Published online Jan 19, 2024. doi: 10.5498/wjp.v14.i1.76
Peer-review started: November 20, 2023
First decision: December 5, 2023
Revised: December 20, 2023
Accepted: December 25, 2023
Article in press: December 25, 2023
Published online: January 19, 2024
Processing time: 60 Days and 4 Hours
Occult breast cancer (OBC) has traditionally been considered to be a carcinoma of unknown primary origin with a favorable prognosis and can be treated as stage II-III breast cancer. Due to the small number of cases and limited clinical ex-perience, treatments vary greatly around the world and no standardized treat-ment has yet been established.
To investigate the clinicopathological features, psychological status and prog-nostic features of patients with OBC.
The clinicopathological data of 33 OBC patients diagnosed and treated in the Affiliated Hospital of Xuzhou Medical University and Xuzhou Central Hospital from November 2015 to November 2022 were retrospectively analyzed. The psychological status of OBC patients was evaluated by the Self-rating Anxiety Scale and Self-rating Depression Scale. Patients’ emotions, stress perception and psychological resilience were evaluated by the Positive and Negative Affect Schedule, the Chinese Perceived Stress Scale, and the Connor-Davidson Resilience Scale (CD-RISC), respectively. Patient survival was calculated using the Kaplan-Meier method, and survival curves were plotted for analysis with the log-rank test. Univariate and multivariate survival analyses were performed using the Cox regression model.
The 33 OBC patients included 32 females and 1 male. Of the 33 patients, 30 (91%) had axillary tumors, 3 (9%) had a neck mass as the primary symptom; 18 (54.5%) had estrogen receptor-positive tumors, 17 (51.5%) had progesterone receptor-positive tumors, and 18 (54.5%) had Her-2-positive tumors; 24 (72.7%) received surgical treatment, including 18 patients who underwent modified radical mastectomy, 1 patient who underwent breast-conserving surgery plus axillary lymph node dissection (ALND), and 5 patients who underwent ALND alone; 12 patients received preoperative neoadjuvant therapy. All 30 patients developed anxiety and depression, with low positive affect scores and high negative affect scores, accompanied by a high stress level and poor psychological resilience. There were no differences in the psychological status of patients according to age, body mass index, or menopausal status. The overall survival and disease-free survival (DFS) of all the patients were 83.3% and 55.7%, respectively. Univariate analysis demonstrated that the initial tumor site (P = 0.021) and node stage (P = 0.020) were factors that may affect patient prognosis. The 5-year DFS rate of OBC patients who received radiotherapy was greater (P < 0.001), while the use of different surgical methods (P = 0.687) had no statistically significant effect on patient outcomes. Multivariate analysis revealed that radiotherapy (P = 0.031) was an independent prognostic factor. Receiving radiotherapy had a significant effect on the CD-RISC score (P = 0.02).
OBC is a rare breast disease whose diagnosis and treatment are currently controversial. There was no significant difference in the efficacy of other less invasive surgical procedures compared to those of modified radical mastectomy. In addition, radiotherapy can significantly improve patient outcomes. We should pay attention to the psychological state of patients while they receive antitumor therapy.
Core Tip: Occult breast cancer (OBC) has traditionally been considered to be a carcinoma of unknown primary origin with a favorable prognosis and can be treated as stage II-III breast cancer. This study aimed to investigate the clinicopathological features, psychological status and prognostic factors of patients with OBC. The authors found that radiotherapy can significantly improve patient outcomes. The psychological state of patients while they receive antitumor therapy should be paid more attention.
- Citation: Wang HM, Yu AY, Li LL, Ma LY, Cao MH, Yang YL, Qin XB, Tang JJ, Han ZX. Clinicopathological features, psychological status, and prognosis of 33 patients with occult breast cancer. World J Psychiatry 2024; 14(1): 76-87
- URL: https://www.wjgnet.com/2220-3206/full/v14/i1/76.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i1.76
Occult breast cancer (OBC) is a rare form of BC that usually presents as axillary lymph node metastasis without a clear primary breast lesion[1]. OBC is a rare breast disease. According to the foreign literature, the incidence of BC accounts for approximately 0.3%-1% of all BCs[2], with a peak incidence occurring at approximately 55 years of age.
With atypical clinical manifestations, OBC usually presents with painless axillary masses initially, but a few patients seek medical help for neck masses[3]. For patients with the above symptoms, core needle biopsy or incisional biopsy should be performed first to clarify the pathology, along with immunohistochemistry to determine its possible source[4]. Mammography and breast ultrasound are commonly used tools for diagnosing breast lesions in patients with lymph node metastases of unknown origin, but the detection rate of the primary focus of OBC is low[5]. With the development of advanced diagnostic modalities, the incidence of OBC has been decreasing [6]. The 2022 Breast Cancer Diagnosis and Treatment Guidelines suggest that breast magnetic resonance imaging (MRI) has the advantage of high sensitivity compared with other breast imaging methods; additionally, breast MRI can display multifocal, multicenter or bilateral BC lesions as well as axillary lymph node metastasis at the same time and is therefore recommended for identifying the primary focus of patients with axillary lymph node metastasis[7]. Positron emission tomography-computed tomography (PET-CT) is also used to diagnose OBC. However, there is only one relevant report in the literature thus far[8], which, coupled with its high price, limits its application in OBC detection.
Although OBC is generally accepted to have similar biological behavior to lymph node-positive non-OBC, the clinicopathological features of this disease are unclear[9,10]. Several previous studies have suggested that estrogen receptor (ER) status, triple-negative status, and at least four positive lymph nodes are individual prognostic factors for occult breast cancer[11,12]. Survival outcomes in patients with OBC are also controversial. Patients with OBC have similar or less unfavorable outcomes than non-OBC patients[13], while others have reached the opposite conclusion[14]. Therefore, this study aimed to better reveal the clinicopathological features and prognostic factors of OBC patients through a retrospective analysis of clinicopathological data.
A retrospective analysis of the clinical data of 33 OBC patients admitted to the Affiliated Hospital of Xuzhou Medical University and Xuzhou Central Hospital was conducted from November 2015 to November 2022. Approximately 0.48% (33/6835) of the 33 OBC patients were included in this study, of whom 20 (60%) were from the Affiliated Hospital of Xuzhou Medical University and 13 (40%) were from Xuzhou Central Hospital. The patient cohort consisted of 32 females and 1 male. Pathology revealed invasive breast cancer (intermediate/poorly differentiated).
No obvious primary breast lesions were found by physical examination, breast color ultrasound, mammography or breast CT, MRI, PET-CT or other examinations. Any tumors occurring in any part of the body were confirmed to be metastatic carcinoma by pathological examination and indicated to be of breast origin by histological and immunohistochemical methods.
A total of 30 patients were scored on the Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS) for anxiety and depression assessments, and their scores were analyzed.
Patients’ emotions, stress perception and psychological resilience were evaluated by the Positive and Negative Affect Schedule (PANAS), the Chinese Perceived Stress Scale (CPSS), and the Connor-Davidson Resilience Scale (CD-RISC), respectively.
Patients were followed up for recurrence, metastasis and death through inpatient or outpatient information or telephone follow-up. Patients were followed up until November 2022 (5 to 226 months), for a median follow-up of 38 months. Disease-free survival (DFS) and overall survival (OS) were used as follow-up indicators, where the former was defined as the time from the start of follow-up to disease recurrence or metastasis.
R 4.3.2 and SPSS27.0 statistical software were used for data analysis, with P < 0.05 as the threshold of statistical significance. Nonparametric comparisons between parameters were conducted using the Mann-Whitney U test. The survival rate was calculated by using the Kaplan-Meier method, and survival curves were drawn. The difference in survival rate between subgroups was tested by the log-rank test, and the factors with statistical significance in the univariate analysis were further tested by multivariate Cox regression analysis.
The age of onset ranged from 28 to 66 years (median age: 53). There were 9 patients (27.0%) aged ≤ 50 years and 24 patients (73.0%) aged > 50 years. There were 1 (3.0%) male and 32 (97.0%) female patients, including 22 (66.7%) menopausal and 10 (30.3%) non-menopausal women. While 24 patients (72.7%) had a body mass index (BMI) < 24.0 and 9 patients (27.2%) had a BMI ≥ 24.0, patients’ BMI, which was associated with occult breast cancer, was not related to survival (Table 1).
| Clinical features | n | 5-year DFS rate (%) | χ2 | P value |
| Whole patient cohorts | 33 | 55.7 | ||
| Age (yr) | 0.20 | 0.887 | ||
| < 50 | 9 | 58.9 | ||
| ≥ 50 | 24 | 55.8 | ||
| BMI | 0.294 | 0.588 | ||
| < 23.9 | 24 | 51.7 | ||
| ≥ 23.9 | 9 | 87.5 | ||
| Menopause | 2.923 | 0.087 | ||
| Yes | 23 | 46.9 | ||
| No | 10 | 80.0 | ||
| N staging | 5.448 | 0.020a | ||
| 1 | 15 | 92.3 | ||
| ≥ 2 | 18 | 34.3 | ||
| Initial tumor site | 5.333 | 0.021a | ||
| Armpit | 30 | 60.8 | ||
| Neck | 3 | - | ||
| M staging | 1.486 | 0.223 | ||
| 0 | 24 | 46.2 | ||
| 1 | 9 | 53.3 | ||
| Ki67 | 0.317 | 0.573 | ||
| ≤ 14% | 8 | 60.0 | ||
| > 14% | 25 | 54.1 | ||
| ER | 0.003 | 0.953 | ||
| Positive | 18 | 55.1 | ||
| Negative | 15 | 56.0 | ||
| PR | 2.355 | 0.125 | ||
| Positive | 17 | 67.1 | ||
| Negative | 16 | 34.8 | ||
| Her-2 | 0.111 | 0.739 | ||
| Positive | 18 | 66.4 | ||
| Negative | 15 | 42.2 | ||
| Molecular subtyping | 1.690 | 0.639 | ||
| Luminal A | 15 | 73.4 | ||
| Luminal B | 12 | 27.8 | ||
| Her-2 enriched | 3 | 50.0 | ||
| TNBC | 3 | - |
Thirty patients (91%) had an axillary mass at the first symptom, and only three (9.1%) patients had a neck mass at the first symptom. There were 22 patients (66.7%) in whom the lesions were located on the left side and 11 (33.3%) on the right side. There were 15 patients (45.5%) whose axillary lymph node stage was the N1, 18 patients (54.5%) whose axillary lymph node stage was N2 or N3, and 6 patients (18.2%) whose axillary lymph node stage was IV (distant metastases) (Table 1).
Immunohistochemical tests were performed on all patients. There were 18 (54.5%) OBC patients who were ER-positive and 17 (51.5%) patients who were progesterone receptor (PR) positive. There were 18 HER-2-positive patients (including 13 HER-2 IHC 3+ patients and 5 IHC 2+ patients with positive amplification according to the FISH test) and 15 HER-2-negative patients. Ki67 was detected in all patients; 8 had Ki67 ≤ 14%, and 25 had Ki67 > 14% (Table 1).
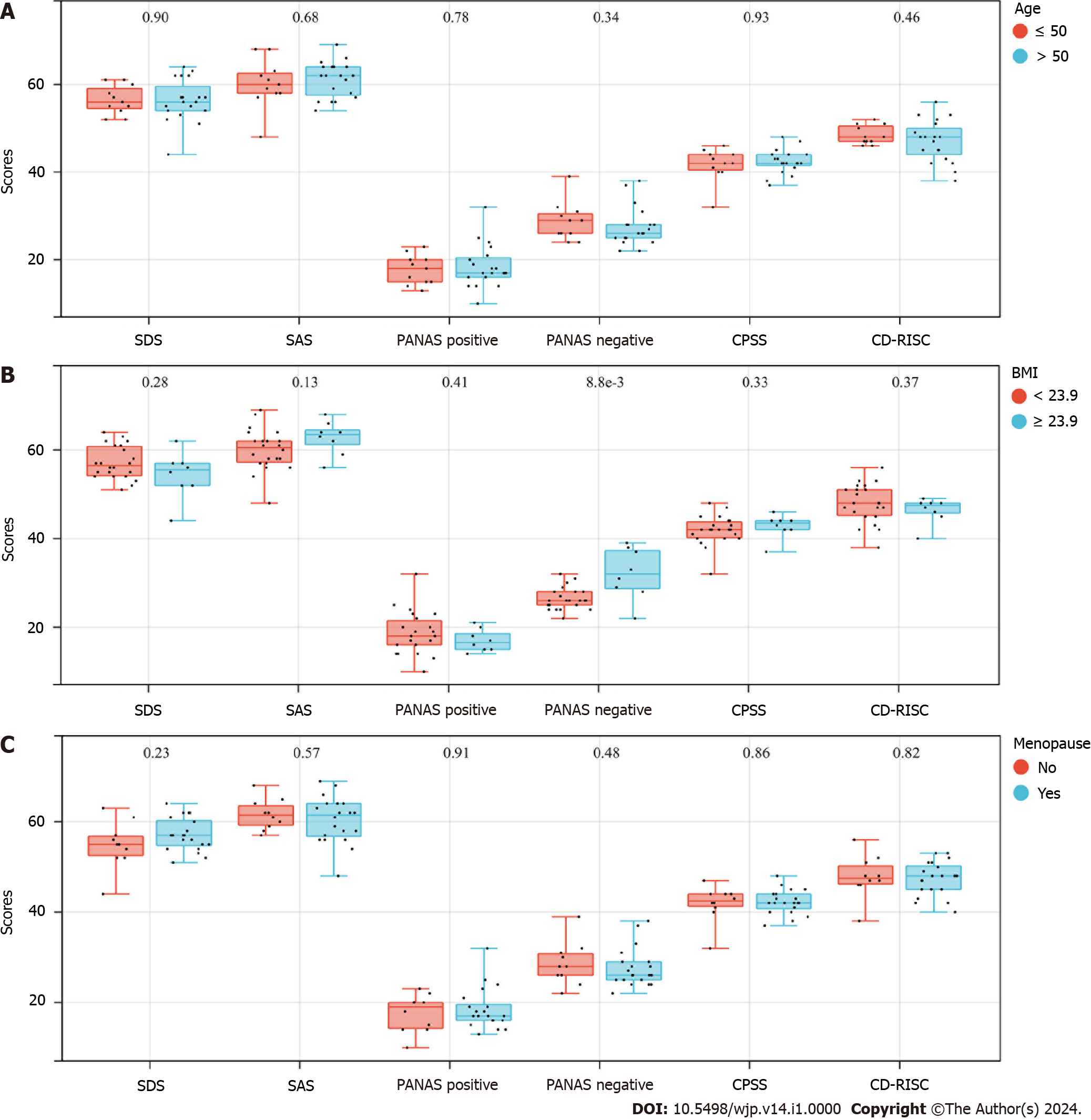
All 30 patients developed anxiety and depression, with low positive affect scores and high negative affect scores, accompanied by a high stress level and poor psychological resilience (Tables 2 and 3).
| Categories | Minimum | Maximum | Score |
| SDS | 44 | 64 | 56.40 ± 4.29 |
| SAS | 48 | 69 | 60.77 ± 4.58 |
| Categories | Minimum | Maximum | Score |
| Positive and negative affect schedule | |||
| Positive | 10 | 32 | 18.23 ± 4.38 |
| Negative | 22 | 39 | 27.97 ± 4.37 |
| Chinese Perceived Stress Scale | 32 | 48 | 42.13 ± 3.12 |
| Connor-Davidson Resilience Scale | 38 | 56 | 47.53 ± 4.03 |
There were no differences in the psychological status of patients according to age, BMI, or menopausal status (Figure 1).
Twenty-four of the 33 patients underwent surgical treatment, with 18 patients receiving modified radical mastectomy (MRM), 1 patient receiving breast-conserving surgery (BCS) plus axillary lymph node dissection (ALND), and 5 receiving ALND only. Twelve patients received neoadjuvant therapy before surgery, including 4 patients receiving 6 cycles of PC regimen (docetaxel + carboplatin), 4 receiving 4 cycles of TE regimen (docetaxel + epirubicin), 3 receiving PC regimen for 4 cycles and sequential EC regimen for 4 cycles (docetaxel + carboplatin sequential epirubicin + cyclophosphamide), and 1 receiving TE regimen for 4 cycles (docetaxel + epirubicin). All patients received adjuvant therapy after surgery, and the treatment schemes were selected based on different factors, such as age, tumor stage and molecular subtyping. There were 20 hormone receptor-positive patients in this cohort, all of whom received endocrine therapy.
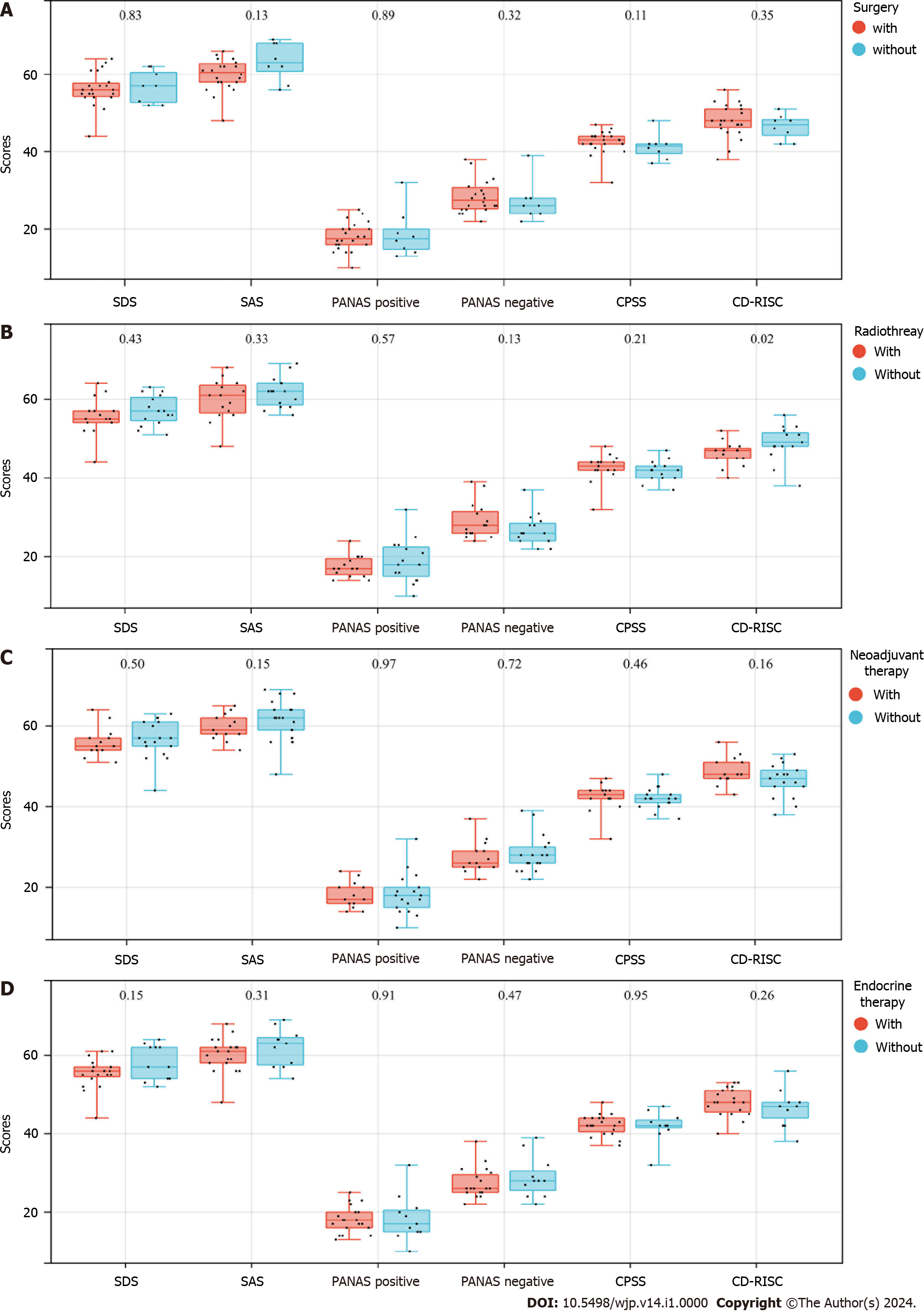
As of the follow-up date, a total of 30 OBC patients underwent SDS, SAS, PANAS, CPSS, and CD-RISC testing. The results revealed that patients who received radiotherapy had lower CD-RISC scores (P = 0.02) (Figure 2B). Surgery, neoadjuvant chemotherapy, and endocrine therapy had no significant impact on the psychological status of the OBC patients (Figure 2A, C, and D).
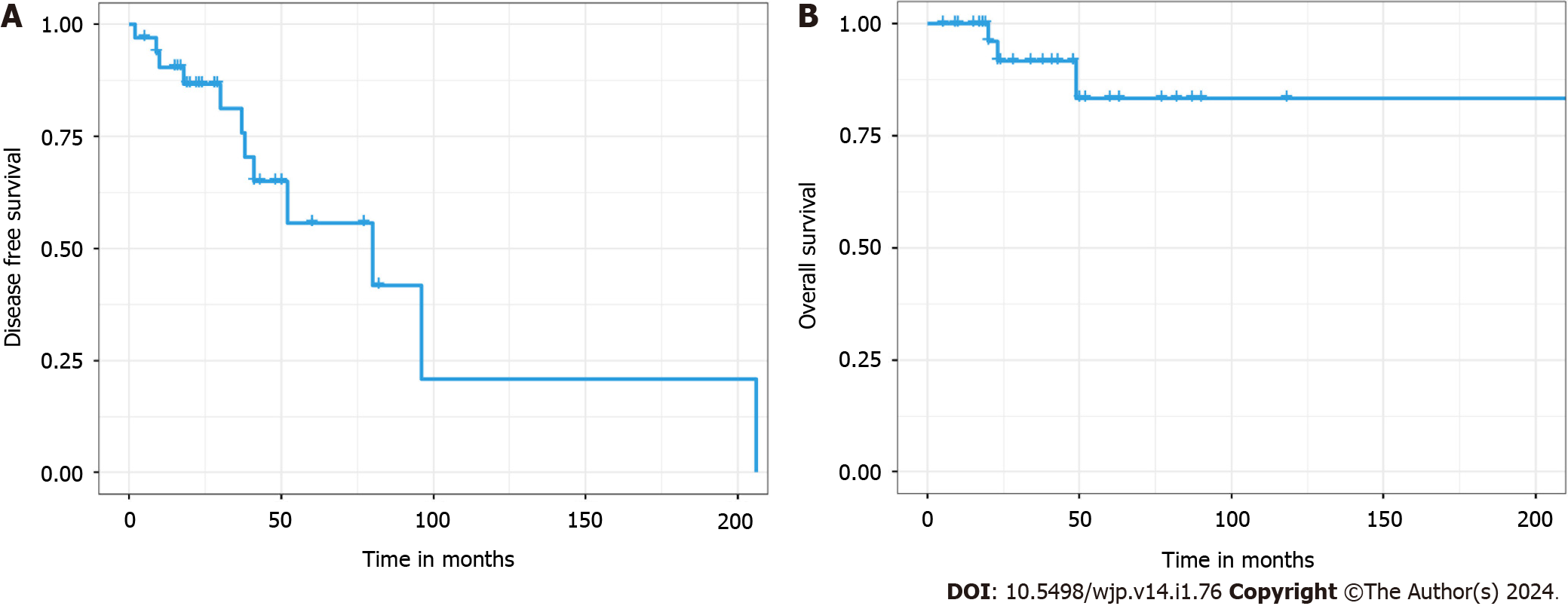
Overall survival (OS) curve of 33 patients with occult breast cancer. 5-year OS rate: 83.3%. Disease-free survival (DFS) curve of 33 patients with occult breast cancer. 5-year DFS rate: 55.7% (Figure 3).
The patients were followed up for 5-226 months (median: 38 months). A total of 12 patients relapsed and metastasized, three of whom died. Among the patients with metastasis, 4 had simple lung metastasis, 3 had lung and brain metastases, 2 had simple brain metastasis, 2 had simple skeletal metastasis, and 1 had lung and skeletal metastases.
However, there were no significant differences in 5-year DFS according to age, BMI, menopausal status, presence of distant metastasis, Ki67 index, or hormone receptor status. OBC patients with an initial tumor site in the axilla had a greater 5-year DFS rate than did those with an initial lesion in the neck (P = 0.021) (Table 1). Five-year DFS was greater in patients with few involved lymph nodes (P = 0.020) (Table 1). Age, BMI, menopausal status, node staging, initial tumor site, M staging, Ki67, ER, PR, HER-2, molecular subtyping, surgery and radiotherapy (with/without) were selected for univariate analysis using the log-rank test. The results showed that the 5-year DFS rate of OBC patients who received radiotherapy was greater than that of patients who did not receive radiotherapy (P < 0.001), while there was no difference in the 5-year DFS of patients who received different surgeries, endocrine therapies, or neoadjuvant chemotherapy (Table 4).
| Treatment method | n | 5-year DFS rate (%) | χ2 | P value |
| Total | 33 | |||
| Surgery | 1.486 | 0.223 | ||
| With | 24 | 46.2 | ||
| Without | 9 | 53.1 | ||
| Radiotherapy | 10.450 | < 0.001 | ||
| With | 16 | 88.9 | ||
| Without | 17 | 21.4 |
Furthermore, the 5-year DFS rate of the 24 patients who underwent surgical treatment was analyzed by the log-rank test, revealing no significant difference among patients who underwent different surgical treatments or neoadjuvant therapy (with/without) (Table 5).
| Surgical modalities used for patients | n | 5-year DFS rate (%) | χ2 | P value |
| Total | 24 | |||
| Surgical method | 0.751 | 0.687 | ||
| Modified radical mastectomy | 18 | 35.7 | ||
| Axillary lymph node dissection | 5 | 50.0 | ||
| Breast-conserving surgery + axillary lymph node dissection | 1 | - | ||
| Neoadjuvant therapy | 0.168 | 0.682 | ||
| With | 12 | 0.571 | ||
| Without | 12 | 0.458 |
In our case series, there were 20 receptor-positive patients, all of whom received endocrine therapy. Univariate analysis using the log-rank test also revealed no significant difference in DFS among patients receiving different endocrine therapies (Table 6).
| Endocrine therapy for hormone receptor-positive patients | n | 5-year DFS rate (%) | χ2 | P value |
| Total | 20 | |||
| Drugs used for endocrine therapy | 1.669 | 0.434 | ||
| Tamoxifen | 10 | 0.370 | ||
| Goserelin | 6 | 1.000 | ||
| Anastrozole/letrozole | 4 | 0.667 |
Moreover, statistically significant factors were included in the multivariate survival analysis, and it was found that radiotherapy (with/without; P = 0.031) was an independent prognostic factor.
In this study, 33 patients were reported in this cohort, accounting for 0.48% (33/6835) of all patients included in this study; these findings are basically consistent with the literature. All 33 OBC patients underwent relevant examinations after admission, and no primary lesions were found by mammography or ultrasound; 6 patients underwent PET-CT, and no suspicious primary lesions were detected; however, breast MRI detected 6 suspicious cancer lesions, which was significantly greater than the number of other imaging tests. A total of 18 patients underwent MRM, and 11 (11/18) patients were found to have primary lesions in ipsilateral breast tissue after surgery, for a percentage of 61.1%, which was similar to the pathological findings of 51 patients with OBC reported by Wang et al in 2010[15]. According to the results of the SEER database-based analysis reported by Zhu et al[16], ER-positive and PR-positive patients accounted for 54.1% and 50.8%, respectively, of the total OBC cases[16], which supports our findings. In addition, approximately 54.5% of the patients were Her-2 positive, which was higher than approximately 20% of the general types of BC[17].
Due to the low incidence of OBC and the lack of sufficient evidence, the choice of treatment methods is still controversial. A number of retrospective studies have shown that using MRM and choosing combined chemoradiotherapy or endocrine therapy according to individual differences are the traditional treatments for OBC[18,19]. However, recent research by Sohn et al[20] confirmed that there was no significant difference in the prognosis of patients who received ALND only, BCS plus ALND, or MRM. Tsai et al[21] argued that the curative effect of MRM is similar to that of radiotherapy alone. Other relevant studies have also demonstrated that less intensive surgery does not negatively affect the prognosis of OBC patients, adjuvant radiotherapy is beneficial for prolonging OS, and ALND combined with radiotherapy may be the most suitable surgical modality for OBC[22,23]. Eighteen of the 33 patients received MRM, and their prognosis was not significantly different from that of patients who underwent other surgical methods. As an independent prognostic factor, radiotherapy (P = 0.031) could become a mainstay treatment for OBC.
OBC is a rare disease, and male OBC is even rarer than female OBC and generally has a poor prognosis regardless of sex. At present, most of the related cases reported internationally progress within a few years, which may be related to the difficulty in diagnosis and the lack of standardized treatment[24-26]. This study included one male OBC patient whose DFS and OS were significantly lower than those of the other patients in the group. Recent relevant studies have demonstrated the efficacy of immunotherapy for male OBC, and anti-androgen therapy can achieve effective control of disease progression with minimal toxicity[25], which provides a new idea for future male OBC management.
The psychological condition of OBC patients, a rare disease group, deserves further study. We analyzed the results of psychological questionnaires administered to OBC patients. The results showed that all 30 patients developed anxiety and depression, with low PA scores and high NA scores, accompanied by a high stress level and poor psychological resilience. It is well known that the diagnosis and treatment of cancer have both negative physical and psychological long-term side effects that affect the quality of life of patients and survivors[27,28]. Breast cancer patients and survivors experience significant changes in their evaluations of their appearance and their attitudes toward their bodies, particularly with regard to femininity[29]. For example, mastectomy or breast retention may threaten overall self-satisfaction and trigger multiple changes in body perception mediated by sensations within the breast and chest[30]. We also found significant differences in Conner-Davidson resilience scores between patients who did and did not receive radiotherapy. In this nuclear age, people have been repeatedly explained and made aware of the dangers of exposure to radiation and the need to avoid it. Therefore, when patients are receiving radiation cancer treatment, stress and anxiety can ensue. In addition, during radiation therapy, patients must lie alone on a table with a large machine above them, which can create fear, isolation, and anxiety[31]. In addition, 60% of patients have significant anxiety before treatment, and 80% have anxiety after treatment[32]. These findings remind us that, although patients receiving radiation therapy often have a better prognosis, the psychological issues associated with radiation therapy should not be overlooked. Therefore, psychological counseling is needed while patients receive antitumor therapy. We hope that patients can be physically and mentally healthy and return to society normally.
The incidence of OBC is low, and diagnosis is difficult, limiting its use in clinical practice. Our research showed that the diagnostic sensitivity of breast MRI is high, which is helpful for clinical diagnosis. In addition, a less invasive surgical modality can be selected according to the individual differences of patients, which has no obvious influence on patient disease progression. Moreover, postoperative adjuvant radiotherapy can obviously improve patient outcomes. To improve the quality of life of patients, appropriate treatment methods should be selected. Moreover, psychological problems need to be considered. The advantage of this study lies in the use of bicenter case data, but the present study still has limitations. Most of the studies were retrospective in design and included cohort selection, the impact of previous exposure to risk variables, treatment approaches, follow-up, reporting, complications, and genetic mutations. Additionally, the overall sample size was still small, and the follow-up time was short, warranting a prospective study with a large sample size to further guide the diagnosis and treatment of OBC.
Occult breast cancer (OBC) is a rare breast disease. With atypical clinical manifestations, OBC usually presents with painless axillary masses initially, but a few patients seek medical help for neck masses.
Although it is generally accepted that OBC has a similar biological behavior to lymph node-positive non-OBC, the clinicopathological features of the disease are unclear.
This study aims at better reveal the clinicopathological features and prognostic factors of OBC patients through a retrospective analysis of their clinicopathological data.
The clinicopathological data of 33 diagnosed OBC patients were retrospectively analyzed. The psychological status of OBC patients was evaluated by self-rating Anxiety Scale and self-rating Depression Scale. Patients’ emotions, stress perception and psychological resilience were evaluated by the Positive and Negative Affect Schedule, the Chinese Perceived Stress Scale, and the Connor-Davidson Resilience Scale, respectively. Patient survival was calculated. Univariate and multivariate survival analyses were performed using the COX regression model.
There were 30 (91%) with axillary tumor and 3 (9%) with Neck mass as the first symptom; 18 (54.5%) were ER-positive, 17 (51.5%) were PR-positive, and 18 (54.5%) were Her-2-positive; 24 (72.7%) received surgical treatment, including 18 cases of modified radical mastectomy, 1 case of breast-conserving surgery plus axillary lymph node dissection (ALND), and 5 cases of ALND alone; 12 cases received preoperative neoadjuvant therapy. All the 30 patients developed certain anxiety and depression, with low positive affect scores and high negative affect scores, accompanied by a high stress level and poor psychological resilience. The overall survival and disease-free survival of all the patients was 83.3% and 55.7%, respectively. Multivariate analysis revealed that radiotherapy was an independent prognostic factor.
OBC is a rare breast disease whose diagnosis and treatment are currently controversial. There was no significant difference in the efficacy of other less invasive surgical procedures compared to the modified radical mastectomy, and radiotherapy can significantly improve patient outcomes.
The incidence of OBC is low and the diagnosis is difficult, which is easy to be ignored in clinical practice. In order to improve the quality of life of patients, appropriate treatment methods should be selected. At the same time, psychological problems also need to be concerned about.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Essa MM, India; Insel PS, Sweden S-Editor: Wang JL L-Editor: A P-Editor: Yuan YY
| 1. | Huang KY, Zhang J, Fu WF, Lin YX, Song CG. Different Clinicopathological Characteristics and Prognostic Factors for Occult and Non-occult Breast Cancer: Analysis of the SEER Database. Front Oncol. 2020;10:1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 2. | Ofri A, Moore K. Occult breast cancer: Where are we at? Breast. 2020;54:211-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Ge XC, Yin DY, Zhang Q, Liu WJ, Xin Q. Diagnosis and treatment of occult breast cancer. Zhonghua Ruxian Bing Zazhi. 2021;15:381-383. [DOI] [Full Text] |
| 4. | Guo MQ, Song W. Controversy and prospect of the diagnosis and treatment of occult breast cancer. Zhonghua Ruxian Bing Zazhi. 2016;10:305-309. [DOI] [Full Text] |
| 5. | Kim H, Park W, Kim SS, Ahn SJ, Kim YB, Kim TH, Kim JH, Choi JH, Park HJ, Chang JS, Choi DH. Outcome of breast-conserving treatment for axillary lymph node metastasis from occult breast cancer with negative breast MRI. Breast. 2020;49:63-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Urban D, Rao A, Bressel M, Lawrence YR, Mileshkin L. Cancer of unknown primary: a population-based analysis of temporal change and socioeconomic disparities. Br J Cancer. 2013;109:1318-1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | National Health Commission of the People’s Republic of China. Guidelines for the Diagnosis and Treatment of Breast Cancer (2022 edition). Zhongguo Heli Yongyao Tansuo. 2022;19:1-26. [DOI] [Full Text] |
| 8. | Liu M, Liu B, Song Y, Ding L, Dong L. FDG PET/CT reveals the primary tumor in a patient with occult breast carcinoma undetected by other modalities. Clin Nucl Med. 2014;39:755-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Hessler LK, Molitoris JK, Rosenblatt PY, Bellavance EC, Nichols EM, Tkaczuk KHR, Feigenberg SJ, Bentzen SM, Kesmodel SB. Factors Influencing Management and Outcome in Patients with Occult Breast Cancer with Axillary Lymph Node Involvement: Analysis of the National Cancer Database. Ann Surg Oncol. 2017;24:2907-2914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Ye X, Yang L, He Q, Lin X, Wang J, Cui R, Xu C. Reconceptualizing the clinicopathological features, locoregional therapy and prognostic factors of occult breast cancer in the era of molecular subtyping. Women Health. 2023;63:105-114. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | He M, Tang LC, Yu KD, Cao AY, Shen ZZ, Shao ZM, Di GH. Treatment outcomes and unfavorable prognostic factors in patients with occult breast cancer. Eur J Surg Oncol. 2012;38:1022-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Montagna E, Bagnardi V, Rotmensz N, Viale G, Cancello G, Mazza M, Cardillo A, Ghisini R, Galimberti V, Veronesi P, Monti S, Luini A, Raviele PR, Mastropasqua MG, Goldhirsch A, Colleoni M. Immunohistochemically defined subtypes and outcome in occult breast carcinoma with axillary presentation. Breast Cancer Res Treat. 2011;129:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Ge LP, Liu XY, Xiao Y, Gou ZC, Zhao S, Jiang YZ, Di GH. Clinicopathological characteristics and treatment outcomes of occult breast cancer: a SEER population-based study. Cancer Manag Res. 2018;10:4381-4391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Svastics E, Rónay P, Bodó M. Occult breast cancer presenting with axillary metastasis. Eur J Surg Oncol. 1993;19:575-580. [PubMed] |
| 15. | Wang X, Zhao Y, Cao X. Clinical benefits of mastectomy on treatment of occult breast carcinoma presenting axillary metastases. Breast J. 2010;16:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Zhu HY, Dai M, Liu CG. Treatment options and prognosis evaluation of stage Ⅱ/Ⅲ occult breast cancer: A study based on SEER database. Shiyong Zhongliu Zazhi. 2020;35:166-172. [DOI] [Full Text] |
| 17. | Lebert J, Lilly EJ. Developments in the Management of Metastatic HER2-Positive Breast Cancer: A Review. Curr Oncol. 2022;29:2539-2549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Yan TT, Jiang JR. Experience in diagnosis and treatment of 21 cases of occult breast cancer. Xiandai Linchuang Yixue. 2019;45:174-176. [DOI] [Full Text] |
| 19. | Shen HY, Hu CH, Han YT, Peng DJ, Yu YL, Xu YB, Peng P, Liu CH, Hou YF. The exploration of clinical pathological characteristics and the diagnosis and treatment strategy of 56 patients with occult breast cancer. Zhongguo Aizheng Zazhi. 2018;28:429-434. [DOI] [Full Text] |
| 20. | Sohn G, Son BH, Lee SJ, Kang EY, Jung SH, Cho SH, Baek S, Lee YR, Kim HJ, Ko BS, Lee JW, Ahn SH. Treatment and survival of patients with occult breast cancer with axillary lymph node metastasis: a nationwide retrospective study. J Surg Oncol. 2014;110:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Tsai C, Zhao B, Chan T, Blair SL. Treatment for occult breast cancer: A propensity score analysis of the National Cancer Database. Am J Surg. 2020;220:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Zhao Z, Zhang T, Yao Y, Lu X. Clinicopathological characteristics and treatment outcomes of occult breast cancer: a population-based study. BMC Surg. 2022;22:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Macedo FI, Eid JJ, Flynn J, Jacobs MJ, Mittal VK. Optimal Surgical Management for Occult Breast Carcinoma: A Meta-analysis. Ann Surg Oncol. 2016;23:1838-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Hur SM, Cho DH, Lee SK, Choi MY, Bae SY, Koo MY, Kim S, Nam SJ, Lee JE, Yang JH. Occult breast cancers manifesting as axillary lymph node metastasis in men: a two-case report. J Breast Cancer. 2012;15:359-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Wang XH, Zhang J, Wu J, He XH, Shen YR, Peng YG, An YZ. Case Report: Response to Immunotherapy and Anti-Androgen Therapy in Male Occult Triple-Negative Breast Cancer. Front Oncol. 2022;12:840453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Li SJ, Zhang J, Liu X, Luo QW, Li QJ. A case of male occult breast cancer. Yixue Lunli Yu Shijian. 2021;34:1014-1015. [DOI] [Full Text] |
| 27. | Sterba KR, Burris JL, Heiney SP, Ruppel MB, Ford ME, Zapka J. "We both just trusted and leaned on the Lord": a qualitative study of religiousness and spirituality among African American breast cancer survivors and their caregivers. Qual Life Res. 2014;23:1909-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Ahmad S, Fergus K, McCarthy M. Psychosocial issues experienced by young women with breast cancer: the minority group with the majority of need. Curr Opin Support Palliat Care. 2015;9:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Sherman KA, Przezdziecki A, Alcorso J, Kilby CJ, Elder E, Boyages J, Koelmeyer L, Mackie H. Reducing Body Image-Related Distress in Women With Breast Cancer Using a Structured Online Writing Exercise: Results From the My Changed Body Randomized Controlled Trial. J Clin Oncol. 2018;36:1930-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 30. | Paterson CL, Lengacher CA, Donovan KA, Kip KE, Tofthagen CS. Body Image in Younger Breast Cancer Survivors: A Systematic Review. Cancer Nurs. 2016;39:E39-E58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 31. | Mungase M, Chaudhury S, Patil AA, Jagtap B, Jain V. Stress, anxiety, depression, and resilience in cancer patients on radiotherapy. Ind Psychiatry J. 2021;30:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Chen AM, Daly ME, Vazquez E, Courquin J, Luu Q, Donald PJ, Farwell DG. Depression among long-term survivors of head and neck cancer treated with radiation therapy. JAMA Otolaryngol Head Neck Surg. 2013;139:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |