Published online Oct 19, 2024. doi: 10.5498/wjp.v14.i10.1592
Revised: September 2, 2024
Accepted: September 13, 2024
Published online: October 19, 2024
Processing time: 58 Days and 0.8 Hours
Cognitive impairment, which manifests as a limited deterioration of specific functions associated with a particular disease, can lead to a general deterioration of the patient’s standard of living. Transcranial magnetic stimulation, a non-invasive neuromodulation technique, is frequently employed to treat cognitive impairment in neuropsychiatric disorders.
To analyzed the state of international research on neuromodulation methods for treating cognitive impairment between 2014 and 2023, with the aim of exploring the state of research worldwide and the most recent developments in this par
Articles and reviews pertaining to neuromodulation methods for cognitive impairment were examined using the web of science database between January 2014 and December 2023. Publications, nations, organizations, writers, journals, citations, and keywords data from the identified studies were systematically analyzed using the CiteSpace 6.3. R1 software.
A total of 2371 documents with 11750 authors and 9461 institutions, with some co-occurrences, were retrieved. The quantity of yearly publications is showing an increasing trend. The United States and China have emerged as important contributors. Among the institutes, Harvard University had the highest number of publications, while Rossi S an author who is frequently cited. Initially, the primary keywords included human motor cortex, placebo-controlled trials, and serotonin reuptake inhibitors. However, the emphasis gradually moved to substance use disorders, supplementary motor areas, neural mechanisms, and exercise.
The use of neuromodulation techniques to treat cognitive impairment has drawn interest from academics all around the world. This study revealed hotspots and new trends in the research of transcranial magnetic stimulation as a cognitive impairment rehabilitation treatment. These findings are hold significant potential to guide further research and thus promote transcranial magnetic stimulation as a treatment method for cognitive impairment.
Core Tip: The study examines 2371 documents from a wide range of writers and institutions around the world, covering a sizable amount of literature between 2014 and 2023. This breadth ensures a comprehensive overview of the field’s evolution and current trends. Through an analysis of submissions from 9461 institutions worldwide, the study offers an international viewpoint on the field of neuromodulation for cognitive impairment research. In addition to highlighting trends, important contributors, and changing research interests, the paper offers a useful overview of global research on neuromodulation techniques for cognitive impairment. The topic is also highly relevant, and the manuscript is well-organized.
- Citation: Zhang Q, Zhu PP, Yang L, Guo AS. Research hotspots and trends in transcranial magnetic stimulation for cognitive impairment: A bibliometric analysis from 2014 to 2023. World J Psychiatry 2024; 14(10): 1592-1604
- URL: https://www.wjgnet.com/2220-3206/full/v14/i10/1592.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i10.1592
Cognition is a broad concept referring to the various mental processes that encompass “all forms of awareness and consciousness”, including multiple dimensions such as executive functioning, working memory, focus, speed of information processing, fluency in speech, verbal learning, and social cognition[1]. Cognitive impairment (CI) can influence several cognitive domains, such as attention, working memory, information processing, and executive function[2]. CI is a common feature of many psychiatric and neurological disorders. For example, cognitive control dysfunction has been found to play a significant role in the pathology of obsessive-compulsive disorder (OCD)[3]. Cognitive functions can be affected by many brain disorders[4], in certain cases, such as in Alzheimer’s disease or Parkinson’s disease, the observed CI can be severe, while in other conditions (e.g. depression) it can be relatively mild[5]. This is important, as cognition largely determines a person’s social and professional achievement as well as self-sufficiency[6]; thus, CI not only manifests itself as a limited deterioration of specific functions associated with a particular disease, but may additionally result in a general deterioration in the well-being of patients[7].
Physical activity, cognitive-enhancing medications, cognitive remediation, and different brain stimulation methods are the available treatments for CI[8]. Despite recent developments in pharmacological and rehabilitative approaches, the treatments for neuropsychiatric disorders have shown little effect on CI, posing practical and challenging problems for specialists[9]. Brain stimulation methods have gained popularity and acceptance as promising nonpharmacological interventions for the treatment of a variety of neuropsychiatric disorders. Non-invasive transcranial magnetic stimulation (TMS), which has shown consistent antidepressant properties during and after the daily treatment phase, is one example of such an approach[10]. The high tolerability and scarce side effects of TMS are important advantages compared to pharmacological treatments[11]. Notably, TMS requires very little engagement from the patient compared to cognitive restoration or exercises and training, which makes it an important treatment option for those suffering from fatigue, apathy, or diminished motivation.
TMS, a non-invasive brain stimulation technique based on Faraday’s law of electromagnetic induction, in which a brief, high-intensity magnetic field generates electrical currents that depolarize neurons in cortical-related areas involved in mood regulation and depressive symptoms in the neurocircuitry regulation, thus affecting their neuro electrophy
Bibliometric analysis uses visual instruments for examining a lot of academic literature that has been published, and can be utilized to investigate the contributions of writers, nations/regions, institutions, and their partners both quantitatively and qualitatively. Bibliometric analyses can further pinpoint frontiers and hotspots for research, forecast trends in a particular research field, and provide important indicators for subsequent research[20]. CiteSpace is a commonly used visualization and analysis software, originally researched and designed by professor Chen at Drexel University (United States)[21]. The co-citation analysis theory serves as the foundation for CiteSpace and the pathfinding algorithm for networks, which facilitates the analysis and exploration of development trends, as well as research hotspots in allied fields[22]. Recently, numerous investigators have carried out visual evaluations within the domain of TMS and cognitive dysfunction. For example, Yang et al[23] carried out a visual examination of acupuncture therapy for mild CI, revealing the significant potential of acupuncture treatment for mild CI. Similarly, a study using bibliometric methods to assess the breadth of literature based on TMS assessed developments over the past 30 years, contributing to an understanding of the historical progression of TMS over the past few decades[24].
Over the past decade, numerous researchers and academic journals have published articles on the use of TMS to improve CI. However, to date, no study has yet offered a comprehensive overview of the use of TMS in cognitive function rehabilitation through a bibliometric analysis. Therefore, a bibliometric analysis of TMS for CI was performed in this study using records that were published between the start of 2014 and the end of 2023. Publication patterns and trends were subsequently analyzed using CiteSpace 6.3. R1 derived from the web of science core collection (WOSCC) database. The objective of this study was to assist medical professionals and investigators in understanding the concerns and study focus areas pertaining to TMS for CI treatment, with the intention of offering fresh perspectives for upcoming studies and applications.
To retrieve relevant articles, we searched the web of science (WOS) database (version 2022 Clarivate), a well-known platform for scientific data services created by Clarivate. Using this strategy, we were able to retrieve the impact factor of Clarivate journals from the WOS over the last 10 years. Publications on relevant topics from the beginning of 2014 to the end of 2023 were retrieved from the WOSCC databases [science citation index expanded (SCIE) and social science citation index (SSCI)]. SCIE and SSCI are sub-databases of WOSCC, consisting of scientific research journals from around the world, covering publications in the fields of research in neurology and medicine pertaining to the subject of this investigation, “TMS for CI”.
The data from SCIE were obtained on the 1st of April, 2024. We conducted a topical search using the query TypeScript (TS) = [“Transcranial Magnetic Stimulation” OR “Magnetic Stimulation, Transcranial” OR “Magnetic Stimulations, Transcranial” OR “Stimulation, Transcranial Magnetic” OR “Stimulations, Transcranial Magnetic” OR “Transcranial Magnetic Stimulations” OR “Transcranial Magnetic Stimulation, Single Pulse” OR “Transcranial Magnetic Stimulation, Paired Pulse” OR “Transcranial Magnetic Stimulation, Repetitive” OR “TMS” OR “Theta burst stimulation (TBS)”] and TS = ( “Cognitive Impairment” OR “Cognitive Dysfunction” OR “Cognitive Function” OR “Cognitive Competence” OR “Cognitive Disorder” OR “Cognitive Disorder Disease” OR “Cognitive Deline”) to find papers that specifically used the problematic terms.
In terms of inclusion criteria, we only considered English-language articles and reviews, excluding letters, conference abstracts, editorial materials, news items, book chapters, etc., for the search period from January 1, 2014 to December 31, 2023, with a retrieval date of April 1, 2024. Data retrieval and deduplication of all included documents were peer-reviewed, and 2371 relevant documents were obtained in the final analysis. These articles were saved as plain-text files in a ‘download-text’ format for further reference and analysis.
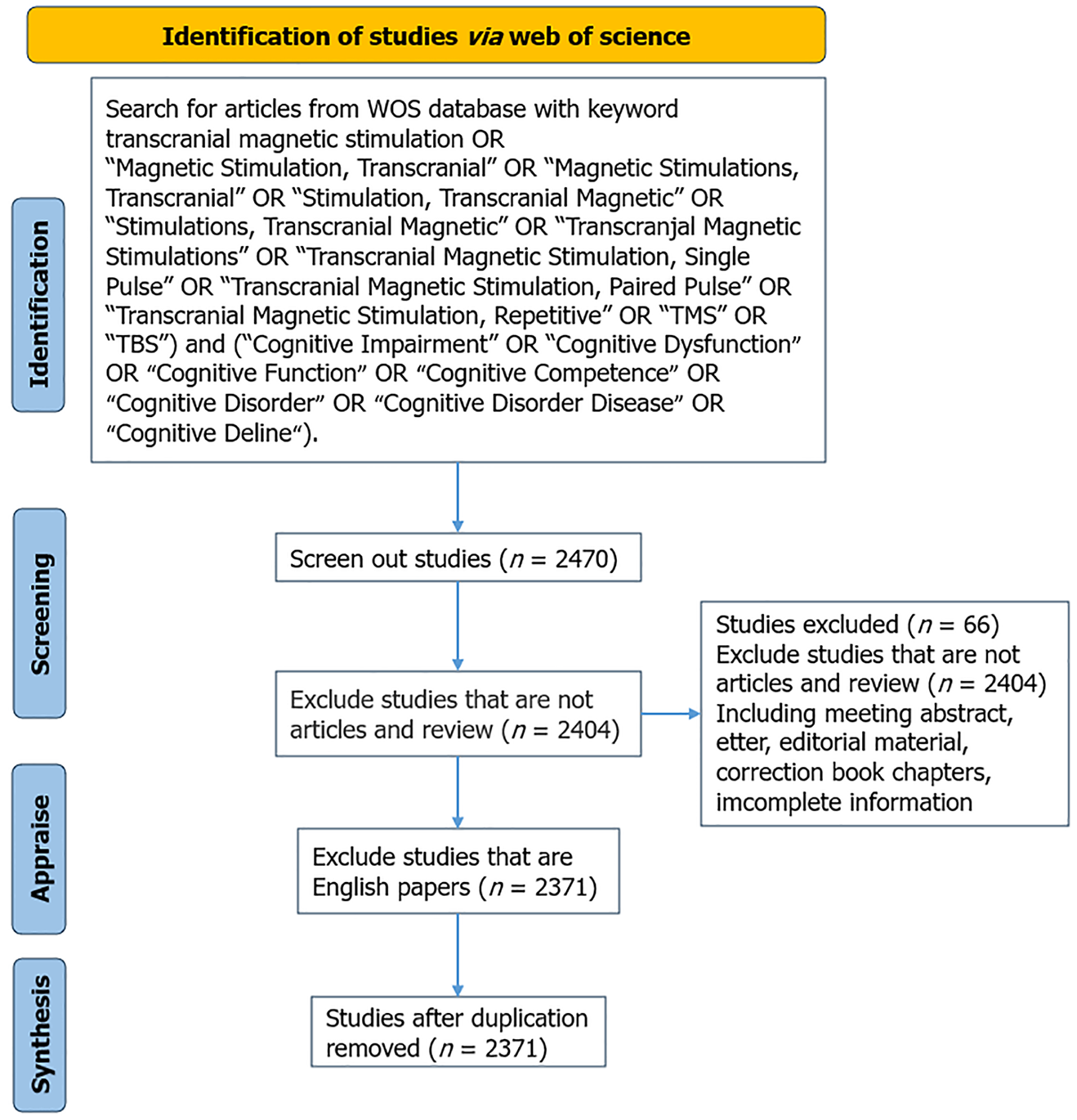
The Java-based application CiteSpace 6.3. R1 facilitated data analysis by visualizing the knowledge structure, patterns, and distribution. The parameters were set as follows: Time slice: January 1, 2014 to December 31, 2023; Node type: Select institution, author, reference, and keyword; Selection criterion: G-index (k = 25) for each node; and Pruning: No clipping method was selected. The nodes in network mapping represent institutions, authors, references, and keywords. The thresholds were adjusted according to the nodes: The nodes’ dimensions indicates the quantity or regularity; The connecting lines represent the cooperation, co-occurrence or relationship of co-citation between the nodes; The degree of cooperation is indicated by the thickness of the connecting lines; and The outer circle of the nodes represents the centrality, defined as a measure of the node’s significance within the co-occurrence network; The greater the node’s influence, the higher its centrality. Figure 1 shows a flow chart of the study process.
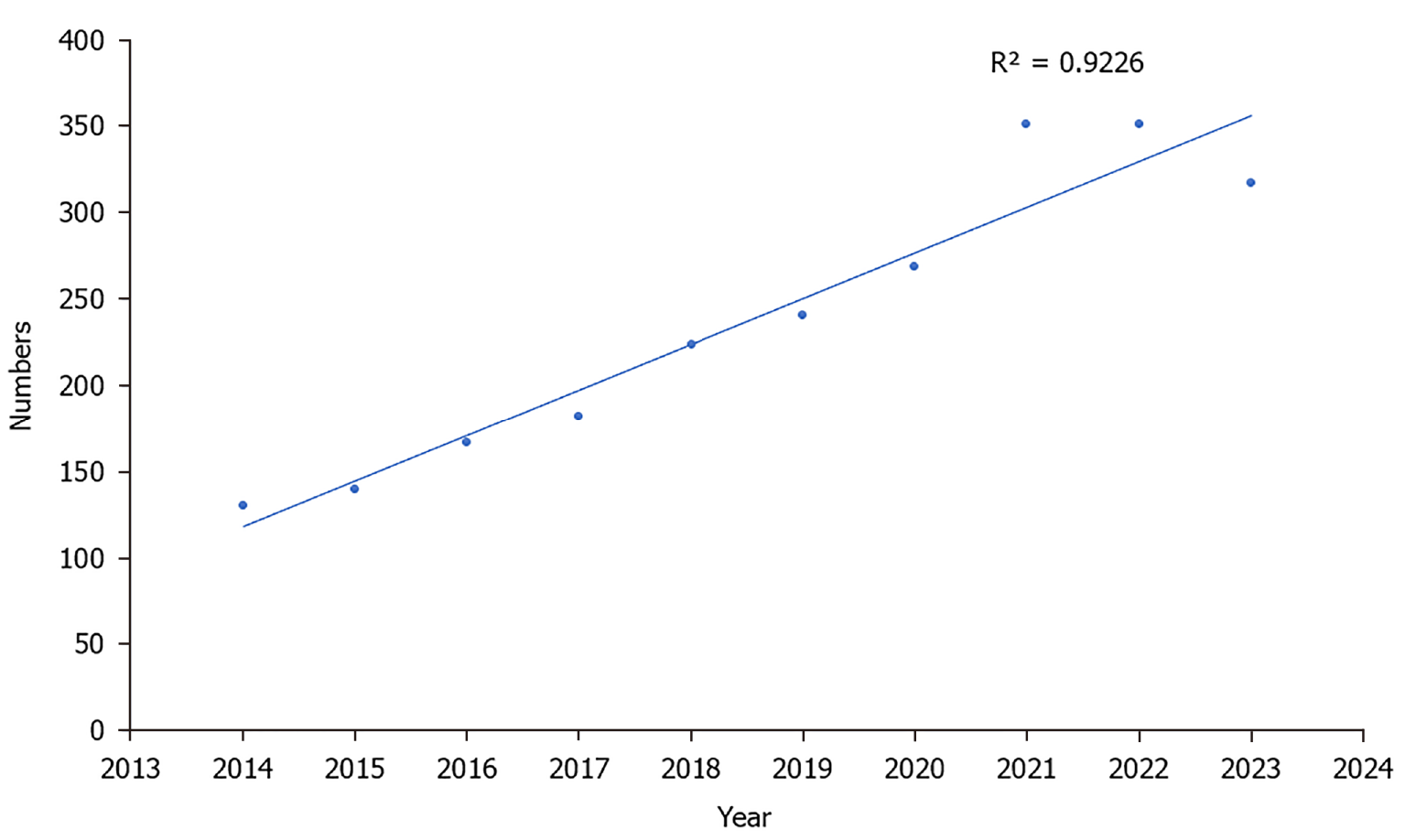
The publication trend in the volume of literature as a whole depicts the time series and curve of variations in the volume of research papers published in the field of TMS in cognition. Figure 2 shows the steadily increasing trend in publications observed between 2014 and 2023. The highest number of publications was published in 2022, with 285 papers. Despite declining after 2022, the temporal trend of paper publications nevertheless exhibited a strong relationship between the yearly amount of publications and the year in which papers were published over the last ten years (R2 = 0.9226; P < 0.0001).
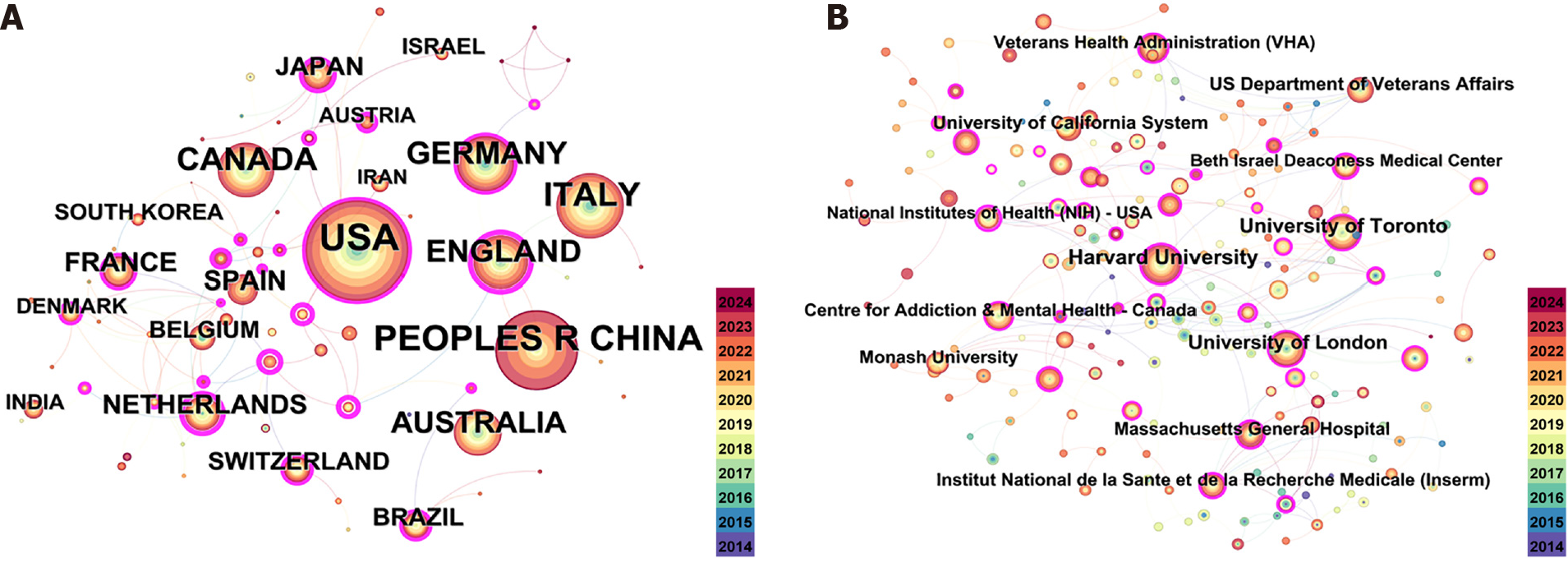
The top ten countries/regions in terms of publications are listed in Table 1. Figure 3 shows a diagram showing the nations participating in TMS and cognitive function research. The United States topped the list of countries/regions with the greatest quantity of publications with 715 publications, as shown in Table 1. The other countries shown in Table 1 all had more than 100 publications. The year in which research began in each country is indicated by the circles’ various color shades. In the realm of TMS and cognitive function research, the United States initiated research the earliest, as depicted in network map illustrating the participating countries (Figure 3). Overall, these results demonstrate the early engagement of the United States in this field, and a subsequent increase in publications from China. The strength of the connections a node has with other nodes in the network is indicated by its centrality. A high degree of centrality suggests that the important nodes have a significant impact on the connections within the network. In Figure 3A, an elevated degree of centrality is indicated by the pink outer circle, which signifies that the centrality exceeds 0.1, while Table 2 in this regard indicates that Germany (0.34) has the greatest centrality influence and China (0.05) has the lowest. Figure 3B shows a network diagram for each research institution (further shown in Table 2) for the 2371 publications comprised this analysis. When it came to publications, Harvard University (146) was the highest ranked institution, followed by the University of Toronto (113), the University of London (100), and the University of California System (71).
| Count | Betweenness centrality | Country | |
| 1 | 715 | 0.21 | United States |
| 2 | 461 | 0.05 | China |
| 3 | 331 | 0.12 | Italy |
| 4 | 246 | 0.08 | Canada |
| 5 | 243 | 0.21 | England |
| 6 | 227 | 0.34 | Germany |
| 7 | 169 | 0.08 | Australia |
| 8 | 108 | 0.02 | Netherlands |
| 9 | 106 | 0.07 | Spain |
| 10 | 102 | 0.14 | France |
| Count | Betweenness centrality | Institution | |
| 1 | 146 | 0.08 | Harvard University |
| 2 | 113 | 0.03 | University of Toronto |
| 3 | 100 | 0.09 | University of London |
| 4 | 71 | 0.06 | University of California System |
| 5 | 68 | 0.02 | United States Department of Veterans Affairs |
| 6 | 67 | 0.02 | Veterans Health Administration |
| 7 | 66 | 0.02 | Centre for Addiction and Mental Health-Canada |
| 8 | 61 | 0.08 | Massachusetts General Hospital |
| 9 | 60 | 0.04 | Monash University |
| 10 | 57 | 0.05 | National de la Sante et de la Recherche Medicale (Inserm) |
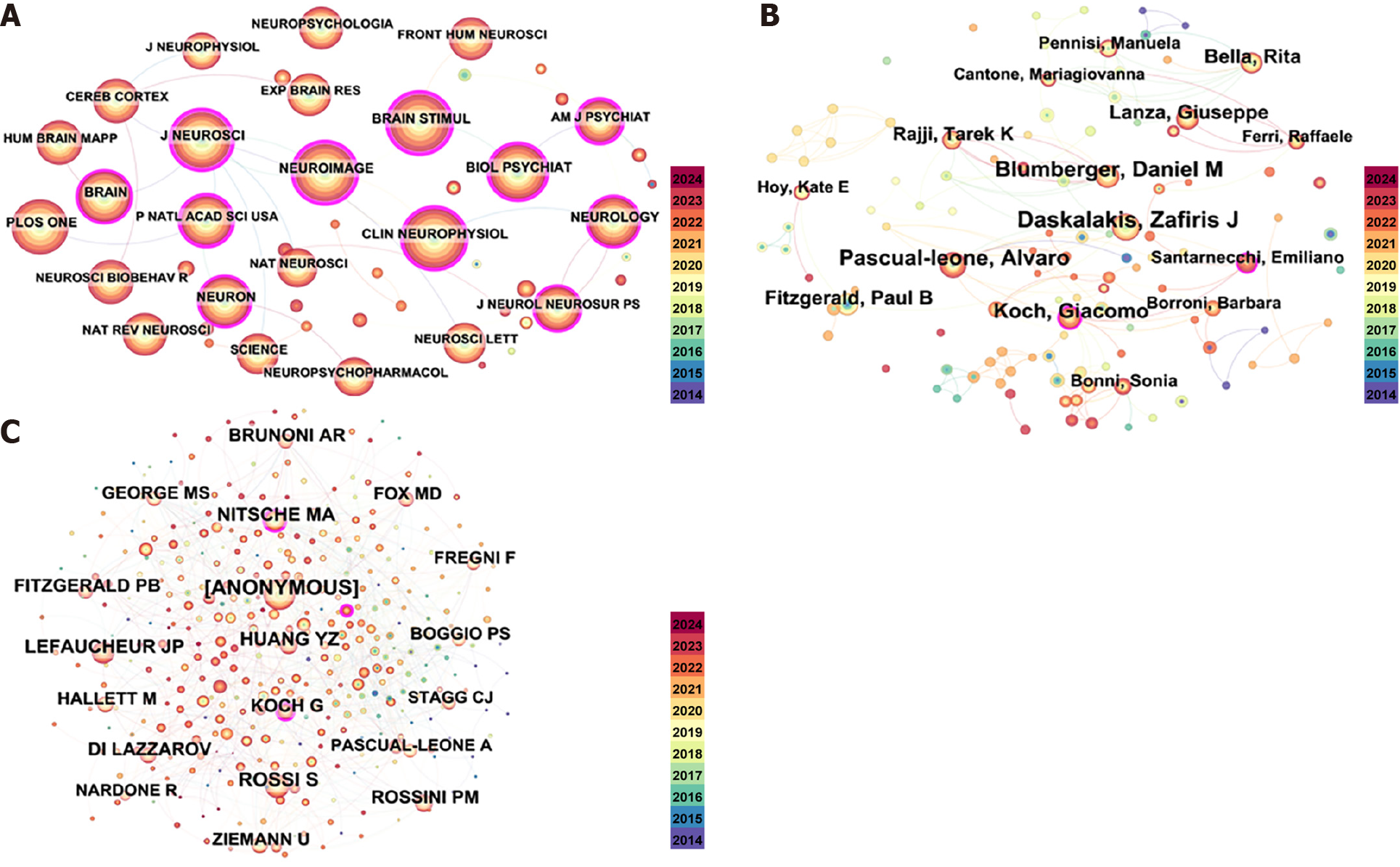
Figure 4A presents a co-occurrence graph of the journals. The most cited journal among the top ten cited journals in Table 3 was Brain Stimul, with 1493 citations, followed by Clin Neurophysiol (1480 citations), Neuroimage (1444 citations), J Neurosci (1370 citations), and Plos One (1349 citations), all with more than 1000 citations.
| Count | Journal name | |
| 1 | 1493 | Brain Stimul |
| 2 | 1480 | Clin Neurophysiol |
| 3 | 1444 | Neuroimage |
| 4 | 1370 | J Neurosci |
| 5 | 1349 | Plos One |
| 6 | 1174 | Brain |
| 7 | 1143 | Biol Psychiat |
| 8 | 1099 | Neuron |
| 9 | 1097 | Neurology |
| 10 | 1043 | P Natl Acad Sci United States |
Figure 4B shows the co-occurrence analysis of co-authors. Notable researchers such as Daskalakis ZJ and Pascual-Leone A were notable in the author distribution analysis (Figure 4B and Table 4). In terms of the number of citations, Rossi S (453 citations) was the most cited author, followed by Lefaucheur JP (391 citations), Nitsche MA (345 citations), and Huang YZ (315 citations) (Figure 4C).
| Count | Betweenness centrality | Author name | |
| 1 | 39 | 0.06 | Daskalakis ZJ |
| 2 | 35 | 0.07 | Pascual-Leone A |
| 3 | 31 | 0.02 | Blumberger DM |
| 4 | 27 | 0.06 | Koch G |
| 5 | 26 | 0.01 | Fitzgerald PB |
| 6 | 26 | 0.01 | Lanza G |
| 7 | 23 | 0.01 | Bella R |
| 8 | 20 | 0 | Cantone M |
| 9 | 18 | 0.01 | Rajji TK |
| 10 | 16 | 0.01 | Downar J |
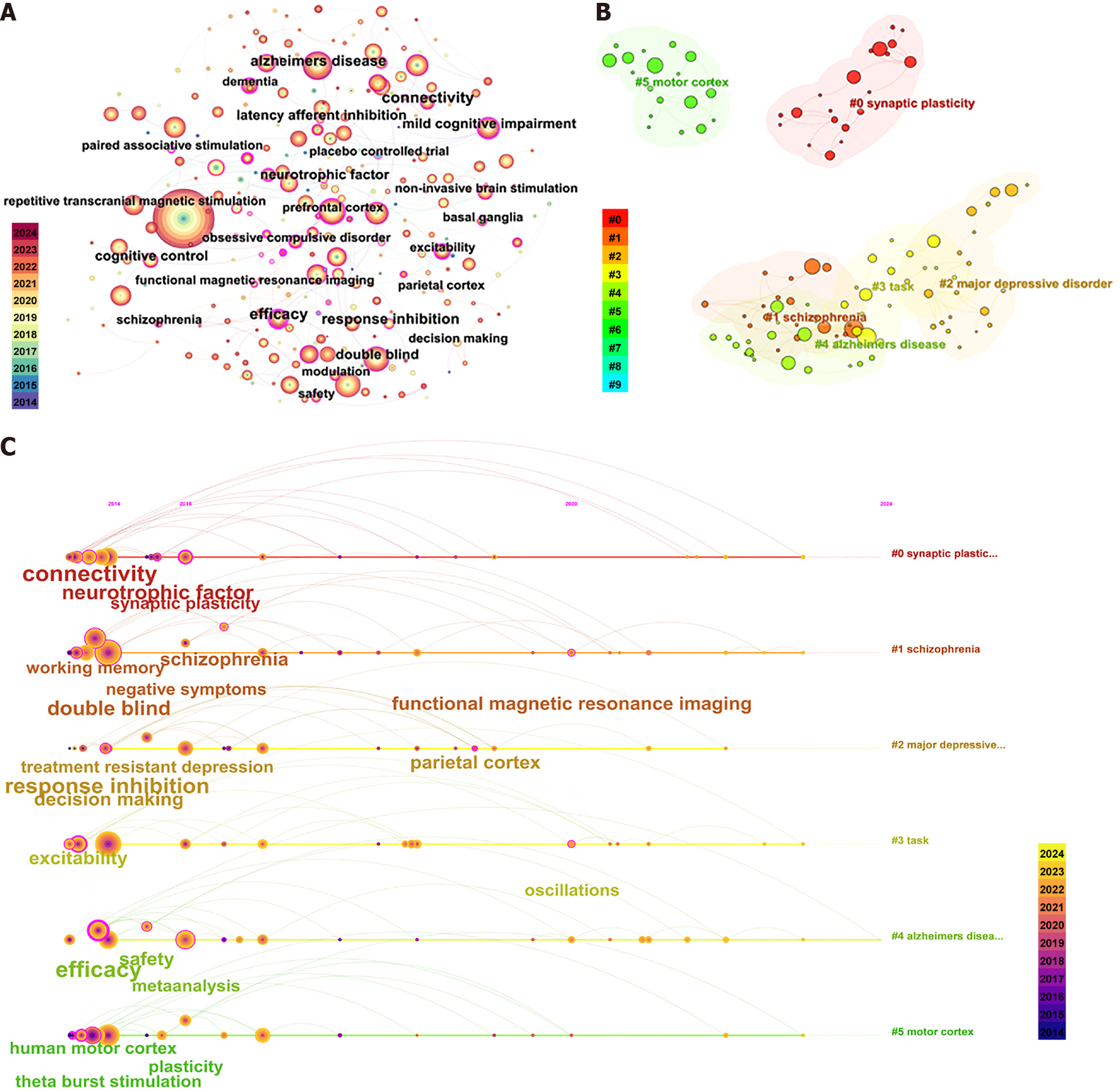
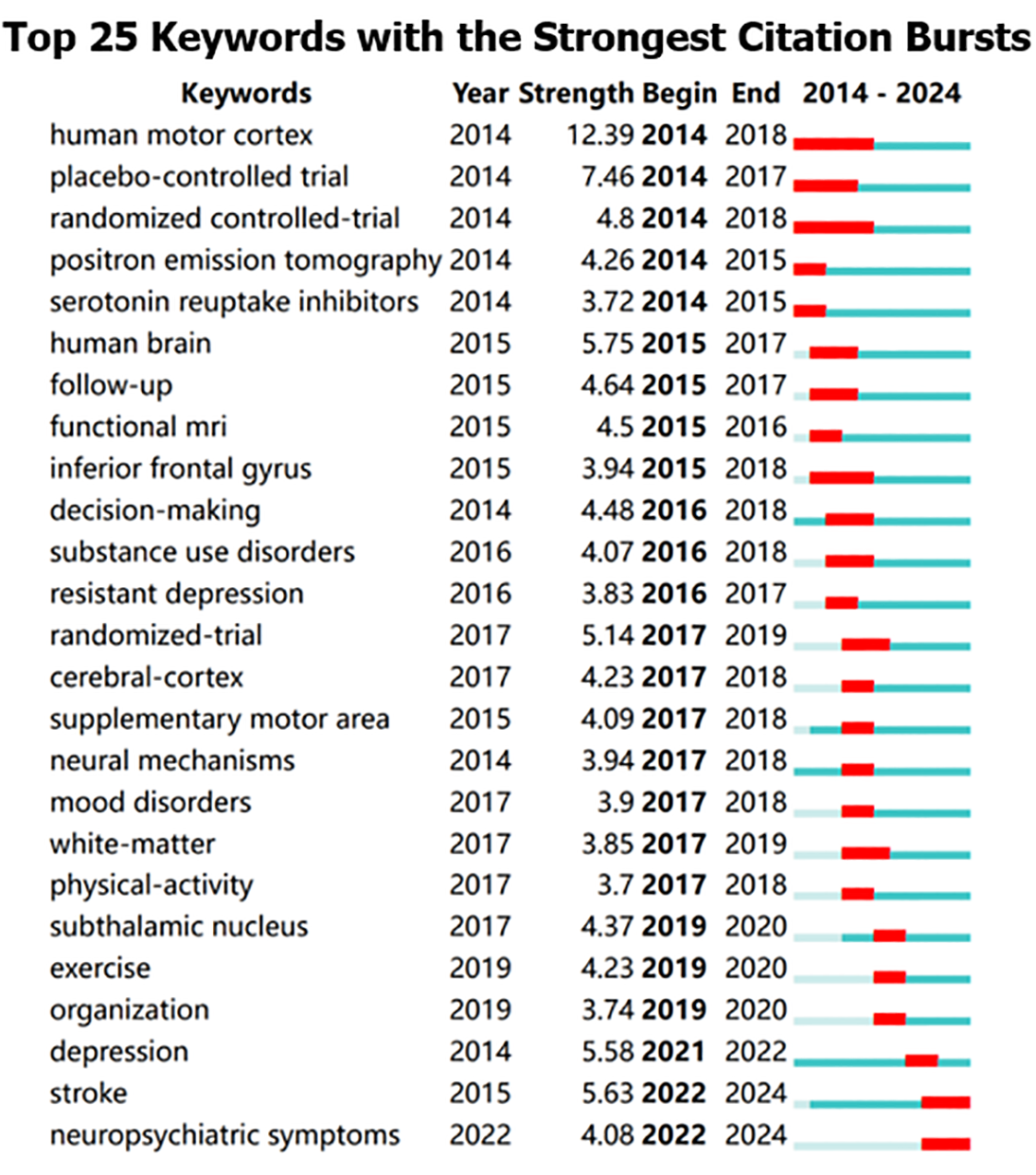
Keywords condense and distill the content of an article and represent its core topics. Scholars typically employ high-frequency keywords to reflect the prevailing issues in the research domain. In this study, keywords with synonymous meanings, such as “repetitive transcranial magnetic stimulation” and “rTMS,” are amalgamated. Figure 5A illustrates the combined keyword co-occurrence graph. In this analysis, after excluding “transcranial magnetic stimulation” and “repetitive transcranial magnetic stimulation,” the primary keywords were “Alzheimer’s disease” (frequency = 361, centrality = 0.03). Subsequently, “double-blind” (300, 0.03), “dorsolateral prefrontal cortex” (297, 0.02), “prefrontal cortex” (290, 0.02), “non-invasive brain stimulation” (281, 0.01), “functional connectivity” (233, 0.02), “transcranial direct current stimulation” (213, 0.02), and “theta burst stimulation” (208, 0.03) (Table 5). Table 5 further underscores the pivotal role of keywords in encapsulating article themes, with “Alzheimer’s disease” standing out as a primary keyword due to its high frequency and centrality within the network. Subsequently, we employed the log-likelihood ratio algorithm to identify six keyword clusters, reflecting emerging trends in the field (Figure 5B and C). These included: 0: Synaptic plasticity; 1: Schizophrenia; 2: Major depressive disorder; 3: Task; 4: Alzheimer’s disease; and 5: Motor cortex. These results align with the results of the keyword co-occurrence analysis. Additionally, burst keywords serve as indicators of emerging trends, with Figure 6 illustrating the keywords pinpointed as having the largest spurts of citations, including “stroke” and “neuropsychiatric symptoms”, which are indicative of recent research foci.
| Count | Betweenness centrality | Keywords | |
| 1 | 1538 | 0.01 | Transcranial magnetic stimulation |
| 2 | 361 | 0.03 | Alzheimer’s disease |
| 3 | 352 | 0.02 | Repetitive transcranial magnetic stimulation |
| 4 | 300 | 0.03 | Double-blind |
| 5 | 297 | 0.02 | Dorsolateral prefrontal cortex |
| 6 | 290 | 0.02 | Prefrontal cortex |
| 7 | 281 | 0.01 | Noninvasive brain stimulation |
| 8 | 233 | 0.02 | Functional connectivity |
| 9 | 213 | 0.02 | Transcranial direct current stimulation |
| 10 | 208 | 0.03 | Theta burst stimulation |
Over the previous few decades, TMS has drawn a lot of interest from scholars, and the quantity of relevant research has accordingly shown an annual increase. This bibliometric study used CiteSpace to analyze and visualize 2371 papers investigating the use of TMS for CI over the past decade, with the aim of identifying the recent trends and hotspots for research in this area. As TMS and receiving increasing attention from the medical community and researchers, it has become more widely used and popularized, and the quantity of related documents has progressively grown, with high-quality studies confirming the feasibility of improving cognitive function in neurological and psychiatric disorders. The last ten years’ variations in productivity and research activity, which could be broken down into three stages, were evident in our investigation of the publications on related topics. Studies on the modalities of transcranial magnetic research programs and treatment of the cortical area predominated from 2014 to 2015; while from 2016-2018, studies primarily focused on neuropsychiatric disorders, substance use disorders, and mood disorders; finally, a large number of studies on depression panic disorders, and therapeutic regional programs were published from 2019 to 2024. This trend suggests an increase in the use of TMS as a secure and non-invasive neuromodulation method for the management of neurological CI in patients with psychiatric disorders and stroke victims, and indicate that it has great research potential and has received increasing attention from scholars[25,26]. Therefore, we predict that the popularity of future research in this field will continue.
Nine developed nations made up the top 10 list of nations with the most publications in this field of study, with China being the only developing country. The United States, Italy, Canada, the United Kingdom, and Germany are outstanding leaders in this area. Despite China’s research efforts in this area began late, it is developing rapidly. Over the past decade, publications have multiplied dramatically in recent years. Nonetheless, the low centrality of China (0.05) suggests that developing countries, such as China, have fewer connections with developed nations in this research field, reflecting a lack of international collaboration. The institutional collaboration networks depicted in Figure 3 and Table 2 further show that national universities in Europe, the United States, and Canada, including Harvard University, the University of Toronto, and the University of London, continue to lead the research field in TMS for CI. The nations and institutions’ visual analysis makes it abundantly evident that national institutions in the United States and Europe continue to dominate the TMS for CI research field. Further, the institutional and national visual analysis reveals which the most active and prominent institutions are almost exclusively prestigious universities from academically-rich developed countries, and that there exists a certain disparity in the transfer of scholarly materials between developing and developed nations. This phenomenon may have several explanations: Firstly, industrialized nations initiated TMS research for cognitive therapy earlier, whereas developing countries, such as China, have only begun to publish more articles and establish some links with neighboring nations in the last few years; Secondly, financial constraints and insufficient attention in developing countries have prevented them from sustaining investment in the use of TMS technology in healthcare administration. This may have resulted in a lack of high-quality research in developing countries.
The distribution of author clusters was found to be similar to that of the country and institutional clusters. The majority of the top ten authors in Table 4 are affiliated with research facilities in developed nations. Further, Figure 4B's author cluster network diagram shows that Daskalakis ZJ, Blumberger DM, Pascual-Leone A and Fitzgerald PB constitute a broad cluster. Daskalakis ZJ and Blumberger DM reviewed the therapeutic management of refractory depression, which helped to advance research on burst vs high-frequency repetitive TMS in the treatment of depression and cognitive functioning[27]. Further, Daskalakis ZJ and Fitzgerald PD reviewed the therapeutic use of Repetitive TMS (rTMS) for the treatment of tobacco, alcohol, and illicit drug addictions, suggesting that rTMS is a promising treatment drug addiction[28]. Pascual-Leone A and Fitzgerald PD investigated and analyzed the large-scale inter-individual variability in single- and double-pulse TMS data[29] and inter-individual variability in theta burst stimulation data[30], providing a theoretical basis for improved research on transcranial magnetism in the future. In addition, Daskalakis ZJ, Blumberger DM, Pascual-Leone A and Fitzgerald PD reviewed the clinical neurophysiology of TMS combined with electroencephalogram, providing evidence for an emerging tool for studying clinical populations probing brain function in virtually all areas of the cortical coat membrane and associated cortical networks[31].
Information gathered from citations and publication outputs revealed that brain stimulation, a premier journal publishing studies in the neuromodulation field, whose impact factor in 2023 is 7.7, is the most influential journal in the field. This journal covers both non-invasive and invasive techniques, exploring methods to alter brain function, including electricity, magnetism, radio waves, sound, light, and targeted pharmacological stimulation. In addition, it features high-level research on the biophysical and biopsychophysical aspects of stimulation. These techniques are utilized as probes to delineate the patterns of neural connectivity. Besides Brain Stimul, several other neuroscience journals including Clinical Neurophysiology, Neuroscience, and the J Neurosci, all provide the field with excellent research contributions, with more than one-third of the papers published overall in the top 10 journals. Meanwhile, all of the top four active journals had impact factors greater than 3. Therefore, leading journals on TMS for cognitive dysfunction feature robust research methodologies and publish rigorous clinical trials, offering researchers dependable evidence.
Analysis of keywords can help to recognize new trends and areas for future investigation in a particular field[32]. In our study, the following observations were made regarding the hotspots of future research directions of TMS for CI based on the analysis of keywords.
Keyword bursting and cluster analysis indicated that the treatment of neuropsychiatric disorders has drawn more and more attention from scholars. Neuropsychiatric disorders reduce the patients’ cognitive abilities and quality of life. Recent research has demonstrated that TMS, a non-invasive and painless technique that can stimulate and modulate cortical function[33], is a promising approach for the treatment of psychiatric and neurological disorders. rTMS involves the administration of sequences of TMS pulses at varying frequencies and intensities. This method of neurostimulation and modulation has long-lasting effects. The effects of rTMS on cognitive functioning have been mainly studied in treatment-resistant MDD patients, in whom it has shown clear antidepressant effects[34]. Specifically, ten open-label studies in depressed patients have shown that high-frequency magnetic stimulation (≥ 5 Hz, predominantly 18-20 Hz) rTMS achieved significant effects. Improvements in sustained attention, visuospatial and working memory, psychomotor skills, and information processing speed were observed in four open-label studies[35-38]. Specifically, according to Blumberger et al[39], elderly patients with depression responded well to a standard sequential bilateral repetitive TMS combined with bilateral θ burst stimulation. In addition, many basic and clinical studies have shown that rTMS can improve the symptoms of Alzheimer’s disease, and has significant potential as a therapeutic approach[40-43]. Koch et al[44] further investigated the modulation of neuronal excitability and inter cerebral connectivity in the brains of patients with Alzheimer’s disease using 20 Hz rTMS precuneus stimulation. They found that rhythmic beta neural oscillations showed increased energy and phase synchrony, improved connectivity with the medial frontal cortex, and alleviated the symptoms of cognitive dysfunction in Alzheimer’s disease patients[44,45]. Lately, numerous researches have also demonstrated the effectiveness of beta-rhythm TMS combined with electroencephalography (EEG) in stroke aphasia and the recovery of cortical cognitive function after stroke[46]. The selection of brain sites for the control and treatment of patients with neuropsychiatric diseases, as well as the parameters and modes of TMS, require more investigation, according to a study conducted using key word and co-citation analysis in the literature.
The visual analyses of keywords in the last decade revealed that the effects of stimulation with various parameters and in different brain regions are no longer the only focus of researchers’ attention, instead, increased focus has been placed on the investigation of the mechanism of action of TMS combined with electromyography, as well as the prospects of its application. The qualitative insight of researchers or clinicians working in the field of treating cognitive disorders is to combine TMS with methods like EEG and functional magnetic resonance imaging (fMRI) in order to assess the modulatory effect of TMS on neuronal activity in the brain. The results demonstrate that the combination of TMS and fMRI can regulate the connectivity within the stimulated network. When TMS acts on the motor cortex, the peripheral nerves innervated by the motor cortex are activated, causing changes in the potential of the peripheral neurons, i.e., motor-evoked potential, the magnitude of which depicts the corticospinal pathways’ general excitability[47,48], while reflecting the functional integrity of the connections in the corpus callosum is the ipsilateral silent period[49]. TMS combined with EEG methods can directly assess the modulation of neuronal activity in the brain by TMS, which modulates the neurons’ electrical activity in the stimulation target area; further, the stimulus response is transmitted to neural circuits outside the target area of stimulation. When TMS is combined with fMRI, it is not possible to assess how well stimulus responses are transmitted in the associated brain regions, owing to the influence of temporal resolution, whereas TMS-EEG has a better temporal resolution and can record neuronal activities in multiple brain regions simultaneously, thereby evaluating the transmission of stimulus responses in the brain regions. The distribution of TMS-evoked potentials[45] in the cerebral cortex revealed that the stimulus response was transferred from the stimulation target region to the cerebral cortices on the ipsilateral and contralateral sides over time[50]. Therefore, one study of inter-area connectivity using TMS-EEG was conducted to explore the inter-area correlations in cognitive activities and identify the faulty connections that lead to damage to brain function in clinical disorders[51,52]. An understanding of the response characteristics of TMS-EEG would help achieve more accurate regulation of neuronal activity in the cerebral cortex. Stimulus response characteristics are relatively stable during cognitive activities; however, abnormal responses have been noted in mental and neurological illnesses[53,54]. In the future, further research progress in cognitive sciences, as well as in the diagnosis and treatment of clinical neuropsychiatric disorders, should be achieved through the use of TMS-EEG, TMS, and fMRI technologies.
Overall, this study utilized bibliometric analysis to investigate the trends for the treatment of cognitive dysfunction through neuromodulation techniques between 2013 and 2024. The current state of research on neuromodulation techniques in the treatment of cognitive dysfunction, as well as hotspots and research frontiers, are revealed by further analyzing the relationships between the 2371 articles we obtained from the WOS and the notable publications, authors, journals, institutions, and countries. Our comprehensive analysis revealed that despite the fact that North America and Europe have a considerable academic influence, some institutions in developing countries, particularly China, have shown clear potential in this area. Nevertheless, rich nations are home to the majority of influential organizations and writers, indicating an imbalance in academic development. Additionally, high impact factor journals are the norm in this field, indicating that they are excellent resources for scholarly references. Keyword hotspots will be the subject of further research in cognitive science and in the diagnosis and treatment of clinical psychiatric disorders. However, future studies will need to address possible drawbacks, like linguistic biases in the chosen literature or regional variations in citation styles. Acknowledging these difficulties will lead to a more balanced view of the research landscape.
The authors express their gratitude to the researchers who are employed by Nantong University’s Affiliated Hospital.
| 1. | Laskov O, Klírová M. Effects of deep transcranial magnetic stimulation (dTMS) on cognition. Neurosci Lett. 2021;755:135906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Higuchi Y, Sumiyoshi T, Seo T, Suga M, Takahashi T, Nishiyama S, Komori Y, Kasai K, Suzuki M. Associations between daily living skills, cognition, and real-world functioning across stages of schizophrenia; a study with the Schizophrenia Cognition Rating Scale Japanese version. Schizophr Res Cogn. 2017;7:13-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Hajcak G, Simons RF. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Res. 2002;110:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 232] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Robbins TW. Cognition: the ultimate brain function. Neuropsychopharmacology. 2011;36:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Douglas KM, Gallagher P, Robinson LJ, Carter JD, McIntosh VV, Frampton CM, Watson S, Young AH, Ferrier IN, Porter RJ. Prevalence of cognitive impairment in major depression and bipolar disorder. Bipolar Disord. 2018;20:260-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 6. | Benedict RH, Zivadinov R. Predicting neuropsychological abnormalities in multiple sclerosis. J Neurol Sci. 2006;245:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Toyoshima K, Kako Y, Toyomaki A, Shimizu Y, Tanaka T, Nakagawa S, Inoue T, Martinez-Aran A, Vieta E, Kusumi I. Associations between cognitive impairment and quality of life in euthymic bipolar patients. Psychiatry Res. 2019;271:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Palm U, Schiller C, Fintescu Z, Obermeier M, Keeser D, Reisinger E, Pogarell O, Nitsche MA, Möller HJ, Padberg F. Transcranial direct current stimulation in treatment resistant depression: a randomized double-blind, placebo-controlled study. Brain Stimul. 2012;5:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 9. | Lee WK. Effectiveness of computerized cognitive rehabilitation training on symptomatological, neuropsychological and work function in patients with schizophrenia. Asia Pac Psychiatry. 2013;5:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Berlim MT, Van den Eynde F, Tovar-Perdomo S, Chachamovich E, Zangen A, Turecki G. Augmenting antidepressants with deep transcranial magnetic stimulation (DTMS) in treatment-resistant major depression. World J Biol Psychiatry. 2014;15:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Lage C, Wiles K, Shergill SS, Tracy DK. A systematic review of the effects of low-frequency repetitive transcranial magnetic stimulation on cognition. J Neural Transm (Vienna). 2016;123:1479-1490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, Siebner HR, Classen J, Cohen LG, Rothwell JC. Consensus: Motor cortex plasticity protocols. Brain Stimul. 2008;1:164-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 473] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 13. | Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. Int J Neuropsychopharmacol. 2002;5:73-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 287] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Valero-Cabré A, Amengual JL, Stengel C, Pascual-Leone A, Coubard OA. Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neurosci Biobehav Rev. 2017;83:381-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 240] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 15. | Begemann MJ, Brand BA, Ćurčić-Blake B, Aleman A, Sommer IE. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis. Psychol Med. 2020;50:2465-2486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 16. | Pope PA, Miall RC. Restoring cognitive functions using non-invasive brain stimulation techniques in patients with cerebellar disorders. Front Psychiatry. 2014;5:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Filipčić I, Šimunović Filipčić I, Milovac Ž, Sučić S, Gajšak T, Ivezić E, Bašić S, Bajić Ž, Heilig M. Efficacy of repetitive transcranial magnetic stimulation using a figure-8-coil or an H1-Coil in treatment of major depressive disorder; A randomized clinical trial. J Psychiatr Res. 2019;114:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Carmi L, Alyagon U, Barnea-Ygael N, Zohar J, Dar R, Zangen A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 170] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 19. | Minichino A, Bersani FS, Capra E, Pannese R, Bonanno C, Salviati M, Delle Chiaie R, Biondi M. ECT, rTMS, and deepTMS in pharmacoresistant drug-free patients with unipolar depression: a comparative review. Neuropsychiatr Dis Treat. 2012;8:55-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Wei YX, Tu LD, He L, Qiu YT, Su W, Zhang L, Ma RT, Gao Q. Research hotspots and trends of transcranial magnetic stimulation in Parkinson's disease: a bibliometric analysis. Front Neurosci. 2023;17:1280180. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Chen Y, Guo JJ, Zhan S, Patel NC. Treatment effects of antidepressants in patients with post-stroke depression: a meta-analysis. Ann Pharmacother. 2006;40:2115-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Chen C, Song M. Visualizing a field of research: A methodology of systematic scientometric reviews. PLoS One. 2019;14:e0223994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 520] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 23. | Yang W, Liu X, Zhang X, Li C, Li Z, Li Y, Li M. Bibliometric analysis of acupuncture and moxibustion treatment for mild cognitive impairment. Front Neurosci. 2023;17:1209262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 24. | Lawson McLean A. Publication trends in transcranial magnetic stimulation: a 30-year panorama. Brain Stimul. 2019;12:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Alyagon U, Barnea-Ygael N, Carmi L, Zangen A. Modifications of cognitive performance in the stroop task following deep rTMS treatment course in OCD patients. Brain Stimul. 2021;14:48-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Iudicello JE, Woods SP, Vigil O, Scott JC, Cherner M, Heaton RK, Atkinson JH, Grant I; HIV Neurobehavioral Research Center (HNRC) Group. Longer term improvement in neurocognitive functioning and affective distress among methamphetamine users who achieve stable abstinence. J Clin Exp Neuropsychol. 2010;32:704-718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Voineskos D, Daskalakis ZJ, Blumberger DM. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr Dis Treat. 2020;16:221-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 268] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 28. | Barr MS, Farzan F, Wing VC, George TP, Fitzgerald PB, Daskalakis ZJ. Repetitive transcranial magnetic stimulation and drug addiction. Int Rev Psychiatry. 2011;23:454-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Corp DT, Bereznicki HGK, Clark GM, Youssef GJ, Fried PJ, Jannati A, Davies CB, Gomes-Osman J, Kirkovski M, Albein-Urios N, Fitzgerald PB, Koch G, Di Lazzaro V, Pascual-Leone A, Enticott PG; 'Big TMS Data Collaboration'. Large-scale analysis of interindividual variability in single and paired-pulse TMS data. Clin Neurophysiol. 2021;132:2639-2653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Corp DT, Bereznicki HGK, Clark GM, Youssef GJ, Fried PJ, Jannati A, Davies CB, Gomes-Osman J, Stamm J, Chung SW, Bowe SJ, Rogasch NC, Fitzgerald PB, Koch G, Di Lazzaro V, Pascual-Leone A, Enticott PG; ‘Big TMS Data Collaboration’. Large-scale analysis of interindividual variability in theta-burst stimulation data: Results from the 'Big TMS Data Collaboration'. Brain Stimul. 2020;13:1476-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 31. | Tremblay S, Rogasch NC, Premoli I, Blumberger DM, Casarotto S, Chen R, Di Lazzaro V, Farzan F, Ferrarelli F, Fitzgerald PB, Hui J, Ilmoniemi RJ, Kimiskidis VK, Kugiumtzis D, Lioumis P, Pascual-Leone A, Pellicciari MC, Rajji T, Thut G, Zomorrodi R, Ziemann U, Daskalakis ZJ. Clinical utility and prospective of TMS-EEG. Clin Neurophysiol. 2019;130:802-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 296] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 32. | Brunoni AR, Ekhtiari H, Antal A, Auvichayapat P, Baeken C, Benseñor IM, Bikson M, Boggio P, Borroni B, Brighina F, Brunelin J, Carvalho S, Caumo W, Ciechanski P, Charvet L, Clark VP, Cohen Kadosh R, Cotelli M, Datta A, Deng ZD, De Raedt R, De Ridder D, Fitzgerald PB, Floel A, Frohlich F, George MS, Ghobadi-Azbari P, Goerigk S, Hamilton RH, Jaberzadeh SJ, Hoy K, Kidgell DJ, Zonoozi AK, Kirton A, Laureys S, Lavidor M, Lee K, Leite J, Lisanby SH, Loo C, Martin DM, Miniussi C, Mondino M, Monte-Silva K, Morales-Quezada L, Nitsche MA, Okano AH, Oliveira CS, Onarheim B, Pacheco-Barrios K, Padberg F, Nakamura-Palacios EM, Palm U, Paulus W, Plewnia C, Priori A, Rajji TK, Razza LB, Rehn EM, Ruffini G, Schellhorn K, Zare-Bidoky M, Simis M, Skorupinski P, Suen P, Thibaut A, Valiengo LCL, Vanderhasselt MA, Vanneste S, Venkatasubramanian G, Violante IR, Wexler A, Woods AJ, Fregni F. Digitalized transcranial electrical stimulation: A consensus statement. Clin Neurophysiol. 2022;143:154-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Ni Z, Chen R. Transcranial magnetic stimulation to understand pathophysiology and as potential treatment for neurodegenerative diseases. Transl Neurodegener. 2015;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, Filipović SR, Grefkes C, Hasan A, Hummel FC, Jääskeläinen SK, Langguth B, Leocani L, Londero A, Nardone R, Nguyen JP, Nyffeler T, Oliveira-Maia AJ, Oliviero A, Padberg F, Palm U, Paulus W, Poulet E, Quartarone A, Rachid F, Rektorová I, Rossi S, Sahlsten H, Schecklmann M, Szekely D, Ziemann U. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clin Neurophysiol. 2020;131:474-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 1245] [Article Influence: 249.0] [Reference Citation Analysis (0)] |
| 35. | Levkovitz Y, Harel EV, Roth Y, Braw Y, Most D, Katz LN, Sheer A, Gersner R, Zangen A. Deep transcranial magnetic stimulation over the prefrontal cortex: evaluation of antidepressant and cognitive effects in depressive patients. Brain Stimul. 2009;2:188-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Isserles M, Rosenberg O, Dannon P, Levkovitz Y, Kotler M, Deutsch F, Lerer B, Zangen A. Cognitive-emotional reactivation during deep transcranial magnetic stimulation over the prefrontal cortex of depressive patients affects antidepressant outcome. J Affect Disord. 2011;128:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Naim-Feil J, Bradshaw JL, Sheppard DM, Rosenberg O, Levkovitz Y, Dannon P, Fitzgerald PB, Isserles M, Zangen A. Neuromodulation of Attentional Control in Major Depression: A Pilot DeepTMS Study. Neural Plast. 2016;2016:5760141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Levkovitz Y, Rabany L, Harel EV, Zangen A. Deep transcranial magnetic stimulation add-on for treatment of negative symptoms and cognitive deficits of schizophrenia: a feasibility study. Int J Neuropsychopharmacol. 2011;14:991-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Blumberger DM, Mulsant BH, Thorpe KE, McClintock SM, Konstantinou GN, Lee HH, Nestor SM, Noda Y, Rajji TK, Trevizol AP, Vila-Rodriguez F, Daskalakis ZJ, Downar J. Effectiveness of Standard Sequential Bilateral Repetitive Transcranial Magnetic Stimulation vs Bilateral Theta Burst Stimulation in Older Adults With Depression: The FOUR-D Randomized Noninferiority Clinical Trial. JAMA Psychiatry. 2022;79:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 40. | Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipović SR, Hummel FC, Jääskeläinen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, Padberg F, Poulet E, Rossi S, Rossini PM, Rothwell JC, Schönfeldt-Lecuona C, Siebner HR, Slotema CW, Stagg CJ, Valls-Sole J, Ziemann U, Paulus W, Garcia-Larrea L. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125:2150-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1225] [Cited by in RCA: 1370] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 41. | Cheng CPW, Wong CSM, Lee KK, Chan APK, Yeung JWF, Chan WC. Effects of repetitive transcranial magnetic stimulation on improvement of cognition in elderly patients with cognitive impairment: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2018;33:e1-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Benussi A, Di Lorenzo F, Dell'Era V, Cosseddu M, Alberici A, Caratozzolo S, Cotelli MS, Micheli A, Rozzini L, Depari A, Flammini A, Ponzo V, Martorana A, Caltagirone C, Padovani A, Koch G, Borroni B. Transcranial magnetic stimulation distinguishes Alzheimer disease from frontotemporal dementia. Neurology. 2017;89:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 43. | Anderkova L, Rektorova I. Cognitive effects of repetitive transcranial magnetic stimulation in patients with neurodegenerative diseases - clinician's perspective. J Neurol Sci. 2014;339:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Koch G, Spampinato D. Alzheimer disease and neuroplasticity. Handb Clin Neurol. 2022;184:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 45. | Tăuƫan AM, Casula EP, Pellicciari MC, Borghi I, Maiella M, Bonni S, Minei M, Assogna M, Palmisano A, Smeralda C, Romanella SM, Ionescu B, Koch G, Santarnecchi E. TMS-EEG perturbation biomarkers for Alzheimer's disease patients classification. Sci Rep. 2023;13:7667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 46. | Menardi A, Rossi S, Koch G, Hampel H, Vergallo A, Nitsche MA, Stern Y, Borroni B, Cappa SF, Cotelli M, Ruffini G, El-Fakhri G, Rossini PM, Dickerson B, Antal A, Babiloni C, Lefaucheur JP, Dubois B, Deco G, Ziemann U, Pascual-Leone A, Santarnecchi E. Toward noninvasive brain stimulation 2.0 in Alzheimer's disease. Ageing Res Rev. 2022;75:101555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 47. | Somaa FA, de Graaf TA, Sack AT. Transcranial Magnetic Stimulation in the Treatment of Neurological Diseases. Front Neurol. 2022;13:793253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 48. | Hill AT, Rogasch NC, Fitzgerald PB, Hoy KE. TMS-EEG: A window into the neurophysiological effects of transcranial electrical stimulation in non-motor brain regions. Neurosci Biobehav Rev. 2016;64:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 49. | Welch JF, Argento PJ, Mitchell GS, Fox EJ. Reliability of diaphragmatic motor-evoked potentials induced by transcranial magnetic stimulation. J Appl Physiol (1985). 2020;129:1393-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Werhahn KJ, Classen J, Benecke R. The silent period induced by transcranial magnetic stimulation in muscles supplied by cranial nerves: normal data and changes in patients. J Neurol Neurosurg Psychiatry. 1995;59:586-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Kähkönen S, Wilenius J, Komssi S, Ilmoniemi RJ. Distinct differences in cortical reactivity of motor and prefrontal cortices to magnetic stimulation. Clin Neurophysiol. 2004;115:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Pellegrino G, Schuler AL, Cai Z, Marinazzo D, Tecchio F, Ricci L, Tombini M, Di Lazzaro V, Assenza G. Assessing cortical excitability with electroencephalography: A pilot study with EEG-iTBS. Brain Stimul. 2024;17:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 53. | Trajkovic J, Sack AT, Romei V. EEG-based biomarkers predict individual differences in TMS-induced entrainment of intrinsic brain rhythms. Brain Stimul. 2024;17:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 54. | Mimura Y, Tobari Y, Nakajima S, Takano M, Wada M, Honda S, Bun S, Tabuchi H, Ito D, Matsui M, Uchida H, Mimura M, Noda Y. Decreased short-latency afferent inhibition in individuals with mild cognitive impairment: A TMS-EEG study. Prog Neuropsychopharmacol Biol Psychiatry. 2024;132:110967. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |