INTRODUCTION
Compared with their normal-hearing peers, hearing-impaired children tend to demonstrate delayed language development[1,2]. However, the intricacies of the underlying mechanism responsible for this developmental divergence are not yet fully understood. This gap in knowledge highlights the urgent need for more thorough research into the factors that contribute to differences in early brain development in participants with hearing loss. This research aimed to elucidate these complicated processes and ultimately improve our understanding of early language acquisition in this unique population.
Behavioral studies have revealed that participants with sensorineural hearing loss (SNHL) lag behind their peers in various aspects of language development[3-5]. A study by Moeller et al[4] revealed that the onset of babbling in participants with SNHL occurs approximately 7 months later than that in participants with normal hearing (NH). At the syllable level, participants with SNHL produced fewer multisyllables than did participants with NH[3]. In addition, Tomblin et al[5] examined the language outcomes of children with mild to severe hearing loss. Compared with children with NH, children with SNHL lagged behind their peers in terms of both morphosyntax and semantic comprehension, with morphosyntax being more delayed. These studies suggest that both groups have comparable timetables for language development, although hearing-impaired children lag behind their normal-hearing peers in various language-related aspects.
The concept of prenatal experiences impacting later language acquisition has garnered significant attention in related research[6,7]. Newborns exhibit the ability to recognize sounds they hear frequently in the womb, such as stories[8], music[9], and atypical prosody contours[10]. Newborns also prefer their mothers’ native languages[11] and voices[12], which provides evidence of the important role of prenatal experiences. Notably, the influence of prenatal experience on subsequent language development has been extensively examined[13,14]. Benavides-Varela et al[13] performed 2 studies using the manipulation of prosodical contours. In the first study, 4-word sequences recorded with a list intonation were played for newborn participants, whereas in the second study, the same 4-word sequences recorded with a well-formed utterance-level prosodic contour were played. The results showed that newborns could detect violations of the word order in the list prosody condition but not in the utterance prosody condition. This study suggested that native prosody, with which newborns are already familiar owing to prenatal experience, is a stronger cue at birth than word order is, thus indicating linguistic units. However, no study to date has examined the effect of insufficient prenatal experience, particularly that caused by a lack of sufficient hearing, on language development.
Multiple neuroimaging studies have documented early alterations in functional connectivity (FC) or brain networks in children with hearing impairment[15-17]. Independent component analysis (ICA) of functional magnetic resonance imaging (fMRI) data revealed that children younger than 3 years with SNHL exhibited reduced FC in the default mode network, increased connectivity in the auditory and salience networks, and no significant changes in the visual and sensorimotor networks[18]. Using functional near-infrared spectroscopy (fNIRS), participants aged 3 to 10 months with unilateral hearing loss or single-sided deafness exhibited increased FC, particularly involving the right middle temporal gyrus, compared with normal-hearing participants[15]. A study using diffusion tensor imaging and fMRI revealed that participants aged 18 months with SNHL had increased connectivity in areas associated with auditory and language networks, suggesting potential reorganization and compensatory activation due to early auditory deprivation[17]. Although these studies contribute to further understanding how infants with hearing loss develop their language ability, hearing age, an important variable, was not considered in the above studies. Hearing age refers to the accumulated amount of language exposure. At birth, infants with hearing loss have three months fewer of hearing experience than NH infants. These deficits will persist until hearing is partially or completely restored[19]. Therefore, direct chronological comparisons between infants with hearing loss and controls with NH cannot disentangle the effects of maturation and experiential factors on language development. In the present study, a hearing-matched group was included to fill this gap.
As an imaging technique, fNIRS can be used to investigate the functional activation of the infant brain in awake, engaged participants. One of the main domains of fNIRS research is language development because fNIRS can help identify linguistic knowledge in the immature brain and the ways in which this knowledge changes with time and experience[20]. Using graph theory to analyze the connectivity between different regions of the brain in fNIRS studies has become a promising approach[21,22]. Graph theory has been widely applied in the research of complex systems, including markets, ecosystems, computer circuits, and gene-gene interactions[23,24]. Many studies have demonstrated that graph theory is a powerful approach that can be used to understand the evolution and development of clinical disorders and the corresponding brain systems[21,22,25]. Importantly, Cai et al[21] used graph theory to examine the development of the functional organization network between early childhood and early adolescence. The study revealed that significant and subtle topological changes in functional network organization occurred during both critical stages. Jia et al[23] provided an example of how to identify biological markers with fNIRS data using graph theory. Furthermore, graph theory has been widely used to investigate brain connectivity in specific populations, such as patients with Parkinson’s disease[26], Alzheimer’s disease[27], and autism spectrum disorders[11]. These studies suggest that graph theory can be used to identify changes in the functional organization of brain networks and information transmission efficiency among brain regions.
In this study, using multichannel fNIRS, we examined developmental changes in both global and regional nodal topological properties in newborns and 4-month-old participants with and without SNHL. We hypothesized that there would be a significant linear improvement in certain topological characteristics of brain networks, such as local and global efficiency (Eglob), revealing functional segregation and integration between 4-month-old participants with SNHL and age-matched infants without SNHL and between 4-month-old participants with NH and healthy newborns. Additionally, we anticipated that the developmental effects observed in 4-month-old normal-hearing infants compared with newborns may vary in infants with SNHL because of the absence of auditory input.
MATERIALS AND METHODS
Participants
A total of 65 participants registered at North Sichuan Medical University participated in this study. Among these participants, 28 were newborns (1-2 days), 22 were 4-month-old participants with SNHL (6 males, mean age = 135 days), and 15 were 4-month-old healthy participants (5 males, mean age = 122 days). Parents signed written informed consent forms before the start of the experiments. The experiment was conducted at North Sichuan Medical Affiliated Hospital with the approval of the institutional review board of North Sichuan Medical University.
The inclusion criteria for the neonatal group were: (1) Successfully passing the infant hearing screening examination and (2) Not meeting the criteria for sensory hearing loss. The inclusion criteria for the SNHL group were: (1) Not successfully passing the newborn hearing screening examination and (2) A lack of clearance in the auditory brainstem response examination at approximately four months of age, with a threshold exceeding 40 dB. The inclusion criteria for the 4-month-old healthy control group were: (1) Passing the auditory brainstem response test with hearing thresholds less than 30 dB and (2) No neural pathologies as confirmed by the parents’ reports.
fNIRS data acquisition
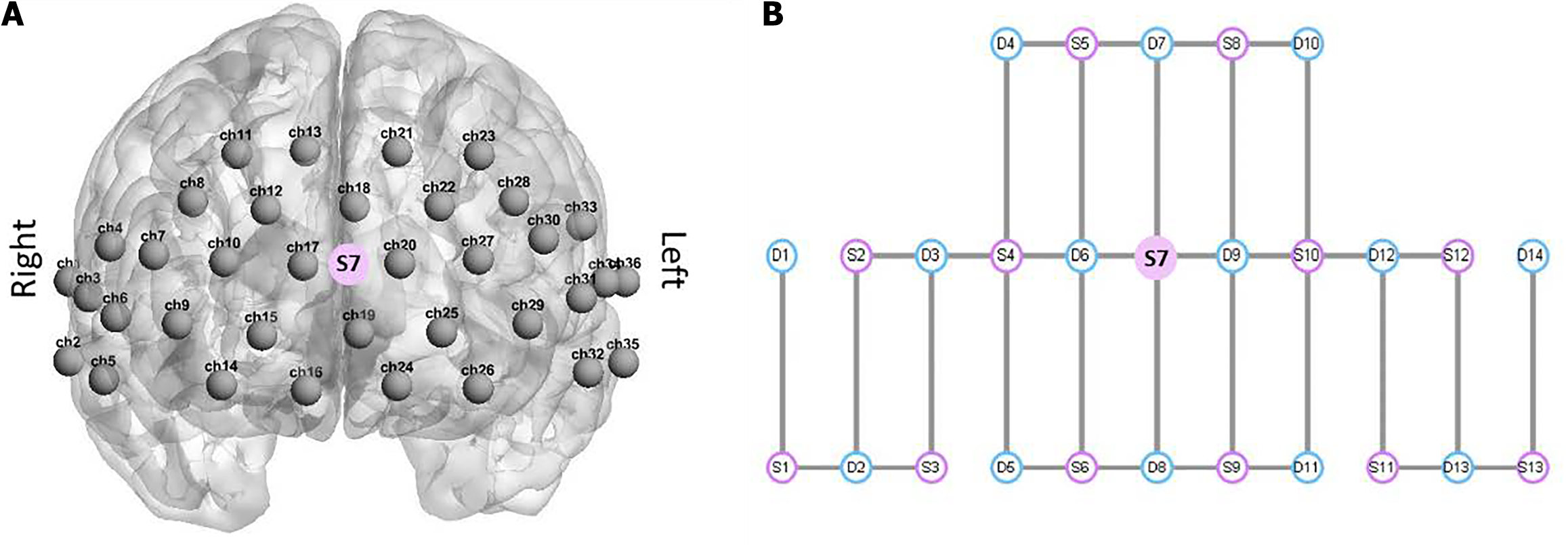
Resting-state data were collected by a multichannel fNIRS imaging system (NirSmart II-3000A) equipped with 14 detectors and 13 sources, resulting in a total of 36 measuring channels covering the frontal, temporal and parietal cortices (Figure 1), regions that have been found to play important roles in early cognitive and language development[28]. The distance of source-detector separation was approximately 1.5 cm. The absorption of near-infrared light at two wavelengths (730 nm and 850 nm) was recorded with a sampling rate of 11 Hz. The opcodes were positioned according to the international 10-20 coordinate system. All infants had an 8-minute rest period in a softly lit, quiet room while either seated in a baby chair or cradled by their accompanying parent. Prior to conducting fNIRS measurements, the head circumference of each baby was measured to ensure the selection of an appropriately sized cap.
Figure 1 Overview of functional near-infrared spectroscopy channel positions and optode placement.
A: Spatial arrangement of 63 channels on a head template with channel numbers ranging from 1 to 36; B: Layout of the functional near-infrared spectroscopy (fNIRS) sources and detectors, with the fNIRS source depicted in red and the detector shown in blue. Source 7 is positioned at the center of the layout design and corresponds to the frontal region of the brain, as indicated in A.
Preprocessing of fNIRS data
The collected fNIRS data were preprocessed according to the methods of Wang et al[29]. The FC-NIRS package was used to preprocess the fNIRS data[30]. First, the raw data were converted into optical density (OD) values, after which a bandpass filter (0.01 Hz to 0.1 Hz) was used to eliminate the effects of low-frequency drift and high-frequency neurophysiological noise. Temporal ICA was conducted to remove systematic physiological noise (e.g., superficial noise) and motion-induced artifacts. The OD values were then converted into concentrations of oxyhemoglobin and deoxyhemoglobin (HbR) using a modified Beer-lambert law[31], with a differential path length factor of 6[32]. Continuous data were extracted in 6-minute epochs for each participant for the network analyses. For our subsequent brain network analysis, we utilized the concentration of HbR as the primary index. This choice aligns with established practices in fMRI studies that rely on the blood oxygen level-dependent signal[33,34]. Furthermore, the HbR concentration has demonstrated good test-retest reliability in fNIRS-based brain network investigations, as demonstrated by the findings of Niu et al[35].
FC calculations and brain network construction
For each participant, FC was calculated using Pearson correlation analyses of the time series of hemoglobin concentrations for every pair of nodes, where the nodes were the measurement channels. This analysis generated a 36 × 36 correlation matrix for each participant. The correlation coefficients (r) were normalized to z values with Fisher’s r-to-z transformation with a predetermined sparsity (1% < s < 50%, step size = 1%), which denotes the number of actual connections divided by the maximum possible number of connections in the network. The correlation matrix was then thresholded into a binary matrix that described the topological organization of the functional networks. As in previous studies[21,29], a sparsity value of 0.20 was chosen to construct the brain network. To investigate whether there were significant differences in FC among the three groups, analysis of variance (ANOVA) tests were performed. Before conducting the analysis of variance, we performed a Shapiro-Wilk test for normality, which confirmed that the data were normally distributed (P > 0.05). We also conducted Levene’s test to check for homogeneity of variance among the groups, and the results indicated that the variances were equal. Post hoc, we applied false discovery rate correction to the FC strengths. In addition, our data were continuous, and we confirmed the applicability of the data distribution for correlation analysis after passing the normality test.
Brain network analysis
A graph theory approach was used to characterize the topological organization of the brain functional networks in the newborn group, 4-month SNHL group, and 4-month healthy group. In fNIRS-derived brain network studies, topological network efficiency has been frequently used to characterize the capacity for parallel information processing within a brain network. To investigate the topological organization of the brain network, we calculated both global properties and regional node statistics[29,36]. Notably, there are four key global network properties within a network: The clustering coefficient (Cp), the characteristic path length (Lp), the Eglob, and the local efficiency (Eloc). The Cp offers a measure of the degree of connection between spatially closer brain regions or the local connectedness of the network. The Cp signifies the extent to which nodes in a graph tend to form clusters, whereas the Lp measures parallel information transfer. These global network properties were also calculated for random networks. In addition, the small work index (sigma), which has a higher Cp and a shorter Lp than other indices as well as high efficiency, was estimated[37]. Typically, a network is considered to have small-world properties when sigma is greater than 1[38]. To determine regional nodal properties, we computed the nodal degree and efficiency across groups. We used the functional hub to represent the local network integrity, which was identified as a node with a high degree and efficiency that exceeded one standard deviation above the average.
RESULTS
Resting-state FC
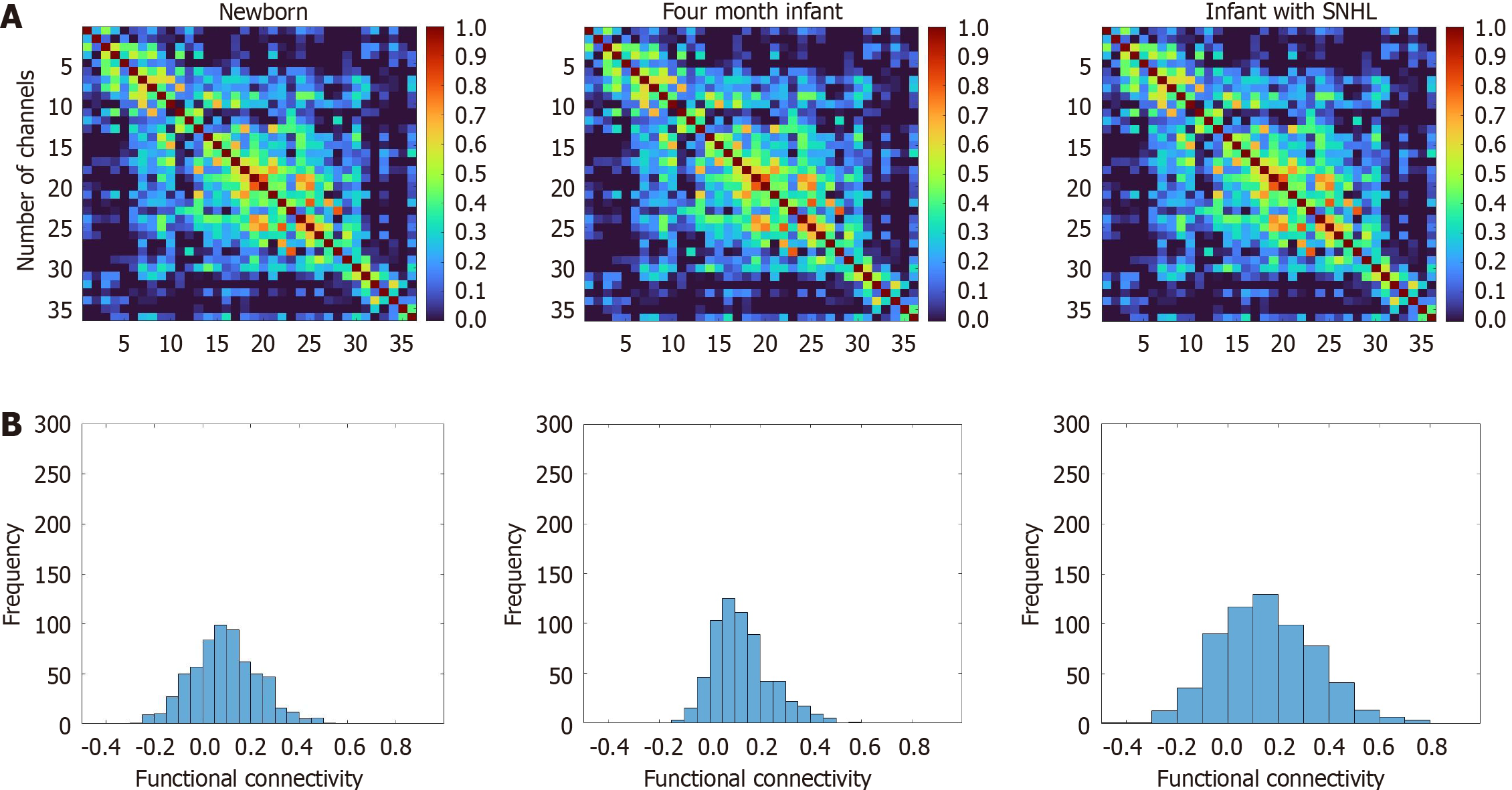
The FCs at the group level in newborns, 4-month-old participants with SNHL, and 4-month-old participants without SNHL are illustrated in Figure 2. One-way ANOVA revealed significant differences among the three groups [F (2.63) = 10.83, P < 0.001, η2 = 0.14). Post hoc analysis revealed significantly lower FC (P = 0.05, t = 1.79) in participants with SNHL (mean ± SD, 0.13 ± 0.04) than in age-matched healthy participants (mean ± SD, 0.16 ± 0.08). In addition, we observed greater connectivity in participants with SNHL (P = 0.034, t = -3.6228) than in newborns (mean ± SD, 0.098 ± 0.04), as well as greater FC in 4-month-old healthy participants than in newborns (P < 0.000, t = -4.192). The three groups exhibited different FC organization.
Figure 2 Tests for the newborn, 4-month sensorineural hearing loss, and 4-month healthy groups.
A: Average resting-state functional connectivity at the group level, as represented by correlation matrices with r value indices, for the newborn, 4-month sensorineural hearing loss, and 4-month healthy groups. The numerical values within these matrices denote the correlation strength between pairs of measurement channels; B: Z values of the correlations observed across the three distinct groups. SNHL: Sensorineural hearing loss.
Properties of global networks
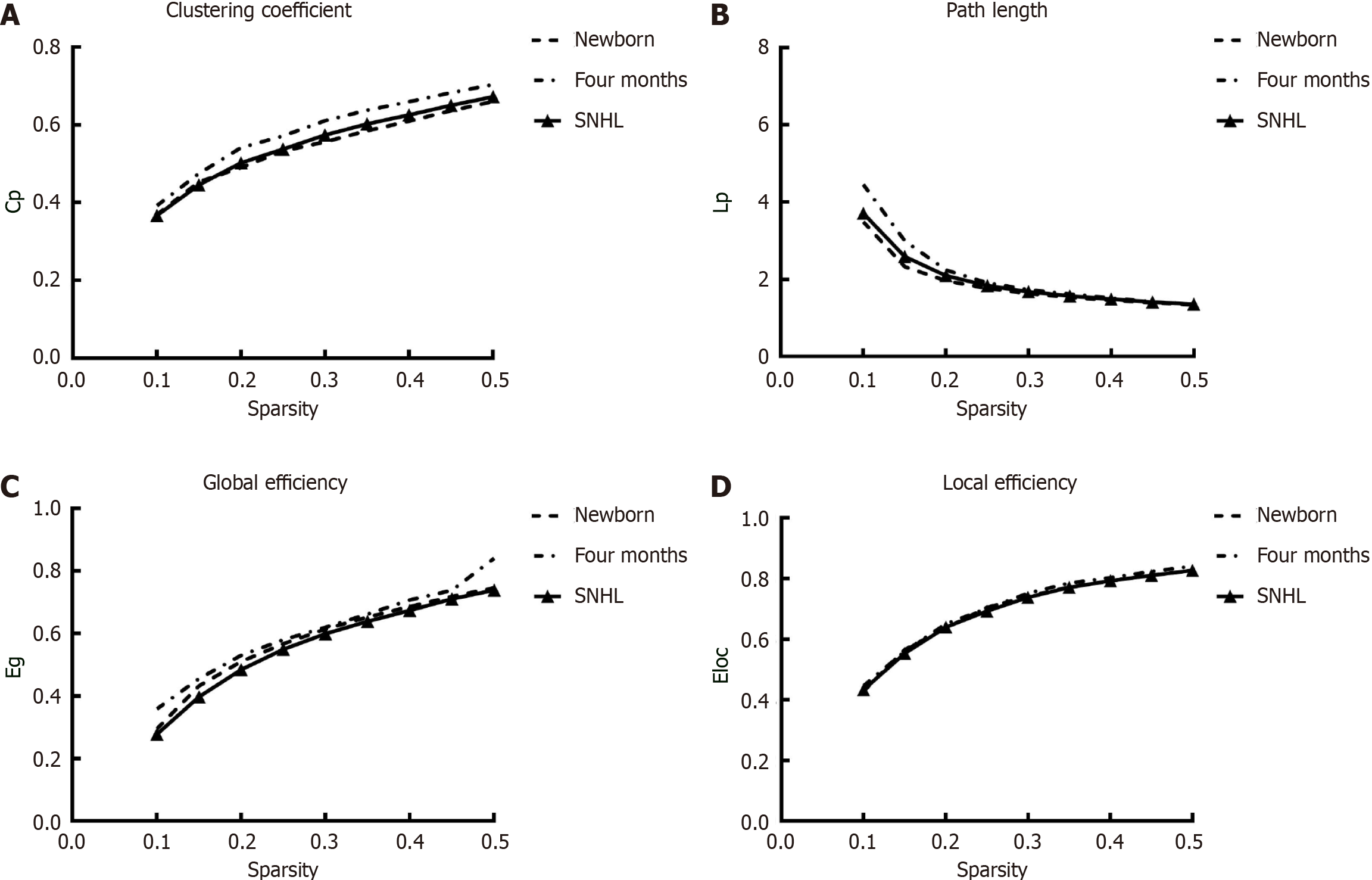
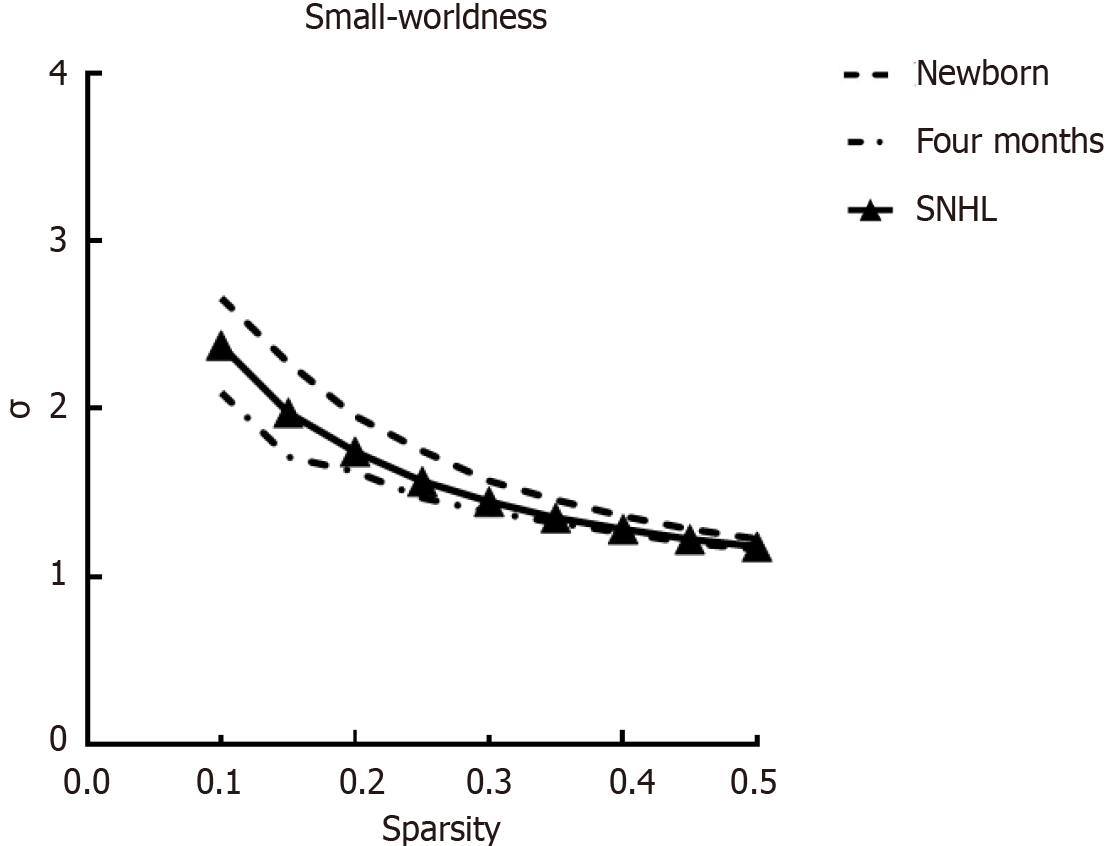
To further investigate the intrinsic organization of cortical networks, we next analyzed the metrics of graph theory, such as the Cp, Lp, Eglob, Eloc and small-world parameters, for the three groups. ANOVA revealed significant group differences in the Cp [F (2.63) = 4.298, P = 0.018, η2 = 0.12] and Lp [F (2.63) = 7.516, P = 0.001, η2 = 0.195) and significant differences in the small-world property [F (2.63) = 4.215, P = 0.019, η2 = 0.12). Figure 3 shows the global network properties as functions of the sparsity thresholds (ranging from 10% to 50%, with a 5% step size), and Figure 4 shows the small-world property sigma. There was a significant difference in Cp (P = 0.0430, t = 2.1004) and Lp (P = 0.000, t = 4.0969) between participants with SNHL and age-matched controls. However, no significant differences were detected in Eloc and Eglob. Regarding the developmental effect, we observed significant differences in Cp (P = 0.017, t = -2.4786), Lp (P = 0.000, t = 4.1817) and Eglob (P = 0.000, t = -4.2146) between 4-month-old healthy participants and neonates. Interestingly, we observed no difference between 4-month-old participants with SNHL and neonates for Cp (P > 0.1 in these global matrices), demonstrating high between-modality similarities between infants with SNHL and newborns. The P values were not corrected for multiple comparisons. According to graph theory, greater cognitive performance was associated with brain graphs globally configured for greater efficiency-speed and fidelity of parallel information transfer between regional nodes. These findings suggest that the information processing ability of infants in the healthy group is different from that of infants with SNHL.
Figure 3 Global network metrics across a range of sparsity thresholds (1%-50%) for newborn and 4-month-old participants and the corresponding random networks.
A: Clustering coefficient; B: Path length; C: Global efficiency; D: Local efficiency. Cp: Clustering coefficient; Lp: Path length; Eg: Global efficiency; Eloc: Local efficiency; SNHL: Sensorineural hearing loss.
Figure 4 Global network index sigma across a range of sparsity thresholds (1%-50%) for newborns.
SNHL: Sensorineural hearing loss.
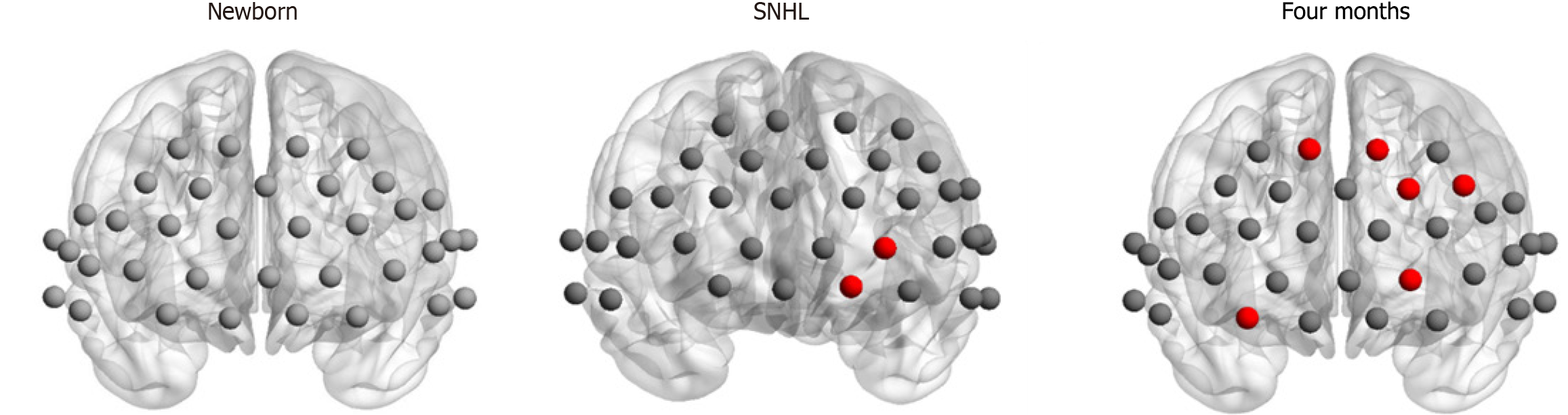
In our investigation of regional nodal characteristics, specifically nodal degree and efficiency, within the brain network, we identified two prominent central hubs (channel 24 and channel 25) in the 4-month SNHL group and six hubs in the 4-month healthy group. However, notably, no hubs were identified in the newborn group, as illustrated in Figure 5.
Figure 5 Network regional properties of newborn and 4-month-old participants with and without sensorineural hearing loss.
The red circles represent hubs identified in 4-month-old participants with and without sensorineural hearing loss (P < 0.05, corrected by false discovery rate). No hubs were identified in the newborns. SNHL: Sensorineural hearing loss.
DISCUSSION
In this study, we aimed to use fNIRS and graph theory to investigate the differences in brain networks and developmental changes in 4-month-old participants with SNHL, 4-month-old age-matched healthy participants and newborns. By exploring brain connectivity, we found that there was a significant difference in resting FC patterns between participants with and without SNHL, which indicates that brain functional development in infants with SNHL is different from that in normally hearing infants. A significant difference was also identified in the FC pattern between 4-month-old healthy participants and newborns, demonstrating age-related increases in brain FC. We also observed differences between participants with SNHL and newborns. However, among the 3 groups, the FC value was the highest in the 4-month-old healthy group, which suggested that the developmental effect might differ between 4-month-old participants with and without SNHL and newborns.
Using graph theory, we found that the Cp, Eglob, and Eloc were significantly greater in 4-month-old healthy participants than in participants with SNHL and in newborns. Cp, Eglob and Eloc are important parameters in graph theory, demonstrating that the enhanced brain functional organization and integration in 4-month-old healthy participants are greater than those in participants with SNHL and in newborns. This finding indicates that relative to their peers, participants with SNHL exhibit neural dysfunction and different developmental trajectories, which are induced by hearing impairment. Moreover, there was no significant difference in Cp between participants with SNHL and newborns. This finding suggests that the ability of SNHL patients to integrate information processing is similar to that in newborns. In future studies, experiments on hearing loss should not be limited to comparisons between participants with hearing loss and age-matched controls. Hearing should be considered according to age group.
The FC pattern differed significantly between participants with SNHL and age-matched healthy participants. The resting-state FC patterns often reflect changes in the intrinsic functional organization of the brain. A review by Gervain et al[6] revealed that the hearing ability of fetuses at approximately the 20th week of gestation is similar to that of adults. Fetuses can hear the melody and rhythm of speech, although the intrauterine signals are attenuated by maternal tissues. After birth, the hearing stimuli detected by participants with SNHL differ from those detected by their peers. This difference results in a different age of hearing onset in participants with SNHL than in age-matched normally hearing participants. These different hearing-onset ages are represented by different intrinsic functional organizations of the brain. These findings are consistent with those of previous studies[39-41]. Smyser et al[40] used the resting-state FC pattern to categorize the development of brain networks in preterm infants. They reported that the brain networks in preterm infants were significantly different from those in full-term infants.
There was also a significant difference in the FC pattern between participants with SNHL and newborns. This difference can be explained by hearing-onset age. Although participants with SNHL were subjected to more hearing stimuli during their first 4 months of life than newborns were, these hearing stimuli were limited in vivo and after birth. Hearing input for four months after birth may compensate for impaired hearing in the womb. Our findings indicated that participants with SNHL lag behind their peers, which is consistent with the review by Lovcevic et al[19]. We also found that the mean FC value in participants with SNHL was significantly greater than that in newborns, demonstrating that participants with SNHL still undergo cognitive development, although this development is affected by hearing impairment. This result suggested that FC in participants with SNHL might be a good biomarker for the early diagnosis of SNHL. In future studies, an FC study with machine learning should be performed to identify participants with SNHL during infancy.
We also found that the value of Eglob was lower in participants with SNHL than in age-matched participants without SNHL, demonstrating that hearing loss may impact language development and cognitive development. We also found that Eglob was significantly greater in 4-month-old participants than in newborns, which may be due to maturity. No increase was found between newborns and participants with SNHL, suggesting that impaired hearing impacts the development of brain functional networks and language. These findings are consistent with previous results on brain network development.
For nodal efficiency, we compared the hub distribution patterns across the three groups. In the newborn group, no central hub was observed, whereas there were two hubs in infants with SNHL and six hubs in age-matched infants. The remaining hearing ability can also contribute to the development and detection of melodies and rhythm in SNHL infants. The two identified hubs were located in the right hemisphere of SNHL infants. Since the right hemisphere plays a role in prosody processing, this finding suggests that infants with SNHL show developmental progress in prosody processing compared with newborns, which needs to be confirmed by further studies at the behavioral level. According to the study by Hwang et al[42], hub regions play a central role in global information integration between parallel and distributed networks. In other words, the hub region can indicate the ability to integrate local information. However, in 4-month-old healthy infants, 6 hubs were identified, covering both the left and right hemispheres. These brain areas are related to the language network according to previous studies[43-46]. For example, the dorsolateral prefrontal cortex (DLPFC) is a core language area located in the middle frontal gyrus, comprising parts of Brodmann areas 46 and 9[47]. The functions of the DLPFC include discourse management and the integration of prosody. In addition, the DLPFC is involved in both the ventral and dorsal language pathways[46]. Four network hubs in healthy infants were located in the left hemisphere, which is consistent with the left language dominance framework[48-51]. However, in infants with SNHL, only two hubs were identified in the left hemisphere, demonstrating that left lateralization does not develop well. These findings suggest that infants with SNHL display deviated language developmental trajectories. Auditory stimulation is essential for the normative development of the language network. In individuals with SNHL, diminished or absent auditory input results in neurofunctional reorganization, especially within brain regions pivotal to auditory processing and linguistic faculties. Given that language development is contingent upon the intricate interplay of a distributed neural network, atypical FC in the brains of children with SNHL can significantly impact their linguistic and cognitive capabilities. Considering our research as a whole, hearing input is important for the development of the brain functional network and subsequent language development. Inadequate hearing during the early stages of life can potentially be compensated for by focusing on auditory development during the first four months of life for infants with SNHL. This result suggests that if newborns do not pass the initial hearing screening, it is essential to prioritize and emphasize infant-directed speech and auditory stimulation during the crucial early months.
We are conducting further studies. fNIRS has the advantages of being noninvasive, fast-responding, resistant to motion artifacts, and cost-effective. To date, this technology has been widely applied in research involving newborns, infants, and toddlers. We believe that this tool can effectively reflect brain neural activity, thereby assisting in the clinical revelation of the development of cortical brain networks in early life. Moreover, our research group has investigated the therapeutic effects of foot reflexology therapy in female patients with SNHL over a period of six months. We believe that near-infrared spectroscopy can provide a more objective basis and reference for the formulation of clinical interventions or rehabilitation.
Limitations
First, cross-sectional studies can disentangle the effects of maturation and experiential factors on language development; however, longitudinal studies tracking the same group of infants over time, which provides more robust data on how brain connectivity and networks evolve as infants with SNHL grow compared to their normal-hearing peers are encouraged. Second, in addition to comparing infants with SNHL to normal-hearing infants, a control group of patients with other types of developmental delays or impairments should be included. This addition would help in distinguishing the effects specific to SNHL from those related to developmental delays in general. Third, combining fNIRS with other neuroimaging techniques, such as MRI or electroencephalo-graph, could offer a more comprehensive view of brain development in these infants. Finally, this research did not assess specific subgroups of infants with SNHL, such as bilateral vs single-sided hearing loss. These variables have the potential to influence the current findings, and it is advisable for future research to include them in the analysis. Furthermore, promoting the integration of graph theory and machine learning for the early diagnosis of SNHL in infants is an avenue worth exploring.