Published online Jan 19, 2024. doi: 10.5498/wjp.v14.i1.53
Peer-review started: November 6, 2023
First decision: November 16, 2023
Revised: November 25, 2023
Accepted: December 21, 2023
Article in press: December 21, 2023
Published online: January 19, 2024
Processing time: 74 Days and 1.7 Hours
Patients with chronic heart failure (CHF) have a progressive disease that is associated with poor quality of life and high mortality. Many patients experience anxiety and depression (A&D) symptoms, which can further accelerate disease progression. We hypothesized that indicators of myocardial function and inflammatory stress may reflect the severity of A&D symptoms in patients with CHF. Changes in these biomarkers could potentially predict whether A&D symptoms will deteriorate further in these individuals.
To measure changes in cardiac and inflammatory markers in patients with CHF to determine A&D severity and predict outcomes.
We retrospectively analyzed 233 patients with CHF treated at the Jingzhou Hospital, Yangtze University between 2018-2022 and grouped them according to Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS) scores. We compared clinical data in the no-A&D, mild-A&D, moderate-A&D, and severe-A&D groups, the SAS and SDS scores with the New York Heart Association (NYHA) functional classification, and cardiac markers and inflammatory factors between the no/mild-A&D and moderate/severe-A&D groups. Regression analysis was performed on the markers with P < 0.05 to determine their ability to predict A&D severity in patients and the area under the receiver operating characteristic curve (AUROC) was used to evaluate their accuracy.
In the inter-group comparison, the following variables had an effect on A&D severity in patients with CHF: NYHA class, left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter, N-terminal pro-brain natriuretic peptide (NT-proBNP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (P < 0.05). Other variables did not differ significantly between the A&D groups (P > 0.05). In addition, we found that higher NYHA classes were associated with higher the SAS and SDS scores (P < 0.05). Regression analysis showed that LVEF, NT-proBNP, and IL-6 were independent risk factors for A&D severity (P < 0.05). Among them, NT-proBNP had the best predictive ability as a single indicator (AUROC = 0.781). Furthermore, the combination of these three indicators exhibited a good predictive effect toward discriminating the extent of A&D severity among patients (AUROC = 0.875).
Cardiac and inflammatory biomarkers, such as LVEF, NT-proBNP, and IL-6, are correlated with A&D severity in patients with CHF and have predictive value.
Core Tip: We investigated patients with chronic heart failure (CHF) with varying degrees of anxiety and depression (A&D) symptoms and assessed changes in myocardial markers and inflammatory factors to determine their associations with A&D severity. We used independent risk factors as predictive indicators and assessed their discriminative accuracy in predicting A&D severity using the area under the receiver operating characteristic curve. We demonstrated that A&D symptoms can affect the progression of CHF and lead to worse outcomes for patients.
- Citation: Zhang L, Wang Q, Cui HS, Luo YY. Assessing myocardial indices and inflammatory factors to determine anxiety and depression severity in patients with chronic heart failure. World J Psychiatry 2024; 14(1): 53-62
- URL: https://www.wjgnet.com/2220-3206/full/v14/i1/53.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i1.53
Chronic heart failure (CHF), or congestive heart failure, is a progressive disease that commonly manifests as fatigue, reduced physical fitness, shortness of breath, persistent coughing, and arrhythmia. Typically, the New York Heart Association (NYHA) classification is used to evaluate patients according to the severity of their symptoms, alongside cardiovascular functional capacity and blood test results[1]. Currently, treatment aims to ease the impact of these symptoms on daily life activities of patients and improve the quality of life. However, studies have shown that > 50% of patients with CHF develop anxiety and depression (A&D) symptoms[2,3], which has been associated with unfavorable outcomes, such as increased CHF-related hospitalization and mortality rates. However, distinguishing whether certain symptoms are related to A&D or are symptoms of CHF remains a challenge. Therefore, if the A&D status of patients with CHF can be accurately diagnosed and treated, the overall condition and life expectancy of patients is expected to improve[4,5].
In China, the incidence of CHF is on the rise, especially left ventricular dysfunction[6]. Although there has been extensive research on the general demographic characteristics and A&D data of CHF patients, studies on myocardial markers and inflammation factor levels are still limited. Research by Wang et al[7] showed that in the CHF population, white blood cell interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) are higher than those in the healthy population; Yasuhara et al[8] reported that these inflammation factor levels are positively correlated with B-type natriuretic peptide levels; Liu et al[9] showed in subsequent studies that systemic inflammation indicators, such as hypersensitive C-reactive protein (hs-CRP), are supplementary indicators for CHF diagnosis. Therefore, these inflammation factors can well reflect the physiological state of CHF patients. Furthermore, immune abnormalities of inflammatory mediators such as IL-6, TNF-α, etc., may be related to the pathophysiology of depression, and are also potential markers for the classification of A&D states[10,11]. Therefore, these inflammation factors can reflect the physiological state of CHF patients, and also have the potential to classify A&D states in CHF.
Currently, the assessment of A&D relies on subjective patient-reported measures; hence, differential levels of myocardial markers and inflammatory factors could serve as objective measure to assist clinicians in assessing and classifying A&D severity. This measure could facilitate a more precise distinction between CHF patients with different degrees of A&D, enable physicians to better manage and rationally allocate medical resources, and provide the potential for personalized treatment.
Therefore, this retrospective study aimed to explore the differences in myocardial markers, inflammatory factors, and A&D severity in patients of CHF and potentially reveal an association that can used as a reference for clinical assessment of A&D status in patients with CHF in the future.
This retrospective study obtained data from patients with CHF treated at Jingzhou Hospital, Yangtze University, from January 2018 to December 2022. The inclusion criteria were as follows: (1) A confirmed diagnosis of CHF and relevant medical records; (2) Records of A&D symptoms; (3) Patients aged > 18 years; and (4) Information on inflammatory factors and myocardial markers. The exclusion criteria were as follows: (1) Severe cardiovascular disease, such as myocardial infarction or heart valve disease; (2) Severe comorbid systemic diseases, such as cancer or renal failure; (3) History of severe psychological disorders that affect cognition and mood, such as schizophrenia and bipolar disorder; (4) Recent treatments that impact inflammatory and myocardial markers, such as corticosteroid therapy; and (5) History of alcohol or substance abuse. All diagnoses were based on the left ventricular ejection fraction (LVEF) < 50% and N-terminal pro-Brain natriuretic peptide (NT-proBNP) ≥ 125 ng/L one week after hospitalization. The Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS) scales were used to assess A&D, respectively[4,5]. Overall, 233 eligible patients were included in this study. The study was approved by the Institutional Review Board of Jingzhou Hospital, Yangtze University (2023-053-01).
The patients were first grouped according to the degree of A&D indicated by the SAS and SDS scores as follows: No-A&D (SAS < 50 and SDS < 53; n = 65); mild-A&D (SAS 50-59 and SDS 53-62, n = 42); moderate-A&D (SAS 60-69 and SDS 63-72, n = 93); and severe-A&D (SAS > 69 and SDS > 72, n = 33). If the two scores did not meet any of the group requirements simultaneously, the highest SAS or SDS score was used as the grouping criterion.
The demographic and clinical data of the patients in each group were compared to identify indicators of statistically significant differences in A&D. Then, the patients were divided into groups based on the NYHA classification (I, II, III, and IV) and the SAS and SDS scores were compared among the groups[1]. Finally, we determined the effect of A&D on the daily lives of patients based on SAS and SDS scores, with the no/mild-A&D (N/M) group considered not affected and the moderate/severe-A&D (M/S) group considered affected and required targeted intervention[12,13]. Hence, the patients from groups A and B and the patients from groups C and D were combined into the N/M and M/S groups, respectively. We compared the significant indicators between the two groups using regression analysis and evaluated their predictive value using the area under the receiver operating characteristic (AUROC) curve.
The data included in the analysis were as follows: Sex, age, disease course, NYHA classification, myocardial indicators such as LVEF, left ventricular end-diastolic dimension (LVEDd), NT-proBNP, and soluble growth stimulation expressed gene 2 protein, and inflammatory factors such as IL-6, TNF-α, hs-CRP, and high sensitivity cardiac troponin.
All statistical analyses were conducted using SPSS software for Windows (version 26.0; IBM Corp., Armonk, NY, United States). Quantitative data are expressed as the mean ± SD, while qualitative data are expressed as the number of cases (n) and proportions (%). For inter-group comparisons, t-tests, analysis of variance (ANOVA), or non-parametric tests were used depending on whether the quantitative data followed normal distribution and homogeneity of variances. χ2 tests were used for inter-group comparisons of qualitative data. Logistic regression was used for multivariate analysis to calculate the odds ratios (OR) and 95% confidence intervals (CI). P values < 0.05 were considered statistically significant.
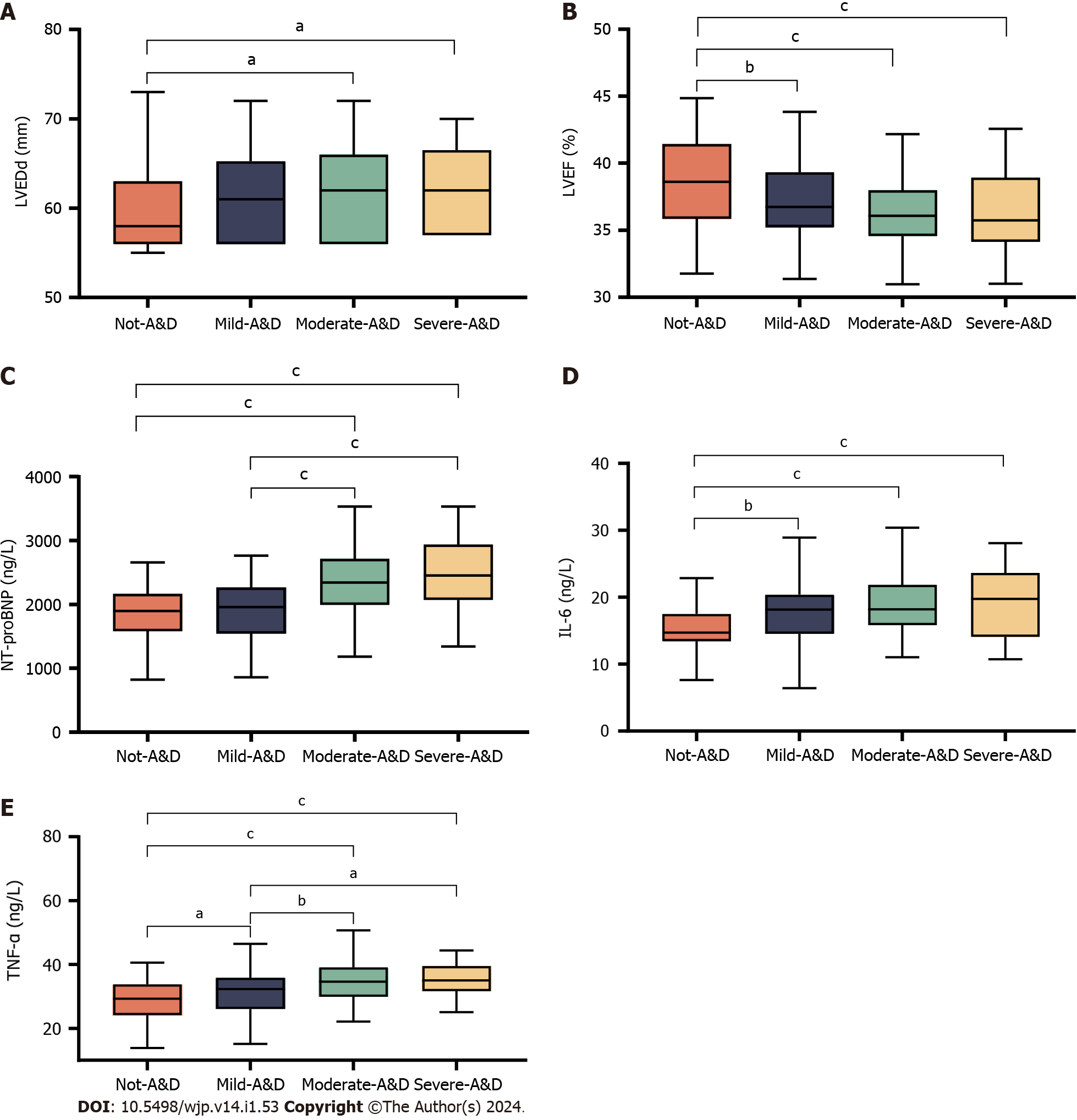
In the inter-group analysis, differences in LVEDd (P < 0.05) and in NYHA class, LVEF, NT-proBNP, IL-6, and TNF-α (P < 0.001) were observed between the no-A&D and severe-A&D groups. No other variables exhibited differences between the two groups (P > 0.05) (Table 1). Since LVEF, NT-proBNP, IL-6, and TNF-α were quantitative variables with large inter-group differences, post hoc multiple comparisons were performed (Figure 1).
| Observation indices | No-A&D (n = 65) | Mild-A&D (n = 42) | Moderate-A&D (n = 93) | Severe-A&D (n = 33) | P value |
| Sex | 0.686 | ||||
| Male | 27 (41.54) | 20 (47.61) | 47 (50.54) | 17 (51.52) | |
| Female | 38 (58.46) | 22 (52.39) | 46 (49.46) | 16 (48.48) | |
| Age (yr, mean ± SD) | 63.20 ± 14.75 | 64.93 ± 12.81 | 66.08 ± 10.99 | 66.67 ± 12.78 | 0.476 |
| Course of disease (month, mean ± SD) | 68.00 ± 17.74 | 66.36 ± 20.49 | 68.53 ± 15.97 | 68.97 ± 20.40 | 0.948 |
| NYHA, n (%) | < 0.001 | ||||
| I | 16 (24.62) | 6 (14.29) | 3 (3.23) | 4 (12.12) | |
| II | 27 (41.54) | 8 (19.05) | 26 (27.96) | 8 (24.24) | |
| III | 21 (32.31) | 24 (57.14) | 54 (58.06) | 17 (51.52) | |
| IV | 1 (1.53) | 4 (9.52) | 10 (10.75) | 4 (12.12) | |
| LVEF (%, mean ± SD) | 38.72 ± 3.32 | 37.08 ± 3.03 | 36.24 ± 2.59 | 36.32 ± 3.16 | < 0.001 |
| LVEDd (mm, mean ± SD) | 59.86 ± 5.22 | 61.38 ± 4.99 | 61.85 ± 4.95 | 62.36 ± 4.52 | 0.047 |
| NT-proBNP (ng/L, mean ± SD) | 1830.32 ± 421.64 | 1913.90 ± 447.93 | 2364.98 ± 490.56 | 2482.70 ± 541.86 | < 0.001 |
| ST2 (μg/L, mean ± SD) | 51.26 ± 10.25 | 53.76 ± 9.92 | 52.97 ± 8.74 | 53.61 ± 8.07 | 0.382 |
| IL-6 (ng/L, mean ± SD) | 15.13 ± 2.98 | 17.66 ± 4.33 | 18.85 ± 3.84 | 19.38 ± 5.17 | < 0.001 |
| TNF-α (ng/L, mean ± SD) | 28.89 ± 5.85 | 31.72 ± 7.08 | 34.68 ± 5.84 | 35.32 ± 5.1 | < 0.001 |
| hs-CRP (ng/L, mean ± SD) | 12.27 ± 2.67 | 12.49 ± 2.71 | 13.03 ± 2.45 | 13.22 ± 2.54 | 0.185 |
| hs-cTn (ng/L, mean ± SD) | 22.94 ± 7.83 | 24.53 ± 7.99 | 24.45 ± 6.22 | 24.40 ± 6.67 | 0.293 |
The LVEF and IL-6 levels in the no-A&D group were clearly different than those of the mild-A&D, moderate-A&D, and severe-A&D groups (P < 0.01), which all had similar levels (P > 0.05) (Figure 1A and D). LVEDd exhibited differences in the no-A&D and moderate-A&D/severe-A&D group comparisons (P < 0.05). However, when the moderate-A&D group was compared with the other groups, the differences were not significant (P > 0.05) (Figure 1B). The NT-proBNP levels showed no significant differences between no-A&D and mild-A&D groups or between the moderate and severe-A&D groups (P > 0.05). However, differences in NT-proBNP levels were observed and highlighted when the no-A&D and mild-A&D groups were compared with the moderate-A&D and severe-A&D groups (P < 0.001) (Figure 1C). Furthermore, significant differences in TNF-α levels were found between the no-A&D/mild-A&D and moderate-A&D/severe A&D groups (P < 0.05), the no-A&D and mild-A&D groups (P < 0.05) but not between the moderate-A&D and severe-A&D groups (P > 0.05) (Figure 1E).
We found that the SAS and SDS scores of patients with CHF increased as the NYHA class improved (P < 0.05) (Table 2). Patients with NYHA class III and IV CHF had higher SAS and SDS scores than those with NYHA class I and II (P < 0.05) (Figure 2).
| Observation indices | I (n = 29) | II (n = 69) | III (n = 116) | IV (n = 19) | P value |
| SAS (mean ± SD) | 51.17 ± 16.54 | 53.84 ± 17.87 | 59.82 ± 15.51 | 65.58 ± 16.76 | 0.003 |
| SDS (mean ± SD) | 53.52 ± 16.85 | 56.72 ± 17.60 | 62.46 ± 14.75 | 68.89 ± 12.40 | 0.001 |
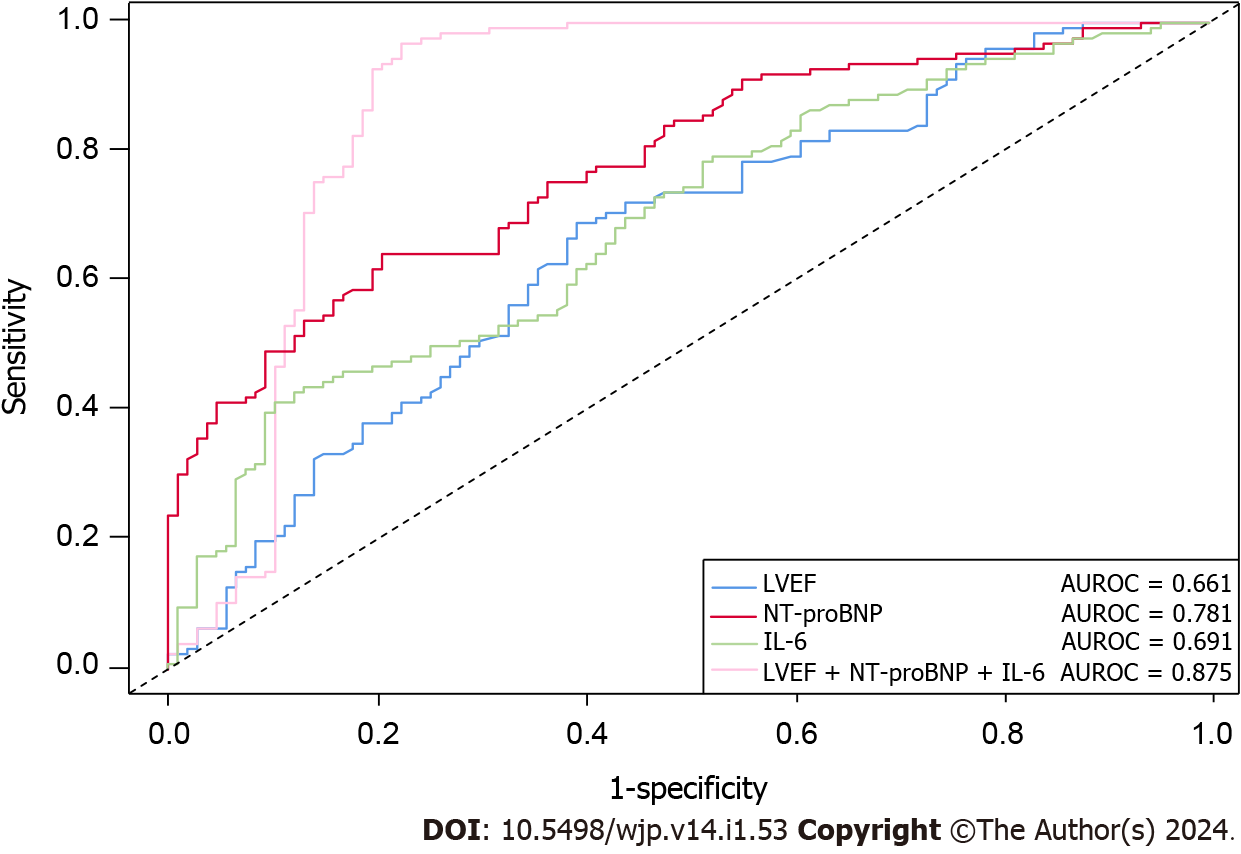
When comparing the myocardial and inflammatory level indicators, we found that the effects of LVEF, LVEDd, NT-proBNP, IL-6, TNF-α, and hs-CRP were larger than other observed indicators between the N/M and M/S groups (P < 0.05). Further details are presented in Table 3. The regression analysis identified LVEF, NT-proBNP, and IL-6 as independent predictors of CHF (P < 0.05), with IL-6 having the strongest predictive value (OR = 1.271, 95%CI: 1.095-1.476), followed by NT-proBNP (OR = 1.004, 95%CI: 1.003-1.006). We also found that LVEF was a protective factor against A&D as it increased (OR = 0.350, 95%CI: 0.226-0.541). The other variables were not deemed statistically significant in the regression analysis. Further details are presented in Table 4. The AUROC curve of the three independent factors showed that NT-proBNP had the best predictive effect, with an AUROC of 0.781 (0.724-0.839), whereas the predictive effects of LVEF and IL-6 were moderate and similar, with AUROCs of 0.661 (0.591-0.732) and 0.691 (0.624-0.758), respectively. The overall combined predictive value of the three indicators was relatively good, with an AUROC of 0.875 (0.820-0.929). Further details are shown in Figure 3.
| Observation indices | No/mild-A&D (N/M) (n = 107) | Moderate/severe-A&D (N/S) (n = 126) | t/Z/χ2 | P value |
| LVEF (%, mean ± SD) | 38.08 ± 3.30 | 36.26 ± 2.72 | 4.536 | < 0.001 |
| LVEDd (mm, mean ± SD) | 60.46 ± 5.16 | 61.98 ± 4.83 | 2.778 | 0.005 |
| NT-proBNP (ng/L, mean ± SD) | 1863.13 ± 432.00 | 2395.81 ± 505.30 | 8.656 | < 0.001 |
| ST2 (μg/L, mean ± SD) | 52.24 ± 10.15 | 53.14 ± 8.54 | 0.908 | 0.364 |
| IL-6 (ng/L, mean ± SD) | 16.12 ± 3.76 | 18.99 ± 4.21 | 5.024 | < 0.001 |
| TNF-α (ng/L, mean ± SD) | 30.00 ± 6.48 | 34.84 ± 5.64 | 6.105 | < 0.001 |
| hs-CRP (ng/L, mean ± SD) | 12.36 ± 2.67 | 13.08 ± 2.46 | 2.144 | 0.033 |
| hs-cTn (ng/L, mean ± SD) | 23.56 ± 7.89 | 24.44 ± 6.31 | 1.301 | 0.193 |
| Observation indices | β | OR | P value | 95%CI |
| LVEF | -1.050 | 0.350 | < 0.001 | 0.226-0.541 |
| LVEDd | -0.182 | 0.834 | 0.095 | 0.673-1.032 |
| NT-proBNP | 0.004 | 1.004 | < 0.001 | 1.003-1.006 |
| IL-6 | 0.240 | 1.271 | 0.002 | 1.095-1.476 |
| TNF-α | -0.002 | 0.957 | 0.998 | 0.928-1.073 |
| hs-CRP | -0.430 | 0.651 | 0.060 | 0.416-1.019 |
Heart failure remains a global public health burden. Since mental health affects the incidence and outcomes of various health conditions[14], the psychological status of patients with CHF warrants attention, in addition to investigating diagnostic and treatment methods.
In this study, when comparing the no-A&D to severe-A&D groups, we observed an increase in LVEDd, NT-proBNP, IL-6 and TNF-α levels as the degree of A&D increased, whereas LVEF levels decreased. This finding indicates that patients with CHF may experience increased A&D symptoms as a result of abnormal myocardial parameters and inflammatory stress, resulting in worse outcomes. Physiologically, A&D symptoms manifest as part of the body’s response to chronic stress exposure, which causes a maladaptive stress response in which the sympathetic nervous system and hypothalamic-pituitary-adrenal axis are activated. This increases the heart rate, output, and load[15,16] and, over time, the blood flow through the coronary arteries decreases, ultimately leading to myocardial ischemia[17]. While CHF is accompanied by some extent of myocardial dysfunction, the stress response aggravates this by causing further myocardial cell injury, impairing myocardial contractile function, and leading to a reduction in LEVF and an increase in LVEDd. When under significant load, myocardial cells secrete NT-proBNP, which functions as a natriuretic, diuretic, and vasodilator, to reduce pressure and restore homeostasis[18]. NT-proBNP levels increase with the degree of A&D symptoms and changes in LVEF and NT-proBNP levels indicate that A&D further aggravates myocardial dysfunction in patients with CHF. Research has shown that higher serum levels of inflammatory factors can lead to weakening of the strength and mass of the myocardium, a specialized type of tissue in the human body[19]. Therefore, patients with CHF are vulnerable to high levels of inflammation. Additionally, A&D can lead to endocrine and immune system imbalances that promote inflammatory responses in the body[20]. In the current study, IL-6 and TNF-α expression levels gradually increased as the extent of A&D increased, which is consistent with Lamers et al[10].
Our results suggest an association between myocardial indicators, inflammatory factor levels, and the degree of A&D. Therefore, when treating patients with both CHF and A&D symptoms, physicians should be aware of the increased risk of myocardial ischemia and inflammation and prepare the appropriate prophylactic interventions, such as administering calcium overload inhibitors and anti-inflammatory drugs. Zhao et al[21] found that the administration of active oxygen nanomaterials effectively improved drug treatment and reduced side effects. The side effects of any pharmacological intervention for CHF and A&D should be considered to avoid worsening the prognosis of patients. Recent studies have indicated that cognitive behavioral therapy and collaborative care have positive effects on alleviating A&D[22,23], and present a safer way to avoid psychotropic drug-induced secondary myocardial damage.
The NYHA classification assesses the self-reported functional status of patients with CHF and indirectly reflects cardiac function. In the present study, the mean SAS and SDS scores consistently increased with each NYHA class, which is consistent with a previous study[24] exploring depression in patients with coronary heart disease. This finding was unsurprising because the NYHA classification closely reflects the patient’s quality of life and the higher the NYHA class, the more restrictions the patient experiences in daily life and mobility, such as inability to work, participate in their hobbies, and socialize, all of which induce A&D[25]. Moreover, A&D can lead to reduced compliance with treatment regimens, which further impacts quality of life, resulting in more individuals progressing to higher NYHA classes, increased hospitalization, and mortality. Hence, medical health providers must consider psychological status in the treatment and management of patients with CHF, in addition to physical symptoms and cardiac function. Providing psychological support, educating patients on disease management, and encouraging them to engage in suitable physical activities and socialization are important measures to improve health outcomes of patients with A&D.
In patients where daily activities have not yet been affected by A&E, we do not recommend targeted interventions such as personalized psychological counselling and cognitive behavioral therapy[13,26]. In this study, we observed different SAS and SDS scores in the N/M and M/S groups. Regression analysis revealed that the combination of LEVF, NT-proBNP, and IL-6 had good predictive and discriminative value between the N/M and M/S groups. These factors comprehensively reflect cardiac function, heart load, and inflammatory state; therefore, compared with single-index predictions, they can more accurately determine A&D severity in patients with CHF. Severe A&D is a complex multifactorial disorder that is influenced by genetic, biological, psychological, and environmental factors. Existing studies have shown the predictive value of conventional biochemical indices, such as leukocytes in the hematopoietic system and uric acid in inflammatory mediators, in discriminating bipolar disorder and depression in the general population, with females exhibiting better predictive effects than males (AUROC = 0.793)[27]. Similarly, in the present study, we constructed a model using myocardial indices and inflammatory factors to predict A&D severity in patients with CHF and demonstrated a relatively good prediction effect (AUROC = 0.875). Perhaps, this was attributable to the targeted groupings used in the analysis.
Overall, the combination of biochemical indicators of A&D severity provides an objective reference for the classification of patients, which is essential for accurate diagnosis. Specialized prediction models for comorbidities, such as patients with both A&D and CHF, are also warranted.
In this study, we found a preliminary association between A&D levels, myocardial indicators, and inflammation in patients with CHF and identified potential indicators and models for classifying A&D on a physiological basis. However, this single-center retrospective study had some limitations in terms of the sample size and reliance on standardized self-reported questionnaires. Future longitudinal studies with larger sample sizes are warranted to validate the association between myocardial indicators, inflammation levels, and A&D.
Cardiac parameters (NT-proBNP and LVEF) and levels of inflammatory factors (IL-6 and TNF-α) significantly vary with the degree of A&D. IL-6, NT-proBNP, and LVEF are independent factors that can distinguish between moderate and severe A&D from no A&D or mild A&D. When combined, they have a high discriminative ability towards A&D.
Anxiety and depression (A&D) are common in patients with chronic heart failure (CHF). While extensive research on the general demographic characteristics and A&D data in patients with CHF has been conducted, research on myocardial markers and inflammatory factor levels remains limited. Uncovering the relationship between myocardial markers, inflammatory factor levels, and the degree of A&D in patients with CHF can further supplement and assist in evaluating and classifying A&D severity in patients.
The differential levels of myocardial markers and inflammatory factors depending on A&D severity in patients with CHF could serve as objective evidence to assist clinicians in assessing and classifying the extent of A&D. This could correct for the subjectivity of the general assessment scales, facilitating a more precise distinction between CHF patients with different degrees of A&D, enabling physicians to better manage and rationally allocate medical resources, and providing the potential for personalized treatment.
We classified patients with CHF with varying degrees of A&D based on myocardial markers and inflammatory factor levels and showed that some factors had good discriminative ability, providing the possibility of establishing and assisting in the evaluation of A&D severity in patients with CHF based on biochemical markers.
We further explored the inter-group differences in myocardial and inflammatory indicators and showed their ability to predict A&D severity in patients with CHF using regression and the area under the receiver operating characteristic curve (AUROC) analyses.
Left ventricular ejection fraction, N-terminal pro-brain natriuretic peptide, and interleukin-6 were used in combination to predict and classify 233 patients with CHF in this study into no/mild- and moderate/severe-A&D and achieved good results (AUROC = 0.875, 95% confidence interval: 0.820-0.929).
Based on our retrospective analysis, we propose that the combination of myocardial and inflammatory factor levels could assist in assessing and classifying the severity of A&D in patients with CHF.
We plan to further combine other biochemical markers, such as hormone levels, with these classification-effective factors and apply targeted interventions to observe patient outcomes and evaluate changes in A&D severity among patients. This will help to validate our preliminary findings and investigate the potential roles of these biochemical markers in guiding CHF management.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Muller DJ, Canada; Pipe AL, Canada S-Editor: Wang JJ L-Editor: A P-Editor: Yuan YY
| 1. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 1249] [Article Influence: 416.3] [Reference Citation Analysis (0)] |
| 2. | Al Shamiri MQ, Almushawah AA, Alsomali AH, Alsuwayegh MB, Aljaffer MA, Hayajneh AM, Prajjwal P. The Prevalence of Depression and Anxiety in Heart Failure Patients in Saudi Arabia: An Original Study. Cureus. 2023;15:e36997. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Manolis TA, Manolis AA, Melita H, Manolis AS. Neuropsychiatric disorders in patients with heart failure: not to be ignored. Heart Fail Rev. 2023;28:821-858. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Alhurani AS, Dekker RL, Abed MA, Khalil A, Al Zaghal MH, Lee KS, Mudd-Martin G, Biddle MJ, Lennie TA, Moser DK. The association of co-morbid symptoms of depression and anxiety with all-cause mortality and cardiac rehospitalization in patients with heart failure. Psychosomatics. 2015;56:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Celano CM, Villegas AC, Albanese AM, Gaggin HK, Huffman JC. Depression and Anxiety in Heart Failure: A Review. Harv Rev Psychiatry. 2018;26:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 312] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 6. | Hao G, Wang X, Chen Z, Zhang L, Zhang Y, Wei B, Zheng C, Kang Y, Jiang L, Zhu Z, Zhang J, Wang Z, Gao R; China Hypertension Survey Investigators. Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012-2015. Eur J Heart Fail. 2019;21:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 7. | Wang Z, Cai Z, Ferrari MW, Liu Y, Li C, Zhang T, Lyu G. The Correlation between Gut Microbiota and Serum Metabolomic in Elderly Patients with Chronic Heart Failure. Mediators Inflamm. 2021;2021:5587428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Yasuhara S, Maekawa M, Bamba S, Kurihara M, Nakanishi N, Yamamoto T, Sakai H, Yagi N, Nakagawa Y, Sasaki M. Energy Metabolism and Nutritional Status in Hospitalized Patients with Chronic Heart Failure. Ann Nutr Metab. 2020;76:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Liu Z, Xv Y, Liu X, Zhou X. Associations of systemic inflammatory markers with the risks of chronic heart failure: A case-control study. Clinics (Sao Paulo). 2022;77:100056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Lamers F, Milaneschi Y, Smit JH, Schoevers RA, Wittenberg G, Penninx BWJH. Longitudinal Association Between Depression and Inflammatory Markers: Results From the Netherlands Study of Depression and Anxiety. Biol Psychiatry. 2019;85:829-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 11. | Zou W, Feng R, Yang Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS One. 2018;13:e0197267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 12. | Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2251] [Cited by in RCA: 2727] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 13. | Zung WW. A SELF-RATING DEPRESSION SCALE. Arch Gen Psychiatry. 1965;12:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5900] [Cited by in RCA: 6024] [Article Influence: 207.7] [Reference Citation Analysis (0)] |
| 14. | Cai W, Mueller C, Li YJ, Shen WD, Stewart R. Post stroke depression and risk of stroke recurrence and mortality: A systematic review and meta-analysis. Ageing Res Rev. 2019;50:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 15. | Mifsud KR, Reul JMHM. Mineralocorticoid and glucocorticoid receptor-mediated control of genomic responses to stress in the brain. Stress. 2018;21:389-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 16. | Ketchesin KD, Stinnett GS, Seasholtz AF. Corticotropin-releasing hormone-binding protein and stress: from invertebrates to humans. Stress. 2017;20:449-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Sara JDS, Toya T, Ahmad A, Clark MM, Gilliam WP, Lerman LO, Lerman A. Mental Stress and Its Effects on Vascular Health. Mayo Clin Proc. 2022;97:951-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 18. | Alawieh H, Chemaly TE, Alam S, Khraiche M. Towards Point-of-Care Heart Failure Diagnostic Platforms: BNP and NT-proBNP Biosensors. Sensors (Basel). 2019;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Tuttle CSL, Thang LAN, Maier AB. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res Rev. 2020;64:101185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 355] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 20. | Qiu W, Cai X, Zheng C, Qiu S, Ke H, Huang Y. Update on the Relationship Between Depression and Neuroendocrine Metabolism. Front Neurosci. 2021;15:728810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Zhao T, Wu W, Sui L, Huang Q, Nan Y, Liu J, Ai K. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact Mater. 2022;7:47-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 155] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 22. | Freedland KE, Skala JA, Carney RM, Steinmeyer BC, Rubin EH, Rich MW. Sequential Interventions for Major Depression and Heart Failure Self-Care: A Randomized Clinical Trial. Circ Heart Fail. 2022;15:e009422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (1)] |
| 23. | Bekelman DB, Allen LA, McBryde CF, Hattler B, Fairclough DL, Havranek EP, Turvey C, Meek PM. Effect of a Collaborative Care Intervention vs Usual Care on Health Status of Patients With Chronic Heart Failure: The CASA Randomized Clinical Trial. JAMA Intern Med. 2018;178:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 24. | Yin H, Liu Y, Ma H, Liu G, Guo L, Geng Q. Associations of mood symptoms with NYHA functional classes in angina pectoris patients: a cross-sectional study. BMC Psychiatry. 2019;19:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Nabdi S, Boujraf S, Benzagmout M. The influence of physical activity, social relationships, and diet intake on depression: a case-series study. Ann Med Surg (Lond). 2023;85:1395-1402. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Plati DK, Tripoliti EE, Bechlioulis A, Rammos A, Dimou I, Lakkas L, Watson C, McDonald K, Ledwidge M, Pharithi R, Gallagher J, Michalis LK, Goletsis Y, Naka KK, Fotiadis DI. A Machine Learning Approach for Chronic Heart Failure Diagnosis. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Trivedi MH. Major Depressive Disorder in Primary Care: Strategies for Identification. J Clin Psychiatry. 2020;81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |