Published online Nov 19, 2024. doi: 10.5498/wjp.v14.i11.1746
Revised: September 6, 2024
Accepted: October 28, 2024
Published online: November 19, 2024
Processing time: 253 Days and 0.3 Hours
Globally, the World Health Organization ranks major depressive disorder (MDD) as the leading cause of disability. However, MDD molecular etiology is still poor
To explore the possible association between mitochondrial ND6 T14502C muta
Clinical data were collected from two pedigrees, and detailed mitochondrial ge
Herein, we reported the clinical, genetic, and molecular profiling of two Chinese families afflicted with MDD. These Chinese families exhibited not only a range of onset and severity ages in their depression but also extremely low penetrances to MDD. Sequence analyses of mitochondrial genomes from these pedigrees have resulted in the identification of a homoplasmic T14502C (I58V) mutation. The polymorphism is located at a highly conserved isoleucine at position 58 of ND6 and distinct mitochondrial DNA (mtDNA) poly
Identifying the T14502C mutation in two individuals with no genetic relation who exhibit symptoms of depression provides compelling evidence that this mutation may be implicated in MDD development. Nonetheless, the two Chinese pedigrees that carried the T14502C mutation did not exhibit any functionally significant mutations in their mtDNA. Therefore, the phenotypic expression of the T14502C mutation related to MDD may be influenced by the nuclear modifier gene(s) or environmental factors.
Core Tip: In this study, we report the clinical, genetic and molecular characterization of two Chinese families with major depressive disorder (MDD). Sequence analyses of mitochondrial genomes from these pedigrees revealed homoplasmic T14502C (I58V) mutation. This observation of the T14502C mutation in two genetically unrelated individuals who suffer from depression strongly suggests that this mutation might play a role in the development of MDD.
- Citation: Jing P, Yu HH, Wu TT, Yu BH, Liang M, Xia TT, Xu XW, Xu T, Liu LJ, Zhang XB. Major depressive disorder is associated with mitochondrial ND6 T14502C mutation in two Han Chinese families. World J Psychiatry 2024; 14(11): 1746-1754
- URL: https://www.wjgnet.com/2220-3206/full/v14/i11/1746.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i11.1746
Major depressive disorder (MDD) is a widespread mental health condition defined by a significant change in mood accompanied by a loss of interest, motivation, or pleasure. Moreover, individuals with MDD may show other symptoms, including changes in appetite, sleep patterns, pain perception, energy levels, and symptoms related to cognitive functions or content[1]. MDD is associated with a significant decrease in functioning, a disruption of normal work-related activities, and substantial public health expenditures. The prevalence of MDD is indeed on the rise globally, and it significantly impacts people’s lives[2-4]. However, MDD molecular etiology is still poorly understood[5]. Extensive studies have shown that the molecular mechanism of depression is highly complex, which is multi-mechanism and multi-pathway rather than a single pathway[6,7]. According to the available literature, various hypotheses and pilot studies have in
The mitochondria are essential organelles in eukaryotic cells and are primarily responsible for producing adenosine-triphosphate (ATP) through the oxidative phosphorylation pathway. In addition, they perform crucial functions in numerous metabolic, regulatory, and developmental mechanisms[13]. Several studies have investigated the association between MDD and mitochondria. The role of mitochondria is crucial in the proper functioning of neurons, and it has been observed that patients with MDD experience impaired mitochondrial function[14-16]. Due to the fact that mi
Patients with MDD have been observed to exhibit a higher incidence of mtDNA deletion mutations compared to control groups or to display mtDNA mutations similar to those observed in mitochondrial disorders[19]. Our previous familial study found that MDD may be associated with mitochondrial T3394C mutations[20]. In this study, we aimed to explore the possible association between mitochondrial ND6 T14502C mutation and MDD. This study was to conduct a characterization of the clinical, genetic, and molecular features of two Chinese families that may have inherited MDD maternally. The ND6 gene mutation T14502C was identified through molecular analysis in two Chinese families. Furthermore, the research employed polymerase chain reaction (PCR) amplification of fragments that covered the entire mitochondrial genome, followed by DNA sequence analysis, for a better understanding of the role of mitochondrial haplotype in the phenotypic expression of the T14502C mutation in the two Chinese families.
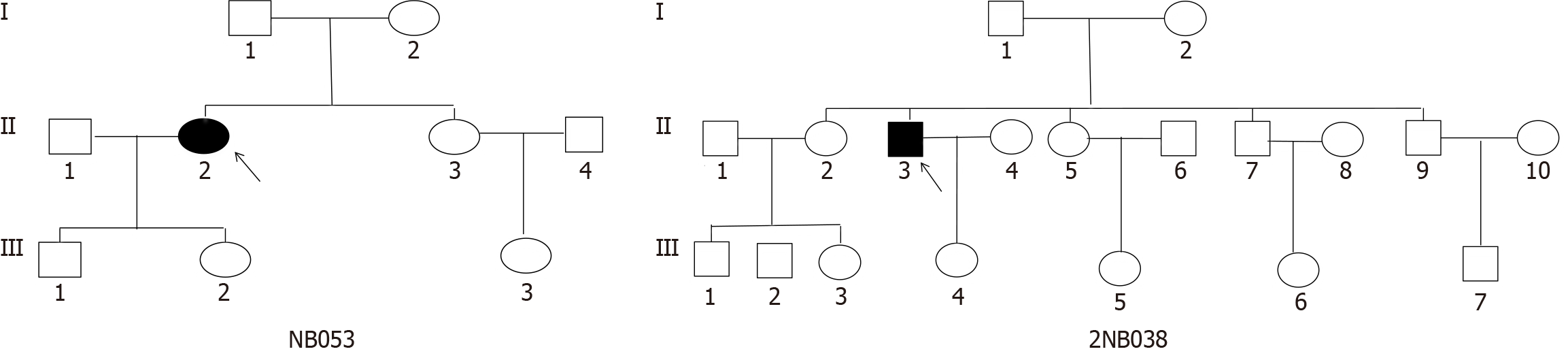
Two Han Chinese families (Figure 1) were identified through the Psychiatric Clinic of Ningbo Kangning Hospital, Zhejiang Province. Structured clinical interviews were utilized to establish MDD diagnosis for the probands who met diagnostic and statistical manual of mental disorders diagnostic criteria[21]. Additionally, a board-certified psychiatrist conducted a separate diagnostic evaluation to verify the diagnosis. The exclusion criteria of this study included sub
The Ningbo Kangning Hospital Ethics Committee approved protocols requiring informed consent, blood samples, as well as clinical assessments from the family members who participated in this study. Members belonging to these pedigrees were extensively interviewed to ascertain their personal or familial MDD medical histories and other clinical abnormalities. The 99 control DNA samples were obtained from the health screening clinic at Ningbo Kangning Hospital with the aim of conducting mtDNA mutation screening. The participants were interviewed to determine the absence of any significant psychiatric disorder in their medical history and to confirm that they were not currently under the influence of psychotropic medication. Upon study entry, all participants were confirmed to be medically healthy except for MDD, with no history of chronic medical conditions, absence of acute infection, and non-pregnant status.
Pure-gene DNA isolation kits (Gentra Systems) were utilized for isolating the genomic DNA from the obtained whole blood samples. The PCR amplification of complete mitochondrial genomes of the two probands was carried out using 24 overlapping fragments and sets of light (L)- and heavy (H)-strand oligonucleotide primers, as described earlier[23]. The fragments were purified and sequenced directly through the Big Dye Terminator Cycle sequencing reaction kit in an applied biosystems 3700 automated DNA sequencer. Subsequently, these results were compared to the updated consensus ambridge sequence (GenBank accession number: NC012920)[24]. The Seqweb program GAP program was utilized for alignments of DNA and protein sequences. The same method was used to analyze blood samples from 99 normal controls.
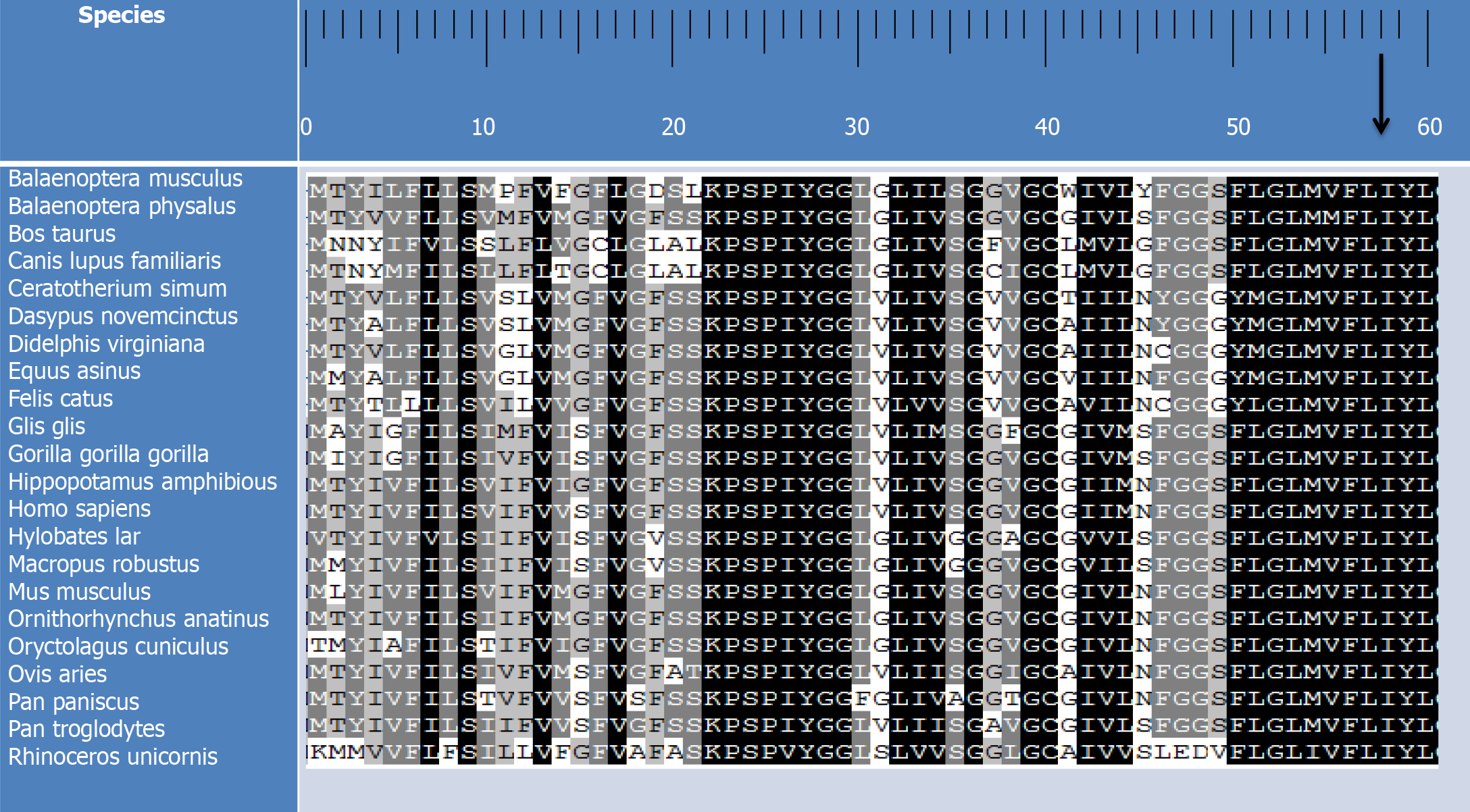
Four vertebrate mtDNA sequences were used in the interspecific analysis, including Homo sapiens, mouse, bovine, and Xenopus laevis[25]. The evaluation of conservation was conducted through a comparative analysis of four different vertebrate species. All mtDNA sequences from the two Chinese probands with the T14502C mutation were assigned to Asian mitochondrial haplogroups through the nomenclature of mitochondrial haplogroups[26].
The proband (II-2) of the family NB053, aged 43 years, as indicated in Table 1, reported experiencing depression and sought treatment at the Psychiatric Clinic at the Ningbo Kangning Hospital. HDRS indicated a score of 26, and the participant did not report any previous suicide attempts, thus exhibiting a typical clinical feature of MDD. No further anomalies were detected during the psychiatric assessment. Additionally, there was no significant medical history present in her records. The family originates from Zhejiang Province in Eastern China. Nonetheless, MDD was not observed in this family’s other five matrilineal relatives.
| Subject | Gender | Age of test (years) | Age of onset (years) | First episode | History of suicide attempts | Psychotic symptoms | HDRS | Level of depression | MtDNA haplogroup |
| NB053-II-2 | F | 43 | 43 | Yes | No | Yes | 26 | Severe | M10 |
| 2NB038-II-3 | M | 53 | 52 | Yes | No | No | 23 | Moderate | H2 |
The proband (III-3) in the 2NB038 pedigree, aged 53 years, came to the Psychiatric Clinic at Ningbo Kangning Hospital. He was diagnosed with MDD one year ago. The participant had a score of 23 on the HDRS with no history of suicide attempts, indicating typical clinical features of MDD. Additionally, there was no significant medical history present in the records. The family originates from Zhejiang Province in Eastern China. MDD was not observed in any of the other matrilineal relatives.
In addition, no substantiated evidence indicates that any individual belonging to these families exhibited any other identifiable factor that could be attributed to MDD. Comprehensive family medical histories of the participants revealed no additional clinical abnormalities (diabetes, hearing impairment, and vision problems).
In order to clarify the molecular basis of MDD, an analysis of mutations in the mitochondrial genome was conducted on the probands. The results of the conducted PCR amplification of fragments revealed that the substitution of valine with isoleucine (I58V) at amino acid position 58, caused by the T-to-C transition at position 14502 (T14502C) in the ND6 gene, has been detected in the subjects. The results also revealed that isoleucine at position 58 in ND6 is highly conserved among 22 organisms (Figure 2). Examination of allele frequency related to the T14502C mutation demonstrated that only one individual (a 45-year-old female) out of a cohort of 99 non-associated Chinese control subjects exhibited the T14502C mutation.
In addition to the T14502C mutation that was found to be identical among the subjects, as presented in Table 2, they showed different mtDNA polymorphisms. The mitochondrial genomes exhibit several nucleotide changes, including 17 variants in the D-loop, 4 variants in the 12S rRNA gene, 5 variants in the 16S rRNA gene, 2 variants in the tRNA gene, 28 silent variants, and 13 missense mutations (1 novel/12 known) in the polypeptide-encoding genes. These missense mutations are GA4569G (Glu34 Lys), TA4573T (Met35 Lys) and A5505G (Ile346Val) in the ND2 gene; T8618C (Ile31Thr), A8701G (Thr59Ala) and A8860G (Thr112Ala) in the ATP6 gene; A10398G (Thr114Ala) in the ND3 gene; C14766T (Thr7Ile), T15071C (Tyr109His), A15326G (Thr194Ala) and G15617A (Val291Ile) in the Cytb gene. Within these, 17 mutations were identified in both probands. Moreover, we conducted a phylogenetic analysis of the variants in RNAs and polypeptides, along with sequences from other organisms, including bovines, mice, and Xenopus laevis. All variants, except for T14502C, did not demonstrate evolutionary conservation. The haplogroup affiliation of each mtDNA was established by utilizing the mtDNA sequence variations among two Chinese probands, depending on the nomenclature of mitochondrial haplogroups. The mtDNA samples obtained from the pedigrees NB053 and 2NB038 have been iden
| Gene | Position | Replacement | Conservation1 (H/B/M/X) | CRS2 | NB053 | 2NB038 | Previously reported3 |
| D-loop | 73 | A to G | A | G | G | Yes | |
| 195 | T to C | T | C | Yes | |||
| 263 | A to G | A | G | G | Yes | ||
| 309 | C to CCT | C | CCT | Yes | |||
| 310 | T to C | T | C | TC | Yes | ||
| 408 | T to A | T | A | Yes | |||
| 459 | C to T | C | T | Yes | |||
| 489 | T to C | T | C | Yes | |||
| 561 | A to C | A | C | Yes | |||
| 567 | A to ACCCC | A | ACCCC | Yes | |||
| 16176 | C to T | C | T | Yes | |||
| 16223 | C to T | C | T | T | Yes | ||
| 16232 | C to T | C | T | Yes | |||
| 16311 | T to C | T | C | Yes | |||
| 16355 | C to T | C | T | Yes | |||
| 16362 | T to C | T | C | Yes | |||
| 16519 | T to C | T | C | C | Yes | ||
| 12S rRNA | 750 | A to G | A/A/A/- | A | G | G | Yes |
| 813 | A to G | A/A/C/T | A | G | Yes | ||
| 884 | T to C | T/C/T/C | T | C | Yes | ||
| 1438 | A to G | A/A/A/G | A | G | G | Yes | |
| 16S rRNA | 1673 | T to C | T/T/T/T | T | C | Yes | |
| 2706 | A to G | A/G/A/A | A | G | G | Yes | |
| 3106 | CN to C | C/-/T/- | CN | C | C | Yes | |
| 3167 | T to TC | T/T/C/C | T | TC | Yes | ||
| 3173 | G to A | G/A/A/A | G | A | Yes | ||
| ND1 | 4140 | C to T | C | T | Yes | ||
| ND2 | 4569 | GA to G (Glu34 Lys) | E/E/E/E | GA | G | No | |
| 4573 | TA to T (Met35 Lys) | M/M/F/I | TA | T | Yes | ||
| 4769 | A to G | A | G | G | Yes | ||
| 5505 | A to G (Ile346Val) | I/L/-/- | A | G | Yes | ||
| CO1 | 5824 | G to A | G | A | Yes | ||
| 6674 | T to C | T | C | Yes | |||
| 7028 | C to T | C | T | T | Yes | ||
| CO2 | 7975 | A to G | A | G | Yes | ||
| tRNALys | 8296 | A to G | A/A/A/A | A | G | Yes | |
| ATP6 | 8618 | T to C (Ile31Thr) | I/F/F/I | T | C | Yes | |
| 8701 | A to G (Thr59Ala) | T/S/L/Q | A | G | Yes | ||
| 8793 | T to C | T | C | Yes | |||
| 8860 | A to G (Thr112Ala) | T/A/A/T | A | G | G | Yes | |
| 8928 | T to C | T | C | Yes | |||
| 9027 | C to T | C | T | Yes | |||
| 9180 | A to G | A | G | Yes | |||
| CO3 | 9540 | T to C | T | C | Yes | ||
| ND3 | 10289 | A to G | A | G | Yes | ||
| 10398 | A to G (Thr114Ala) | T/T/T/A | A | G | Yes | ||
| 10400 | C to T | C | T | Yes | |||
| ND4 | 10873 | T to C | T | C | Yes | ||
| 10908 | T to C (Phe50Ser) | F/L/K/H | T | C | Yes | ||
| 11581 | C to A | C | A | Yes | |||
| 11719 | G to A | G | A | A | Yes | ||
| ND5 | 12549 | C to T | C | T | Yes | ||
| 12705 | C to T | C | T | T | Yes | ||
| 13152 | A to G | A | G | Yes | |||
| 13422 | A to G | A | G | Yes | |||
| ND6 | 14220 | A to G | A | G | Yes | ||
| 14311 | T to C | T | C | Yes | |||
| 14431 | T to C | T | C | Yes | |||
| 14502 | T to C (Ile58Val) | I/I/I/I | T | C | C | Yes | |
| CYTB | 14766 | C to T (Thr7Ile) | T/S/T/S | C | T | T | Yes |
| 14783 | T to C | T | C | Yes | |||
| 15040 | C to T | C | T | Yes | |||
| 15043 | G to A | G | A | Yes | |||
| 15071 | T to C (Tyr109His) | Y/F/F/Y | T | C | Yes | ||
| 15301 | G to A | G | A | Yes | |||
| 15326 | A to G (Thr194Ala) | T/M/I/I | A | G | G | Yes | |
| 15514 | T to C | T | C | Yes | |||
| 15617 | G to A (Val291Ile) | V/V/V/V | G | A | Yes | ||
| tRNAThr | 15900 | T to C | T/C/T/T | T | C | Yes |
Herein, a clinical, genetic, and molecular analysis was conducted on two Chinese families with MDD. The two probands under study were middle-aged individuals who presented with MDD for the first time. Neither of them exhibited suicidal tendencies and both experienced episodes of moderate and severe depression. The pedigrees indicate that MDD is only present in the maternal lineage as a clinical phenotype, which implies that the molecular basis for this disorder is the mtDNA mutation. Distinct sets of mtDNA polymorphisms were identified through sequence analysis of the complete mitochondrial genomes in these pedigrees, along with the identical T14502C (I58V) mutation in the ND6 gene. This mutation has been associated with the occurrence of mitochondrial diseases[27-32]. The T14502C mutation resulted in the change of highly conserved isoleucine at position 58 with valine (I58V) in the ND6 gene, the core component of 58 subunits of complex I. Several studies have suggested that the dysfunction of complex I is involved in the impairment of cellular energy metabolism in the context of a depressive episode[33]. The conserved coefficient of this amino acid was 100% in 22 species. Among them, I58V is located in the third transmembrane region, which is a hydrophobic region. Changes in amino acids may affect the three-dimensional structure of proteins, thus affecting the function of ND6 protein, reducing the activity of nicotinamide adenine dinucleotide-coenzyme Q reductase, and affecting the production of mitochondrial ATP, the primary source of energy, leading to the occurrence of diseases.
Although both families exhibited typical clinical symptoms of MDD, there were variations in terms of penetrance, psychotic symptoms, and age of onset. The penetrance rates observed in the two families were 16.7% and 10.0%, respectively. The present study reveals a low penetrance of MDD among the two Chinese families with the T14502C mutation. Furthermore, the presence of this mutation in only one of 99 controls suggests that the T14502C mutation[34], analogous to other mutations, is inadequate to induce the clinical phenotype. Therefore, the phenotypic expression of the T14502C mutation requires the presence of modifying factors such as nuclear background, environmental factors, and mitochondrial haplotypes. In particular, research has demonstrated that mitochondrial haplotypes affect primary mtDNA mutation-related MDD penetrance and expressivity[35]. These mitochondrial genomes of the NB053 and 2NB038 pedigrees belong to the Eastern Asian haplogroups M10 and H2, respectively.
The homogeneous mutation T14502C has been identified in families with MDD despite having diverse genetic backgrounds. The T14502C mutation may serve as a molecular foundation for the pathogenesis of MDD, as indicated by the low penetrance of MDD in families with this mutation. However, the mutation appears inadequate to induce the phenotypic expression of MDD. This implies that additional mediators contributed to the pathogenesis of these families in a synergistic manner. Furthermore, the complete mitochondrial genome of both families was observed to not exhibit additional mutation sites that were highly conserved and functional. This observation implies that the polymorphic sites that are unique to mitochondrial haplomorphism may not have a significant impact on the pathogenesis of the two families affected by the T14502C mutation and suffering from MDD.
Furthermore, the potential implication of mtDNA epigenetics in the development of MDD remains inadequately explored, and further investigation is warranted to elucidate its role in disease etiology and therapeutic interventions. Therefore, nuclear modifier gene (s) or environmental factor (s) may be involved in these Chinese individuals’ phenotypic expression of MDD-associated T14502C mutation. The ND6 T14502C mutation may represent a mitochondrial gene mutation site correlated to MDD. The results of our study may offer novel perspectives on the pathophysiological mechanisms underlying MDD and provide essential data on therapeutic approaches and interventions for this condition.
In this study, we report the clinical, genetic and molecular characterization of two Chinese families with MDD. Iden
We would like to thank all patients who participated in the study. We are grateful to all the psychiatrists and nurses who participated in our current study and those research staff that contributed to the subjects’ diagnosis and clinical asse
| 1. | Gonda X, Petschner P, Eszlari N, Baksa D, Edes A, Antal P, Juhasz G, Bagdy G. Genetic variants in major depressive disorder: From pathophysiology to therapy. Pharmacol Ther. 2019;194:22-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Kessler RC. The costs of depression. Psychiatr Clin North Am. 2012;35:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 556] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 3. | Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS; National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095-3105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5389] [Cited by in RCA: 5368] [Article Influence: 244.0] [Reference Citation Analysis (0)] |
| 4. | COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700-1712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2518] [Cited by in RCA: 2659] [Article Influence: 664.8] [Reference Citation Analysis (0)] |
| 5. | Fries GR, Saldana VA, Finnstein J, Rein T. Molecular pathways of major depressive disorder converge on the synapse. Mol Psychiatry. 2023;28:284-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 229] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 6. | Gardner A, Boles RG. Beyond the serotonin hypothesis: mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:730-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 7. | Rawdin BJ, Mellon SH, Dhabhar FS, Epel ES, Puterman E, Su Y, Burke HM, Reus VI, Rosser R, Hamilton SP, Nelson JC, Wolkowitz OM. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav Immun. 2013;31:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 8. | Garés-Caballer M, Sánchez-Ortí JV, Correa-Ghisays P, Balanzá-Martínez V, Selva-Vera G, Vila-Francés J, Magdalena-Benedito R, San-Martin C, Victor VM, Escribano-Lopez I, Hernandez-Mijares A, Vivas-Lalinde J, Vieta E, Leza JC, Tabarés-Seisdedos R. Immune-Inflammatory Biomarkers Predict Cognition and Social Functioning in Patients With Type 2 Diabetes Mellitus, Major Depressive Disorder, Bipolar Disorder, and Schizophrenia: A 1-Year Follow-Up Study. Front Neurol. 2022;13:883927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Chung JK, Lee SY, Park M, Joo EJ, Kim SA. Investigation of mitochondrial DNA copy number in patients with major depressive disorder. Psychiatry Res. 2019;282:112616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Xue K, Liang S, Yang B, Zhu D, Xie Y, Qin W, Liu F, Zhang Y, Yu C. Local dynamic spontaneous brain activity changes in first-episode, treatment-naïve patients with major depressive disorder and their associated gene expression profiles. Psychol Med. 2022;52:2052-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 11. | Kuffner K, Triebelhorn J, Meindl K, Benner C, Manook A, Sudria-Lopez D, Siebert R, Nothdurfter C, Baghai TC, Drexler K, Berneburg M, Rupprecht R, Milenkovic VM, Wetzel CH. Major Depressive Disorder is Associated with Impaired Mitochondrial Function in Skin Fibroblasts. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Chang CC, Jou SH, Lin TT, Lai TJ, Liu CS. Mitochondria DNA change and oxidative damage in clinically stable patients with major depressive disorder. PLoS One. 2015;10:e0125855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Zhang G, Xu S, Zhang Z, Zhang Y, Wu Y, An J, Lin J, Yuan Z, Shen L, Si T. Identification of Key Genes and the Pathophysiology Associated With Major Depressive Disorder Patients Based on Integrated Bioinformatics Analysis. Front Psychiatry. 2020;11:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Sharma S, Akundi RS. Mitochondria: A Connecting Link in the Major Depressive Disorder Jigsaw. Curr Neuropharmacol. 2019;17:550-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Scaini G, Mason BL, Diaz AP, Jha MK, Soares JC, Trivedi MH, Quevedo J. Dysregulation of mitochondrial dynamics, mitophagy and apoptosis in major depressive disorder: Does inflammation play a role? Mol Psychiatry. 2022;27:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 16. | Ernst J, Hock A, Henning A, Seifritz E, Boeker H, Grimm S. Increased pregenual anterior cingulate glucose and lactate concentrations in major depressive disorder. Mol Psychiatry. 2017;22:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Burnett BB, Gardner A, Boles RG. Mitochondrial inheritance in depression, dysmotility and migraine? J Affect Disord. 2005;88:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Bergemann ER, Boles RG. Maternal inheritance in recurrent early-onset depression. Psychiatr Genet. 2010;20:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Pratt R, Stapelberg NJC. Early warning biomarkers in major depressive disorder: a strategic approach to a testing question. Biomarkers. 2018;23:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Jing P, Mei X, Zhang YY, Zheng FJ, Luo XM, Liu LJ, Yu HH, Zhang XB. Major depressive disorder is correlated with the mitochondrial ND1 T3394C mutation in two Han Chinese families: Two case reports. World J Psychiatry. 2023;13:75-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (0)] |
| 21. | Bech P, Rasmussen NA, Olsen LR, Noerholm V, Abildgaard W. The sensitivity and specificity of the Major Depression Inventory, using the Present State Examination as the index of diagnostic validity. J Affect Disord. 2001;66:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 691] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 22. | Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21041] [Cited by in RCA: 22811] [Article Influence: 350.9] [Reference Citation Analysis (0)] |
| 23. | Rieder MJ, Taylor SL, Tobe VO, Nickerson DA. Automating the identification of DNA variations using quality-based fluorescence re-sequencing: analysis of the human mitochondrial genome. Nucleic Acids Res. 1998;26:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 409] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 24. | Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet. 1999;23:147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2374] [Cited by in RCA: 2370] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 25. | Liang M, Guan M, Zhao F, Zhou X, Yuan M, Tong Y, Yang L, Wei QP, Sun YH, Lu F, Qu J, Guan MX. Leber's hereditary optic neuropathy is associated with mitochondrial ND1 T3394C mutation. Biochem Biophys Res Commun. 2009;383:286-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Kong QP, Bandelt HJ, Sun C, Yao YG, Salas A, Achilli A, Wang CY, Zhong L, Zhu CL, Wu SF, Torroni A, Zhang YP. Updating the East Asian mtDNA phylogeny: a prerequisite for the identification of pathogenic mutations. Hum Mol Genet. 2006;15:2076-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 318] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 27. | Zhang J, Zhou X, Zhou J, Li C, Zhao F, Wang Y, Meng Y, Wang J, Yuan M, Cai W, Tong Y, Sun YH, Yang L, Qu J, Guan MX. Mitochondrial ND6 T14502C variant may modulate the phenotypic expression of LHON-associated G11778A mutation in four Chinese families. Biochem Biophys Res Commun. 2010;399:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Zhang S, Wang L, Hao Y, Wang P, Hao P, Yin K, Wang QK, Liu M. T14484C and T14502C in the mitochondrial ND6 gene are associated with Leber's hereditary optic neuropathy in a Chinese family. Mitochondrion. 2008;8:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Zhao F, Guan M, Zhou X, Yuan M, Liang M, Liu Q, Liu Y, Zhang Y, Yang L, Tong Y, Wei QP, Sun YH, Qu J, Guan MX. Leber's hereditary optic neuropathy is associated with mitochondrial ND6 T14502C mutation. Biochem Biophys Res Commun. 2009;389:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Ozawa T, Tanaka M, Sugiyama S, Ino H, Ohno K, Hattori K, Ohbayashi T, Ito T, Deguchi H, Kawamura K. Patients with idiopathic cardiomyopathy belong to the same mitochondrial DNA gene family of Parkinson's disease and mitochondrial encephalomyopathy. Biochem Biophys Res Commun. 1991;177:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Guo H, Li S, Dai L, Huang X, Yu T, Yin Z, Bai Y. Genetic analysis in a cohort of patients with hereditary optic neuropathies in Southwest of China. Mitochondrion. 2019;46:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Jin X, Wang L, Gong Y, Chen B, Wang Y, Chen T, Wei S. Leber's Hereditary Optic Neuropathy is Associated with Compound Primary Mutations of Mitochondrial ND1 m.3635G > A and ND6 m.14502 T > C. Ophthalmic Genet. 2015;36:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Zvěřová M, Hroudová J, Fišar Z, Hansíková H, Kališová L, Kitzlerová E, Lambertová A, Raboch J. Disturbances of mitochondrial parameters to distinguish patients with depressive episode of bipolar disorder and major depressive disorder. Neuropsychiatr Dis Treat. 2019;15:233-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Ji Y, Zhang J, Yu J, Wang Y, Lu Y, Liang M, Li Q, Jin X, Wei Y, Meng F, Gao Y, Cang X, Tong Y, Liu X, Zhang M, Jiang P, Zhu T, Mo JQ, Huang T, Jiang P, Guan MX. Contribution of mitochondrial ND1 3394T>C mutation to the phenotypic manifestation of Leber's hereditary optic neuropathy. Hum Mol Genet. 2019;28:1515-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Zhou X, Wei Q, Yang L, Tong Y, Zhao F, Lu C, Qian Y, Sun Y, Lu F, Qu J, Guan MX. Leber's hereditary optic neuropathy is associated with the mitochondrial ND4 G11696A mutation in five Chinese families. Biochem Biophys Res Commun. 2006;340:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |