damage of neighboring structures near the site of ablation. With the main target site being the thalamus, the surgical depth required carries an increased risk for damaging surrounding structures such as those of the optic tract, internal capsule, and hypothalamus[89]. Despite these concerns, recent studies emphasize the non-invasive nature of MRgFUS, highlighting the precision allowing for ablation of target tissue without damage to surrounding structures[90-93]. MRgFUS uses focused ultrasound waves to generate heat at the target area, resulting in coagulative necrosis which non-invasively destroys the targeted tissue. This is guided with high-resolution MRI imaging to allow clinicians to precisely visualize the target tissue and surrounding structures. The continuous monitoring and adjustment during the procedure maximizes safety and accuracy. Additionally, the development of dysarthria is an important, potentially irreversible side effect that may occur with bilateral ablation, most likely attributable to ventrolateral thalamic damage[52,94,95]. With the advent of DBS, surgical ablation may still be indicated in cases where patients are not eligible for DBS, such as limited access to postoperative programming, previous hardware complications, or previous infections[48]. In such cases, evaluation of the benefits and drawbacks of different surgical ablation techniques is required.
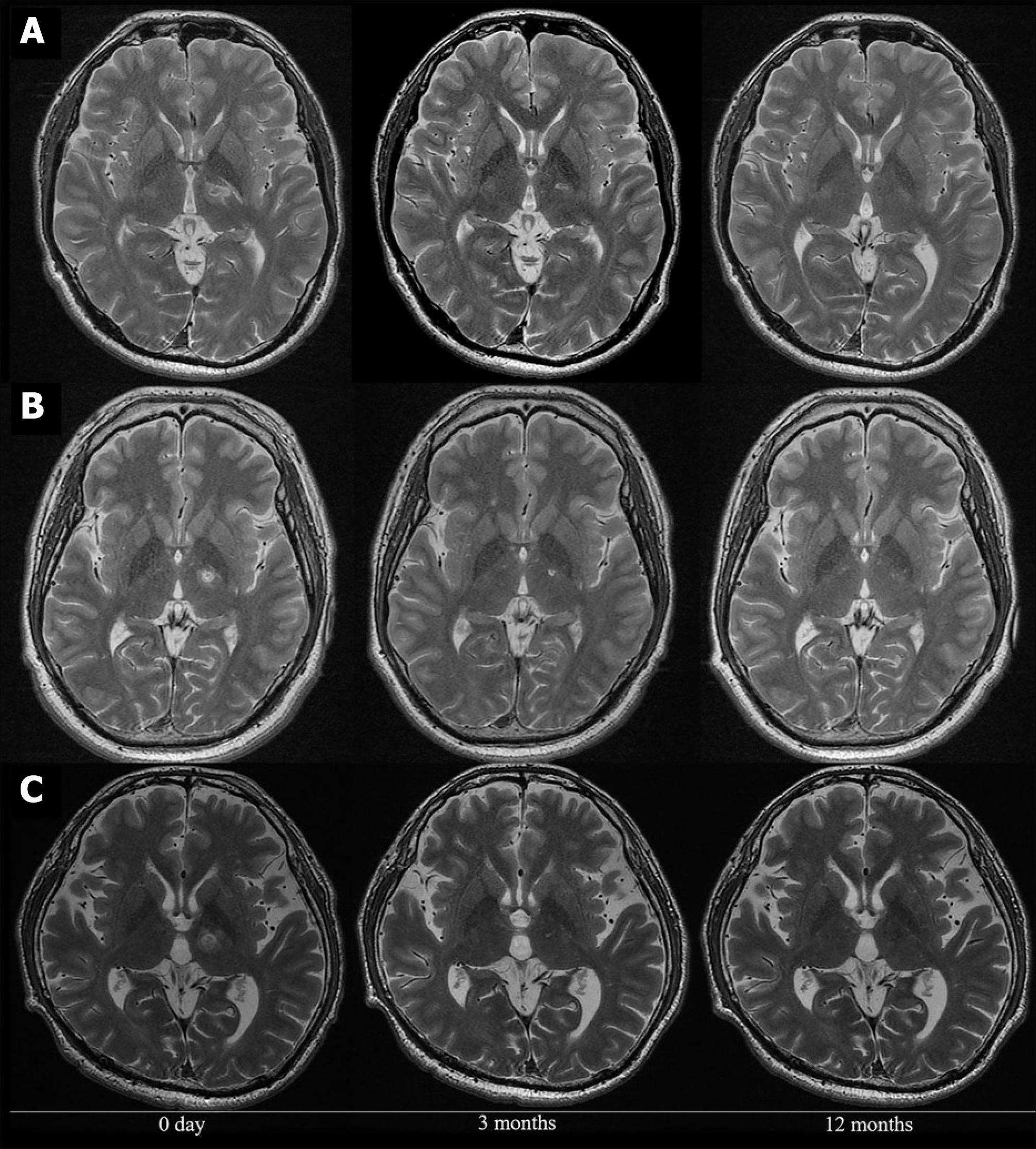
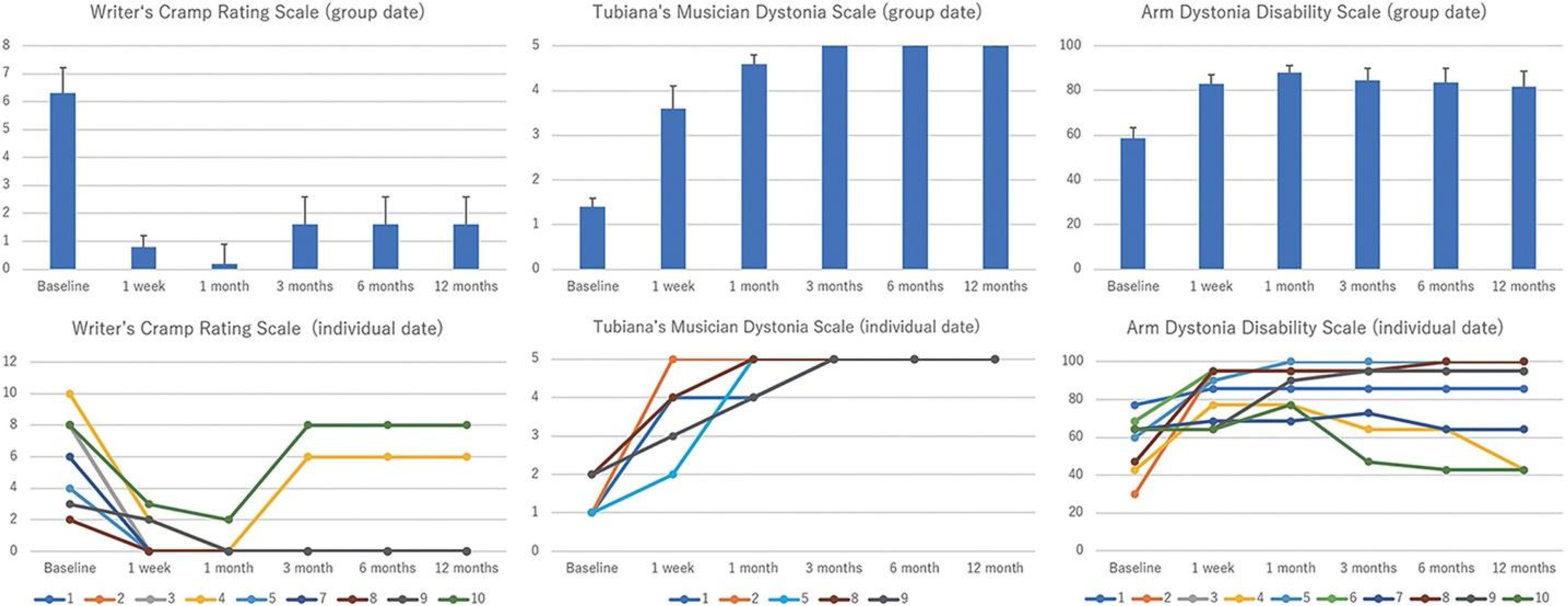
MRgFUS is a common option for surgical ablation, allowing for preoperative region mapping, non-invasive administration, and attenuation of damage to neighboring structures[96,97]. However, gait disturbances, paresthesias, and limb dysmetria are considerable side effects associated with this procedure, often due to the primary lesion itself or to perilesional edema[98-100]. MRgFUS may be the preferred technique for surgical ablation as more transient and less severe adverse effects have been reported compared with radiofrequency ablation[86,101,102]. For radiofrequency ablation, electrode placement requires craniotomy or a small burr hole[103,104]. However, recent trials have demonstrated safe bilateral lesioning with this technique[104]. Additionally, the capacity for region mapping prior to the procedure gives radiofrequency ablation an upper hand over stereotactic radiosurgery in preoperative surgical preparation. Although stereotactic radiosurgery provides a non-invasive alternative, radiation induced neurotoxicity is an additional accompanying drawback of the procedure[105-107]. MRgFUS coalesces both the region mapping abilities of radiofrequency ablations and decreased adverse effect profile of the stereotactic technique, placing it at the forefront of therapeutic options for patients with dystonia[108]. A pilot study investigated the efficacy of MRgFUS thalamotomy of the ventro-oral nucleus of the thalamus in 10 patients with focal hand dystonia (Figure 2). They reported significantly improved symptoms and only one serious adverse event (suicide attempt) related to previously concealed attempts and depression, in addition to mild dysarthria in one patient at 12 months (Figure 3). The study sample included professional musicians, writers, and dart-related dystonia. Researchers utilized three scales for quantifying patient clinical improvement: Writer’s Cramp Rating Scale, Tubiana Musician’s Dystonia Scale, and Arm Dystonia Disability Scale. All three scales demonstrated statistically significant improvement 12 months after treatment. These findings are critical in suggesting the potential benefits of MRgFUS utilization in patients with dystonia, underpinning its clinical efficacy and safety profile.
Thalamotomy constitutes the main surgical ablation procedure for dystonia and has been evaluated in many controlled studies[109]. Small lesions of the thalamus appear reliable and even provide less risk of infection in comparison to DBS, the primary treatment for focal dystonia. Thalamotomy also provides permanent effects and is a more financially achievable technique than DBS. Pallidotomy has not been evaluated in any controlled studies for dystonia but has demonstrated efficacy in a number of case series[52,88,94]. Irreversible side effects remain a potential complication for any type of ablation procedure[109]. Because ablations for dystonia involve the severing of efferent nerve tracts including the pallidothalamic tract and ventral intermediate nucleus, absolute precision is required to mitigate the risk of dysphagia, dysesthesia, and local muscle atrophy can be a result of the procedure[110]. Besides the ventro-oral nucleus, other common sites of thalamotomy include the ventral intermediate nucleus and the globus pallidus pars interna.
The use of peripheral denervation surgery has demonstrated efficacy for treating cervical dystonia in several case series[111-114]. Peripheral denervation is a selective surgery that resects and avulses the specific nerves innervating the muscles responsible for dystonia. Short electrical stimulations are then used to identify other small nerve branches that could be supplementing dystonia symptoms. This is all done with respect to the accessory nerve to avoid post-operative shoulder weakness[111]. Targeted muscles are based on the dominant subtype contributing to symptoms and include muscles of the posterior neck and the sternocleidomastoid muscle[115-117]. Since it is an invasive option, it is typically implemented after conservative measures of treatment have proven ineffective or inappropriate. Additionally, the practice of peripheral denervation has been available for longer than other surgical management options but clinical preference remains unestablished, with one review of 18 studies including both DBS and peripheral denervation use suggesting a current lack of identification of patient subpopulations benefiting more from either approach[118]. Adverse effects of peripheral denervation include dysphagia and return of symptoms following denervation[112].
The current management options for patients with dystonia aim to optimally minimize the motor effects of dystonia on afflicted patients. While the exact etiology is unclear in most cases, potential interventions lie in surgical and pharmacological symptom alleviation. Advancements in precise neurosurgical procedures such as DBS have allowed for better long-term patient outcomes. For different types of dystonia, specific brain regions, such as the output efferent fibers of the globus pallidus pars interna and subthalamic nucleus, have been identified for optimal targeting. Previously used ablative surgeries have also improved through the advent of radiofrequency, stereotaxis, and ultrasound. Even with the use of state-of-the-art technologies, adverse effects remain a significant consideration when weighing these interventions. As a result, the use of pharmacological agents to modulate the aversive effects of dystonia on patient life is prevalent. Careful analysis of the etiology, associated symptoms, and potential side effects should be undertaken before beginning any individual medication. Continued research in the hopes of further symptom alleviation and cause identification is needed to better control the clinical presentation of dystonia and provide patients with more diverse, robust options to consider in their treatment.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country of origin: United States
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Viani-Walsh D, Ireland S-Editor: Chen YL L-Editor: A P-Editor: Zhao S
| 1. | Grütz K, Klein C. Dystonia updates: definition, nomenclature, clinical classification, and etiology. J Neural Transm (Vienna). 2021;128:395-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 2. | Jinnah HA, Factor SA. Diagnosis and treatment of dystonia. Neurol Clin. 2015;33:77-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 3. | Albanese A, Di Giovanni M, Lalli S. Dystonia: diagnosis and management. Eur J Neurol. 2019;26:5-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 4. | Defazio G, Gigante AF, Abbruzzese G, Bentivoglio AR, Colosimo C, Esposito M, Fabbrini G, Guidubaldi A, Girlanda P, Liguori R, Marinelli L, Morgante F, Santoro L, Tinazzi M, Livrea P, Berardelli A. Tremor in primary adult-onset dystonia: prevalence and associated clinical features. J Neurol Neurosurg Psychiatry. 2013;84:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Bailey GA, Rawlings A, Torabi F, Pickrell O, Peall KJ. Adult-onset idiopathic dystonia: A national data-linkage study to determine epidemiological, social deprivation, and mortality characteristics. Eur J Neurol. 2022;29:91-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Müller J, Kiechl S, Wenning GK, Seppi K, Willeit J, Gasperi A, Wissel J, Gasser T, Poewe W. The prevalence of primary dystonia in the general community. Neurology. 2002;59:941-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Steeves TD, Day L, Dykeman J, Jette N, Pringsheim T. The prevalence of primary dystonia: a systematic review and meta-analysis. Mov Disord. 2012;27:1789-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 8. | Lalli S, Albanese A. The diagnostic challenge of primary dystonia: evidence from misdiagnosis. Mov Disord. 2010;25:1619-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Lenka A, Jankovic J. Sports-Related Dystonia. Tremor Other Hyperkinet Mov (N Y). 2021;11:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 10. | Stephen CD, Perez DL, Chibnik LB, Sharma N. Functional dystonia: A case-control study and risk prediction algorithm. Ann Clin Transl Neurol. 2021;8:732-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Pérez-de-Heredia-Torres M, García-Bravo C, Huertas-Hoyas E, Martínez-Piédrola MR, Serrada-Tejeda S, Martínez-Castrillo JC. Sensitivity and pain in focal dystonia of the hand. Neurologia (Engl Ed). 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 12. | Avanzino L, Tinazzi M, Ionta S, Fiorio M. Sensory-motor integration in focal dystonia. Neuropsychologia. 2015;79:288-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Siokas V, Aloizou AM, Tsouris Z, Michalopoulou A, Mentis AA, Dardiotis E. Risk Factor Genes in Patients with Dystonia: A Comprehensive Review. Tremor Other Hyperkinet Mov (N Y). 2018;8:559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 14. | Vidailhet M, Grabli D, Roze E. Pathophysiology of dystonia. Curr Opin Neurol. 2009;22:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Frucht L, Perez DL, Callahan J, MacLean J, Song PC, Sharma N, Stephen CD. Functional Dystonia: Differentiation From Primary Dystonia and Multidisciplinary Treatments. Front Neurol. 2020;11:605262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Demircioglu FE, Sosa BA, Ingram J, Ploegh HL, Schwartz TU. Structures of TorsinA and its disease-mutant complexed with an activator reveal the molecular basis for primary dystonia. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Liu C, Scorr L, Kilic-Berkmen G, Cotton A, Factor SA, Freeman A, Tran V, Liu K, Uppal K, Jones D, Jinnah HA, Sun YV. A metabolomic study of cervical dystonia. Parkinsonism Relat Disord. 2021;82:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Macerollo A, Edwards MJ, Huang HC, Lu MK, Chen HJ, Tsai CH, Chen JC. Peripheral trauma and risk of dystonia: What are the evidences and potential co-risk factors from a population insurance database? PLoS One. 2019;14:e0216772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Shetty AS, Bhatia KP, Lang AE. Dystonia and Parkinson’s disease: What is the relationship? Neurobiol Dis. 2019;132:104462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 20. | Jankovic J. Medical treatment of dystonia. Mov Disord. 2013;28:1001-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Termsarasab P, Frucht SJ. Dystonias. Ref Mod Bio Sci. 2021;. [DOI] [Full Text] |
| 22. | Cloud LJ, Jinnah HA. Treatment strategies for dystonia. Expert Opin Pharmacother. 2010;11:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Harvey AR, Baker LB, Reddihough DS, Scheinberg A, Williams K. Trihexyphenidyl for dystonia in cerebral palsy. Cochrane Database Syst Rev. 2018;5:CD012430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Downs AM, Fan X, Donsante C, Jinnah HA, Hess EJ. Trihexyphenidyl rescues the deficit in dopamine neurotransmission in a mouse model of DYT1 dystonia. Neurobiol Dis. 2019;125:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Woo KA, Kim HJ, Yoo D, Choi JH, Shin J, Park S, Kim R, Jeon B. Patient-reported responses to medical treatment in primary dystonia. J Clin Neurosci. 2020;75:242-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Romeo DM, Specchia A, Fasano A, Leoni C, Onesimo R, Brogna C, Veltri S, Zampino G. Treatment of Dystonia Using Trihexyphenidyl in Costello Syndrome. Brain Sci. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Sarangi A, Lance Mwangi, Domingo-johnson E, Arham Siddiqui. Management of dystonia in patients with hypersensitivity to anticholinergics. The Chronicles. 2021;9:35-40. [DOI] [Full Text] |
| 28. | Ertzgaard P, Campo C, Calabrese A. Efficacy and safety of oral baclofen in the management of spasticity: A rationale for intrathecal baclofen. J Rehabil Med. 2017;49:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Langlois M, Richer F, Chouinard S. New perspectives on dystonia. Can J Neurol Sci. 2003;30 Suppl 1:S34-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Tan EK, Chan LL, Wong MC. Levodopa-induced oromandibular dystonia in progressive supranuclear palsy. Clin Neurol Neurosurg. 2003;105:132-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Modreanu R, Özdemir G, Buhmann C, Hauptmann B. Levodopa-induced dystonia in a patient with possible progressive supranuclear palsy with progressive gait freezing. J Neurol Sci. 2018;388:139-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Calabresi P, Standaert DG. Dystonia and levodopa-induced dyskinesias in Parkinson’s disease: Is there a connection? Neurobiol Dis. 2019;132:104579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Okamoto N, Konishi Y, Tesen H, Ikenouchi A, Yoshimura R. A Low Clozapine Dose Improved Refractory Tardive Dystonia without Exacerbating Psychiatric Symptoms: A Case Report. Int Med Case Rep J. 2021;14:237-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Karp BI, Goldstein SR, Chen R, Samii A, Bara-Jimenez W, Hallett M. An open trial of clozapine for dystonia. Mov Disord. 1999;14:652-657. [PubMed] [DOI] [Full Text] |
| 35. | Joe S, Park J, Lim J, Park C, Ahn J. Remission of irreversible aripiprazole-induced tardive dystonia with clozapine: a case report. BMC Psychiatry. 2015;15:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Van Harten PN, Kampuis DJ, Matroos GE. Use of clozapine in tardive dystonia. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Seeman P. Clozapine, a fast-off-D2 antipsychotic. ACS Chem Neurosci. 2014;5:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 38. | Spiegel LL, Ostrem JL, Bledsoe IO. FDA Approvals and Consensus Guidelines for Botulinum Toxins in the Treatment of Dystonia. Toxins (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Ataov G. Treatment of tardive dystonia due to risperidone use with single-dose botulinum toxin. Dusunen Adam. 2022;. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Albanese A, Asmus F, Bhatia KP, Elia AE, Elibol B, Filippini G, Gasser T, Krauss JK, Nardocci N, Newton A, Valls-Solé J. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. 2011;18:5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 275] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 41. | Zoons E, Dijkgraaf MG, Dijk JM, van Schaik IN, Tijssen MA. Botulinum toxin as treatment for focal dystonia: a systematic review of the pharmaco-therapeutic and pharmaco-economic value. J Neurol. 2012;259:2519-2526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Kojovic M, Caronni A, Bologna M, Rothwell JC, Bhatia KP, Edwards MJ. Botulinum toxin injections reduce associative plasticity in patients with primary dystonia. Mov Disord. 2011;26:1282-1289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Dressler D, Adib Saberi F, Rosales RL. Botulinum toxin therapy of dystonia. J Neural Transm (Vienna). 2021;128:531-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 44. | Kassavetis P, Lungu C, Ehrlich D, Alter K, Karp BI. Self-reported benefit and weakness after botulinum toxin in dystonia. Parkinsonism Relat Disord. 2020;80:10-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Hok P, Veverka T, Hluštík P, Nevrlý M, Kaňovský P. The Central Effects of Botulinum Toxin in Dystonia and Spasticity. Toxins (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 46. | Fietzek UM, Nene D, Schramm A, Appel-Cresswell S, Košutzká Z, Walter U, Wissel J, Berweck S, Chouinard S, Bäumer T. The Role of Ultrasound for the Personalized Botulinum Toxin Treatment of Cervical Dystonia. Toxins (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Greene P, Fahn S, Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov Disord. 1994;9:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 255] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Thenganatt MA, Jankovic J. Treatment of dystonia. Neurotherapeutics. 2014;11:139-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 49. | Volkmann J, Wolters A, Kupsch A, Müller J, Kühn AA, Schneider GH, Poewe W, Hering S, Eisner W, Müller JU, Deuschl G, Pinsker MO, Skogseid IM, Roeste GK, Krause M, Tronnier V, Schnitzler A, Voges J, Nikkhah G, Vesper J, Classen J, Naumann M, Benecke R; DBS study group for dystonia. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. Lancet Neurol. 2012;11:1029-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 50. | Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, Lagrange C, Tézenas du Montcel S, Dormont D, Grand S, Blond S, Detante O, Pillon B, Ardouin C, Agid Y, Destée A, Pollak P; French Stimulation du Pallidum Interne dans la Dystonie (SPIDY) Study Group. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 732] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 51. | Kupsch A, Benecke R, Müller J, Trottenberg T, Schneider GH, Poewe W, Eisner W, Wolters A, Müller JU, Deuschl G, Pinsker MO, Skogseid IM, Roeste GK, Vollmer-Haase J, Brentrup A, Krause M, Tronnier V, Schnitzler A, Voges J, Nikkhah G, Vesper J, Naumann M, Volkmann J; Deep-Brain Stimulation for Dystonia Study Group. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 675] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 52. | Ondo WG, Desaloms JM, Jankovic J, Grossman RG. Pallidotomy for generalized dystonia. Mov Disord. 1998;13:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 126] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Yelnik J, Bardinet E, Benabid AL, Navarro S, Dormont D, Grand S, Blond S, Ardouin C, Pillon B, Dujardin K, Hahn-Barma V, Agid Y, Destée A, Pollak P; French SPIDY Study Group. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol. 2007;6:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 272] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 54. | Panov F, Gologorsky Y, Connors G, Tagliati M, Miravite J, Alterman RL. Deep brain stimulation in DYT1 dystonia: a 10-year experience. Neurosurgery. 2013;73:86-93; discussion 93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 55. | Schjerling L, Hjermind LE, Jespersen B, Madsen FF, Brennum J, Jensen SR, Løkkegaard A, Karlsborg M. A randomized double-blind crossover trial comparing subthalamic and pallidal deep brain stimulation for dystonia. J Neurosurg. 2013;119:1537-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 56. | Sun B, Chen S, Zhan S, Le W, Krahl SE. Subthalamic nucleus stimulation for primary dystonia and tardive dystonia. Acta Neurochir Suppl. 2007;97:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Cao C, Pan Y, Li D, Zhan S, Zhang J, Sun B. Subthalamus deep brain stimulation for primary dystonia patients: a long-term follow-up study. Mov Disord. 2013;28:1877-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Ostrem JL, Racine CA, Glass GA, Grace JK, Volz MM, Heath SL, Starr PA. Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology. 2011;76:870-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 59. | Morishita T, Foote KD, Haq IU, Zeilman P, Jacobson CE, Okun MS. Should we consider Vim thalamic deep brain stimulation for select cases of severe refractory dystonic tremor. Stereotact Funct Neurosurg. 2010;88:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 60. | Tagliati M, Krack P, Volkmann J, Aziz T, Krauss JK, Kupsch A, Vidailhet AM. Long-Term management of DBS in dystonia: response to stimulation, adverse events, battery changes, and special considerations. Mov Disord. 2011;26 Suppl 1:S54-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Zauber SE, Watson N, Comella CL, Bakay RA, Metman LV. Stimulation-induced parkinsonism after posteroventral deep brain stimulation of the globus pallidus internus for craniocervical dystonia. J Neurosurg. 2009;110:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Blahak C, Capelle HH, Baezner H, Kinfe TM, Hennerici MG, Krauss JK. Micrographia induced by pallidal DBS for segmental dystonia: a subtle sign of hypokinesia? J Neural Transm (Vienna). 2011;118:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 63. | Chiken S, Nambu A. Mechanism of Deep Brain Stimulation: Inhibition, Excitation, or Disruption? Neuroscientist. 2016;22:313-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 64. | Montgomery EB Jr, Gale JT. Mechanisms of action of deep brain stimulation(DBS) . Neurosci Biobehav Rev. 2008;32:388-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 65. | Birdno MJ, Grill WM. Mechanisms of deep brain stimulation in movement disorders as revealed by changes in stimulus frequency. Neurotherapeutics. 2008;5:14-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 66. | Shroff S. Globus Pallidus. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York: Springer, 2011: 1159. [DOI] [Full Text] |
| 67. | Au KLK, Wong JK, Tsuboi T, Eisinger RS, Moore K, Lemos Melo Lobo Jofili Lopes J, Holland MT, Holanda VM, Peng-Chen Z, Patterson A, Foote KD, Ramirez-Zamora A, Okun MS, Almeida L. Globus Pallidus Internus (GPi) Deep Brain Stimulation for Parkinson’s Disease: Expert Review and Commentary. Neurol Ther. 2021;10:7-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 68. | Zhang J, Wang T, Zhang CC, Zeljic K, Zhan S, Sun BM, Li DY. The safety issues and hardware-related complications of deep brain stimulation therapy: a single-center retrospective analysis of 478 patients with Parkinson’s disease. Clin Interv Aging. 2017;12:923-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 69. | Malatt C, Tagliati M. Long-Term Outcomes of Deep Brain Stimulation for Pediatric Dystonia. Pediatr Neurosurg. 2022;57:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 70. | DiFrancesco MF, Halpern CH, Hurtig HH, Baltuch GH, Heuer GG. Pediatric indications for deep brain stimulation. Childs Nerv Syst. 2012;28:1701-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Gillies MJ, Joint C, Forrow B, Fletcher C, Green AL, Aziz TZ. Rechargeable vs. nonrechargeable internal pulse generators in the management of dystonia. Neuromodulation. 2013;16:226-9; discussion 229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Kaminska M, Lumsden DE, Ashkan K, Malik I, Selway R, Lin JP. Rechargeable deep brain stimulators in the management of paediatric dystonia: well tolerated with a low complication rate. Stereotact Funct Neurosurg. 2012;90:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 73. | Waln O, Jankovic J. Bilateral globus pallidus internus deep brain stimulation after bilateral pallidotomy in a patient with generalized early-onset primary dystonia. Mov Disord. 2013;28:1162-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | Fonoff ET, Campos WK, Mandel M, Alho EJ, Teixeira MJ. Bilateral subthalamic nucleus stimulation for generalized dystonia after bilateral pallidotomy. Mov Disord. 2012;27:1559-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 75. | Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum Brain Mapp. 2002;17:48-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 76. | Wilke M, Schmithorst VJ, Holland SK. Normative pediatric brain data for spatial normalization and segmentation differs from standard adult data. Magn Reson Med. 2003;50:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Gelineau-Morel R, Kruer MC, Garris JF, Abu Libdeh A, Barbosa DAN, Coffman KA, Moon D, Barton C, Zea Vera A, Bruce AB, Larsh T, Wu SW, Gilbert DL, O’Malley JA. Deep Brain Stimulation for Pediatric Dystonia: A Review of the Literature and Suggested Programming Algorithm. J Child Neurol. 2022;37:813-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 78. | Kuiper MJ, Vrijenhoek L, Brandsma R, Lunsing RJ, Burger H, Eggink H, Peall KJ, Contarino MF, Speelman JD, Tijssen MAJ, Sival DA. The Burke-Fahn-Marsden Dystonia Rating Scale is Age-Dependent in Healthy Children. Mov Disord Clin Pract. 2016;3:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Hale AT, Monsour MA, Rolston JD, Naftel RP, Englot DJ. Deep brain stimulation in pediatric dystonia: a systematic review. Neurosurg Rev. 2020;43:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 80. | Gimeno H, Tustin K, Selway R, Lin JP. Beyond the Burke-Fahn-Marsden Dystonia Rating Scale: deep brain stimulation in childhood secondary dystonia. Eur J Paediatr Neurol. 2012;16:501-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 81. | Kaminska M, Perides S, Lumsden DE, Nakou V, Selway R, Ashkan K, Lin JP. Complications of Deep Brain Stimulation (DBS) for dystonia in children - The challenges and 10 year experience in a large paediatric cohort. Eur J Paediatr Neurol. 2017;21:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 82. | Koy A, Bockhorn N, Kühn AA, Schneider GH, Krause P, Lauritsch K, Witt K, Paschen S, Deuschl G, Krauss JK, Saryyeva A, Runge J, Borggraefe I, Mehrkens JH, Horn A, Vesper J, Schnitzler A, Siegert S, Freilinger M, Eckenweiler M, Coenen VA, Tadic V, Voges J, Pauls KAM, Wirths J, Timmermann L, Hellmich M; GEPESTIM consortium. Adverse events associated with deep brain stimulation in patients with childhood-onset dystonia. Brain Stimul. 2019;12:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 83. | Krauss JK, Lipsman N, Aziz T, Boutet A, Brown P, Chang JW, Davidson B, Grill WM, Hariz MI, Horn A, Schulder M, Mammis A, Tass PA, Volkmann J, Lozano AM. Technology of deep brain stimulation: current status and future directions. Nat Rev Neurol. 2021;17:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 425] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 84. | Steigerwald F, Müller L, Johannes S, Matthies C, Volkmann J. Directional deep brain stimulation of the subthalamic nucleus: A pilot study using a novel neurostimulation device. Mov Disord. 2016;31:1240-1243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 85. | Horn A, Reich MM, Ewert S, Li N, Al-Fatly B, Lange F, Roothans J, Oxenford S, Horn I, Paschen S, Runge J, Wodarg F, Witt K, Nickl RC, Wittstock M, Schneider GH, Mahlknecht P, Poewe W, Eisner W, Helmers AK, Matthies C, Krauss JK, Deuschl G, Volkmann J, Kühn AA. Optimal deep brain stimulation sites and networks for cervical vs. generalized dystonia. Proc Natl Acad Sci U S A. 2022;119:e2114985119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 86. | Horisawa S, Yamaguchi T, Abe K, Hori H, Fukui A, Iijima M, Sumi M, Hodotsuka K, Konishi Y, Kawamata T, Taira T. Magnetic Resonance-Guided Focused Ultrasound Thalamotomy for Focal Hand Dystonia: A Pilot Study. Mov Disord. 2021;36:1955-1959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 87. | Kosmin M, Rees J. Radiation and the nervous system. Pract Neurol. 2022;22:450-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 88. | Centen LM, Oterdoom DLM, Tijssen MAJ, Lesman-Leegte I, van Egmond ME, van Dijk JMC. Bilateral Pallidotomy for Dystonia: A Systematic Review. Mov Disord. 2021;36:547-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 89. | Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med. 2012;2:a009621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 489] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 90. | Jolesz FA. MRI-guided focused ultrasound surgery. Annu Rev Med. 2009;60:417-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 91. | Quadri SA, Waqas M, Khan I, Khan MA, Suriya SS, Farooqui M, Fiani B. High-intensity focused ultrasound: past, present, and future in neurosurgery. Neurosurg Focus. 2018;44:E16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 92. | Xiong Y, Lin J, Bian X, Lu H, Zhou J, Zhang D, Pan L, Lou X. Treatment-Specific Network Modulation of MRI-Guided Focused Ultrasound Thalamotomy in Essential Tremor: Modulation of ET-Related Network by MRgFUS Thalamotomy. Neurotherapeutics. 2022;19:1920-1931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Hynynen K, Jones RM. Image-guided ultrasound phased arrays are a disruptive technology for non-invasive therapy. Phys Med Biol. 2016;61:R206-R248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 94. | Gross RE. What happened to posteroventral pallidotomy for Parkinson’s disease and dystonia? Neurotherapeutics. 2008;5:281-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 95. | Fritsch M, Rangus I, Nolte CH. Thalamic Aphasia: a Review. Curr Neurol Neurosci Rep. 2022;22:855-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 96. | Jamora RDG, Khu KJO, Sy MCC, Pascual JSG, Legaspi GD, Aguilar JA. Transcranial magnetic resonance-guided focused ultrasound pallidothalamic tractotomy for patients with X-linked dystonia-parkinsonism: a study protocol. BMC Neurol. 2023;23:306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 97. | Pouratian N, Sheth SA. Stereotactic and Functional Neurosurgery. Springer Cham: 2020. [DOI] [Full Text] |
| 98. | Halpern CH, Santini V, Lipsman N, Lozano AM, Schwartz ML, Shah BB, Elias WJ, Cosgrove GR, Hayes MT, McDannold N, Aldrich C, Eisenberg HM, Gandhi D, Taira T, Gwinn R, Ro S, Witt J, Jung NY, Chang JW, Rosenberg J, Ghanouni P. Three-year follow-up of prospective trial of focused ultrasound thalamotomy for essential tremor. Neurology. 2019;93:e2284-e2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 99. | Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W, Schwartz M, Hynynen K, Lozano AM, Shah BB, Huss D, Dallapiazza RF, Gwinn R, Witt J, Ro S, Eisenberg HM, Fishman PS, Gandhi D, Halpern CH, Chuang R, Butts Pauly K, Tierney TS, Hayes MT, Cosgrove GR, Yamaguchi T, Abe K, Taira T, Chang JW. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N Engl J Med. 2016;375:730-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 750] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 100. | Paff M, Boutet A, Germann J, Elias GJB, Chow CT, Loh A, Kucharczyk W, Fasano A, Schwartz ML, Lozano AM. Focused Ultrasound Thalamotomy Sensory Side Effects Follow the Thalamic Structural Homunculus. Neurol Clin Pract. 2021;11:e497-e503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Franzini A, Moosa S, Servello D, Small I, DiMeco F, Xu Z, Elias WJ, Franzini A, Prada F. Ablative brain surgery: an overview. Int J Hyperthermia. 2019;36:64-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 102. | Agrawal M, Garg K, Samala R, Rajan R, Naik V, Singh M. Outcome and Complications of MR Guided Focused Ultrasound for Essential Tremor: A Systematic Review and Meta-Analysis. Front Neurol. 2021;12:654711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 103. | Dey S, Ghosh S. Cervical Dystonia Refractory to Botulinum Toxin Responding to Radiofrequency Ablation: A Case Report. J Pain Res. 2020;13:2313-2316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 104. | Horisawa S, Azuma K, Akagawa H, Nonaka T, Kawamata T, Taira T. Radiofrequency ablation for DYT-28 dystonia: short term follow-up of three adult cases. Ann Clin Transl Neurol. 2020;7:2047-2051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 105. | Friehs GM, Park MC, Goldman MA, Zerris VA, Norén G, Sampath P. Stereotactic radiosurgery for functional disorders. Neurosurg Focus. 2007;23:E3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 106. | Kwon Y, Whang CJ. Stereotactic Gamma Knife radiosurgery for the treatment of dystonia. Stereotact Funct Neurosurg. 1995;64 Suppl 1:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 107. | Tripathi M, Sharan S, Mehta S, Deora H, Yagnick NS, Kumar N, Ahuja CK, Batish A, Gurnani J. Gamma Knife Radiosurgical Pallidotomy for Dystonia: Not a Fallen Angel. Neurol India. 2019;67:1515-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Natera-Villalba E, Matarazzo M, Martinez-Fernandez R. Update in the clinical application of focused ultrasound. Curr Opin Neurol. 2022;35:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 109. | Jinnah HA. Medical and Surgical Treatments for Dystonia. Neurol Clin. 2020;38:325-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 110. | Horisawa S, Kim K, Sakaguchi M, Kawamata T, Taira T. Radiofrequency ablation of the pallidothalamic tract and ventral intermediate nucleus for dystonic tremor through the parietal approach. Surg Neurol Int. 2023;14:390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 111. | Cohen-Gadol AA, Ahlskog JE, Matsumoto JY, Swenson MA, McClelland RL, Davis DH. Selective peripheral denervation for the treatment of intractable spasmodic torticollis: experience with 168 patients at the Mayo Clinic. J Neurosurg. 2003;98:1247-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 112. | Braun V, Richter HP. Selective peripheral denervation for spasmodic torticollis: 13-year experience with 155 patients. J Neurosurg. 2002;97:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 113. | Münchau A, Palmer JD, Dressler D, O’Sullivan JD, Tsang KL, Jahanshahi M, Quinn NP, Lees AJ, Bhatia KP. Prospective study of selective peripheral denervation for botulinum-toxin resistant patients with cervical dystonia. Brain. 2001;124:769-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 114. | Ford B, Louis ED, Greene P, Fahn S. Outcome of selective ramisectomy for botulinum toxin resistant torticollis. J Neurol Neurosurg Psychiatry. 1998;65:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 115. | Alkarras M, Nabeeh A, El Molla S, El Gayar A, Fayed ZY, Ghany WA, Raslan AM. Evaluation of outcome of different neurosurgical modalities in management of cervical dystonia. Egypt J Neurol Psychiatry Neurosurg. 2022;58:56. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 116. | Bertrand CM. Selective peripheral denervation for spasmodic torticollis: surgical technique, results, and observations in 260 cases. Surg Neurol. 1993;40:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 80] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 117. | Wilson TJ, Spinner RJ. Selective Cervical Denervation for Cervical Dystonia: Modification of the Bertrand Procedure. Oper Neurosurg (Hagerstown). 2018;14:546-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 118. | Ravindran K, Ganesh Kumar N, Englot DJ, Wilson TJ, Zuckerman SL. Deep Brain Stimulation Versus Peripheral Denervation for Cervical Dystonia: A Systematic Review and Meta-Analysis. World Neurosurg. 2019;122:e940-e946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |