Published online Mar 19, 2024. doi: 10.5498/wjp.v14.i3.342
Peer-review started: December 20, 2023
First decision: January 11, 2024
Revised: January 16, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 19, 2024
Processing time: 89 Days and 17.6 Hours
Recent studies highlight the strong correlation between infectious diseases and the development of neuropsychiatric disorders. In this editorial, we comment on the article “Anti-infective therapy durations predict psychological stress and laparoscopic surgery quality in pelvic abscess patients” by Zhang et al, published in the recent issue of the World Journal of Psychiatry 2023; 13 (11): 903-911. Our discussion highlighted the potential consequences of anxiety, depression, and psychosis, which are all linked to bacterial, fungal, and viral infections, which are relevant to the impact of inflammation on the sequelae in mental health as those we are observing after the coronavirus disease 2019 pandemic. We focus specifically on the immune mechanisms triggered by inflammation, the primary contributor to psychiatric complications. Importantly, pathophysiological mechanisms such as organ damage, post-injury inflammation, and infection-induced endocrine alterations, including hypocortisolism or autoantibody formation, significantly contribute to the development of chronic low-grade inflammation, promoting the emergence or development of psychiatric alterations in susceptible individuals. As inflammation can have long-term effects on patients, a multidisciplinary treatment plan can avoid complications and debilitating health issues, and it is crucial to recognize and address the mental health implications.
Core Tip: In recent years there has been increasing evidence that infectious diseases increase the risk of developing psychiatric disorders due to acute or chronic inflammation. This manuscript offers a detailed summary and discussion that will provide valuable insights on the mechanisms behind psychiatric complications observed in infectious conditions as a commentary to the article “Anti-infective therapy durations predict psychological stress and laparoscopic surgery quality in pelvic abscess patients”.
- Citation: Ferat-Osorio E, Maldonado-García JL, Pavón L. How inflammation influences psychiatric disease. World J Psychiatry 2024; 14(3): 342-349
- URL: https://www.wjgnet.com/2220-3206/full/v14/i3/342.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i3.342
The coronavirus disease 2019 (COVID-19) pandemic has made it clearer that infectious diseases can cause psychiatric complications. These complications may occur during an infection or, in either case, afterward as a result of the inflammatory response[1]. Although psychiatric symptoms have been commonly associated with systemic (e.g., sepsis) and central nervous system infections (e.g., viral encephalitis or toxoplasmosis)[2], they may occur even without a brain infection[3]. The advances in understanding sepsis’s molecular pathophysiology play a crucial role in implementing therapeutic actions, leading to increased patient survival. Nonetheless, the mortality rate is still considerably high, and there are many challenges because of the global burden of sepsis[4,5]. Even though pathophysiology mainly refers to alterations caused by the microorganisms, the host’s inflammatory response may contribute to prolonged immune dysfunction, leading to immunosuppression, persistent inflammation, and catabolism[6]. The early phase of sepsis involves organ failure and lasts one to two weeks; this constitutes the pro-inflammatory phase, which is followed by the compensatory (anti-inflammatory) phase. If the anti-inflammatory phase fails to reach homeostasis, a low-grade or cronic persistent inflammatory state can develop. A more severe problem is that patients who survive the first phase experience increasing symptoms at 2 to 3 months. One possible reason for this condition is the advanced intensive care unit care that continues to keep elderly and comorbid patients despite ongoing immunological and metabolic issues[7,8]. Non-encephalic systemic infections can affect the central nervous system, resulting in neurological symptoms such as altered consciousness, disorientation, cognitive deficits, seizures, and coma. Sepsis-associated encephalopathy is a condition that affects the brain, and it can occur in up to 70% of patients with sepsis[9]. Furthermore, it can be acute or chronic[10]. The etiology of sepsis-associated encephalopathy can be caused by almost any systemic infection, including those in the respiratory, urinary, gastrointestinal, biliary (such as cholangitis), and genital tract.
Pelvic inflammatory disease (PID) is less frequent but also critical. PID is an infection that occurs in the upper female genital tract and primarily affects sexually active young women. Although the actual incidence and prevalence of PID are unknown, data from 2013 suggest that 4.4% of sexually active women report a history of PID. Typically, PID is caused by a sexually transmitted infection by Chlamydia trachomatis and Neisseria gonorrhoeae, but it can also be caused by Mycoplasma genitalium and Actinomyces species. Microorganisms present in the gastrointestinal or respiratory tract may also play a role in the development of PID (E. coli, B. fragilis, Pepto-streptococcus spp, Haemophilus influenzae). Clinical manifestations may range from mild to severe and require in-hospital management using parenteral antibiotics. Antibiotic treatment can prove efficacious in 34% to 88%. Interventional approaches may provide the definitive treatment for those who do not improve with medical therapy. Interventional radiology (percutaneous drain with drains placed) or laparoscopy are options to treat PID complications like tubo-ovarian abscess (TOA). In this case, it is important to begin the treatment early for optimal results (48 h to 72 h). TOA occurs when pus accumulates in the fallopian tubes and ovaries, leading to inflammation and severe pain. Unfortunately, around 25% to 30% of women with TOA will require surgical drainage to relieve the symptoms. Untreated PID in women with TOA can lead to long-term consequences such as chronic pain (29%), infertility (18%), or ectopic pregnancy (0.6%)[11].
PID presents itself in two scenarios. In the first one, the acute form of the condition can be managed conservatively, but if it does not respond to treatment, it may result in an abscess. If left untreated, this abscess can lead to serious complications such as sepsis, septic shock, and death. The molecular pathophysiology of this condition begins with the host recognizing of the offending agent. Recognition of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) present in immune cells like neutrophils, monocytes, and dendritic cells can activate signaling cascades that induce the transcription of inflammatory mediators in response to microorganisms. Subsequently, mediators act locally, either in a paracrine or autocrine way[12].
The second scenario of the PID is the chronic form. It has been observed that some women may have mild symptoms or lower suspicion levels, which can lead to the possibility of missing out on identifying an inflammatory-infectious problem from a gynecological perspective. In these cases, chronic infection may result in the previously mentioned sequelae. Infertility due to recurrent or chronic infections seems to be associated with cell death by pyroptosis[13]. This type of cellular death is linked to inflammasome activation, which leads to caspase-1 activation following the recognition of PAMPs and damage associated molecular patterns by different PRRs (intracellular and extracellular, depending on the microorganism). Caspase-1 activation, in turn, triggers a protein called gasdermin D, inducing the formation of pores in the host cell’s membrane, and releasing intracellular content that can act as alarmins (e.g., high mobility group box 1 proteins or HMGB1). Caspase-1 also facilitates the cleavage of Pro-IL-1 and Pro-IL-18, releasing them into the extracellular space as IL-1β and IL-18[14]. In the case of PID, there is a process of endometritis that may be associated with pyroptosis[15].
According to several reports, HMGB1-mediated macrophage pyroptosis is involved in the molecular pathophysiology of chronic endometritis[16]. HMGB1 is an intranuclear protein that can be released due to cellular damage associated with multiple causes, sterile inflammation, or infectious processes[17,18]. In the extracellular environment, HMGB1 acts as an alarmin, binds to its receptor RAGE, and subsequently induces pyroptosis, amplifying the inflammatory response following the release of IL-1 and IL-18[19].
Related to central nervous system disorders induced by infectious processes mentioned earlier, HMGB1, through binding with MD-2, is known to promote NLRP3-induced neuroinflammation, resulting in cognitive impairment in cases of sepsis-associated encephalopathy in murine models[20]. In the manuscript of Zhang et al[21] “Anti-infective therapy durations predict psychological stress and laparoscopic surgery quality in pelvic abscess patients”, serum levels of inflammatory mediators like HMGB1 or pro- and anti-inflammatory cytokines have not been determined. Knowing the concentrations of these proteins could provide insights into cognitive impairments in the group of patients with PID who exhibited neurological symptoms. Psychiatric symptoms may occur without neurological symptoms, as in some cases of viral encephalitis[3]. Psychosis or mood symptoms may feature as a component of the clinical presentation secondary to brucellosis or toxoplasmosis[22]. Late-onset neuropsychiatric complications, such as subacute sclerosing panencephalitis caused by measles, have been reported years after acute infection[23]. Some studies suggest that viral infections like influenza virus or HSV-1 may increase the risk of developing schizophrenia and psychosis, indicating a possible link between psychiatric disorders and infectious diseases[24]. Furthermore, psychiatric symptoms can also be reactivated because of chronic, complicated, and severe infections, such as HIV, that can cause an individual to experience depression, anxiety, or adjustment disorders[25].
Stress plays a crucial role in the development of major depressive disorder (MDD), particularly stress in early life and chronic stress in susceptible individuals[26]. Stress response is modulated by the hypothalamus-pituitary-adrenal (HPA) axis, which connects the nervous and endocrine systems and is formed by the hypothalamus and pituitary and adrenal glands[27]. A stressor, such as an infection, activates the HPA axis and promotes the release of corticotropin hormone release by the hypothalamus, which stimulates the pituitary gland to release corticotropin (ACTH), which enables the adrenal glands to release cortisol and catecholamines to trigger the flight or fight response[28]. Chronic stress affects hippocampus functions, and it has been reported that cortisol is an important mediator. Consequently, if the stress lasts longer, chronic stress can generate changes in the hippocampus, ranging from modification of plasticity to neurotoxicity and neuronal death[29,30]. As a result, chronic stress can induce glucocorticoid resistance[31], which is characterized by alterations in glucocorticoid receptor (GR) function, changes in GR expression, alterations in glucocorticoid bioavailability through modification of serum protein binding, deficiencies in HPA axis feedback and immune system inhibition[32,33]. In chronic stress conditions, MDD patients have higher levels of circulating glucocorticoids compared to healthy individuals. They may coexist with elevated concentrations of proinflammatory cytokines such as IL-1β, tumour necrosis factor alpha (TNF-α), and IL-6[34-36]. The simultaneous presence of elevated levels of glucocorticoids and cytokines creates a complex interaction between the immune system and the HPA axis, a paradoxical phenomenon characterized by chronic inflammation[37,38].
In systemic or chronic inflammatory diseases (e.g., chronic infections), it has been reported that circulating proinflammatory cytokines stimulate the brain and cause anxiety, anhedonia, fatigue, and sleep disturbances. In addition, sickness behavior characterized by the presence of a febrile response, anorexia, lack of motivation, social deprivation, and reduced movement is also present[39-41]. One of the first associations of cytokines with neuropsychiatric complications was observed in hepatitis C treatment with interferon-alpha (IFN-α); the presence of depressive symptoms and even suicidal ideation was observed in patients receiving IFN-α treatment[42,43].
Proinflammatory cytokines produced in the brain can stimulate the brain through different pathways: (1) Stimulating receptors in the blood-brain barrier (BBB) and producing metabolites in the brain; (2) accessing the brain through the circumventricular organs; (3) being carried through transporters in the BBB; and (4) through stimulation of afferent fibers of the vagus nerve[44,45].
As previously described, peripheral proinflammatory cytokines stimulate the brain and generate a neuroinflammatory response caused by the activation of neurons, microglia, and astrocytes[46]. Proinflammatory cytokines induce changes in the metabolism of tryptophan, a precursor of serotonin in both the periphery and the brain, thereby increasing inflammation and decreasing serotonin production[47]. One of the mechanisms involved in this metabolic pathway change is the activation of indolamine 2,3-dioxygenase in macrophages and microglia cells, whereby tryptophan is metabolized in the kynurenine pathway; it causes a decrease in serotonin levels and an increase in kynurenine in the body and brain[40,48]. Moreover, peripheral kynurenine crosses the BBB and is metabolized in activated astrocytes and microglia by kynurenine aminotransferase II (KAT II)[49]. The kynurenine metabolism generates quinolinic acid and induces a decrease in dopamine and glutamate production and blockade of α7nAChR cholinergic receptors; these changes are associated with cognitive dysfunction[49,50]. Similarly, activated microglia metabolize kynurenine through the enzymes kynurenine 3-monooxygenase and 3-hydroxyanthranilicoxygenase, which generate metabolites such as kynurenic acid, which stimulates NMDA receptors and causes lipid peroxidation, oxidative stress, excitotoxicity, and neurodegeneration[49,50]. In addition, chronic stress decreases the function of the serotonergic system, characterized by increased SERT and p11 expression in peripheral blood mononuclear cells[51].
On the other hand, inflammation causes oxidative stress, which reduces the production of tetrahydrobiopterin (BH), a necessary cofactor for synthesizing serotonin, dopamine, and norepinephrine. As a result, inflammation leads to a deficiency in the production of monoamines[49,52]. All metabolic changes together are related to the development of disease behavior in patients with systemic inflammatory responses caused by injury or infection, and these symptoms remit as soon as the inflammation is resolved[39].
As mentioned above, inflammation decreases dopamine and serotonin synthesis in the brain and periphery. Inflammation associated with infection has different sources, including antigen persistence, hypocortisolism or HPA axis dysfunction, chronic organ dysfunction or worsening of pre-existent dysfunction, persistent tissue or end-organ damage, and persistent cytokine release, among others[1,53,54].
The immune system usually resolves pathogens that cause acute infections. Still, ample evidence indicates that some pathogens can cause persistent and sometimes lifelong infections[55]. Some bacteria that cause chronic infections are phylogenetically diverse[55]. However, they share common characteristics that allow a prolonged period of colonization and share strategies to evade elimination by the immune system, thus causing chronic intracellular infections[56]. An example is brucellosis and typhoid fever, which are characterized by a long incubation period leading to a regular, sometimes lifelong illness, which is debilitating and can cause severe clinical manifestations[57]. Recently, our group has characterized the neurochemical, hormonal, and inflammatory alterations present in a murine model of brucellosis and behavioral alterations. We have reported that brucellosis infection induces decreased motivation and physical performance, as well as increased hopelessness and anxiety. These findings are complemented by a decrease in dopamine and serotonin in the hippocampus and frontal cortex and elevated levels of IL-6, IFN-γ, and TNF-α in serum[58]. Subsequently, we observed that administration of imipramine in mice infected with Brucella abortus 2308 causes an improvement in hopelessness, anxiety, physical performance, and motivation even though the infection has not been entirely eliminated[59].
Chronic infections have a significant impact on public health due to the use of resources for the long-term treatment of patients. In addition, chronic infections can lead to disability as they can cause the development of psychiatric illnesses like depression or anxiety. These illnesses can significantly impact the patient’s ability to generate economic resources to support their families, ultimately resulting in a substantial socioeconomic burden on countries affected by such infections[57].
Solid evidence shows that some infections can induce hypocortisolism due to adrenal insufficiency[60]. Bacterial infections such as Mycobacterium tuberculosis, Pseudomonas aeruginosa, Group A Streptococcus, or Haemophilus influenzae can cause hypocortisolism[60]. Similarly, viral agents such as HIV and cytomegalovirus, and fungal infections by Pneumocystis carinii, Coccidioides immitis, Cryptococcus neoformans, and Histoplasma capsulatum can induce adrenal insufficiency which results in hypocortisolism[60]. Low circulating cortisol levels observed in hypocortisolism have been associated with a chronic inflammatory state; this is explained by the inhibition of the proinflammatory cytokines production in leukocytes such as macrophages and lymphocytes induced by cortisol[61,62]. In this way, the generation of autoantibodies against ACTH has been observed during coronavirus infections, and it has been proposed that this mechanism is the cause of the post-infection hypocortisolism observed in patients[63,64]. Furthermore, in the case of COVID-19, hypocortisolism has been proposed as one of the mechanisms associated with the development of chronic inflammation and long-COVID[53].
Another mechanism of damage associated with infections that induce chronic inflammation is target organ damage or worsening of pre-existing damage[65]. Infections that induce chronic inflammation also have been found to cause a mechanism of damage known as target organ damage or the exacerbation of pre-existing damage; for example, in post-COVID-19 patients, it has been observed that damage to the pancreas can occur and induce hyperglycemia due to a deficit in insulin production; such increased hyperglycemia may lead to chronic low-grade inflammation[1,53]. Another example can be observed in patients who have recovered from sepsis. These patients may experience chronic immunosuppression and inflammation due to changes in T lymphocytes and hematopoiesis. They may also suffer from complications arising from damage to their kidneys, heart, or endothelium[66,67].
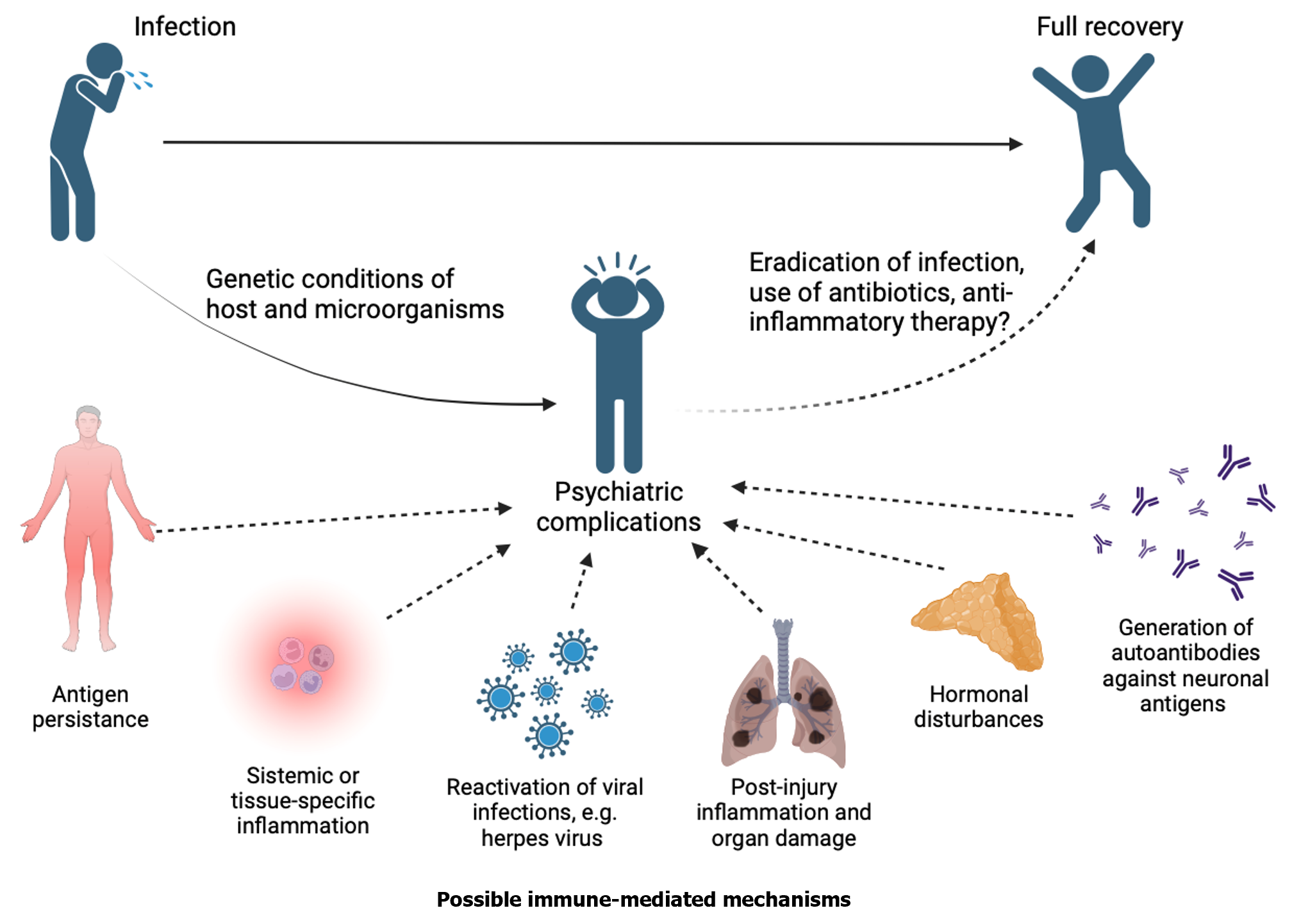
Finally, it has been observed that antibodies produced during an infection can trigger neuropsychiatric complications due to a cross-reaction in which antibodies recognize pathogen antigens but may also recognize self-antigens[68,69]. It has been proposed that genetic factors of the host and infectious agent influence the development of these complications[70]. Cross-reacting antibodies can directly recognize neuronal antigens, as in streptococcal infections, and trigger neuropsychiatric symptoms[71]; however, antibodies can cross-react against cortisol, as in the case of coronavirus infections, and thus inhibit the regulation of inflammation and generate neuropsychiatric complications secondary to chronic inflammation[64]. Figure 1 summarizes the proposed mechanisms by which infections may cause psychiatric disorders.
Even after injury or infection has been resolved, persistent inflammatory parameters may continue to affect body levels of inflammatory, hormonal, and neurochemical molecules. In some individuals, exposure to stressors like surgery or infections can contribute to the development of psychiatric disorders, such as anxiety and depression, due to chronic stress. It can also exacerbate pre-existing psychiatric conditions. Clinicians must take into account infection-associated factors such as microorganisms, host, and treatment characteristics when treating patients. These factors may lead to the development of psychiatric complications, so it is imperative to offer more holistic therapeutic options that consider the primary problem and its psychiatric complications. Further investigation is crucial for future studies to understand better the mechanisms by which infection causes psychiatric complications.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liu XQ, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
| 1. | Peluso MJ, Deeks SG. Early clues regarding the pathogenesis of long-COVID. Trends Immunol. 2022;43:268-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 87] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 2. | Smith ML, Gradus JL. Psychiatric disorders and risk of infections: early lessons from COVID-19. Lancet Healthy Longev. 2020;1:e51-e52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Müller N. Infectious Diseases and Mental Health. Key Issues Ment Heal. 2015;179:99-113. [DOI] [Full Text] |
| 4. | Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K; International Forum of Acute Care Trialists. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1639] [Cited by in RCA: 2251] [Article Influence: 250.1] [Reference Citation Analysis (0)] |
| 5. | Fleischmann-Struzek C, Rudd K. Challenges of assessing the burden of sepsis. Med Klin Intensivmed Notfmed. 2023;118:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 537] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 7. | Moore FA, Moore EE. Evolving concepts in the pathogenesis of postinjury multiple organ failure. Surg Clin North Am. 1995;75:257-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 383] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, Zawistowski C, Bemis-Dougherty A, Berney SC, Bienvenu OJ, Brady SL, Brodsky MB, Denehy L, Elliott D, Flatley C, Harabin AL, Jones C, Louis D, Meltzer W, Muldoon SR, Palmer JB, Perme C, Robinson M, Schmidt DM, Scruth E, Spill GR, Storey CP, Render M, Votto J, Harvey MA. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1316] [Cited by in RCA: 1387] [Article Influence: 106.7] [Reference Citation Analysis (34)] |
| 9. | Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 497] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 10. | Ren C, Yao RQ, Zhang H, Feng YW, Yao YM. Sepsis-associated encephalopathy: a vicious cycle of immunosuppression. J Neuroinflammation. 2020;17:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 11. | Frock-Welnak DN, Tam J. Identification and Treatment of Acute Pelvic Inflammatory Disease and Associated Sequelae. Obstet Gynecol Clin North Am. 2022;49:551-579. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Greydanus DE, Cabral MD, Patel DR. Pelvic inflammatory disease in the adolescent and young adult: An update. Dis Mon. 2022;68:101287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Jennings LK, Krywko DM. Pelvic Inflammatory Disease. 2023 Mar 13. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 14. | Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277:61-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1195] [Article Influence: 149.4] [Reference Citation Analysis (0)] |
| 15. | Huang Y, Li R, Hu R, Yao J, Yang Y. PEG2-Induced Pyroptosis Regulates the Expression of HMGB1 and Promotes hEM15A Migration in Endometriosis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 16. | Yang G, Zhang Q, Tan J, Xiong Y, Liang Y, Yan J, Gu F, Xu Y. HMGB1 induces macrophage pyroptosis in chronic endometritis. Int Immunopharmacol. 2023;123:110706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Arriaga-Pizano L, Boscó-Gárate I, Martínez-Ordaz JL, Wong-Baeza I, Gutiérrez-Mendoza M, Sánchez-Fernandez P, López-Macías C, Isibasi A, Pelaez-Luna M, Cérbulo-Vázquez A, Torres-González R, Ferat-Osorio E. High Serum Levels of High-Mobility Group Box 1 (HMGB1) and Low Levels of Heat Shock Protein 70 (Hsp70) are Associated with Poor Prognosis in Patients with Acute Pancreatitis. Arch Med Res. 2018;49:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Zhou M, Aziz M, Wang P. Damage-Associated Molecular Patterns As Double-Edged Swords in Sepsis. Antioxid Redox Signal. 2021;35:1308-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Shang J, Zhao F, Cao Y, Ping F, Wang W, Li Y. HMGB1 mediates lipopolysaccharide-induced macrophage autophagy and pyroptosis. BMC Mol Cell Biol. 2023;24:2. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Xiong Y, Yang J, Tong H, Zhu C, Pang Y. HMGB1 augments cognitive impairment in sepsis-associated encephalopathy by binding to MD-2 and promoting NLRP3-induced neuroinflammation. Psychogeriatrics. 2022;22:167-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Zhang RR, Zhang L, Zhao RH. Anti-infective therapy durations predict psychological stress and laparoscopic surgery quality in pelvic abscess patients. World J Psychiatry. 2023;13:903-911. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 22. | Obuaya CC, Gangatharan GT, Karra E. Brucella-Induced Acute Psychosis: A Novel Cause of Acute Psychosis. Case Rep Infect Dis. 2021;2021:6649717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Munjal S, Ferrando SJ, Freyberg Z. Neuropsychiatric Aspects of Infectious Diseases: An Update. Crit Care Clin. 2017;33:681-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Kotsiri I, Resta P, Spyrantis A, Panotopoulos C, Chaniotis D, Beloukas A, Magiorkinis E. Viral Infections and Schizophrenia: A Comprehensive Review. Viruses. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Nedelcovych MT, Manning AA, Semenova S, Gamaldo C, Haughey NJ, Slusher BS. The Psychiatric Impact of HIV. ACS Chem Neurosci. 2017;8:1432-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Tafet GE, Nemeroff CB. The Links Between Stress and Depression: Psychoneuroendocrinological, Genetic, and Environmental Interactions. J Neuropsychiatry Clin Neurosci. 2016;28:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 210] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 27. | Mikulska J, Juszczyk G, Gawrońska-Grzywacz M, Herbet M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 177] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 28. | Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol. 2016;6:603-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 1127] [Article Influence: 125.2] [Reference Citation Analysis (0)] |
| 29. | Kim EJ, Pellman B, Kim JJ. Stress effects on the hippocampus: a critical review. Learn Mem. 2015;22:411-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 336] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 30. | Kim EJ, Kim JJ. Neurocognitive effects of stress: a metaparadigm perspective. Mol Psychiatry. 2023;28:2750-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 31. | Hassamal S. Chronic stress, neuroinflammation, and depression: an overview of pathophysiological mechanisms and emerging anti-inflammatories. Front Psychiatry. 2023;14:1130989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 125] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 32. | Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012;109:5995-5999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 828] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 33. | Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 489] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 34. | Pariante CM. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. Eur Neuropsychopharmacol. 2017;27:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 251] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 35. | Perrin AJ, Horowitz MA, Roelofs J, Zunszain PA, Pariante CM. Glucocorticoid Resistance: Is It a Requisite for Increased Cytokine Production in Depression? A Systematic Review and Meta-Analysis. Front Psychiatry. 2019;10:423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 36. | Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:722-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 382] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 37. | Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 464] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 38. | Weber MD, Godbout JP, Sheridan JF. Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal. Neuropsychopharmacology. 2017;42:46-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 209] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 39. | Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29:247-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 548] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 40. | Miller AH. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: psychoneuroimmunology at the translational interface. Brain Behav Immun. 2009;23:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 41. | Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1963] [Cited by in RCA: 2144] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 42. | Amodio P, De Toni EN, Cavalletto L, Mapelli D, Bernardinello E, Del Piccolo F, Bergamelli C, Costanzo R, Bergamaschi F, Poma SZ, Chemello L, Gatta A, Perini G. Mood, cognition and EEG changes during interferon alpha (alpha-IFN) treatment for chronic hepatitis C. J Affect Disord. 2005;84:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Chiu WC, Su YP, Su KP, Chen PC. Recurrence of depressive disorders after interferon-induced depression. Transl Psychiatry. 2017;7:e1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Bauer ME. Accelerated immunosenescence in rheumatoid arthritis: impact on clinical progression. Immun Ageing. 2020;17:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 45. | Becher B, Spath S, Goverman J. Cytokine networks in neuroinflammation. Nat Rev Immunol. 2017;17:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 467] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 46. | Corrigan M, O’Rourke AM, Moran B, Fletcher JM, Harkin A. Inflammation in the pathogenesis of depression: a disorder of neuroimmune origin. Neuronal Signal. 2023;7:NS20220054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 47. | Herselman MF, Bailey S, Bobrovskaya L. The Effects of Stress and Diet on the "Brain-Gut" and "Gut-Brain" Pathways in Animal Models of Stress and Depression. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 48. | Huang YS, Ogbechi J, Clanchy FI, Williams RO, Stone TW. IDO and Kynurenine Metabolites in Peripheral and CNS Disorders. Front Immunol. 2020;11:388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 49. | Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 685] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 50. | Mithaiwala MN, Santana-Coelho D, Porter GA, O’Connor JC. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 51. | Becerril-Villanueva E, Olvera-Alvarez MI, Alvarez-Herrera S, Maldonado-García JL, López-Torres A, Ramírez-Marroquín OA, González-Ruiz O, Nogueira-Fernández JM, Mendoza-Contreras JM, Sánchez-García HO, José-Alfallo JA, Valencia Baños A, Torres-Serrano AB, Jiménez-Genchi J, Mendieta-Cabrera D, Pérez-Sánchez G, Pavón L. Screening of SERT and p11 mRNA Levels in Airline Pilots: A Translational Approach. Front Psychiatry. 2022;13:859768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Vancassel S, Capuron L, Castanon N. Brain Kynurenine and BH4 Pathways: Relevance to the Pathophysiology and Treatment of Inflammation-Driven Depressive Symptoms. Front Neurosci. 2018;12:499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 53. | Bansal R, Gubbi S, Koch CA. COVID-19 and chronic fatigue syndrome: An endocrine perspective. J Clin Transl Endocrinol. 2022;27:100284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 54. | Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 901] [Article Influence: 225.3] [Reference Citation Analysis (0)] |
| 55. | Petitdemange C, Funderburg N, Zaunders J, Corbeau P. Editorial: Infectious Agent-Induced Chronic Immune Activation: Causes, Phenotypes, and Consequences. Front Immunol. 2021;12:740556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 56. | Cohen SP, Wang EJ, Doshi TL, Vase L, Cawcutt KA, Tontisirin N. Chronic pain and infection: mechanisms, causes, conditions, treatments, and controversies. BMJ Med. 2022;1:e000108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Schwab JJ. Psychiatric aspects of infectious diseases. Curr Psychiatr Ther. 1982;21:225-239. [PubMed] |
| 58. | Maldonado-García JL, Pérez-Sánchez G, Becerril Villanueva E, Alvarez-Herrera S, Pavón L, Gutiérrez-Ospina G, López-Santiago R, Maldonado-Tapia JO, Pérez-Tapia SM, Moreno-Lafont MC. Behavioral and Neurochemical Shifts at the Hippocampus and Frontal Cortex Are Associated to Peripheral Inflammation in Balb/c Mice Infected with Brucella abortus 2308. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Maldonado-García JL, Pérez-Sánchez G, Becerril-Villanueva E, Alvarez-Herrera S, Pavón L, Sánchez-Torres L, Gutiérrez-Ospina G, Girón-Pérez MI, Damian-Morales G, Maldonado-Tapia JO, López-Santiago R, Moreno-Lafont MC. Imipramine Administration in Brucella abortus 2308-Infected Mice Restores Hippocampal Serotonin Levels, Muscle Strength, and Mood, and Decreases Spleen CFU Count. Pharmaceuticals (Basel). 2023;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 60. | Alevritis EM, Sarubbi FA, Jordan RM, Peiris AN. Infectious causes of adrenal insufficiency. South Med J. 2003;96:888-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Moutsopoulos NM, Madianos PN. Low-grade inflammation in chronic infectious diseases: paradigm of periodontal infections. Ann N Y Acad Sci. 2006;1088:251-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 62. | Arabi YM, Chrousos GP, Meduri GU. The ten reasons why corticosteroid therapy reduces mortality in severe COVID-19. Intensive Care Med. 2020;46:2067-2070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 63. | Leow MK, Kwek DS, Ng AW, Ong KC, Kaw GJ, Lee LS. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin Endocrinol (Oxf). 2005;63:197-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 64. | Pérez-Torres D, Díaz-Rodríguez C, Armentia-Medina A. Anti-ACTH antibodies in critically ill Covid-19 patients: A potential immune evasion mechanism of SARS-CoV-2. Med Intensiva (Engl Ed). 2022;46:472-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 65. | Sauaia A, Moore FA, Moore EE. Postinjury Inflammation and Organ Dysfunction. Crit Care Clin. 2017;33:167-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 66. | Caraballo C, Jaimes F. Organ Dysfunction in Sepsis: An Ominous Trajectory From Infection To Death. Yale J Biol Med. 2019;92:629-640. [PubMed] |
| 67. | Liu D, Huang SY, Sun JH, Zhang HC, Cai QL, Gao C, Li L, Cao J, Xu F, Zhou Y, Guan CX, Jin SW, Deng J, Fang XM, Jiang JX, Zeng L. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res. 2022;9:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 189] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 68. | Johnson D, Jiang W. Infectious diseases, autoantibodies, and autoimmunity. J Autoimmun. 2023;137:102962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 69. | Rivera-Correa J, Rodriguez A. Autoantibodies during infectious diseases: Lessons from malaria applied to COVID-19 and other infections. Front Immunol. 2022;13:938011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 70. | Puel A, Bastard P, Bustamante J, Casanova JL. Human autoantibodies underlying infectious diseases. J Exp Med. 2022;219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 71. | Mader S, Brimberg L, Diamond B. The Role of Brain-Reactive Autoantibodies in Brain Pathology and Cognitive Impairment. Front Immunol. 2017;8:1101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |