Published online Jan 19, 2024. doi: 10.5498/wjp.v14.i1.102
Peer-review started: August 26, 2023
First decision: September 29, 2023
Revised: October 30, 2023
Accepted: December 21, 2023
Article in press: December 21, 2023
Published online: January 19, 2024
Processing time: 145 Days and 11.4 Hours
Several studies have reported that the walking trail making test (WTMT) com
To explore the performance in the WTMT in older people with WMH.
In this single-center, observational study, 25 elderly WMH patients admitted to our hospital from June 2019 to June 2020 served as the WMH group and 20 participants matched for age, gender, and educational level who were undergoing physical examination in our hospital during the same period served as the control group. The participants completed the WTMT-A and WTMT-B to obtain their gait parameters, including WTMT-A completion time, WTMT-B completion time, speed, step length, cadence, and stance phase percent. White matter lesions were scored according to the Fazekas scale. Multiple neuropsychological assessments were carried out to assess cognitive function. The relationships between WTMT performance and cognition and motion in elderly patients with WMH were analyzed by partial Pearson correlation analysis.
Patients with WMH performed significantly worse on the choice reaction test (CRT) (0.51 ± 0.09 s vs 0.44 ± 0.06 s, P = 0.007), verbal fluency test (VFT, 14.2 ± 2.75 vs 16.65 ± 3.54, P = 0.012), and digit symbol substitution test (16.00 ± 2.75 vs 18.40 ± 3.27, P = 0.010) than participants in the control group. The WMH group also required significantly more time to complete the WTMT-A (93.00 ± 10.76 s vs 70.55 ± 11.28 s, P < 0.001) and WTMT-B (109.72 ± 12.26 s vs 82.85 ± 7.90 s, P < 0.001). WTMT-A completion time was positively correlated with CRT time (r = 0.460, P = 0.001), while WTMT-B completion time was negatively correlated with VFT (r = -0.391, P = 0.008). On the WTMT-A, only speed was found to statistically differ between the WMH and control groups (0.803 ± 0.096 vs 0.975 ± 0.050 m/s, P < 0.001), whereas on the WTMT-B, the WMH group exhibited a significantly lower speed (0.778 ± 0.111 vs 0.970 ± 0.053 m/s, P < 0.001) and cadence (82.600 ± 4.140 vs 85.500 ± 5.020 steps/m, P = 0.039), as well as a higher stance phase percentage (65.061 ± 1.813% vs 63.513 ± 2.465%, P = 0.019) relative to controls.
Older adults with WMH showed obviously poorer WTMT performance. WTMT could be a potential indicator for cognitive and motor deficits in patients with WMH.
Core Tip: A new modified trail making test [walking trail making test (WTMT)], was used to explore the cognitive and motor deficits in older adults with WMH. In addition, wearable sensors were selected firstly in the WTMT to analyze the gait features of subjects. The results implied that WTMT could be a potential indicator for the cognitive and motor deficits in WMH patients.
- Citation: Zhao HY, Zhang ZQ, Huang YH, Li H, Wei FY. Performance of the walking trail making test in older adults with white matter hyperintensities. World J Psychiatry 2024; 14(1): 102-110
- URL: https://www.wjgnet.com/2220-3206/full/v14/i1/102.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i1.102
With continued advances in medical technologies and improvement in life expectancy in modern society, cognitive impairment and gait disturbance have become common symptoms negatively affecting the daily life of the growing elderly population. In the last decade, an increasing number of studies have confirmed that cognitive impairment and gait abnormalities in older adults should not to be explored in isolation[1]. On the contrary, impairments in cognitive and physical dimensions are frequently concurrent[2]. Kelaiditi et al[3] proposed the concept of “cognitive frailty” in 2013, and “motor cognitive risk syndrome” was reported by Verghese et al[4] a year later. Recent findings have even demonstrated the synergistic effects of cognitive and motor dysfunction in patients with cerebral small vessel disease (CSVD)[5].
White matter hyperintensities (WMH), together with cerebral microbleeds, recent subcortical lacunar infarcts (clinically symptomatic), lacunes (clinically silent), prominent perivascular spaces, atrophy lacunar infarcts, etc are known to be common signs of CSVD on conventional magnetic resonance imaging (MRI)[6]. WMH represent a common condition in older adults, occurring in approximately 80% adults in the general population over the age of 60 years[7]. Gait disorders and cognitive dysfunction (especially executive dysfunction) are the main symptoms of WMH[8]. Longitudinal studies revealed that WMH are associated with a high risk of falling, disability, and mortality due to the persistent deterioration of cognitive and motor function[9,10]. However, the early detection of the above symptoms is difficult in clinical practice. Recent studies inferred that a well-designed cognitive-motor dual walking task could be a useful tool for detecting cognitive and motor impairment in patients with WMH[11]. Under dual task conditions, the motor and/or cognitive task performance of older people can deteriorate due to competing demands when the available central resource capacity is exceeded[12].
The traditional trail making test (TMT) is a commonly used paper-and-pencil cognitive function test that can reflect a person’s ability in terms of executive function, attention, and processing speed. Recent studies have attempted to modify the traditional TMT to create the walking TMT (WTMT)[13-15]. In contrast to the ordinary dual walking task tests based on a cognitive task separate from a motor task, the WTMT incorporates a cognitive task into walking. In addition, recently published findings have implied that cognitive tasks involving internal interfering factors (e.g., mental tracking) impair gait performance more than those involving external interfering factors (e.g., reaction time)[13]. Multiple studies have reported that the WTMT completion time is significantly higher in patients with developmental coordination disorders and mild cognitive impairment[14,16]. Thus, the aim of the current study was to assess the gait characteristics of elderly individuals with WMH using the WTMT task.
A total of 25 older adults with WMH (WMH group) and 20 healthy individuals matched for age, gender, and educational level (HE group) were recruited from the Department of Neurology, the Seventh Medical Center of PLA General Hospital (which also receives older individuals in Aged Cadre Convalescent subdepartments). These patients were recruited consecutively from June 1, 2021, to April 1, 2022. Participants in the HE group, who had no record of a WMH diagnosis and who had regular rest and recuperation plans, were recruited from the Aged Cadre Convalescent subdepartment. Each participant voluntarily signed an informed consent form to participate in the current study.
All participants underwent screening by 3.0 T MRI of the brain and were grouped based on a method previously described by our group[11]. White matter lesions were graded using the Fazekas scale, as previously described[17]. Briefly, we rated WMH severity as grade 1 (punctate lesions), grade 2 (early confluent lesions), or grade 3 (confluent lesions). Only individuals with a Fazekas score of 0 were included in the HE group.
The exclusion criteria were history of major stroke; presence of multiple lacunar infarcts, other reasons for leukoencephalopathy (including immune, demyelination, and genetic); major psychiatric disorders (diagnosed using the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV); use of psychotropic medications or drugs with the side effect of risk of falling (e.g., tranquillizers/sedatives, diuretics, antiparkinsonian drugs); MRI contraindications; dementia (diagnosed using an International Classification of Diseases-10 code); a mini-mental state examination (MMSE) score lower than 23 points[18]; and use of walking aids.
A 3.0 T MRI brain scan (Discovery MR750; GE Healthcare, United States) displayed white matter lesions, which indicated the degree of CSVD. Brain MRI (slice and interslice thicknesses of 5 mm and 1.5 mm, respectively) was carried out as follows: T1 fluid-attenuated inversion recovery (TR, 1750 ms; TE, 23 ms; TI, 780 ms; FOV, 24 cm) and T2-weighted imaging (TR, 7498 ms; TE, 105 ms; FOV, 24 cm) sequences. The researchers who assessed gait were blinded to the imaging findings.
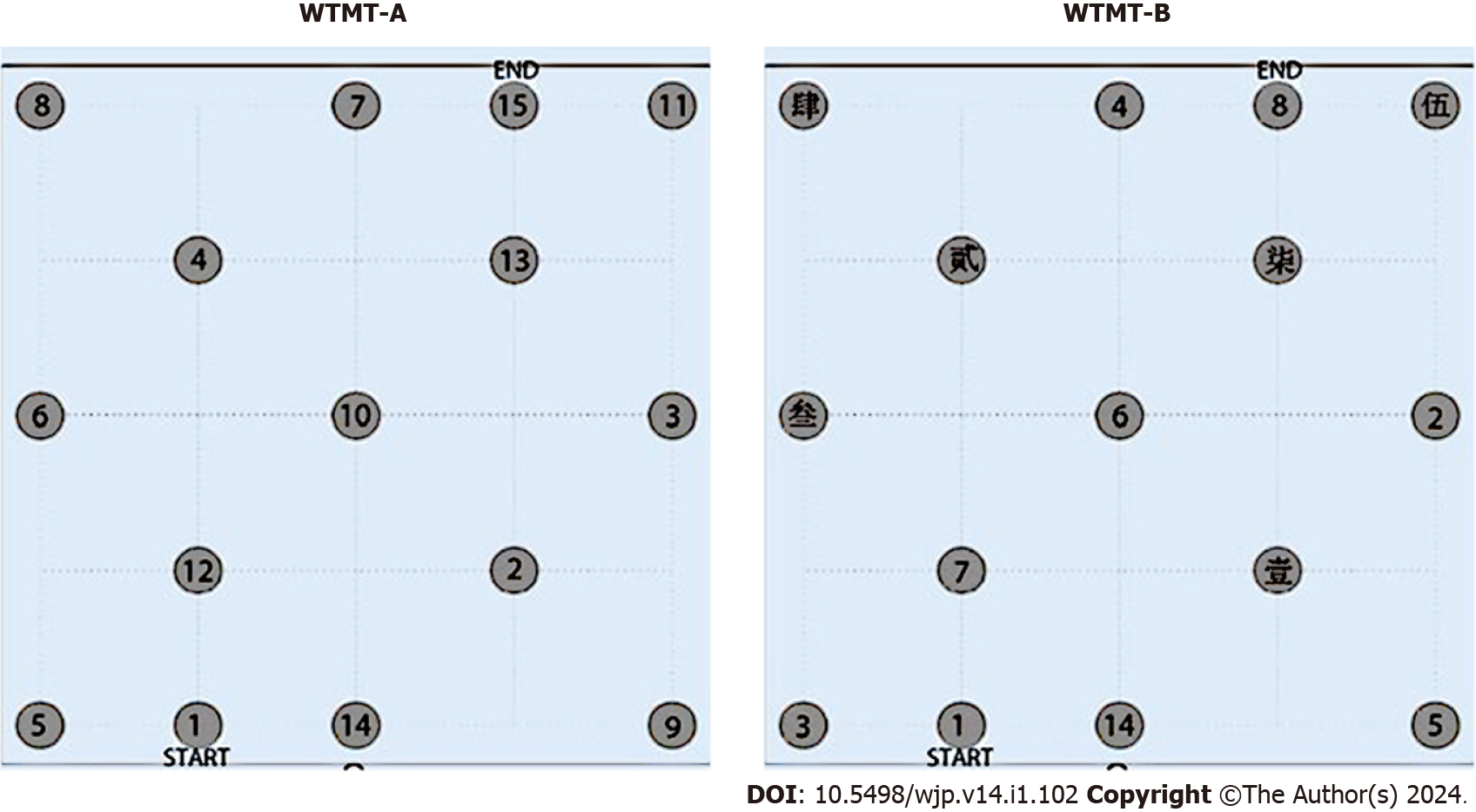
The WTMT was conducted in a quiet and comfortable environment. For the WTMT-A, randomly distributed coins with a 30-cm diameter and labeled with Arabic numbers (1-15) were positioned in a 16 m2 square area (4 m × 4 m). Participants were instructed to step as quickly and accurately as possible. Experimenters instructed the participants as follows: “Please walk on numbered targets in a sequential order as rapidly as possible, joining consecutive numbers (i.e., 1 to 2 to 3…15) in the coins randomly distributed on the floor.” When participants stepped on an incorrect number, the experimenter indicated the error, instructing them to step on the correct number as time continued to be measured. The WTMT-A was performed only once.
For the WTMT-B, the arrangement and procedure were similar to those of WTMT-A, except the Arabic numbers 1-15 were replaced with Arabic numbers (1-8) and Chinese characters (壹, 贰, 叁, 肆, 伍, 陆, 柒). Experimenters instructed the participants as follows: “Please walk on numbered targets in a sequential order as rapidly as possible joining consecutive numbers (i.e., 1 to 壹 to 2 to 贰 to 3 to 叁…8) in the coins randomly distributed on the floor.”
The arrangements of numbers in the WTMT are detailed in Figure 1 and were similar to the method reported by Schott et al[16].
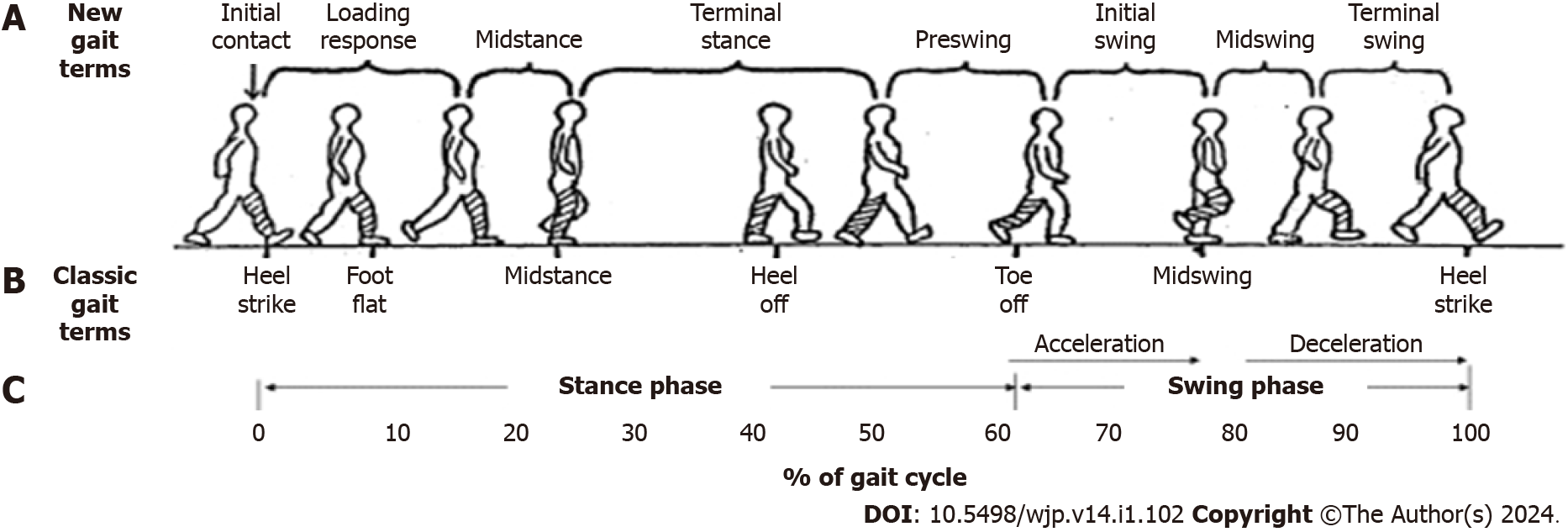
Participants’ gait characteristics during the WTMT were captured and analyzed using the Intelligent Device for Energy Expenditure and Activity (IDEEA) (Minisun, United States). The IDEEA comprises five motion sensors and a microcomputer. The device was calibrated and used as indicated by the manufacturer and as depicted previously[1].
The parameters captured by the IDEEA are detailed in Figure 2 and listed below: (1) Speed (m/s), as the mean velocity for two successive strides; (2) Step length (m), representing the half distance between consecutive points of initial contact of the same foot; (3) Cadence (steps/min), representing the number of steps/stairs per minute; and (4) Stance phase percentage (%), reflecting the duration of the stance phase (starting from initial contact and ending at toe-off for a particular foot) divided by stride time.
All participants completed a series of neuropsychological assessments, including the MMSE (reflecting global cognitive level), choice reaction test (CRT, reflecting attention and concentration), digit symbol substitution test (DSST, reflecting processing speed), category verbal fluency test (cVFT, reflecting psychomotor speed, attention, and semantic memory), and auditory verbal learning test-huashan (AVLTh, reflecting immediate memory performance)[19].
Student’s t-test was carried out for comparison of continuous parametric variables. Categorical variables were compared using the Chi-square or Fisher exact test. Partial Pearson correlation analysis was performed to calculate the correlation between the WTMT completion time and neuropsychological performance, controlled for age, sex, and educational level.
As shown in Table 1, age, sex, and educational level were similar between the WMH and HE groups (P > 0.05). The participants in the WMH group had an average Fazekas score of 1.52. Overall, the WMH group performed significantly worse on the CRT (0.51 ± 0.09 s vs 0.44 ± 0.06 s, P = 0.007), VFT (14.2 ± 2.75 vs 16.65 ± 3.54, P = 0.012), and DSST (16.00 ± 2.75 vs 18.40 ± 3.27, P = 0.010) than the HE group. In addition, the WHM group had significantly longer completion times for the WTMT-A (93.00 ± 10.76 s vs 70.55 ± 11.28 s, P < 0.001) and WTMT-B (109.72 ± 12.26 s vs 82.85 ± 7.90 s, P < 0.001; Table 1).
| WMH group (n = 25) | HE group (n = 20) | P value | |
| Age, years | 74.00 ± 5.40 | 75.30 ± 4.26 | 0.385 |
| Male, % | 48% | 35% | 0.392 |
| Education, years | 8.00 ± 2.34 | 8.05 ± 2.28 | 0.943 |
| MMSE, score | 28.00 ± 1.15 | 28.10 ± 1.10 | 0.883 |
| CRT, seconds | 0.51 ± 0.09 | 0.44 ± 0.06 | 0.007 |
| cVFT, words | 14.20 ± 2.75 | 16.65 ± 3.54 | 0.012 |
| DSST, counts | 16.00 ± 2.75 | 18.40 ± 3.27 | 0.010 |
| AVLTh, words | 7.60 ± 1.83 | 8.25 ± 1.20 | 0.178 |
| WTMT-A, seconds | 93.00 ± 10.76 | 70.55 ± 11.28 | < 0.001 |
| WTMT-B, seconds | 109.72 ± 12.26 | 82.85 ± 7.90 | < 0.001 |
| Fazekas, score | 1.52 ± 0.71 | 0.00 ± 0.00 | < 0.001 |
The results for the correlation between WTMT completion time and performance on neuropsychological tests for the WMH group are presented in Table 2. WTMT-A completion time was positively correlated with CRT time (r = 0.460, P = 0.001), while WTMT-B completion time was negatively correlated with VFT (r = -0.391, P = 0.008).
| WTMT-A | WTMT-B | |||
| r value | P value | r value | P value | |
| MMSE | 0.060 | 0.697 | 0.190 | 0.212 |
| CRT | 0.460 | 0.001 | 0.254 | 0.092 |
| cVFT | -0.157 | 0.303 | -0.391 | 0.008 |
| DSST | -0.264 | 0.080 | -0.207 | 0.172 |
| AVLTh | -0.024 | 0.874 | -0.267 | 0.076 |
Furthermore, we explored the gait features during the WTMT, and only speed was found to differ statistically between the two groups (0.803 ± 0.096 vs 0.975 ± 0.050 m/s, P < 0.001). On the WTMT-B, the WMH group exhibited significantly lower speed (0.778 ± 0.111 vs 0.970 ± 0.053 m/s, P < 0.001) and cadence (82.600 ± 4.140 vs 85.500 ± 5.020 steps/m, P = 0.039), as well as a higher stance phase percentage (65.061 ± 1.813% vs 63.513 ± 2.465%, P = 0.019) relative to the HE group (Table 3).
| WMH group (n = 25) | HE group (n = 20) | P value | |
| WTMT-A | |||
| Speed, m/s | 0.803 ± 0.096 | 0.975 ± 0.050 | < 0.001 |
| Step length, m | 0.486 ± 0.035 | 0.484 ± 0.038 | 0.820 |
| Cadence, steps/min | 86.520 ± 5.730 | 89.050 ± 5.671 | 0.127 |
| Stance phase percentage, % | 63.189 ± 1.147 | 63.737 ± 1.231 | 0.130 |
| WTMT-B | |||
| Speed, m/s | 0.778 ± 0.111 | 0.970 ± 0.054 | < 0.001 |
| Step length, m | 0.468 ± 0.041 | 0.473 ± 0.041 | 0.713 |
| Cadence, steps/min | 82.600 ± 4.140 | 85.500 ± 5.020 | 0.039 |
| Stance phase percentage, % | 65.061 ± 1.813 | 63.513 ± 2.465 | 0.019 |
The clinical presentation of WMH can be asymptomatic, silent, or covert[20] until a threshold is reached and “malignant” symptoms (such as stroke) appear[21]. Thus, much research effort has been devoted to identifying indicators for earlier recognition of WMH[19]. The present study revealed that patients with WMH exhibited remarkably worse performance on the WTMT compared with healthy individuals, as reflected in by the completion times for both the WTMT-A and WTMT-B. Considering its simplicity, non-invasiveness, and low cost, the WTMT represents a potentially useful assessment tool for patients with WMH.
Our previous studies confirmed that patients with WMH display cognitive deficits and gait abnormalities[11,22]. According to the consensus on shared measures of mobility and cognition from the Canadian Consortium on Neurodegeneration in Aging[23], both the TMT and the dual task gait speed task were included as proposed “core battery” tests. These findings, together with those of the present study, support the modification of the TMT into the WTMT, for evaluation of characteristics in aspects of gait and cognition. Furthermore, as the WTMT was designed to be an incorporated cognitive task, instead of an addition to a motor task, WTMT performance could be a better indicator of cognitive impairment than gait or cognitive tests alone[24].
The current study also investigated the relationship between WTMT completion time and cognitive function in older adults with WMH. Our analyses showed that the WTMT-A completion time was correlated with attention and concentration, while the WTMT-B completion time was correlated with psychomotor speed, attention, and semantic memory. These results should not be surprising, as the WTMT, a type of cognitive-motor dual task, is considered a useful “brain stress test” for predicting cognitive deficits[25]. For example, Perrochon and Kemoun[14] reported that poor WTMT performance is associated with executive dysfunction (in particular, mental flexibility) in patients with mid-cognitive impairment. Among community-dwelling older adults, Osuka et al[13] discovered that the WTMT completion time is associated with a series of executive functions, such as performance on the DSST and the traditional TMT. The disruption of crucial subcortical connections in the frontal and other lobes, as well as the basal ganglia area, following multiple pathophysiological changes could be the possible mechanism through which WMH affect cognition and WTMT per-formance[26,27].
To the best of our knowledge, this is the first study to assess the utility of a wearable sensor for gait analysis during the WTMT. Gait speed was not the only parameter found to be affected in the WTMT-B. Older people with WMH exhibited significantly lower speed and cadence, as well as a higher stance phase percentage. The discrepancy between the WTMT-A and WTMT-B might also imply that WTMT-B performance reflects sophisticated processing and problem solving aspects of executive functioning, which may be necessary to deal with more challenging terrain[28]. Similar trends were also reported for the traditional WTMT and other variations of the TMT[29].
Several limitations of the present study warrant consideration. First, the sample size was small. Second, some aspects, such as delayed recall of the AVLTh, were not chosen in the present study, because patients with WMH were previously found to not show deficits in this domain[30]. In addition, 3T-WMH volume should be used to quantify WMH in future research.
Notably, the TMT has been modified in different ways by multiple research groups previously (e.g., WTMT, oral TMT[31], driving TMT[32]), and alternative evaluation systems for the TMT also have been reported (e.g., error analysis[33], derived TMT indices[34,35]). From our point of view, delta TMT is a good indicator of executive function. Thus, delta WTMT might be another effective tool for detecting the cognitive profile of WMH and neuropsychological features of subcortical vascular dementia in the future.
In the present study, older adults with WMH showed obviously poorer WTMT performance than healthy control participants. The WTMT completion time was associated with aspects of cognitive function. Therefore, WTMT performance represents a potential indicator for early identification of the cognitive and mobility decline induced by WMH.
The early detection of the white matter hyperintensities (WMH) is difficult in clinical practice, and dual task has been confirmed as a useful tool.
Trail making test (TMT), a commonly used paper-and-pencil cognitive function test, is now modified into different versions. Walking TMT (WTMT) is a modified TMT incorporates a cognitive task and concurrent walking.
The aim of the current study was to assess the gait characteristics of elderly individuals with WMH using the WTMT task.
The WTMT was conducted in a 16 m2 square area (4 m × 4 m). Each participant need to walk according to the coins randomly distributed as TMT-A and TMT-B to complete this task.
The WMH group also required significantly more time to complete the WTMT-A and WTMT-B.
Older adults with WMH showed obviously poorer WTMT performance.
Notably, the TMT has been modified in different ways by multiple research groups previously (e.g., WTMT, oral TMT, driving TMT), and alternative evaluation systems for the TMT also have been reported (e.g., error analysis, derived TMT indices). From our point of view, delta TMT is a good indicator of executive function. Thus, delta WTMT might be another effective tool for detecting the cognitive profile of WMH and neuropsychological features of subcortical vascular dementia in the future.
We thank Mr. Cheng-Gang Gu for technical support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arboix A, Spain; Bernstein HG, Germany S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY
| 1. | Zhào H, Wei W, Xie H, Huang Y. Motoric Cognitive Risk Syndrome Among Chinese Older Adults with White Matter Lesions: A Cross-Sectional Observational Study. J Alzheimers Dis. 2023;91:925-931. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Lauretani F, Longobucco Y, Ferrari Pellegrini F, De Iorio AM, Fazio C, Federici R, Gallini E, La Porta U, Ravazzoni G, Roberti MF, Salvi M, Zucchini I, Pelà G, Maggio M. Comprehensive Model for Physical and Cognitive Frailty: Current Organization and Unmet Needs. Front Psychol. 2020;11:569629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Kelaiditi E, Cesari M, Canevelli M, van Kan GA, Ousset PJ, Gillette-Guyonnet S, Ritz P, Duveau F, Soto ME, Provencher V, Nourhashemi F, Salvà A, Robert P, Andrieu S, Rolland Y, Touchon J, Fitten JL, Vellas B; IANA/IAGG. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. 2013;17:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 697] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 4. | Verghese J, Ayers E, Barzilai N, Bennett DA, Buchman AS, Holtzer R, Katz MJ, Lipton RB, Wang C. Motoric cognitive risk syndrome: Multicenter incidence study. Neurology. 2014;83:2278-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Jokinen H, Laakso HM, Ahlström M, Arola A, Lempiäinen J, Pitkänen J, Paajanen T, Sikkes SAM, Koikkalainen J, Lötjönen J, Korvenoja A, Erkinjuntti T, Melkas S. Synergistic associations of cognitive and motor impairments with functional outcome in covert cerebral small vessel disease. Eur J Neurol. 2022;29:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Rudilosso S, Rodríguez-Vázquez A, Urra X, Arboix A. The Potential Impact of Neuroimaging and Translational Research on the Clinical Management of Lacunar Stroke. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 7. | Moran C, Phan TG, Srikanth VK. Cerebral small vessel disease: a review of clinical, radiological, and histopathological phenotypes. Int J Stroke. 2012;7:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Siejka TP, Srikanth VK, Hubbard RE, Moran C, Beare R, Wood A, Phan T, Callisaya ML. Frailty and Cerebral Small Vessel Disease: A Cross-Sectional Analysis of the Tasmanian Study of Cognition and Gait (TASCOG). J Gerontol A Biol Sci Med Sci. 2018;73:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Taylor ME, Lord SR, Delbaere K, Wen W, Jiang J, Brodaty H, Kurrle SE, Stefanie Mikolaizak A, Close JCT. White matter hyperintensities are associated with falls in older people with dementia. Brain Imaging Behav. 2019;13:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Del Brutto OH, Rumbea DA, Recalde BY, Mera RM. The association between white matter hyperintensities of presumed vascular origin and disability is mediated by age: a population-based study in stroke-free older adults. Aging Clin Exp Res. 2023;35:887-892. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Ma R, Zhào H, Wei W, Liu Y, Huang Y. Gait characteristics under single-/dual-task walking conditions in elderly patients with cerebral small vessel disease: Analysis of gait variability, gait asymmetry and bilateral coordination of gait. Gait Posture. 2022;92:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Piche E, Chorin F, Gerus P, Jaafar A, Guerin O, Zory R. Effects of age, sex, frailty and falls on cognitive and motor performance during dual-task walking in older adults. Exp Gerontol. 2023;171:112022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Osuka Y, Kojima N, Sakurai R, Watanabe Y, Kim H. Reliability and construct validity of a novel motor-cognitive dual-task test: A Stepping Trail Making Test. Geriatr Gerontol Int. 2020;20:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Perrochon A, Kemoun G. The Walking Trail-Making Test is an early detection tool for mild cognitive impairment. Clin Interv Aging. 2014;9:111-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Yamada M, Ichihashi N. Predicting the probability of falls in community-dwelling elderly individuals using the trail-walking test. Environ Health Prev Med. 2010;15:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Schott N, El-Rajab I, Klotzbier T. Cognitive-motor interference during fine and gross motor tasks in children with Developmental Coordination Disorder (DCD). Res Dev Disabil. 2016;57:136-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2813] [Cited by in RCA: 3619] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
| 18. | Blumen HM, Allali G, Beauchet O, Lipton RB, Verghese J. A Gray Matter Volume Covariance Network Associated with the Motoric Cognitive Risk Syndrome: A Multicohort MRI Study. J Gerontol A Biol Sci Med Sci. 2019;74:884-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Zhào H, Teulings HL, Xia C, Huang Y. Aged Patients With Severe Small Vessel Disease Exhibit Poor Bimanual Coordination During the Anti-Phase Horizontal Line Drawing Task. Percept Mot Skills. 2023;130:750-769. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Clancy U, Appleton JP, Arteaga C, Doubal FN, Bath PM, Wardlaw JM. Clinical management of cerebral small vessel disease: a call for a holistic approach. Chin Med J (Engl). 2020;134:127-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Lau AYL, Ip BYM, Ko H, Lam BYK, Shi L, Ma KKY, Au LWC, Soo YOY, Leung TWH, Wong A, Mok VCT. Pandemic of the aging society - sporadic cerebral small vessel disease. Chin Med J (Engl). 2021;134:143-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Zhào H, Wei W, Do EY, Huang Y. Assessing Performance on Digital Clock Drawing Test in Aged Patients With Cerebral Small Vessel Disease. Front Neurol. 2019;10:1259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Montero-Odasso M, Almeida QJ, Bherer L, Burhan AM, Camicioli R, Doyon J, Fraser S, Muir-Hunter S, Li KZH, Liu-Ambrose T, McIlroy W, Middleton L, Morais JA, Sakurai R, Speechley M, Vasudev A, Beauchet O, Hausdorff JM, Rosano C, Studenski S, Verghese J; Canadian Gait and Cognition Network. Consensus on Shared Measures of Mobility and Cognition: From the Canadian Consortium on Neurodegeneration in Aging (CCNA). J Gerontol A Biol Sci Med Sci. 2019;74:897-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 24. | Osuka Y, Kim H, Watanabe Y, Taniguchi Y, Kojima N, Seino S, Kawai H, Sakurai R, Inagaki H, Awata S, Shinkai S. A Stepping Trail Making Test as an Indicator of Cognitive Impairment in Older Adults. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Osuka Y, Kim H, Watanabe Y, Taniguchi Y, Kojima N, Seino S, Kawai H, Sakurai R, Inagaki H, Awata S, Shinkai S. A combined stepping and visual tracking task predicts cognitive decline in older adults better than gait or visual tracking tasks alone: a prospective study. Aging Clin Exp Res. 2021;33:1865-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Biesbroek JM, Weaver NA, Biessels GJ. Lesion location and cognitive impact of cerebral small vessel disease. Clin Sci (Lond). 2017;131:715-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 27. | Joutel A, Chabriat H. Pathogenesis of white matter changes in cerebral small vessel diseases: beyond vessel-intrinsic mechanisms. Clin Sci (Lond). 2017;131:635-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Alexander NB, Ashton-Miller JA, Giordani B, Guire K, Schultz AB. Age differences in timed accurate stepping with increasing cognitive and visual demand: a walking trail making test. J Gerontol A Biol Sci Med Sci. 2005;60:1558-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Guo Y. A selective review of the ability for variants of the Trail Making Test to assess cognitive impairment. Appl Neuropsychol Adult. 2022;29:1634-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 30. | Tao W, Liu J, Ye C, Kwapong WR, Wang A, Wang Z, Chen S, Liu M. Relationships between cerebral small vessel diseases markers and cognitive performance in stroke-free patients with atrial fibrillation. Front Aging Neurosci. 2022;14:1045910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 31. | Mrazik M, Millis S, Drane DL. The oral trail making test: effects of age and concurrent validity. Arch Clin Neuropsychol. 2010;25:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Lee S, Lee JA, Choi H. Driving Trail Making Test part B: a variant of the TMT-B. J Phys Ther Sci. 2016;28:148-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Mahurin RK, Velligan DI, Hazleton B, Mark Davis J, Eckert S, Miller AL. Trail making test errors and executive function in schizophrenia and depression. Clin Neuropsychol. 2006;20:271-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Drane DL, Yuspeh RL, Huthwaite JS, Klingler LK. Demographic characteristics and normative observations for derived-trail making test indices. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:39-43. [PubMed] |
| 35. | Hobert MA, Meyer SI, Hasmann SE, Metzger FG, Suenkel U, Eschweiler GW, Berg D, Maetzler W. Gait Is Associated with Cognitive Flexibility: A Dual-Tasking Study in Healthy Older People. Front Aging Neurosci. 2017;9:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |