Published online Oct 19, 2024. doi: 10.5498/wjp.v14.i10.1437
Revised: August 27, 2024
Accepted: September 6, 2024
Published online: October 19, 2024
Processing time: 91 Days and 0.6 Hours
Androgenetic alopecia (AGA) is a common form of hair loss that can be influ
To investigate the impact of mental stress on neurotrophic factors in patients with AGA and correlate the findings with the progression of AGA.
A total of 120 patients with AGA were analyzed in this study, which were divided into a non-stress group (n = 30) and a stress group (n = 90) on the basis of the presence or absence of psychological stress confirmed by Depression Anxiety Stress Scale-21 scale. The baseline demographic characteristics, serum cortisol levels, hair growth parameters, neurotrophic factors, and AGA progression scores between the non-stress and stress groups were compared. Correlation analyses were conducted to assess the relationships among stress, neurotrophic factors, hair loss progression, and AGA progression.
This study revealed significantly higher cortisol levels throughout the day in the stress group than in the non-stress group. The stress group exhibited lower levels of nerve growth factor, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor and higher expression levels of neurotrophin (NT)-3 and NT-4 than the non-stress group. Hair parameters indicated lower hair diameter, decreased hair density, and more severe AGA grading in the stress group, whereas follicle count and terminal/vellus hair ratio showed no significant differences between the two groups. After 1 year of treatment with 5% minoxidil, efficacy was observed to be lower but AGA progression was notably more pronounced in the stress group than in the non-stress group. Disease progression was positively correlated with high stress and NT-4 levels.
This study provides compelling evidence of the influence of mental stress on neurotrophic factors and its correlation with the progression of AGA. The findings underscore the need for a comprehensive approach to the management of AGA that considers the physiological and psychosocial aspects. Further research is warranted to validate the findings and explore targeted therapeutic interventions for individuals with stress-related AGA.
Core Tip: This study investigates the impact of mental stress on neurotrophic factors in patients with androgenetic alopecia (AGA) and its correlation with AGA progression. Findings reveal that patients experiencing psychological stress have elevated serum cortisol levels and altered neurotrophic factor profiles, including reduced nerve growth factor, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor. The stress group also exhibited more severe AGA progression despite treatment. These results highlight the need for integrated management approaches that address both physiological and psychological aspects of AGA to improve treatment outcomes.
- Citation: Cheng Y, Lv LJ, Cui Y, Han XM, Zhang Y, Hu CX. Psychological stress impact neurotrophic factor levels in patients with androgenetic alopecia and correlated with disease progression. World J Psychiatry 2024; 14(10): 1437-1447
- URL: https://www.wjgnet.com/2220-3206/full/v14/i10/1437.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i10.1437
Androgenetic alopecia (AGA), also known as male or female pattern baldness, is the most common form of hair loss, affecting millions of individuals worldwide[1]. AGA is characterized by a progressive, genetically determined pattern of hair thinning and loss, typically manifesting as a receding hairline in men and as diffuse thinning over the crown in women[2]. The condition can have significant psychosocial implications, leading to decreased self-esteem and quality of life for those affected[3]. The pathogenesis of AGA involves a complex interplay of genetic, hormonal, and environmental factors[4]. Genetic predisposition, particularly the inheritance of certain androgen receptor gene polymorphisms, plays a pivotal role in the development of AGA. Furthermore, androgens, particularly dihydrotestosterone (DHT), have been implicated in the pathogenesis of AGA due to their influence on hair follicle miniaturization[5]. DHT is derived from the conversion of testosterone by the enzyme 5-alpha-reductase within the hair follicles. In individuals with a genetic predisposition to AGA, hair follicles in specific regions of the scalp are genetically programmed to be sensitive to the deleterious effects of DHT[6]. The binding of DHT to androgen receptors within these genetically predisposed hair follicles triggers a process known as follicular miniaturization. This process leads to the progressive shrinking of the hair follicles, resulting in shorter, finer hairs that are unable to achieve their full growth potential[7]. Over time, the miniaturization of hair follicles contributes to the characteristic pattern of hair thinning and loss observed in AGA, typically manifesting as a receding hairline in men and diffuse thinning over the crown in women[8]. While several factors contribute to the development and progression of AGA, recent research suggests that mental stress may play a significant role in exacerbating the condition[9].
Chronic stress has been associated with alterations in neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), which are known to modulate hair follicle activity and play a critical role in hair growth cycles[10]. Neurotrophic factors are essential for the growth, development, and maintenance of neurons, and they have significant effects outside the nervous system, including on hair follicles[11]. BDNF, for example, has been shown to promote the survival and growth of dermal papilla cells, which play a key role in the regulation of hair follicle cycling and hair growth[12]. Similarly, NGF has been implicated in promoting hair growth and maintaining the hair growth cycle[13]. In addition, other neurotrophic factors, such as glial cell line-derived neurotrophic factor (GDNF), neurotrophin (NT)-3, and NT-4, have significant implications in the context of hair growth and AGA[14]. GDNF is known to promote the proliferation of hair follicle cells and to inhibit the transition of hair follicles from the anagen (growth) phase to the catagen (regression) phase, thereby playing a crucial role in sustaining the active growth of hair follicles[15]. On the other hand, NT-3 is involved in various physiological processes regulating neuronal survival, but it has also been shown to affect hair follicle cycling, influencing the timing of catagen onset[16]. Finally, NT-4 is considered an inducer of catagen, and increasing levels of NT-4 have been linked to androgen activity that may contribute to AGA pathology[15].
Neurotrophic factors play a crucial role in the regulation of hair follicle biology and are essential for the growth, maintenance, and survival of various cell types within the hair follicle[16]. The modulation of these neurotrophic factors in response to psychological stress may provide deeper insights into the underlying mechanisms that exacerbate AGA, highlighting the necessity of exploring their roles further within the context of stress-related hair disorders. The impact of stress on neurotrophic factors and their subsequent influence on hair follicle activity is an area of increasing interest in dermatological research. Stress-induced alterations in neurotrophic factor levels may disrupt the delicate balance of signaling pathways involved in hair growth regulation, potentially contributing to conditions such as AGA[17].
This connection among stress, neurotrophic factors, and hair follicle activity underscores the complex interplay between psychological and physiological factors in dermatological conditions, making it a compelling avenue for further investigation[18]. Understanding the mechanisms through which stress-related changes in neurotrophic factors influence hair follicle biology is crucial for elucidating the pathogenesis of stress-related hair disorders and may offer insights into novel therapeutic approaches for these conditions.
A total of 120 cases of patients with AGA diagnosed first at our institution from January 2022 to December 2023 were analyzed. This study aimed to investigate the impact of mental stress on neurotrophic factors in patients with AGA and link these findings with the progression of AGA. This study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 2021KY036), and all patients signed informed consent forms.
Inclusion criteria: Patients with a confirmed diagnosis of AGA and those who provided informed consent for par
Exclusion criteria: The following patients were excluded: Those with a diagnosis of alopecia areata, telogen effluvium, or other forms of non-AGA; those with a history of chronic systemic diseases, such as chronic kidney disease, liver disease, or endocrine disorders, that may impact cortisol levels and neurotrophic factor expression; those with a history of psychiatric disorders or receiving psychotropic medications that may affect stress perception and neurotrophic factor levels; those with a history of recent significant life events or trauma that could confound the assessment of mental stress; those with a history of recent major surgical procedures or acute medical conditions within the past 6 months; pregnant or lactating women, because hormonal fluctuations during pregnancy and lactation may impact neurotrophic factors and stress perception; those with a history of recent use of medications known to impact cortisol levels, including corticosteroids and hormonal therapies.
Data were collected through the completion of relevant questionnaires, including general hair loss information and the Depression Anxiety Stress Scale (DASS-21). The collected data were organized and analyzed using Excel. The DASS-21 questionnaire is a scoring tool used to assess human behavior and emotions, comprising 21 items, each associated with specific behaviors or emotional states. This questionnaire includes three subscales: Depression, anxiety, and stress. Each subscale consists of seven items, with a total score range of 0 to 63. Different score ranges are used to determine an individual’s emotional state: 0–9 points indicate normal levels, 10–13 points indicate mild abnormality, 14–20 points indicate moderate abnormality, and 21 points or higher indicate severe abnormality. In this study, individuals were classified into the non-stress group if their scores ranged from 0 to 9 and into the stress group if their scores were 10 or above.
For plasma cortisol detection, 5 mL of fasting venous blood was drawn from the patients at 8: 00 AM, 7: 00 PM, and 12 midnight and placed in heparin anticoagulant tubes. The diurnal variation was defined as the absolute value of cortisol at 12 midnight minus at 8: 00 a.m. The blood samples were immediately centrifuged, and the plasma was extracted for testing using the operational procedures provided in the nzyme-linked immunosorbent assays (ELISA) Kit for Cortisol purchased from Beijing Beierkang Biotechnology Co., Ltd.
Collect 5 mL of fasting venous blood from patients before 8: 00 AM. Centrifuge the samples at a speed of 3000 rpm for 5 minutes, and then extract the supernatant for the analysis of NGF, BDNF, NT-3, NT-4, and GDNF. The concentrations of these neurotrophic factors will be determined using ELISA. The specific kits employed for the assays include NGF (ab155436, Abcam, United States), BDNF (ab212166, Abcam, United States), NT-3 (ab277461, Abcam, United States), NT-4 (ab277242, Abcam, United States), and GDNF (ab100525, Abcam, United States).
Minoxidil solution [Shanxi Zhendong Ate Biological Pharmaceutical Co., Ltd.; National Drug Approval Number: H20060626; 90 mL (5%)] was used for treatment for 1 year. One millimeter of minoxidil solution was taken, applied to the alopecia area of the scalp, and locally massaged for 3–5 minutes once a day.
Norwood–Hamilton classification: Primarily used for male AGA, the Norwood–Hamilton Classification can be classified into stages 1–7. Stages below 3 indicate mild alopecia, stages 3–5 indicate moderate alopecia, and stages 6 and above indicate severe alopecia.
Ludwig classification: Applicable to female AGA, the Ludwig Classification primarily assesses the width of the parting to determine the severity of the AGA. In stage 1, the parting is not particularly wide; in stage 2, the parting is wider; and in stage 3, the entire scalp is very sparse.
Hair growth parameters: Hair Assessment Overview: Prior to treatment and at week 52, photographs of the crown area were captured using a digital camera (EOS200D, Canon Inc., Japan). These images were then subject to comprehensive evaluation by two associate chief dermatologists. Hair diameter and density were assessed using a dermatoscope (Nanjing Beineng: BN-PFMF-8001) positioned at the intersection of the sagittal midline of the crown and the highest points of both auricles, magnified 30 times. Hair image data were collected from a targeted area measuring 2 cm × 2 cm to measure hair diameter, while simultaneously counting the number of hairs and hair follicles within a 1 cm² target area. This provided data on hair density (roots/cm²) and follicle density (per 1 cm²). Additionally, the counts of terminal and vellus hairs in the target area were recorded to calculate the terminal to vellus hair ratio.
The data were analyzed using SPSS (version 25.0) statistical software. Count data were expressed as n (%). For sample sizes of ≥ 40 and theoretical frequencies (Ts) ≥ 5, χ2 test was employed with the basic formula, and the test statistic was χ2. For sample sizes of ≥ 40 but 1 ≤ Ts < 5, χ2 test was adjusted using the correction formula. If the sample size was < 40 or T < 1, Fisher’s exact probability method was used for statistical analysis. Normally distributed continuous data were expressed as (mean ± SD). For non-normally distributed data, statistical analysis was performed after variable transformation to achieve normal distribution, and t-test was used. Spearman’s correlation analysis was employed for correlation analysis to identify statistically significant differences between the two groups.
On the basis of the presence or absence of psychological stress, the patients were divided into a non-stress group (n = 30) and a stress group (n = 90). The non-stress group consisted of 18 males and 12 females, with a mean age of 29.03 ± 2.39 years, and the stress group comprised 58 males and 32 females, with a mean age of 29.79 ± 4.81 years. The baseline demographic characteristics of both groups were comparable, demonstrating no statistically significant differences in age (t = 1.131, P = 0.261), gender distribution (χ2 = 0.048, P = 0.827), BMI (t = 1.051, P = 0.299), smoking history (t = 0.604, P = 0.549), alcohol intake (t = 1.319, P = 0.194), prevalence of hypertension (χ2 = 0, P = 1), diabetes (χ2 = 0, P = 1), family history of alopecia (χ2 = 0.011, P = 0.916), educational status (χ2 = 0, P = 0.1), marital status (χ2 = 0.316, P = 0.957), and duration of AGA (χ2 = 4.538, P = 0.209), as shown in Table 1. The non-significant differences in these demographic parameters indicate a balanced distribution of baseline characteristics between the two groups, supporting the comparability of the study cohorts for further analyses.
| Parameter | Non-stress group (n = 30) | Stress group (n = 90) | t/χ² | P value |
| Age (years) | 29.03 ± 2.39 | 29.79 ± 4.81 | 1.131 | 0.261 |
| Gender | 0.048 | 0.827 | ||
| Male | 18 (60.00) | 58 (64.44) | ||
| Female | 12 (40.00) | 32 (35.56) | ||
| BMI (kg/m2) | 25.36 ± 2.12 | 25.82 ± 1.92 | 1.051 | 0.299 |
| Smoking history (pack-years) | 0.78 ± 1.66 | 0.89 ± 2.03 | 0.254 | 0.800 |
| Alcohol intake (g/week) | 20.18 ± 4.56 | 18.96 ± 3.81 | 1.319 | 0.194 |
| Comorbidities | ||||
| Hypertension | 2 (6.67) | 5 (5.56) | 0 | 1 |
| Diabetes | 2 (6.67) | 7 (7.78) | 0 | 1 |
| Family history of alopecia | 17 (56.67) | 48 (53.33) | 0.011 | 0.916 |
| Educational status | 0 | 1 | ||
| Junior high and below | 4 (13.33) | 13 (14.44) | 0 | 1 |
| Above junior high school | 26 (86.67) | 77 (85.56) | ||
| Marital status | ||||
| Unmarried | 10 (33.33) | 35 (38.8) | ||
| Married | 14 (46.67) | 41 (45.56) | ||
| Divorced | 5 (16.67) | 12 (13.33) | ||
| Widowed | 1 (3.33) | 2 (2.22) | 0.924 | 0.863 |
| Duration of AGA | 4.538 | 0.209 | ||
| < 1 year | 9 | 21 | ||
| 1–5 years | 15 | 32 | ||
| 5–10 years | 4 | 28 | ||
| > 10 years | 2 | 9 |
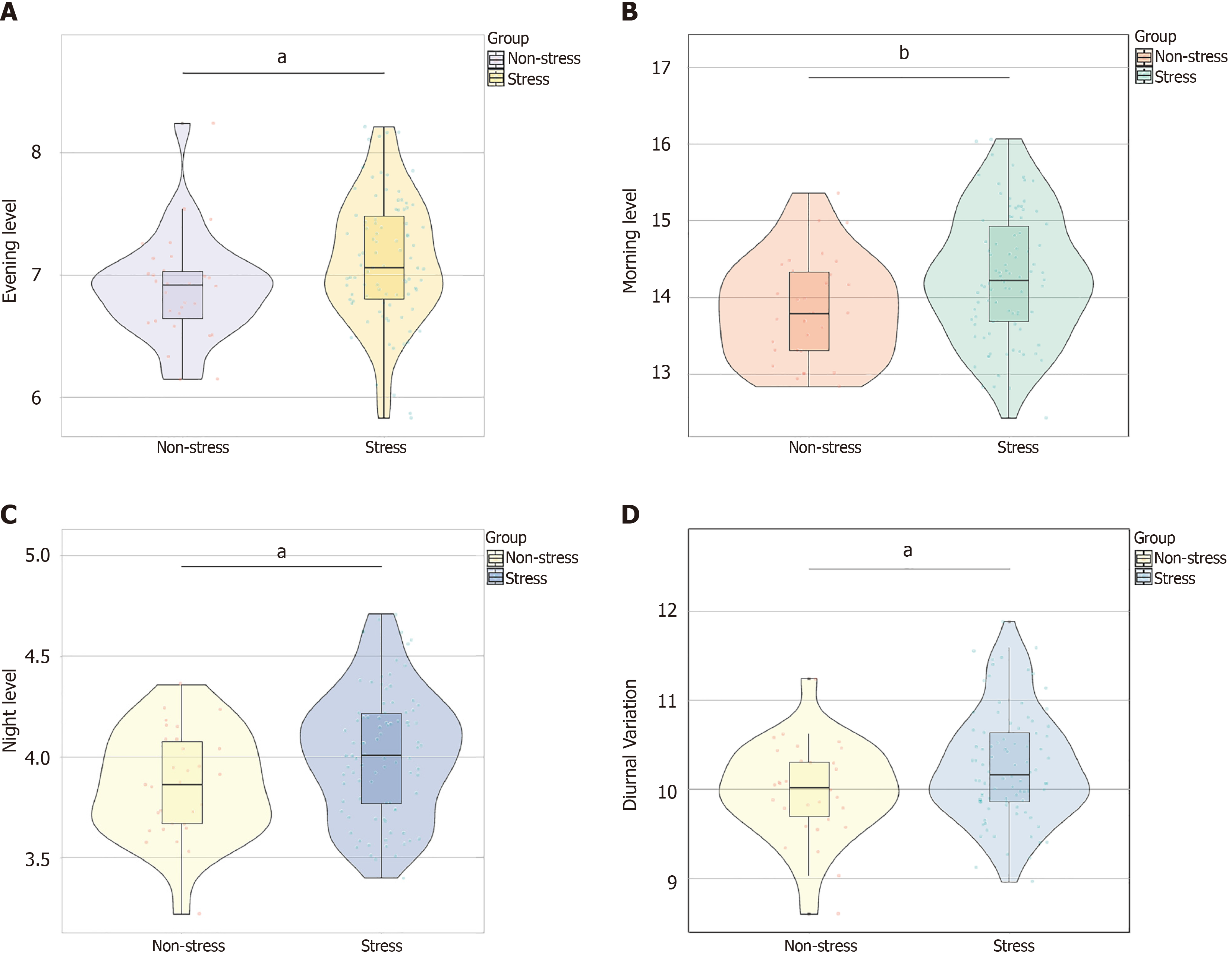
As shown in Figure 1, the morning (t = 2.790, P = 0.007), evening (t = 2.469, P = 0.016), and night (t = 2.552, P = 0.013) levels of serum cortisol and the diurnal variation (t = 2.447, P = 0.018) of the two groups were compared. The stress group exhibited higher cortisol levels across all timepoints than the non-stress group, indicating a clear association between stress and increased serum cortisol levels throughout the day and highlighting the physiological impact of stress on cortisol secretion.
Hair growth parameters were assessed and compared between the two groups, as outlined in Table 2. The stress group exhibited a statistically significant decrease in mean hair diameter and hair density in comparison to the non-stress group (P < 0.05). However, no statistically significant variances were noted in terms of hair follicle count and the ratio of terminal to vellus hair between the two groups (P > 0.05 for all comparisons).
| Hair growth parameters | Non-stress group (n = 30) | Stress group (n = 90) | t-value | P value |
| Mean hair diameter (μm) | 59.81 ± 6.40 | 50.047 ± 7.36 | 6.492 | 0.000 |
| Hair density (hairs/cm²) | 105.24 ± 21.38 | 93.11 ± 28.69 | 2.124 | 0.036 |
| Hair follicle count (per 1 cm²) | 60.14 ± 10.89 | 56.47 ± 15.83 | 1.416 | 0.161 |
| Terminal/vellus hair ratio | 7.16 ± 0.61 | 7.25 ± 0.72 | 0.649 | 0.519 |
The levels of neurotrophic factors in the two groups were analyzed, and the results showed the levels of NGF (t = 2.264, P = 0.028), BDNF (t = 2.637, P = 0.011), NT-3 (t = 2.077, P = 0.044), NT-4 (t = 3.44, P = 0.001), and GDNF (t = 2.244, P = 0.030, Table 3). The stress group exhibited lower levels of NGF, BDNF, and GDNF and higher levels of NT-3 and NT-4 than the non-stress group. These findings indicate a potential association between stress and altered neurotrophic factor levels, suggesting a potential impact of stress on neurotrophic support and signaling pathways.
| Neurotrophic factor | Non-stress group (n = 30) | Stress group (n = 90) | t-value | P value |
| NGF (pg/mL) | 24.65 ± 5.32 | 22.18 ± 4.76 | 2.264 | 0.028 |
| BDNF (ng/mL) | 9.61 ± 2.14 | 8.46 ± 1.85 | 2.637 | 0.011 |
| NT-3 (pg/mL) | 15.89 ± 3.76 | 17.48 ± 3.21 | 2.077 | 0.044 |
| NT-4 (ng/mL) | 7.73 ± 1.64 | 8.89 ± 1.47 | 3.44 | 0.001 |
| GDNF (pg/mL) | 12.45 ± 2.78 | 11.18 ± 2.35 | 2.244 | 0.030 |
In the evaluation of AGA progression, a clear association between stress and AGA progression was observed in male and female patients. Among the male and female patients assessed using the Norwood–Hamilton scale and the Ludwig scale, respectively, a statistically significant difference was found before treatment (χ2 = 12.801, P = 0.002), with a higher proportion of individuals in the stress group exhibiting higher AGA stages than the non-stress group. However, after 12 months of treatment, the difference in AGA progression scores between the two groups was statistically insignificant (χ2 = 5.106, P = 0.078), suggesting a potential impact of treatment on AGA progression related to stress levels (Table 4).
| Subgroup | Non-stress group (n = 30) | Stress group (n = 90) | χ2 | P value |
| Before treatment | 12.801 | 0.002 | ||
| < 3/I | 18 (60) | 22 (24.44) | ||
| 3–5/II | 8 (26.67) | 45 (50) | ||
| 6 or 7/III | 4 (13.33) | 23 (25.56) | ||
| After 12 months of treatment | 5.106 | 0.078 | ||
| < 3/I | 23 (76.67) | 48 (53.33) | ||
| 3–5/II | 4 (13.33) | 22 (24.44) | ||
| 6 or 7/III | 3 (10) | 20 (22.22) |
Furthermore, the overall AGA progression was significantly higher in the stress group, with 14.44% of individuals showing a progression of at least 1 class compared with 6.67% in the non-stress group (χ2 = 7.589, P = 0.022). Additionally, the proportion of individuals showing improvement was notably lower in the stress group (47.78%) than in the non-stress group (76.67%), indicating a potential influence of stress on the efficacy of treatment (Table 5). These findings suggest a compelling association between stress and AGA progression, highlighting the importance of considering stress management in the treatment of patients with AGA.
| Progress | Non-stress group (n = 30) | Stress group (n = 90) | χ2 | P value |
| Progress ≥ 1 class | 2 (6.67) | 13 (14.44) | 7.589 | 0.022 |
| Ineffective | 5 (16.67) | 34 (37.78) | ||
| Improved | 23 (76.67) | 43 (47.78) |
Correlation analyses were conducted to assess the relationship between stress and specific neurotrophic factors to investigate the impact of mental stress on neurotrophic factors in patients with AGA and its correlation with the progression of AGA. Table 6 demonstrates the correlation coefficients (r) for NGF, BDNF, GDNF, NT-3, and NT-4. The results revealed statistically significant negative correlations between stress and the levels of NGF (r = −0.215, P = 0.018), BDNF (r = −0.252, P = 0.005), and GDNF (r = −0.219, P = 0.016) and positive correlations between stress and the levels of NT-3 (r = 0.237, P = 0.009) and NT-4 (r = 0.236, P = 0.01). These findings suggest a significant association between mental stress and altered levels of these neurotrophic factors in patients with AGA, indicating a potential mechanistic link among stress, neurotrophic factors, and the progression of AGA. Further exploration into the underlying pathways and clinical implications of these findings is warranted.
| Parameter | r | P value |
| NGF (pg/mL) | −0.215 | 0.018 |
| BDNF (ng/mL) | −0.252 | 0.005 |
| NT-3 (pg/mL) | 0.237 | 0.009 |
| NT-4 (ng/mL) | 0.236 | 0.01 |
| GDNF (pg/mL) | −0.219 | 0.016 |
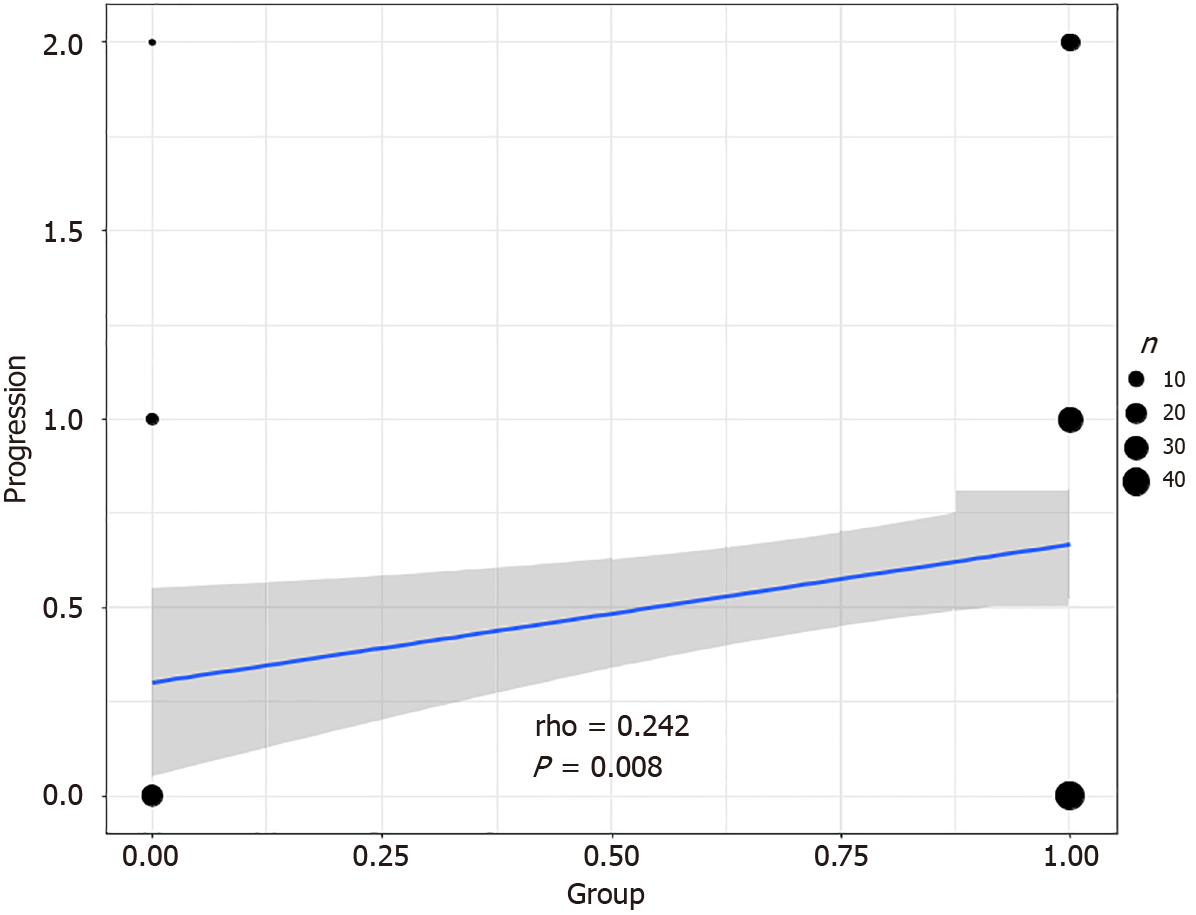
Correlation analysis was conducted to evaluate the relationship between stress and hair loss progression to investigate the impact of mental stress on neurotrophic factors in patients with AGA and its correlation with the progression of AGA. As shown in Figure 2, the results revealed a significant positive correlation (r = 0.242, P = 0.008) between stress and an increase in hair loss progression by at least one class. This finding indicates a strong association between mental stress and the progression of AGA, underscoring the potential role of stress as a contributing factor to the exacerbation of AGA.
An in-depth correlation analysis was performed to assess the relationship between hair loss progression and specific neurotrophic factors to investigate the impact of mental stress on neurotrophic factors in patients with AGA and its correlation with the progression of AGA. As outlined in Table 7, the findings unveiled noteworthy positive correlations between NT-4 Levels and the progression of AGA. This finding suggests the potential role of NT-4 in the stress-related advancement of hair loss in patients with AGA.
| Parameter | r | P value |
| NGF (pg/mL) | −0.121 | 0.189 |
| BDNF (ng/mL) | −0.118 | 0.198 |
| NT-3 (pg/mL) | −0.114 | 0.214 |
| NT-4 (ng/mL) | 0.341 | < 0.001 |
| GDNF (pg/mL) | −0.12 | 0.193 |
This study revealed a potential link between mental stress and altered levels of neurotrophic factors, including BDNF, NGF, NT-3, NT-4, and GDNF, in patients with AGA. Chronic stress has been associated with alterations in neurotrophic factors, with potential implications for hair follicle activity and hair growth cycles[19,20]. Notably, BDNF has been shown to promote the survival and growth of dermal papilla cells, which play a key role in the regulation of hair follicle cycling and hair growth[21], and NGF has been implicated in promoting hair growth and maintaining hair growth cycle[22]. Studies suggest that GDNF promotes hair follicle cell proliferation and retards hair follicle catagen regression[23,24]. Botchkarev et al[25] found that NT-3-overexpressing transgenic mice showed precocious catagen development during the postnatal initiation of hair follicle cycling, whereas heterozygous NT-3 knockout (+/-) mice displayed significant catagen retardation. NT-4 is known to be an inducer of catagen in the hair cycle, and NT-4 activity accelerated by androgen may contribute to the pathogenesis of AGA[26]. The stress-induced alterations in neurotrophic factor levels observed in the present study suggest a potential mechanistic link between stress and hair follicle biology, providing a novel perspective on the pathogenesis of AGA.
The findings demonstrated a clear association between mental stress and increased serum cortisol levels throughout the day, highlighting the physiological impact of stress on cortisol secretion. The stress group exhibited lower levels of NGF, BDNF, and GDNF; higher levels of NT-3 and NT-4; reduced mean hair diameter; lower hair density; and serious AGA grading compared with the non-stress group. These results suggest a negative impact of stress on hair growth parameters and neurotrophic support, potentially contributing to the progression of AGA. Additionally, the correlation analyses revealed significant negative correlations between stress and NGF, BDNF, and GDNF and positive correlations between stress and NT-3 and NT-4, underscoring a notable association between mental stress and altered neurotrophic factor levels in patients with AGA. These findings are in line with those of previous research indicating a potential role of stress in exacerbating AGA[27]. Furthermore, after 1 year of treatment with 5% minoxidil, efficacy was observed to be lower in the stress group, whereas disease progression was notably more pronounced in the stress group than in the non-stress group. The correlation analysis between stress and hair loss progression revealed a significant positive correlation, indicating a strong association between mental stress and the progression of AGA in male and female patients. The correlation analysis of hair loss progression and neurotrophic factors demonstrated significant positive correlations between hair loss progression and NT-4 Levels, highlighting the potential role of neurotrophic factors in mitigating the advancement of AGA. These findings underscore the complex interplay among psychological stress, neurotrophic factors, and the progression of AGA, shedding light on potential targets for therapeutic intervention and emphasizing the need for comprehensive management approaches that address the physiological and psychological factors driving AGA progression. The findings also underscore the potential for psychological stress to directly contribute to the progression of hair loss, potentially affecting the progression of AGA through its influence on neurotrophic factors. The mechanisms underlying this relationship require further investigation in future research.
The relationship among mental stress, altered neurotrophic factor levels, and the progression of AGA presented in this study holds several potential clinical and therapeutic implications. First, the findings underscore the importance of comprehensive patient care that takes into account the physiological and psychological factors contributing to AGA. While current treatment approaches for AGA primarily focus on hormonal manipulation and hair transplantation, the potential influence of stress on hair follicle biology and neurotrophic support suggests the need for an integrated approach that addresses stress management alongside conventional treatment modalities. Psychological interventions, such as mindfulness-based stress reduction, cognitive-behavioral therapy, and relaxation techniques, may offer complementary benefits in managing AGA by mitigating the potential impact of stress on hair health and the progression of AGA[28,29].
Second, the observed alterations in neurotrophic factor levels in response to stress highlight the potential for novel therapeutic approaches that target neurotrophic support in patients with AGA. Strategies aimed at modulating neurotrophic factor signaling pathways, such as the use of neurotrophic factor agonists or enhancers, may offer promising avenues for intervention, potentially complementing existing treatment modalities for AGA[30]. Additionally, the identification of specific neurotrophic factors that exhibit significant correlations with the progression of AGA presents opportunities for targeted therapeutic interventions that aim to restore or enhance the levels of these factors in affected individuals[30,31]. Future research and clinical trials focusing on the modulation of neurotrophic support in the context of AGA hold promise for advancing the development of targeted and personalized treatment approaches for this condition.
While this study provides valuable insights into the impact of mental stress on neurotrophic factors and the progression of AGA, several limitations should be considered. The retrospective nature of the study and the relatively small sample size may limit the generalizability of the findings. The study’s design does not allow for the establishment of causality among mental stress, neurotrophic factors, and the progression of AGA, emphasizing the need for prospective longitudinal studies that could elucidate the temporal and mechanistic relationships among these variables. Additionally, the study’s exclusion criteria, including the exclusion of patients with chronic systemic diseases and psychiatric disorders, may have introduced selection bias and limit the broader applicability of the findings.
The findings of this study provide compelling evidence of an association among mental stress, altered neurotrophic factors, and the progression of AGA, shedding light on the potential influence of stress on hair follicle biology and AGA pathogenesis. The study underscores the potential for psychological stress to directly contribute to the progression of hair loss, potentially affecting the progression of AGA through its influence on neurotrophic factors. The observed correlations among stress, neurotrophic factors, and AGA progression demonstrate the need for an integrated approach to patient care that addresses the physiological and psychological facets of this condition.
| 1. | Aukerman EL, Jafferany M. The psychological consequences of androgenetic alopecia: A systematic review. J Cosmet Dermatol. 2023;22:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 2. | Gupta AK, Cole J, Deutsch DP, Everts PA, Niedbalski RP, Panchaprateep R, Rinaldi F, Rose PT, Sinclair R, Vogel JE, Welter RJ, Zufelt MD, Puig CJ. Platelet-Rich Plasma as a Treatment for Androgenetic Alopecia. Dermatol Surg. 2019;45:1262-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 3. | Wang C, Du Y, Bi L, Lin X, Zhao M, Fan W. The Efficacy and Safety of Oral and Topical Spironolactone in Androgenetic Alopecia Treatment: A Systematic Review. Clin Cosmet Investig Dermatol. 2023;16:603-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 4. | Völker JM, Koch N, Becker M, Klenk A. Caffeine and Its Pharmacological Benefits in the Management of Androgenetic Alopecia: A Review. Skin Pharmacol Physiol. 2020;33:93-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Griggs J, Trüeb RM, Gavazzoni Dias MFR, Hordinsky M, Tosti A. Fibrosing alopecia in a pattern distribution. J Am Acad Dermatol. 2021;85:1557-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Ly NY, Fruechte S, Hordinsky MK, Sadick N, Arruda S, Farah RS. Medical and procedural treatment of androgenetic alopecia - Where are we? J Am Acad Dermatol. 2023;89:S36-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 7. | Gupta AK, Talukder M, Williams G. Comparison of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia. J Dermatolog Treat. 2022;33:2946-2962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 8. | Marks DH, Senna MM. Androgenetic Alopecia in Gender Minority Patients. Dermatol Clin. 2020;38:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Dinkins J, Iwuala C, Akintilo L, Ugonabo N, Shapiro J, Sicco KL, Adotama P. Commonly used hair oils in the Black community: a narrative review in their use to treat androgenetic alopecia. Int J Dermatol. 2023;62:980-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Almeida FB, Barros HMT, Pinna G. Neurosteroids and Neurotrophic Factors: What Is Their Promise as Biomarkers for Major Depression and PTSD? Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Gupta AK, Bamimore MA, Foley KA. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyses, and an assessment of evidence quality. J Dermatolog Treat. 2022;33:62-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Miao Z, Wang Y, Sun Z. The Relationships Between Stress, Mental Disorders, and Epigenetic Regulation of BDNF. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 13. | Ceci FM, Ferraguti G, Petrella C, Greco A, Tirassa P, Iannitelli A, Ralli M, Vitali M, Ceccanti M, Chaldakov GN, Versacci P, Fiore M. Nerve Growth Factor, Stress and Diseases. Curr Med Chem. 2021;28:2943-2959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Förander P, Hoffer B, Strömberg I. Nerve fiber formation and catecholamine content in adult rat adrenal medullary transplants after treatment with NGF, NT-3, NT-4/5, bFGF, CNTF, and GDNF. Cell Tissue Res. 1998;292:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Huber KA, Krieglstein K, Unsicker K. The neurotrophins BDNF, NT-3 and -4, but not NGF, TGF-beta 1 and GDNF, increase the number of NADPH-diaphorase-reactive neurons in rat spinal cord cultures. Neuroscience. 1995;69:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Morcuende S, Muñoz-Hernández R, Benítez-Temiño B, Pastor AM, de la Cruz RR. Neuroprotective effects of NGF, BDNF, NT-3 and GDNF on axotomized extraocular motoneurons in neonatal rats. Neuroscience. 2013;250:31-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Saruta J, To M, Sakaguchi W, Kondo Y, Tsukinoki K. Brain-derived neurotrophic factor is related to stress and chewing in saliva and salivary glands. Jpn Dent Sci Rev. 2020;56:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Gulyaeva NV. Stress-Associated Molecular and Cellular Hippocampal Mechanisms Common for Epilepsy and Comorbid Depressive Disorders. Biochemistry (Mosc). 2021;86:641-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Almashali MA, Alotaibi MA, Alkhanani AH, Al Dera NM, Alwadany MM, Almousa AS, Shadid AM. The psychosocial burden of androgenetic alopecia in Saudi Arabia: A cross-sectional study. J Family Med Prim Care. 2023;12:3374-3379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Kozicka K, Łukasik A, Jaworek A, Pastuszczak M, Spałkowska M, Kłosowicz A, Dyduch G, Wojas-Pelc A. The level of stress and the assessment of selected clinical parameters in patients with androgenetic alopecia. Pol Merkur Lekarski. 2020;48:427-430. [PubMed] |
| 21. | Liu J, Zhang X, Zhang Q, Wang R, Ma J, Bai X, Wang D. Loxhd1b inhibits the hair cell development in zebrafish: Possible relation to the BDNF/TrkB/ERK pathway. Front Cell Neurosci. 2022;16:1065309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Jagadeeshaprasad MG, Govindappa PK, Nelson AM, Noble MD, Elfar JC. 4-Aminopyridine Induces Nerve Growth Factor to Improve Skin Wound Healing and Tissue Regeneration. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 23. | Botchkareva NV, Botchkarev VA, Welker P, Airaksinen M, Roth W, Suvanto P, Müller-Röver S, Hadshiew IM, Peters C, Paus R. New roles for glial cell line-derived neurotrophic factor and neurturin: involvement in hair cycle control. Am J Pathol. 2000;156:1041-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Siah TW, Guo H, Chu T, Santos L, Nakamura H, Leung G, Shapiro J, McElwee KJ. Growth factor concentrations in platelet-rich plasma for androgenetic alopecia: An intra-subject, randomized, blinded, placebo-controlled, pilot study. Exp Dermatol. 2020;29:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Botchkarev VA, Welker P, Albers KM, Botchkareva NV, Metz M, Lewin GR, Bulfone-Paus S, Peters EM, Lindner G, Paus R. A new role for neurotrophin-3: involvement in the regulation of hair follicle regression (catagen). Am J Pathol. 1998;153:785-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Uchiyama C, Ishida K, Tsutsui T, Naito A, Kurita K, Hanihara H, Serizawa T, Fujiwara M, Ohdera M. Effects of Hura crepitans and its active ingredient, daphne factor F3, on dihydrotestosterone-induced neurotrophin-4 activation and hair retardation. Biol Pharm Bull. 2012;35:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Kalner S, Vergilis I. Reversal of Stress-Associated Alopecia. J Drugs Dermatol. 2023;22:300-301. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Maloh J, Engel T, Natarelli N, Nong Y, Zufall A, Sivamani RK. Systematic Review of Psychological Interventions for Quality of Life, Mental Health, and Hair Growth in Alopecia Areata and Scarring Alopecia. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Piraccini BM, Ohyama M, Craiglow B, Bewley A, Ding Y, Chen YF, Dutronc Y, Pierce E, Durand F, Mostaghimi A. Scalp hair regrowth is associated with improvements in health-related quality of life and psychological symptoms in patients with severe alopecia areata: results from two randomized controlled trials. J Dermatolog Treat. 2023;34:2227299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 30. | Dawoud NM, Rajab AZ, El-Hefnawy SM, El-Bayoumy AM, Salem AM, Seleit I. Serum brain-derived neurotrophic factor and vitamin D: Two concordant players controlling depression among alopecia areata and vitiligo patients: A case-control study. J Cosmet Dermatol. 2023;22:2343-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Mohamed NE, Soltan MR, Galal SA, El Sayed HS, Hassan HM, Khatery BH. Female Pattern Hair Loss and Negative Psychological Impact: Possible Role of Brain-derived Neurotrophic Factor (BDNF). Dermatol Pract Concept. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |