Published online Sep 19, 2023. doi: 10.5498/wjp.v13.i9.630
Peer-review started: May 24, 2023
First decision: June 12, 2023
Revised: June 20, 2023
Accepted: July 14, 2023
Article in press: July 14, 2023
Published online: September 19, 2023
Processing time: 114 Days and 9.2 Hours
Exosomal miRNAs play crucial roles in many central nervous system diseases. Cerebral small vessel disease (CVSD) is a small vessel disease that is affected by various factors. This study aimed to investigate the role of exosomal miR-320e in the Wnt/β-catenin pathway stimulated by oxidative stress and assess its clinical correlation with psychiatric symptoms in patients with CVSD.
To explore whether exosomal miR-320e could suppress the Wnt/β-catenin pathway and play a protective role in CVSD progression, as well as examine its potential correlation with cognitive impairment and depression in patients with CVSD.
Differentially expressed exosomal miRNAs were filtered by sequencing plasma exosomes from patients with CVSD and healthy controls. Bioinformatics and dual luciferase analyses were used to confirm the binding of miR-320e to Wnt2, and the mRNA and protein levels of downstream components in the Wnt/β-catenin pathway were evaluated when overexpressed or with knockdown of miR-320e under H2O2-induced oxidative stress. In addition, Wnt2-targeting siRNA was used to confirm the role of miR-320e in the Wnt2-mediated inhibition of the Wnt/β-catenin pathway. A retrospective analysis was conducted among patients with CVSD to confirm the correlation between miR-320e expression and the severity of cognitive impairment and depression, which were quantified using the Montreal Cognitive Assessment (MoCA)/Executive Function Assessment (EFA), and the Hamilton Depression Scale (HAMD)/Beck Depression Inventory (BDI), respectively.
High-throughput sequencing revealed that exosomal miR-320e was downregulated in patients with CVSD. Bioinformatics analysis and dual-luciferase reporter gene experiments showed that exosomal miR-320e inhibited the Wnt/β-catenin pathway in response to oxidative stress by targeting the 3' noncoding region of Wnt2. Uptake of exosomes carrying miR-320e into endothelial cells could also target Wnt2 and inhibit the Wnt2/β-catenin pathway. Elevated miR-320e expression may protect patients with CVSD from relatively severe cognitive impairment and depression, as it was found to have a positive correlation with the MoCA/EFA and HAMD/BDI scores.
Our results suggest that exosomal miR-320e suppresses the Wnt/β-catenin pathway and may play a protective role in CVSD progression.
Core Tip: Exosomal miR-320e is downregulated in patients with cerebral small vessel disease (CVSD), and it inhibits the Wnt/β-catenin pathway by targeting Wnt2 in response to oxidative stress. Uptake of exosomes carrying miR-320e can also target Wnt2 and inhibit the Wnt2/β-catenin pathway. Elevated miR-320e expression may protect patients with CVSD from severe cognitive impairment and depression, as it correlates positively with Montreal Cognitive Assessment/Executive Function Assessment and Hamilton Depression Scale/Beck Depression Inventory scores. Therefore, exosomal miR-320e may play a protective role in CVSD progression by suppressing the Wnt/β-catenin pathway, indicating its potential as a therapeutic target for CVSD.
- Citation: Wang Z, Li XN, Yang SN, Wang Y, Gao KJ, Han B, Ma AJ. Exosomal miR-320e through wnt2targeted inhibition of the Wnt/β-catenin pathway allevisate cerebral small vessel disease and cognitive impairment. World J Psychiatry 2023; 13(9): 630-644
- URL: https://www.wjgnet.com/2220-3206/full/v13/i9/630.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i9.630
Stroke is the deadliest disease in China and the second most disabling disease worldwide, and its socioeconomic burden has increased dramatically in the last three decades[1]. Cerebral small vessel disease (CVSD) accounts for 20%–30% of ischemic stroke cases. CVSD is a series of clinical, imaging, and pathological syndromes caused by various etiologies that affect the small arteries, microarteries, capillaries, microvenules, and small veins in the brain[2]. White matter and deep gray matter damage caused by CVSD is also considered a major cause of cognitive impairment due to damaged crosstalk between the arteries, capillaries, and small veins[3]. Currently, conventional anti-hypertensive, lipid regulatory, and antiplatelet aggregation treatments are not effective in improving cognitive and motor function impairments in patients[4,5]. Thus, new therapeutic targets or mechanisms for CVSD are warranted.
Exosomes, presenting as 30-150 nm extracellular vesicles in diameter, carry rich contents to exert their bioinformatics function near or distantly[6-8] and have been demonstrated to play important roles in vascular diseases, such as atherosclerosis, diabetic vasculopathy, and stroke[9-11]. Moreover, miRNAs (a class of 17–24 nt small, noncoding RNAs) have been identified in exosomes and are involved in the biogenesis, release, and uptake of relevant proteins by mediating post-transcriptional gene silencing by binding to target mRNAs[12]. Exosomal miRNAs participate in numerous biological processes during nerve injuries. Exosomal miR-451a plays a protective role against cerebral ischemia/reperfusion injury[13]. Exosomal miR-124a can be transported to glioblastomas to play a tumor-suppressive role[14]. Therefore, in this study we performed high-throughput sequencing analysis of plasma exosomal miRNAs between patients with CVSD and healthy controls and found that exosomal miR-320e, with the most significant difference, was speculated to be a highly promising target relevant to CVSD pathogenesis.
With aging and the presence of other risk factors, the integrity of the vasculature is gradually lost. This affects various cellular pathways, including the Wnt/β-catenin pathway, which is known to regulate vascular neogenesis, vascular remodeling, and blood-brain barrier maturation in response to harmful stimuli[15,16]. The Wnt family of proteins has been reported to play essential roles in the development of the nervous system[17]. miRNAs have been found to regulate vascular function by targeting the Wnt pathway, as evidenced by the involvement of miR-29b in vascular smooth muscle calcification[18] and the regulation of miR-330 in the process of unstable atherosclerotic plaque formation and vascular endothelial cell proliferation[19]. These studies indicate that the inhibition of the Wnt/β-catenin pathway may delay the progression of some diseases, such as cancer[20], axonal degeneration[21], and chronic kidney disease[22]. However, the mechanism by which exosomal miR-320e regulates the Wnt/β-catenin pathway in cerebrovascular disease has not yet been explored.
Among the secretory glycoproteins belonging to the Wnt family, Wnt2 shows potent antidepressant effects and can inhibit the development of depressive disorders, suggesting the direct binding of miR320e to Wnt2[23]. In this study, we assessed the regulatory effects of miRNA-320e on the Wnt/β-catenin pathway by targeting Wnt2 in endothelial cells under oxidative stress. Furthermore, the predictive value of decreased exosomal-derived miR-320e for the clinical deterioration of depression and impaired cognition in CVSD was analyzed in a retrospective study.
In total, 120 blood samples were collected from patients with CVSD and healthy subjects at the Qingdao University Affiliated Hospital between January 2017 and January 2019 (80 patients with CVSD and 40 controls). Baseline information of the patients with CVSD and controls was shown in the Supplementary Material. The inclusion criteria were based on the STRIVE diagnostic criteria, with the following: (1) Magnetic resonance imaging (MRI) manifestations, including lacunar infarctions, white matter lesions, cerebral microbleeds, and perivascular space enlargement > 2 mm and brain atrophy; (2) Clinical symptoms such as dizziness, fatigue, and cognitive changes; and (3) Age > 18 years. Patients with a definite diagnosis of cardiovascular or cerebrovascular diseases, severe head and neck artery stenosis, arteriosclerosis, incomplete brain MRI, blood system diseases or tumors, or severe liver and kidney insufficiencies were excluded. This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University. All specimens were collected after obtaining authorization from the patients and their families and signing a consent form.
Thermo Fisher Scientific's Total Exosome Precipitation Reagent (from plasma) kit (REF4484451; Waltham, Massachusetts, United States Thermo Fisher Scientific) was used to extract the exosomes. Although ultracentrifugation was often noted for the ability to produce highly purified exosome, filtration could more conveniently generate a higher yield. However, both filtration and ultracentrifugation methods had their unique advantages and were well accepted in the area of exosome extraction.
The frozen plasma was thawed at room temperature, and 200 μL was placed in an EP tube and centrifuged at 2000 × g for 20 min at room temperature. The supernatant was collected in a new EP tube at room temperature and centrifuged again at 10000 × g for 20 min. Then, 100 μL of Phosphate-buffered saline (PBS) was added to the resulting supernatant and vortexed. Then, 10 microliters of proteinase K (Thermo Fisher Scientific 4485229) was added, shaken well and incubated at 37 °C for 10 min. Then, 60 microliters of extraction reagent was added to the incubated liquid, fully vortexed until turbid, and incubated at 4 °C for 30 min. Next, the sample was removed from the 4 °C environment and centrifuged it at 10000 × g for 5 min at room temperature. A pale yellow or colorless precipitate was observed, and the supernatant was discarded. Finally, we resuspended the sample in 100 μL of PBS to obtain an exosome solution and proceeded to the next step immediately or stored it at -80 °C.
The morphology of the exosomes was observed by transmission electron microscopy, and the quality of the isolated exosomes was determined by nanoparticle tracking analysis. Exosome surface proteins were examined using western blotting.
Total RNA was extracted using the miRNeasy Serum/Plasma Kit (50) (Cat. No. 217184). Immediately or after thawing the cryopreserved exosome solution, we took 100 microliters for lysis with 500 microliters of TRIzol and incubated at room temperature for 5 min. After addition of 100 μL of chloroform, the sample was vortexed and incubated at room temperature for 3 min. The samples were centrifuged at 12000 × g for 15 min at 4 °C. We transferred the upper part of the aqueous phase after centrifugation into a new EP tube, added absolute ethanol, mixed well, transferred to a spin column, centrifuged at 8000 × g for 15 s, and discarded the filtrate. Then, we added 700 microliters of RWT to the spin column at room temperature, centrifuged at 8000 × g for 15 s, and discarded the filtrate; added 500 microliters of RPE to the spin column at room temperature, centrifuged at 8000xg for 15 s, and discarded the filtrate; added 500 microliters of 80% ethanol, centrifuged the spin column at room temperature at 8000 × g for 2 min, changed the spin column into a new EP tube, and centrifuged at 12000 × g for 5 min for drying. Finally, 14 μL of enzyme-free water was added, and approximately 14 μL of total RNA was obtained by centrifugation at 12000 × g for 1 min after 1 min.
Human umbilical vein endothelial cells (HUVECs), cell models with stem cell potential commonly used for vascular endothelial cell experiments, were cultured in 90% dulbecco's modified eagle medium (DMEM) containing 10% FBS and 1.5% penicillin-streptomycin. All cells were cultured in 5% CO2 at 37 °C in a humidified atmosphere. DMEM, FBS, and penicillin-streptomycin were purchased from BI (BI, United States), and trypsin was purchased from Thermo Scientific. Oxidative stress stimulation was induced using a 1 mmol/L solution of DEME prepared with hydrogen peroxide for 30 min. R-Spondin-1 (the Wnt pathway agonist) and WIF1 (the Wnt pathway inhibitor) were procured from MCE (MCE, United States) using complete medium diluted to 60 and 20 ng/mL, respectively, for 24 h of cell culture before subsequent treatment as needed. The plasmids of wild-type and mutated Wnt2 3’-UTR and Wnt2-targeting siRNA, as well as the miR-320e mimic and inhibitor required for the transfection of HUVECs, were constructed by Gikai Gene (Shanghai Genechem Co, Ltd.).
We used a DiI (DiI perchlorate, C59H97CIN2O4) cell membrane orange-red fluorescent probe to stain the cell and exosome membranes (Yeasen; 40726ES10, China), and absorbance was detected at 550/567 nm.
Cell lysis buffer (Elabscience Biotechnology Co., Ltd., China) for western blotting was used for total protein extraction. Following centrifugation at 4 °C (12000 × g, 15 min), a BCA kit (Elabscience, China) was used to quantify the protein concentrations. Protein samples were separated using polyacrylamide gel electrophoresis and transferred onto membranes (ACE Biotechnology, China). Freshly prepared Tris-buffered saline (TBS) containing 5% nonfat milk was used to block the membranes for 2 h at room temperature. The blots were probed with primary antibodies at 4 °C overnight at a dilution ratio of 1:1000. The primary antibodies used for the detection of Wnt/β-catenin pathway by western blotting were as follows: Wnt2, FZD2, Axin2, GSK3β, and β-catenin (Proteintech Group, Inc, United States). Membranes coated with primary antibodies were washed three times with TBS-0.05% Tween 20, followed by incubation at room temperature with the corresponding secondary antibodies for 1 h. The blots were then incubated in the dark with ECL and visualized by exposure to enhanced chemiluminescence reagents (GE Healthcare, United States). ImageJ software 1.4.3.67 (National Institutes of Health, United States) was used to analyze the grey scale values of the blots.
Total RNA was isolated from the cells using TRIzol Reagent (Invitrogen, United States). Goldenstar RT6 cDNA Synthesis Kit Ver. 2 (TSINGKE, China) was used to synthesize cDNA from the RNA. Wnt2, FZD2, Axin2, GSK3β, and β-catenin were detected by a 2xT5 Fast real-time polymerase chain reaction (PCR) Mix (SYBR Green Ⅰ) kit (TSINGKE, China). Primer design and synthesis were performed by the Sangon Corporation (Shanghai, China), and the primer sequences were listed in the Supplementary Material. The results of RT-PCR analysis were calculated by the − ΔΔCt method.
Patients and Ethics: Using a retrospective analysis, relevant information on patients with CVSD who met the inclusion criteria was collected between January 2022 and December 2022 at the Department of Neurology of Qingdao University Affiliated Hospital. The inclusion criteria were as follows: (1) Aged–50-80 years; (2) Meeting the diagnostic criteria for CVSD according to the Expert Consensus on Diagnosis and Treatment of CVSD in China (2021); and (3) Agreement to participate in this study and signed an informed consent form. The exclusion criteria were as follows: (1) Other neurological or psychiatric diseases affecting cognitive function; (2) Other systemic diseases or medication history affecting vascular function; and (3) Other factors affecting exosome isolation or detection. Healthy volunteers who underwent physical examinations at the health examination center during the same period were enrolled as controls after obtaining informed consent and met the following conditions: (1) No clinical symptoms or signs; (2) No abnormal laboratory or imaging examinations; and (3) No history of chronic diseases or medication use. This study was approved by the ethics committee of our hospital. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Treatments: Standard therapies were provided to patients with CVSD after a comprehensive assessment of their condition. Doctors developed a personalized treatment plan targeting the risk factors for CSVD in each patient, including anti-hypertensive drugs (such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, diuretics, or β-receptor blockers), antiplatelet drugs (such as aspirin, clopidogrel, or ticagrelor), anticoagulation drugs (such as warfarin, dabigatran etexilate, or rivaroxaban), lipid-lowering therapy (such as simvastatin and atorvastatin), and diabetes controlling drugs (such as insulin and metformin). In addition, lifestyle improvements, such as quitting smoking, limiting alcohol intake, maintaining a healthy diet, and engaging in appropriate exercise, were recommended. For cognitive dysfunction related to CVSD, cholinesterase inhibitors and memantine were chosen to improve depression, anxiety, apathy, and psychiatric symptoms.
Psychiatric Assessments: The following assessment scores of patients were collected: (1) Cognitive function assessment, including the Montreal Cognitive Assessment (MoCA) and the Executive Function Assessment (EFA); and (2) Depression symptom assessment, including the Hamilton Depression Scale (HAMD) and the Beck Depression Inventory (BDI). Utilizing the average scores that patients received as the cutoff point, the CVSD population in this study was divided into two groups: Relatively more severe and relatively better disease.
Statistical analysis was performed using SPSS 26.0. Quantitative data were presented as the mean ± SD and were compared using the Wilcoxon test between the two groups, while categorical data were expressed as frequencies (percentages). For western blotting, the mean normalized gray level in the control group was defined as a relative FC of 1. For qRT-PCR, all data were normalized to the FC of the control group. Correlation analysis was performed using the Spearman’s rank correlation coefficient. Statistical significance was set at P < 0.05. The statistical method of this study was reviewed by Ai-Jun Ma from Qingdao University Affiliated Hospital.
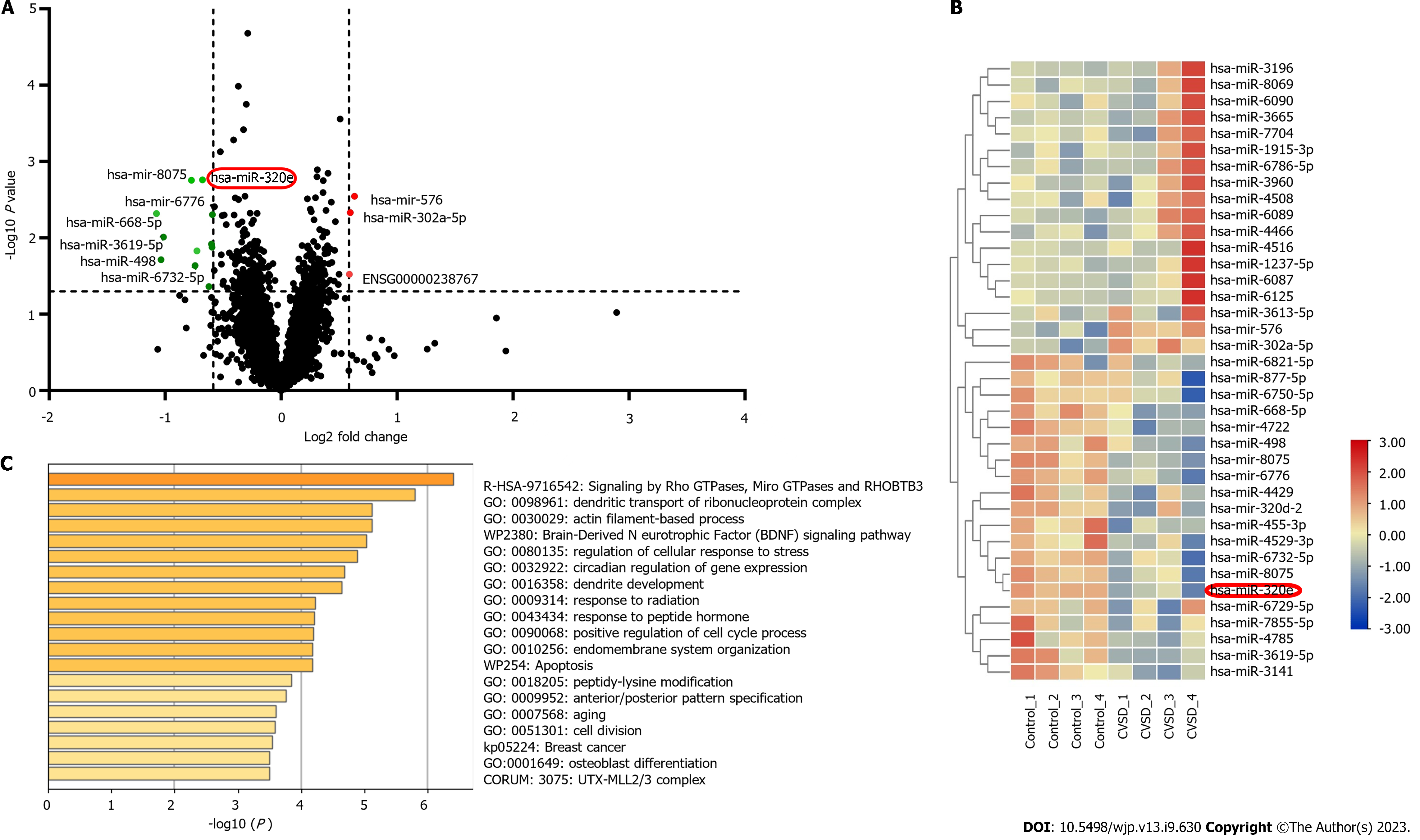
The results of the identification of the extracted exosomes were shown in Supplementary Material. In total, 120 blood samples were collected from 80 patients with CVSD and 40 healthy controls. The expression of miRNAs in plasma exosomes from patients with CVSD and healthy controls was detected by high-throughput detection. As shown in Figure 1A, the results showed that the expression of 38 miRNAs was significantly different in the plasma exosomes of patients with CVSD compared with the controls. Among these differentially expressed miRNAs, 18 miRNAs were significantly upregulated and 20 miRNAs were downregulated. A heatmap of the miRNAs with significant differential expression was shown in Figure 1B. Next, miR320e, miR-498, miR-6776, and miR-455 were verified using real-time PCR. This trend in the real-time PCR results was consistent with that of the miRNA sequencing results. Previous studies have shown that miR-320 regulates the biological behavior of ischemic neurons and changes the state of tissue inflammation and oxidative stress through drug regulation[24,25]. miR-320e was significantly downregulated compared with that in the healthy control group (16.8478, 95%CI = 7.4456–26.2501), and the difference was statistically significant (P < 0.001) (Supplementary Material), indicating that this molecule might play an important role in the pathophysiological process of CVSD.
According to the RNACentral and TargetScan human databases, Wnt2 was one of the target mRNAs of miR-320e. In addition, functional enrichment analysis showed that miR-320e was involved in the regulation of cellular responses to stress, mRNA metabolic processes, cell division, and apoptosis (Figure 1C), which was associated with vascular endothelial structural integrity. In contrast to other differentially expressed miRNAs, miR-320e and its target gene Wnt2 were associated with both leukocyte transendothelial function and vascular structural integrity, leading us to speculate that miR-320e may play an important role in the progression of CVSD development.
To verify the binding relationship between miR-320e and Wnt2, we performed a dual-luciferase reporter assay. The plasmids of wild-type and mutated Wnt2 3’-UTR were constructed and cotransfected with overexpressed miR-320e and control mimics. The results showed that the fluorescence intensity in the WT-Wnt2/miR320e-overexpressed group was significantly reduced (Figure 2A and B), indicating that miR320e specifically bound to Wnt2. A two-factor recovery experiment was conducted to prevent off-target effects. The results showed that the protein expression of Wnt2, Axin2, β-catenin, FZD2, and GSK3β was downregulated in the group that was transfected with the miR-320e mimic. In contrast, the endothelial cells transfected with the inhibitors targeting miR-320e showed higher expression of Wnt2, Axin2, β-catenin, FZD and GSK3β than those in the control group (Figure 2C and D). The fluorescence photos observed after transfection of miR-320e mimic and inhibitor were shown in Figure 2E. These results indicated that miR-320e inhibited the expression of Wnt2 at the post-transcriptional level as well as the activation of downstream β-catenin pathways.
HUVECs were treated by adding H2O2 to the medium to create oxidative stress in the cell culture environment. The expression levels of Wnt2, FZD2, Axin2, GSK3β and total β-catenin in the Wnt/β-catenin pathway were all increased (Figure 3A-C). Oxidized HUVECs were further stimulated with the Wnt pathway inhibitor WIF1 or the agonist R-spondin in parallel with the mock control. We found that the protein expression of Wnt2, FZD2, Axin2, GSK3β and total β-catenin in the WIF1 + H2O2-treated group was similar to that in the WIF1-treated group without oxidative stress, meaning the Wnt2 inhibitor effectively alleviated the H2O2-induced Wnt/β-catenin pathway (Figure 3D-G). Meanwhile, R-spondin activated the Wnt2-mediated β-catenin pathway, and the expression of downstream components in the R-spondin + H2O2 group was enhanced compared to that in the group treated with R-spondin alone (Figure 3D and F-H). The above data indicate that the Wnt/βcatenin pathway and its relevant components were upregulated under H2O2-induced oxidative stress.
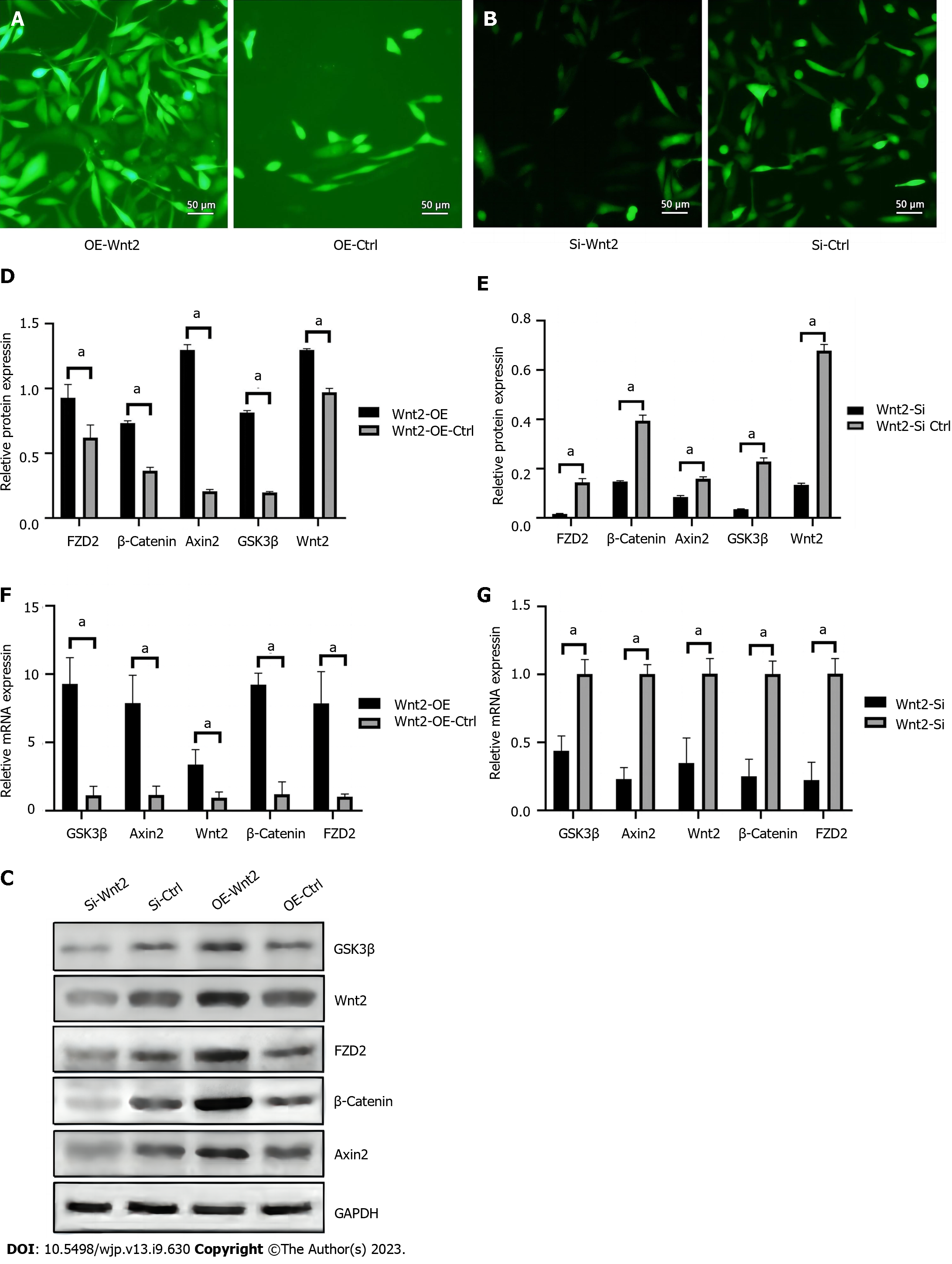
To demonstrate the effects of transfection, we used plasmids or siRNAs with green fluorescent labels and puromycin resistance. The fluorescence images observed after transfection of miR-320e mimics, mimic control, Wnt2-targeting siRNA, and siRNA control were shown in Figure 4A and B. Utilizing H2O2–treated HUVECs, when signaling in the Wnt/β-catenin pathway including Wnt2 was obviously inhibited by miR-320e mimics compared with control mimics, Wnt2-targeting silencing but not the siRNA control reversed the inhibition of the Wnt/β-catenin pathway (Figure 4C). Protein and mRNA expression detected by western blotting and real-time PCR showed similar results under H2O2-induced oxidative stress (Figure 4D-G). These data show that miR-320e inhibited the H2O2–induced Wnt/β-catenin pathway by targeting Wnt2.
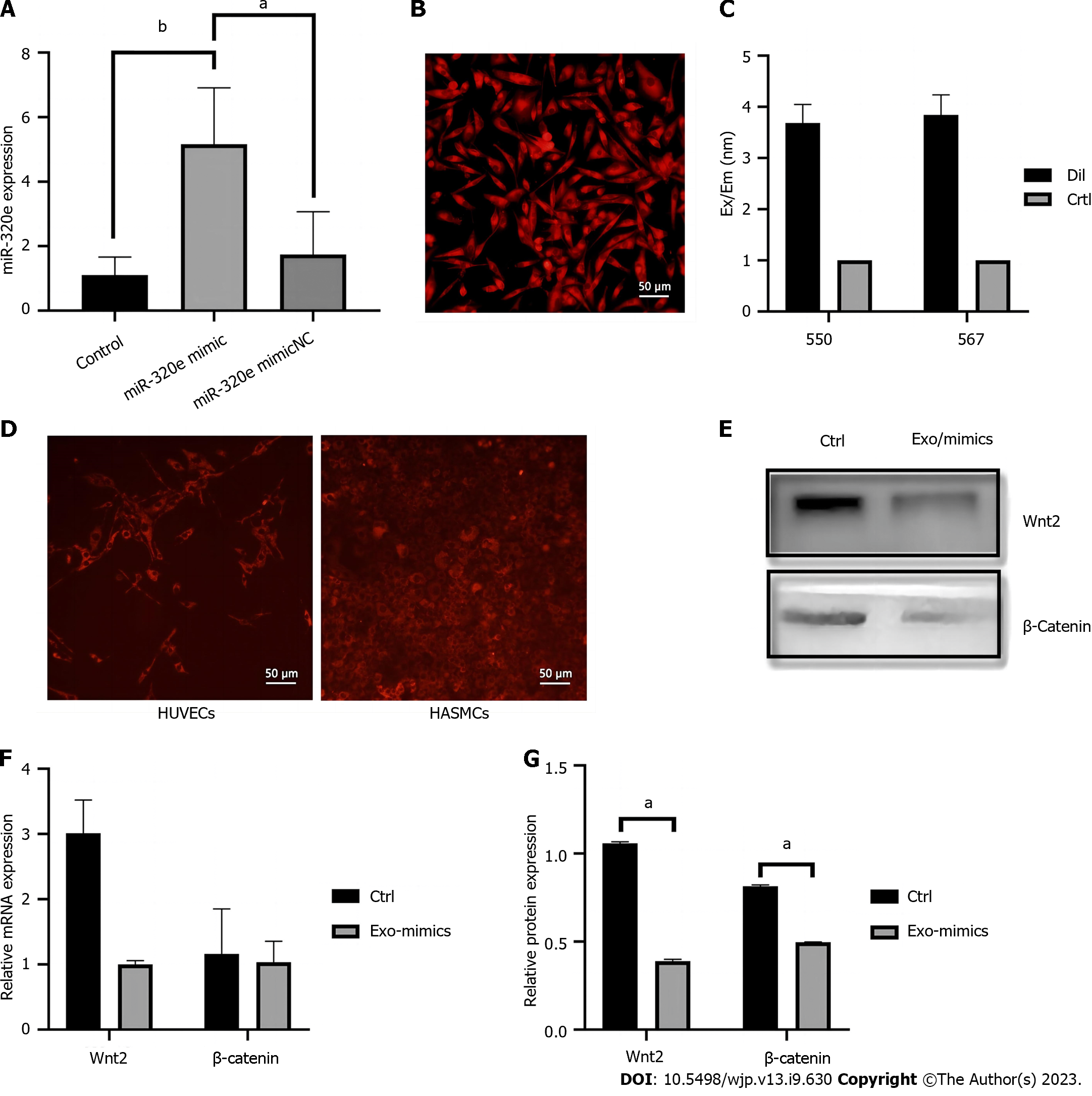
HUVECs were cultured using miR-320e mimics (Figure 5A), and the lipid membranes in the system were stained with DiI, a lipophilic stain with red fluorescence. We collected fresh culture medium and extracted the exosomes secreted by HUVECs to treat another group of HUVECs, and then tested the expression of Wnt2 and β-catenin in the exosome-treated and control groups. The results showed that HUVECs could be stained with DiI (Figure 5B), and we detected the collected exosome suspension in a microplate reader. We observed an increase in the absorbance at 550 and 567 nm (Figure 5C). Using the collected exosomes secreted by DiI-stained endothelial cells to treat unstained and treated endothelial cells, we observed red fluorescence in the unstained cells after exosome treatment. In addition, we used these exosomes to treat human aortic vascular smooth muscle cells (HASMCs) and observed red fluorescence in these HASMCs (Figure 5D), indicating that HUVEC-derived exosomes could be secreted into the extracellular environment and taken up by other cells. In the Exo/miR-320e-mimic group, Wnt2 mRNA and β-catenin protein and mRNA levels were lower than those in the control group (Figure 5E-G). The above information proves that exosomes can be taken up by other cells, and miR-320e loaded into exosomes enters the cells and exerts an inhibitory effect on Wnt/β-catenin pathway activation by targeting Wnt2.
In the same population for exosome extraction, 80 patients with CVSD (42 men and 38 women) with an average age of 66.3 years were included. The average educational level was 9.2 years. Among these patients, 60 patients had hypertension, 24 patients had diabetes, and 36 patients had hyperlipidemia, while the remaining patients did not have respective diseases. Additionally, 28 patients had a history of smoking and 16 patients had a history of alcohol consumption. At the baseline of disease characteristics, 40 patients (50%) had lacunar infarctions, 20 (25%) had cerebral microbleeds, and 20 (25%) had mixed-type CSVD. The white matter hyperintensity burden of the CVSD patient group at enrollment was 12.5 ± 4.2 cm3, and the total CSVD burden score was 3.2 ± 1.1 points.
The correlation between exosomal miR-320e levels and psychiatric symptoms in patients with CVSD was characterized by Pearson’s correlation analysis, which suggested that miR-320e was positively correlated with both cognitive impairmentrelated MoCA/EFA scores and depression-related HAMD/BDI scores (all P < 0.05), as shown in Table 1. Based on the scores for cognitive function and depressive symptoms of patients, we found that patients with relatively severe cognitive impairment and depression had statistically lower expression levels of miR-320e in the extracted exosomes than patients in relatively good condition (all P-values < 0.05), as shown in Table 2.
| Variable | Spearman correlation coefficient | P value |
| MoCA score | 0.328 | 0.003 |
| EFA score | 0.301 | 0.006 |
| HAMD score | -0.296 | 0.007 |
| BDI score | -0.283 | 0.010 |
| Assessment Scale | More severe disease (below the average score) | Less severe disease (beyond the average score) | P value of miRNA-levels | ||
| Patients | miRNA level | Patients | miRNA level | ||
| MoCA | 45 (56.25) | 1.18 ± 0.32 | 35 (43.75) | 2.15 ± 0.45 | 0.010 |
| EFA | 40 (50) | 1.25 ± 0.40 | 40 (50) | 2.30 ± 0.50 | 0.0092 |
| HAMD | 50 (62.5) | 1.10 ± 0.35 | 30 (37.5) | 2.20 ± 0.55 | 0.030 |
| BDI | 38 (47.5) | 1.15 ± 0.38 | 42 (52.5) | 2.25 ± 0.48 | 0.0012 |
The Wnt/β-catenin pathway is involved in the response to oxidative stress stimuli and leads to white matter lesions and central nervous system inflammation and injury[26-28]. Inhibition of the abnormal activation of the Wnt/β-catenin pathway was shown to delay coronary artery disease in the myocardial tissue of rats[29] and the process of renal ischaemia-reperfusion injury involving oxidative stress in the kidney[30]. Inhibition of the Wnt/β-catenin pathway also inhibited the progression of neuroblastoma and attenuated axonal degeneration in Parkinson's disease models[20,21,31]. However, studies on the involvement of the Wnt/β-catenin pathway in CVSD are lacking. Based on the widespreadly applied model of HUVECs in the study of cerebral vasculature, our study showed that miR-320e inhibited the oxidative stress-induced Wnt/β-catenin pathway by targeting Wnt2 in endothelial cells to facilitate a protective role in CVSD. The miR320e expression in patients with CVSD was significantly lower in those with relatively severe cognitive impairment and depression, with a positive correlation with both MoCA/EFA and HAMD/BDI scores. Thus, we speculated that miR-320e could be a potential predictive and therapeutic target for CVSD. However, it was important to note that while HUVECs serve as a useful preliminary model, future investigations employing more directly relevant cell types could potentially further reinforce and expand upon our findings.
Due to the scarcity or absence of smooth muscle content in small arteries, smallvessel diastolic function and substance screening are dependent on endothelial cells to perform normal physiological functions, which are influenced by a combination of factors[32-35]. Recent studies have shown that exosomes transport complex substances that freely reach and cross the blood-brain barrier (BBB), and the miRNAs carried are protected by the exosome membrane from degradation by RNA enzymes so that they can effectively act on target cells. Thus, exosomal miRNAs are considered to affect cerebrovascular disorders by affecting the microenvironment of intracranial vessels[36,37]. Previous studies have found that neuronal-secreted exosomal miR-132 can regulate cerebrovascular integrity[38], exosomal miR-451a may play a protective role in ischemia-reperfusion injury[13], and exosomal miR-124a from mesenchymal stem cells can target and inhibit FOX A2 to suppress glioblastoma[14]. Our study showed that exosomes could carry miR-320e into HUVECs, allowing miR-320e to target Wnt2 to exert inhibition of the Wnt/β-catenin pathway during oxidative stress.
To verify the inhibitory effect of miR-320e on the Wnt/β-catenin pathway, we detected the binding target of Wnt2 and the downstream components of FZD2, Axin2, GSK3β and total cellular β-catenin. Our experiment showed that the oxidative stressinduced enhanced Wnt/β-catenin pathway was significantly inhibited by miR320e, as evidenced by the significant suppression in the above genes. In addition, exosomes carrying miR-320e exerted a similar inhibitory effect on the Wnt/β-catenin pathway. Furthermore, we found that GSK3β was inhibited by miR-320e to a much lesser extent than the other genes in the Wnt/β-catenin pathway. GSK3β is involved in multiple pathways, in addition to its role in the Wnt/β-catenin pathway, representing a class of components that exert pathway interactions during cell signaling[39], which suggests that other miRNAs carried by the experimental exosomes from patients with CVSD may contribute to disease deterioration through other mechanisms.
The Wnt pathway has been suggested to exhibit different responses when subjected to different time frames of oxidative stress, with transiently activated Wnt pathways generally inducing neovascularization and protecting vascular structure and cellular function[40,41], and continuously activated Wnt pathways leading to senescence and basement membrane disruption[42]. In CVSD, when the small vessels that develop lesions are not in critical areas of the white matter or cortex or when CVSD is masked by more severe ischemia and hemorrhage, small vessel lesions can be detected at a more advanced stage, while various aggressive factors may allow sustained activation of Wnt/β-catenin, thus promoting CVSD progression. Because of the ability of exosomal miR-320e to target Wnt2 and thereby inhibit abnormal activation of the Wnt/β-catenin pathway, the significantly lower expression of miR-320e in the plasma exosomes of patients with CVSD may lead to a lack of protection. Moreover, other miRNAs with different expression profiles may exert protective effects when combined with miR-320e, which may guide further investigation and validation.
Normal brain function depends on the integrity of the cerebrovascular system, and several underlying patho-physiological mechanisms have been shown to be involved in CSVD, such as BBB damage, small-vessel stiffness, venous collagen proliferation, inflammation, and myelin damage[43,44]. Endothelial cells are the cornerstone of small vessel function; with the natural onset of aging and the onslaught of various risk factors, intracranial vascular function gradually declines and structural integrity is gradually lost, which has long been seen as a vital process in CSVD[36,45,46]. Currently, there are only a few studies on the mechanisms of action of exosomal miRNAs in CVSD. In summary, using bioinformatics and clinical retrospective analyses, this study predicted and confirmed that the inhibitory role of Wnt2-targeting miR-320e on the Wnt/β-catenin pathway may protect patients with CVSD from relatively severe cognitive impairment and depression, providing a potential novel predictive and therapeutic target for CVSD.
Our study demonstrates that exosomal miR-320e plays a significant role in the pathogenesis of CVSD by regulating the Wnt/β-catenin pathway under oxidative stress. Our findings suggest that exosomal miR-320e may be a potential therapeutic target in CVSD based on its ability to inhibit the Wnt/β-catenin pathway and protect against cognitive impairment and depression. The uptake of exosomes carrying miR-320e can also target Wnt2 and suppress the Wnt2/β-catenin pathway, highlighting the significance of exosomal miRNAs as novel therapeutic agents for CVSD. Further studies are needed to investigate the underlying mechanisms of exosomal miR-320e in CVSD progression and to explore its clinical applications. Overall, this study provides new insights into the complex pathophysiology of CVSD and suggests a promising avenue for developing effective therapies for this devastating disease.
Cerebral small vessel disease (CVSD) is a common neurodegenerative disorder characterized by cognitive impairment and depression. Exosomal miRNAs play an essential role in the pathophysiology of CVSD, and their dysregulation contributes to disease progression. This study aimed to investigate the potential role of exosomal miR-320e in the Wnt/β-catenin pathway stimulated by oxidative stress and its clinical correlation with cognitive impairment and depression in patients with CVSD. The study utilized high-throughput sequencing, bioinformatics analysis, dual-luciferase reporter gene experiments, and retrospective analysis among patients with CVSD. The aim was to explore whether exosomal miR-320e could suppress the Wnt/β-catenin pathway and play a protective role in CVSD progression, as well as examine its potential correlation with cognitive impairment and depression in patients with CVSD. The findings suggest that exosomal miR-320e targets Wnt2 and inhibits the Wnt/β-catenin pathway in response to oxidative stress, potentially playing a protective role in CVSD progression. Elevated miR-320e expression may also correlate with less severe cognitive impairment and depression in patients with CVSD.
CVSD is a complex neurodegenerative disorder that affects cognitive function and mental health, but currently lacks effective treatments. Exosomal miRNAs are emerging as important regulators of physiological and pathological processes in the brain, including CVSD. This study aimed to investigate the potential role of exosomal miR-320e in the Wnt/β-catenin pathway stimulated by oxidative stress and its clinical correlation with cognitive impairment and depression in patients with CVSD. The study utilized high-throughput sequencing, bioinformatics analysis, dual-luciferase reporter gene experiments, and retrospective analysis among patients with CVSD. The aim was to explore whether exosomal miR-320e could suppress the Wnt/β-catenin pathway and play a protective role in CVSD progression, as well as examine its potential correlation with cognitive impairment and depression in patients with CVSD. The findings suggest that exosomal miR-320e has a potentially protective role in CVSD progression and may be a novel therapeutic target. The study provides further insights into the complex pathophysiology of CVSD and highlights the importance of exosomal miRNAs in neurological diseases.
The research objectives of this study were to explore the potential role of exosomal miR-320e in the Wnt/β-catenin pathway stimulated by oxidative stress and its clinical correlation with cognitive impairment and depression in patients with cerebral small vessel disease (CVSD). The study aimed to identify differentially expressed exosomal miRNAs by sequencing plasma exosomes from patients with CVSD and healthy controls, confirm the binding of miR-320e to Wnt2 through bioinformatics and dual luciferase analyses, evaluate the mRNA and protein levels of downstream components in the Wnt/β-catenin pathway when overexpressed or knocked down miR-320e under H2O2-induced oxidative stress, and conduct a retrospective analysis among patients with CVSD to confirm the relationship between miR-320e expression and the severity of cognitive impairment and depression. The study also aimed to investigate whether exosomal miR-320e could inhibit the Wnt/β-catenin pathway and play a protective role in CVSD progression, potentially leading to the development of novel therapeutic targets for this complex neurodegenerative disorder.
The study utilized various methods to investigate the role of exosomal miR-320e in CVSD. Differentially expressed exosomal miRNAs were identified by sequencing plasma exosomes from patients with CVSD and healthy controls. Bioinformatics analysis and dual-luciferase reporter gene experiments were conducted to confirm the binding of miR-320e to Wnt2. The mRNA and protein levels of downstream components in the Wnt/β-catenin pathway were evaluated through overexpression or knockdown of miR-320e under H2O2-induced oxidative stress. Wnt2-targeting siRNA was used to confirm the role of miR-320e in the Wnt2-mediated inhibition of the Wnt/β-catenin pathway. A retrospective analysis was conducted among patients with CVSD to assess the correlation between miR-320e expression and cognitive impairment and depression, which were quantified using the Montreal Cognitive Assessment (MoCA)/Executive Function Assessment (EFA) and the Hamilton Depression Scale (HAMD)/Beck Depression Inventory (BDI), respectively. Overall, these methods provided valuable insights into the potential role of exosomal miR-320e in the pathophysiology of CVSD and its potential clinical significance.
The results of the study indicate that exosomal miR-320e is downregulated in patients with CVSD. Exosomal miR-320e was found to inhibit the Wnt/β-catenin pathway in response to oxidative stress by targeting the 3' noncoding region of Wnt2. Uptake of exosomes carrying miR-320e could also target Wnt2 and inhibit the Wnt2/β-catenin pathway in endothelial cells. The study also found that elevated miR-320e expression may protect patients with CVSD from relatively severe cognitive impairment and depression, as it had a positive correlation with the MoCA/EFA and HAMD/BDI scores. These findings suggest that exosomal miR-320e may have a potentially protective role in CVSD progression and could be a novel therapeutic target for this complex neurodegenerative disorder. Overall, the results provide important insights into the pathophysiology of CVSD and highlight the importance of exosomal miRNAs in neurological diseases.
The study found that exosomal miR-320e is downregulated in patients with CVSD and may play a crucial role in the progression of the disease. Exosomal miR-320e was found to inhibit the Wnt/β-catenin pathway by targeting the 3' noncoding region of Wnt2 in response to oxidative stress. The uptake of exosomes carrying miR-320e could also target Wnt2 and inhibit the Wnt2/β-catenin pathway in endothelial cells. Furthermore, elevated miR-320e expression was found to have a positive correlation with cognitive function and depression scores, suggesting it may protect patients from severe cognitive impairment and depression. The study provides new insights into the pathophysiology of CVSD and highlights the potential clinical significance of exosomal miRNAs in neurological diseases. These findings have implications for the development of novel therapeutic targets for CVSD and may provide hope for patients with this complex neurodegenerative disorder.
The study provides valuable insights into the potential role of exosomal miR-320e in the pathophysiology of CVSD. Future research could investigate the use of exosomal miR-320e as a therapeutic target to prevent or delay the progression of CVSD. Further studies could also investigate the underlying mechanisms by which miR-320e regulates the Wnt/β-catenin pathway and how it affects cognitive function and depression. In addition, the use of animal models could provide further insight into the potential clinical significance of exosomal miRNAs in neurological diseases. Further research could also explore the potential diagnostic and prognostic value of miR-320e in patients with CVSD. Overall, the findings of this study pave the way for future research on the role of exosomal miRNAs in CVSD and other neurodegenerative disorders.
We gratefully acknowledge the support of many individuals who made this study possible and The Affiliated Hospital of Qingdao University for providing the experimental platform.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: McGorry PD, Australia; Wallace D, United States S-Editor: Li L L-Editor: A P-Editor: Chen YX
| 1. | GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4299] [Cited by in RCA: 3596] [Article Influence: 899.0] [Reference Citation Analysis (0)] |
| 2. | Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28:491-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 526] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 3. | Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2376] [Article Influence: 158.4] [Reference Citation Analysis (0)] |
| 4. | Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 1037] [Article Influence: 172.8] [Reference Citation Analysis (0)] |
| 5. | Pavlovic AM, Pekmezovic T, Trajkovic JZ, Tomic G, Cvitan E, Sternic N. Increased risk of cognitive impairment and more severe brain lesions in hypertensive compared to non-hypertensive patients with cerebral small vessel disease. J Clin Hypertens (Greenwich). 2018;20:1260-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Yang B, Chen Y, Shi J. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv Mater. 2019;31:e1802896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 261] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 7. | Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1601] [Cited by in RCA: 1792] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 8. | O'Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21:585-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1071] [Cited by in RCA: 1193] [Article Influence: 238.6] [Reference Citation Analysis (0)] |
| 9. | Wang C, Li Z, Liu Y, Yuan L. Exosomes in atherosclerosis: performers, bystanders, biomarkers, and therapeutic targets. Theranostics. 2021;11:3996-4010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 10. | Chen A, Wang H, Su Y, Zhang C, Qiu Y, Zhou Y, Wan Y, Hu B, Li Y. Exosomes: Biomarkers and Therapeutic Targets of Diabetic Vascular Complications. Front Endocrinol (Lausanne). 2021;12:720466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Chen J, Chopp M. Exosome Therapy for Stroke. Stroke. 2018;49:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 12. | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25833] [Cited by in RCA: 27850] [Article Influence: 1326.2] [Reference Citation Analysis (0)] |
| 13. | Li H, Luo Y, Liu P, Hua W, Zhang Y, Zhang L, Li Z, Xing P, Hong B, Yang P, Liu J. Exosomes containing miR-451a is involved in the protective effect of cerebral ischemic preconditioning against cerebral ischemia and reperfusion injury. CNS Neurosci Ther. 2021;27:564-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Lang FM, Hossain A, Gumin J, Momin EN, Shimizu Y, Ledbetter D, Shahar T, Yamashita S, Parker Kerrigan B, Fueyo J, Sawaya R, Lang FF. Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro Oncol. 2018;20:380-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 15. | Georgakis MK, Malik R, Anderson CD, Parhofer KG, Hopewell JC, Dichgans M. Genetic determinants of blood lipids and cerebral small vessel disease: role of high-density lipoprotein cholesterol. Brain. 2020;143:597-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Mazzoni J, Smith JR, Shahriar S, Cutforth T, Ceja B, Agalliu D. The Wnt Inhibitor Apcdd1 Coordinates Vascular Remodeling and Barrier Maturation of Retinal Blood Vessels. Neuron. 2017;96:1055-1069.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Freese JL, Pino D, Pleasure SJ. Wnt signaling in development and disease. Neurobiol Dis. 2010;38:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Zhang H, Chen J, Shen Z, Gu Y, Xu L, Hu J, Zhang X, Ding X. Indoxyl sulfate accelerates vascular smooth muscle cell calcification via microRNA-29b dependent regulation of Wnt/β-catenin signaling. Toxicol Lett. 2018;284:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Sun J, Yan P, Chen Y, Yang J, Xu G, Mao H, Qiu Y. MicroRNA-26b inhibits cell proliferation and cytokine secretion in human RASF cells via the Wnt/GSK-3β/β-catenin pathway. Diagn Pathol. 2015;10:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Vallée A, Lecarpentier Y, Vallée JN. Curcumin: a therapeutic strategy in cancers by inhibiting the canonical WNT/β-catenin pathway. J Exp Clin Cancer Res. 2019;38:323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | Huang YL, Zhang JN, Hou TZ, Gu L, Yang HM, Zhang H. Inhibition of Wnt/β-catenin signaling attenuates axonal degeneration in models of Parkinson's disease. Neurochem Int. 2022;159:105389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Edeling M, Ragi G, Huang S, Pavenstädt H, Susztak K. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat Rev Nephrol. 2016;12:426-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 325] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 23. | Zhou WJ, Xu N, Kong L, Sun SC, Xu XF, Jia MZ, Wang Y, Chen ZY. The antidepressant roles of Wnt2 and Wnt3 in stress-induced depression-like behaviors. Transl Psychiatry. 2016;6:e892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Xiao C, Yu Y, Liu Y, Yang J. Aerosol inhalation of edaravone can improve inflammation, oxidative stress and pulmonary function of rats with smoke inhalation injury by down-regulating miR-320. Am J Transl Res 2021; 13: 2563-2570. |
| 25. | Shen W, Lu Y, Hu J, Le H, Yu W, Xu W, Zheng J. Mechanism of miR-320 in Regulating Biological Characteristics of Ischemic Cerebral Neuron by Mediating Nox2/ROS Pathway. J Mol Neurosci. 2020;70:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Haider L, Fischer MT, Frischer JM, Bauer J, Höftberger R, Botond G, Esterbauer H, Binder CJ, Witztum JL, Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914-1924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 446] [Cited by in RCA: 541] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 27. | Niu J, Tsai HH, Hoi KK, Huang N, Yu G, Kim K, Baranzini SE, Xiao L, Chan JR, Fancy SPJ. Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation. Nat Neurosci. 2019;22:709-718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 28. | Vallée A, Lecarpentier Y. Crosstalk Between Peroxisome Proliferator-Activated Receptor Gamma and the Canonical WNT/β-Catenin Pathway in Chronic Inflammation and Oxidative Stress During Carcinogenesis. Front Immunol. 2018;9:745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 289] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 29. | Li X, Hou L, Cheng Z, Zhou S, Qi J, Cheng J. Overexpression of GAS5 inhibits abnormal activation of Wnt/β-catenin signaling pathway in myocardial tissues of rats with coronary artery disease. J Cell Physiol. 2019;234:11348-11359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Xu Y, Jiang W, Zhong L, Li H, Bai L, Chen X, Lin Y, Zheng D. circ-AKT3 aggravates renal ischaemia-reperfusion injury via regulating miR-144-5p /Wnt/β-catenin pathway and oxidative stress. J Cell Mol Med. 2022;26:1766-1775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Jo S, Im SH, Seo D, Ryu H, Kim SH, Baek D, Baek A, Cho SR. Low-frequency repetitive magnetic stimulation suppresses neuroblastoma progression by downregulating the Wnt/β-catenin signaling pathway. Bioelectrochemistry. 2022;147:108205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 32. | Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Muñoz Maniega S, Farrall A, Sudlow C, Dennis M, Dhillon B. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 262] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 33. | Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol Rev. 2019;99:21-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1068] [Cited by in RCA: 1459] [Article Influence: 243.2] [Reference Citation Analysis (0)] |
| 34. | Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1535] [Cited by in RCA: 2016] [Article Influence: 288.0] [Reference Citation Analysis (0)] |
| 35. | Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and Dysfunction of the Blood-Brain Barrier. Cell. 2015;163:1064-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 1198] [Article Influence: 133.1] [Reference Citation Analysis (0)] |
| 36. | Wardlaw JM, Doubal FN, Valdes-Hernandez M, Wang X, Chappell FM, Shuler K, Armitage PA, Carpenter TC, Dennis MS. Blood-brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke. 2013;44:525-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 37. | Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 639] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 38. | Xu B, Zhang Y, Du XF, Li J, Zi HX, Bu JW, Yan Y, Han H, Du JL. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. 2017;27:882-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 275] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 39. | Karege F, Perroud N, Burkhardt S, Fernandez R, Ballmann E, La Harpe R, Malafosse A. Protein levels of β-catenin and activation state of glycogen synthase kinase-3β in major depression. A study with postmortem prefrontal cortex. J Affect Disord. 2012;136:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Zhou D, Li Y, Lin L, Zhou L, Igarashi P, Liu Y. Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury in mice. Kidney Int. 2012;82:537-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 41. | Wang Z, Havasi A, Gall JM, Mao H, Schwartz JH, Borkan SC. Beta-catenin promotes survival of renal epithelial cells by inhibiting Bax. J Am Soc Nephrol. 2009;20:1919-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Schunk SJ, Floege J, Fliser D, Speer T. WNT-β-catenin signalling - a versatile player in kidney injury and repair. Nat Rev Nephrol. 2021;17:172-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 43. | Petersen MA, Ryu JK, Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci. 2018;19:283-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 353] [Article Influence: 50.4] [Reference Citation Analysis (1)] |
| 44. | Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 658] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 45. | Kalaria RN, Hase Y. Neurovascular Ageing and Age-Related Diseases. Subcell Biochem. 2019;91:477-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Stevenson SF, Doubal FN, Shuler K, Wardlaw JM. A systematic review of dynamic cerebral and peripheral endothelial function in lacunar stroke vs controls. Stroke. 2010;41:e434-e442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |