Published online Apr 19, 2024. doi: 10.5498/wjp.v14.i4.507
Peer-review started: December 3, 2023
First decision: January 15, 2024
Revised: January 28, 2024
Accepted: March 21, 2024
Article in press: March 21, 2024
Published online: April 19, 2024
Processing time: 135 Days and 15.5 Hours
Anosmia was one of the main symptoms of coronavirus disease 2019 (COVID-19). A psychiatric history (i.e., depression) may be an independent contributor to the risk of COVID-19 diagnosis, and COVID-19 survivors appear to have an increased risk of neuropsychiatric sequelae (bidirectional association).
To compare the rate of psychiatric disorder among post-COVID patients without anosmia vs patients with persistent olfactory complaints.
We conducted a prospective case control study from March 2020 to May 2021. Patients recruited at the ENT department of Nice University Hospital had a subjective olfactory complaint (visual analogue scale) for over 6 wk and a mo
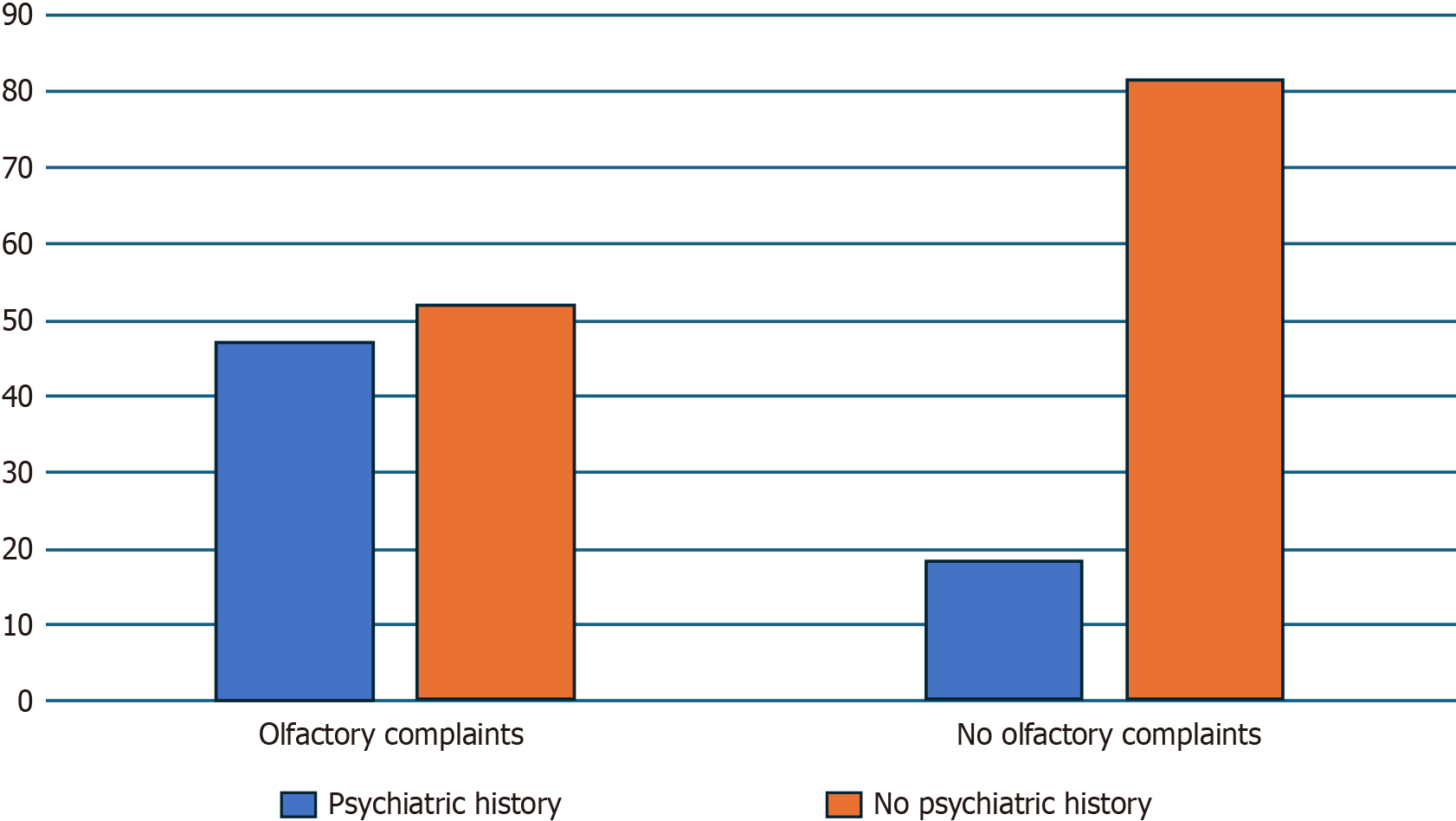
Thirty-four patients with post-COVID-19 olfactory complaints were included in the first group of the study. Fifty percent of the patients were female (n = 17). The group’s mean age was 40.5 ± 12.9 years. The control group included 32 participants, of which 34.4% were female (n = 11), and had a mean age of 61.2 ± 12.2 years. The rate of psychiatric disorder among post-COVID patients with olfactory complaints was significatively higher (41.7%) than among patients without (18.8%) (χ2 = 5.9, P = 0.015).
The presence of a psychiatric history may constitute a potential risk factor for the development of long COVID due to persistent anosmia. It therefore seems important to establish reinforced health monitoring after a COVID 19 infection in at-risk patients. Further prospective, translational, and collaborative studies are needed to extrapolate these results to the general population.
Core Tip: Our study reveals a significant association between a psychiatric history and persistent anosmia in post-coronavirus disease 2019 (COVID-19) patients. With a higher rate of psychiatric disorder observed in individuals experiencing long-COVID symptoms, our findings underscore the need for reinforced health monitoring of at-risk patients. This emphasizes the importance of considering psychiatric factors in the assessment and management of post-COVID-19 sequelae. This study will thus contribute to a broader understanding of the multifaceted impact of the virus on mental health.
- Citation: Metelkina-Fernandez V, Dumas LE, Vandersteen C, Chirio D, Gros A, Fernandez A, Askenazy F, Manera V. Psychiatric outcomes in outpatients affected by long COVID: A link between mental health and persistence of olfactory complaint. World J Psychiatry 2024; 14(4): 507-512
- URL: https://www.wjgnet.com/2220-3206/full/v14/i4/507.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i4.507
The coronavirus disease 2019 (COVID-19) pandemic originated in China. It was first identified in Wuhan (Hubei province) in December 2019 before spreading to other continents[1]. It resulted in a still active global pandemic.
Olfactory loss was one of the main symptoms among European patients with mild-to-moderate COVID-19 (70.2%)[2]. Even if several pathogenic mechanisms of olfactory dysfunction in patients with COVID-19 were postulated, the precise mechanisms remain unclear. Neuroplasticity is known to play a major role in recovery after loss of smell[3]. However, it has been observed that the plasticity of the human brain can be affected by (certain) stressful events, by a psychiatric history (e.g., depression) and by lifetime sensory experiences[4]. Thus, Taquet et al[5] (2021) have suggested bidirectional associations between COVID-19 and psychiatric disorders.
Interestingly, in their study, a psychiatric diagnosis in the previous year was shown to be an independent risk factor of COVID-19 diagnosis[5]. In a further study, Taquet et al[6] (2021) suggested that a COVID-19 diagnosis was associated with psychiatric and neurological outcomes at 6 months in one third of patients.
Based on these results, we can hypothesize that the persistence of an olfactory complaint could also be affected by the patient’s psychiatric history.
The main objective of our study was to compare the psychiatric history within the previous year of post-COVID patients without olfactory complaints (with a total recovery < 1 month) vs patients with persistent post-viral olfactory complaints.
The secondary objectives were: (1) To assess the rate of post-traumatic stress disorder (PTSD) among patients with post-viral olfactory complaints (COVID-19) and to compare it with the rate of PTSD among patients without olfactory complaints (with a total recovery < 1 month); and (2) for patients with persistent olfactory complaints, to correlate the intensity of post-traumatic symptoms with self-reported olfactory recovery.
The study was approved by the institutional review board of Nice University Hospital (CNIL number: 412). This study is part of a large prospective work registered under a ClinicalTrials.gov number (ID: NCT04799977). For this large trial, we prospectively recruited patients of the ENT department of Nice University Hospital, starting in March 2020. All had been contaminated by COVID-19 and had persistent olfactory disorders lasting more than 6 wk (3 to 15 months).
We retrospectively extracted the patients’ demographic data and clinical features, including subjective taste im
In this study, patients with persistent olfactory disorders were recruited at the ENT department of Nice University Hospital during the period from March 2020 to February 2021. Patients were self-referred or referred by colleagues, general practitioners or recommended by the infectiology department that recorded all COVID-19 declared patients (city guidelines). Patients had an olfactory complaint for over 6 weeks and a molecular-proven severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) diagnosis or a CT-proven SARS-CoV-2 diagnosis secondarily confirmed by serology. Patients with other pathologies that could affect the olfactory system were excluded, as confirmed by their medical history and nasofibroscopy results: olfaction disorders, ENT cancer, head radiotherapy history, and post viral (before the pandemic) olfactive history.
Post-COVID patients without persistent olfactory disorders were recruited at the university infectiology department during the same period.
For patients with persistent olfactory complaints, olfactory function was evaluated by an otorhinolaryngologist using a visual analogue scale (VAS) assessing the subjective perceived olfactory recovery.
A psychiatric interview performed by an experienced psychiatrist explored the psychiatric history, the diagnostic categories (according to the DSM 5), the presence of stress factors, and exposure to recent or past psychotrauma. Psychiatric assessments included validated self-report questionnaires for PTSD (PCL-5).
Patients without olfactory complaints were interviewed by a psychiatrist who conducted a medical and psychological evaluation. Special attention was paid to their psychiatric history. They also completed the PCL-5 questionnaire at home using Google Forms.
Data are presented as mean (SD) for quantitative variables and as frequency and percentage for qualitative variables. To compare age between groups (patients with persistent olfactory complaints vs patients without olfactory complaints), we used independent-sample T tests for normally distributed variables (age), and Mann-Whitney U tests for non-normally distributed variables (PCL-5). To investigate gender differences across groups, we performed Chi2 analyses. We also ran an exploratory logistic regression analysis to verify whether the presence of previous mental disorders could have had an impact on the presence of olfactory disorders lasting more than one month.
To investigate correlations between subjective reports (VAS) and PCL-5 scores, we performed bivariate correlation analyses. As data were not normally distributed (as suggested by the Kolmogorov-Smirnov test), non-parametric Spearman’s correlations were made.
The patients’ demographic and clinical features are presented in Table 1. Thirty-four patients with post-COVID-19 olfactory complaints were included in the first group of the study. Fifty percent of the patients were female (n = 17). The patients’ mean age was 40.5 ± 12.9 years. They were interviewed 5.3 ± 2.8 mo after COVID-19 infection. The day of the interview, patients reported having recovered only 37.7% ± 27.5% of their olfaction (ranging from 0% to 90%). The control group included 32 participants, of which 34.4% were female (n = 11), and had a mean age of 61.2 ± 12.2 years. The two groups differed in terms of mean age (t (64) = 6.7, P < 0.001), while gender did not differ between groups (Chi2 (1) = 1.6, P = 0.199).
In the group with olfactory complaints, 47.1% of the subjects (n = 16) reported a psychiatric history prior to SARS-CoV-2 infection. Only 18.8% of subjects in the control group (n = 6) reported a psychiatric history prior to SARS-CoV-2 infection (Figure 1). Chi2 analysis confirmed that the proportion of people with a previous psychiatric history was significantly higher in the patients with persistent olfactory complaints compared to the control group (χ2(1) = 5.9, P = 0.015). Logistic regression analysis suggested that the presence of a previous psychiatric history had a significant impact on the probability of having post-COVID-19 olfactory complaints (B = 1.35, P = 0.018).
Subjects with olfactory complaints had a mean PCL-5 score of 17.8 (SD = 22.4), while control subjects had a mean score of 18.1 (SD = 20.0). The difference was not statistically significant (U = 461.5, P =0.285). In the olfactory complaint group, no significant correlation was found between the percentage of subjective olfactory recovery (VAS) and PCL-5 (rho (32) = 0.02, P = 0.925).
Several factors have been shown to influence the likelihood of developing persistent olfactory disorders after COVID-19 infection, such as belonging to an ethnic minority, socioeconomic deprivation, smoking, and obesity[7]. Here we investigated whether a psychiatric history before SARS-CoV-2 infection was more frequent in patients with and without olfactory complaints. Our results suggest that psychiatric history and certain psychological conditions such as stressful events were more common in patients with persistent olfactory complaints.
Olfactory complaint was one of the main symptoms among European patients with mild-to-moderate COVID-19 (70.2%); in a seminal study that included 1420 patients, Lechien et al[2] (2020) found that olfactory complaints persisted at least 7 d in 37.5% of these cases. Since the beginning of the COVID-19 pandemic, several pathogenic mechanisms of olfactory dysfunction have been postulated. However, the precise mechanisms still remain unclear. Reichert et al. (2018) conducted research on the role of neuroplasticity in recovery after loss of smell, focusing on the decrease in white and grey matter[3]. They also highlighted the efficacy of olfactory training programs. In a large review, McEwen[4] (2007) suggested that the plasticity of the human brain could be affected by stressful life events, a psychiatric history (e.g., depression), lifetime sensory experiences, and stress-related social problems. Taquet et al[5] (2021) suggested bidirectional associations between COVID-19 and psychiatric disorders. They observed that a psychiatric diagnosis in the previous year was an independent risk factor of COVID-19 diagnosis. In a further study, they showed that COVID-19 diagnosis was associated with psychiatric and neurological outcomes in one third of patients 6 months after the infection[6]. These results are supported by evidence that COVID-19 can have an impact on the brain. As mentioned above, McEwen has shown that stress can have a damaging effect on the brain, and that the brain can also respond to stress by manifesting behavioral and physiological symptoms[8]. More broadly, life experiences modify brain function via synaptic tran
The data presented in this study suggests that a psychiatric history and certain psychological conditions, such as stressful events, may have a negative impact on the persistence of an olfactory complaint. These results are consistent with several hypothesized mechanisms of brain involvement in SARS-CoV-2 infection. Indeed, it has been shown that SARS-CoV-2 can infect the Central Nervous System by crossing the neural-mucosal interface and more specifically by crossing the olfactory mucosa and following neuroanatomical structures due to its neurotropism[9]. Moreover, in a large systematic review, Rogers et al[10] (2020) have pointed out that depression, anxiety, PTSD, and other neuropsychiatric syndromes can appear after COVID-19. Once infected, people with pre-existing mental disorders are at high risk of experiencing persistent symptoms of COVID[11]. In our study, we failed to demonstrate that PTSD was a risk factor for developing persistent anosmia, but we did not explore the risk of developing PTSD after COVID infection.
The main limitation of this study is the small sample size, which is not representative of the whole population. Fur
In conclusion, the human brain might be affected by a psychiatric history (including stressful events). This brain damage could partially be an explanation for olfactory complaint persistence months after a SARS CoV-2 infection, showing the key importance of post COVID-19 psychiatric follow-up and of preventive mental health care.
We are grateful to Virginie Carlo-Sifi and Sarah Nakam for their help. We are grateful to Xavier Mondoloni for reviewing the manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tao Y, China S-Editor: Lin C L-Editor: A P-Editor: Zhao YQ
| 1. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17639] [Article Influence: 3527.8] [Reference Citation Analysis (0)] |
| 2. | Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q, Huet K, Plzak J, Horoi M, Hans S, Rosaria Barillari M, Cammaroto G, Fakhry N, Martiny D, Ayad T, Jouffe L, Hopkins C, Saussez S; COVID-19 Task Force of YO-IFOS. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288:335-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 597] [Cited by in RCA: 530] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 3. | Reichert JL, Schöpf V. Olfactory Loss and Regain: Lessons for Neuroplasticity. Neuroscientist. 2018;24:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2555] [Cited by in RCA: 2787] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 5. | Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 736] [Cited by in RCA: 946] [Article Influence: 236.5] [Reference Citation Analysis (0)] |
| 6. | 6 Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 1291] [Article Influence: 322.8] [Reference Citation Analysis (0)] |
| 7. | Subramanian A, Nirantharakumar K, Hughes S, Myles P, Williams T, Gokhale KM, Taverner T, Chandan JS, Brown K, Simms-Williams N, Shah AD, Singh M, Kidy F, Okoth K, Hotham R, Bashir N, Cockburn N, Lee SI, Turner GM, Gkoutos GV, Aiyegbusi OL, McMullan C, Denniston AK, Sapey E, Lord JM, Wraith DC, Leggett E, Iles C, Marshall T, Price MJ, Marwaha S, Davies EH, Jackson LJ, Matthews KL, Camaradou J, Calvert M, Haroon S. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28:1706-1714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 636] [Article Influence: 212.0] [Reference Citation Analysis (0)] |
| 8. | McEwen BS. Allostasis and the Epigenetics of Brain and Body Health Over the Life Course: The Brain on Stress. JAMA Psychiatry. 2017;74:551-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 9. | Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brünink S, Greuel S, Lehmann M, Hassan O, Aschman T, Schumann E, Chua RL, Conrad C, Eils R, Stenzel W, Windgassen M, Rößler L, Goebel HH, Gelderblom HR, Martin H, Nitsche A, Schulz-Schaeffer WJ, Hakroush S, Winkler MS, Tampe B, Scheibe F, Körtvélyessy P, Reinhold D, Siegmund B, Kühl AA, Elezkurtaj S, Horst D, Oesterhelweg L, Tsokos M, Ingold-Heppner B, Stadelmann C, Drosten C, Corman VM, Radbruch H, Heppner FL. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 944] [Article Influence: 236.0] [Reference Citation Analysis (0)] |
| 10. | Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, Zandi MS, Lewis G, David AS. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1673] [Cited by in RCA: 1522] [Article Influence: 304.4] [Reference Citation Analysis (0)] |
| 11. | Molero P, Reina G, Blom JD, Martínez-González MÁ, Reinken A, de Kloet ER, Molendijk ML. COVID-19 risk, course and outcome in people with mental disorders: a systematic review and meta-analyses. Epidemiol Psychiatr Sci. 2023;32:e61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |