Published online Aug 19, 2023. doi: 10.5498/wjp.v13.i8.511
Peer-review started: June 6, 2023
First decision: June 21, 2023
Revised: July 6, 2023
Accepted: July 27, 2023
Article in press: July 27, 2023
Published online: August 19, 2023
Processing time: 71 Days and 20.6 Hours
Alzheimer’s disease (AD) is the most common neurodegenerative disorder, and there are currently no effective drugs to delay progression of the disease. Fer
To investigate the effects of dexmedetomidine (Dex) on ferroptosis in AD mouse hippocampus.
Hippocampal neurons (HNs) HT22 were induced by amyloid β-protein (Aβ) and both in vitro and in vivo AD mouse models were prepared via injections. The cell-counting kit-8 assay and immunofluorescence technique were adopted to determine cell proliferation activity and intracellular Fe2+ levels, and the TBA method and microplate method were employed for malondialdehyde and glutathione measurements, respectively. Hippocampal tissue damage was determined using hematoxylin and eosin and Nissl staining. Mouse learning and memory ability in each group was assessed by the Morris water maze test, and the expression levels of mammalian target of rapamycin (mTOR) signal molecules and ferroptosis-related proteins transferrin receptor 1 (TFR1), SLC7A11 and glutathione peroxidase 4 were examined by western blotting.
Dex enhanced lipid peroxidation and iron influx in mouse HNs in both in vitro and in vivo experiments, while inhibition of the mTOR axis blocked this process. These findings demonstrate that Dex can inhibit ferroptosis-induced damage in mouse HNs by activating mTOR-TFR1 signaling to regulate ferroptosis-associated proteins, thus alleviating cognitive dysfunction in AD mice.
Dex can activate the mTOR-TFR1 axis to inhibit ferroptosis in mouse HNs, thereby improving the learning and memory ability of mice.
Core Tip: Iron death of nerve cells caused by iron overload is an important factor in various neurodegenerative diseases, including Alzheimer’s disease (AD). The classic mammalian target of rapamycin (mTOR) signaling pathway regulates the metabolism of iron ions by regulating transferrin receptor 1 (TFR1), thereby maintaining the intracellular iron balance. It has been shown that dexmedetomidine (Dex) inhibits the release of inflammatory factors and plays a neuroprotective role, thereby improving cognitive dysfunction in elderly rats. The Dex effectively improved hippocampal neuronal loss, cognitive dysfunction, learning and memory abilities in AD mice by regulating the mTOR-TFR1 signaling pathway to reduce iron death.
- Citation: Qiao L, Li G, Yuan HX. Dexmedetomidine mediates the mechanism of action of ferroptosis in mice with Alzheimer’s disease by regulating the mTOR-TFR1 pathway. World J Psychiatry 2023; 13(8): 511-523
- URL: https://www.wjgnet.com/2220-3206/full/v13/i8/511.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i8.511
With the current escalation in global aging, neurodegenerative disorders (NDDs), including Alzheimer’s disease (AD), Parkinson’s disease, and Huntington’s disease, have attracted growing attention from researchers and have become a research hotspot[1]. Ferroptosis is a non-apoptotic programmed cell death associated with cytotoxicity caused by iron-dependent lipid peroxide accumulation, and iron overload is the key to triggering the occurrence of cell ferroptosis[2]. It is reported that iron deposition in brain tissue increases significantly with age, ultimately affecting nerve cell function[3-5]. Immunohistochemical tests confirmed that neuronal ferroptosis caused by iron overload in the cerebellum, cortex and hippocampus was an important factor leading to NDDs. The mammalian target of rapamycin (mTOR) is a classical autophagy-dependent regulatory protein that affects cell death pathways such as autophagy, apoptosis, and ferroptosis[6]. The classic mTOR axis influences iron ion metabolism by modulating transferrin receptor 1 (TFR1), thus maintaining the intracellular iron balance[7]. In addition, the high intracellular Fe2+ level leads to the inhibition of mTOR expression, resulting in intracellular iron homeostasis imbalance and consequently oxidative stress (OS)-induced ferroptosis[8]. Dexmedetomidine (Dex) is a highly selective α2 adrenergic receptor agonist that not only exerts analgesic and sedative effects by inhibiting sympathetic nerve activity, but is also neuroprotective against various brain injuries[9,10]. However, its underlying mechanism is unknown. Several studies have found that, by regulating ferroptosis, Dex can play a protective role in liver injury, myocarditis, cerebral ischemia-reperfusion, and craniocerebral injury[11-13]. In addition, Dex was shown to significantly improve neurocognitive function and effectively inhibit the abnormally high level of OS in NDDs and brain trauma, thus reducing inflammation[14]. Yet, it remains to be confirmed whether and how Dex can be neuroprotective by regulating ferroptosis in AD, a NDD. The experimental results of this study show that Dex can inhibit iron overload by activating the mTOR-TFR1 axis, thus maintaining iron homeostasis, protecting hippocampal neurons (HNs) from ferroptosis, and playing a neuroprotective role in AD mice, which can provide insights into ferroptosis-induced neuronal death.
Forty adult male specific pathogen free C57BL/6 mice, aged 8-10 wk and weighing (23 ± 2) g, were raised under constant temperature (25 ± 1 °C), humidity (60% ± 5%), and an alternating light/dark (12/12 h) cycle. The experiments were carried out after 7 d of adaptive feeding with rat chow and water available ad libitum. All animal experiments were carried out after obtaining approval from the Experimental Animal Ethics Committee of Peking University International Hospital.
Mouse HT22 HNs and dedicated cell culture medium (Procell Life Science & Technology, Wuhan, China); Dex (Chia Tai Tianqing Pharmaceutical Group, Nanjing, China); BCA analysis kit (Boster, China); cell-counting kit-8 (CCK-8; Dojindo, Japan); Perls stain (Thermo Fisher Scientific; United States); Nissl stain (Jiangsu KeyGEN Biotech, Nanjing, China); polyvinylidene fluoride (PVDF) membrane (Millipore, United States); malondialdehyde (MDA) Colorimetric Assay Kit (Abcam, United States); Mito-FerroOrange (Dojindo Molecular Technologies, Tokyo, Japan); 2’,7’-dichlorodihydrofluororescein diacetate (DCFH-DA) fluorescence probe (Solarbio, Beijing); Lipofectamine 2000 (Invitrogen, United States); siRNA mTOR (Shanghai Sangon Biotech, China); specific primary antibodies (Abs) and HRP labeled anti-p-mTOR, TFR1, SLC7A11 and glutathione peroxidase 4 (GPX4), as well as the internal reference GAPDH secondary Ab (Abcam, United States) were used in this study.
The Morris water maze (MWM) system (Noldus, Netherlands); inverted fluorescence biomicroscope (Zeiss, Germany); E0970 Microtome Cryostat (Beyotime Biotech, Beijing, China); enhanced chemiluminescence (ECL) detection system (Bio-Rad, Hercules, CA, United States); Multiskan FC microplate reader (Thermo Scientific, United States); western blot Electrophoresis Instrument and Trans-Blot instrument (Bio-Rad, United States) were used in this study.
Forty C57BL/6 mice were randomized to the sham, amyloid β-protein (Aβ), Dex, and Dex + rapamycin (RAPA) groups, with 10 mice in each group. After anesthesia by intraperitoneal injection of 10% chloral hydrate (dosage: 0.1 mL/10 g body weight) in all C57BL/6 mice, 5 μL of condensed Aβ (80 pmol/μL) was injected as a single bolus into mouse bilateral ventricles using a micro-sampler, and the needle was left in place for 2 min before bandaging. The sham group was injected with an equal amount of normal saline into the bilateral ventricles. The Dex group was intraperitoneally injected with Dex (dosage: 20 μg/kg) dissolved in 0.9% sterile saline, once a day for 4 consecutive weeks; on this basis, the Dex + RAPA group was additionally injected with 2 mg/kg of RAPA dissolved in 2% dimethylsulfoxide. The sham and Aβ groups were given the same amount of 0.9% sodium chloride solution, and the remaining treatment was the same as above.
After the above treatment, the mice underwent the MWM test for 5 d. During the training phase (the first 4 d), the mice were trained once a day at a fixed time, and the time they took to find the platform within 120 s (i.e., escape latency) and the swimming trajectory map were recorded. On the fifth day, the platform was removed for spatial exploration experiments, and the running trajectory, escape latency, number of platform crossings, and target quadrant residence time were recorded.
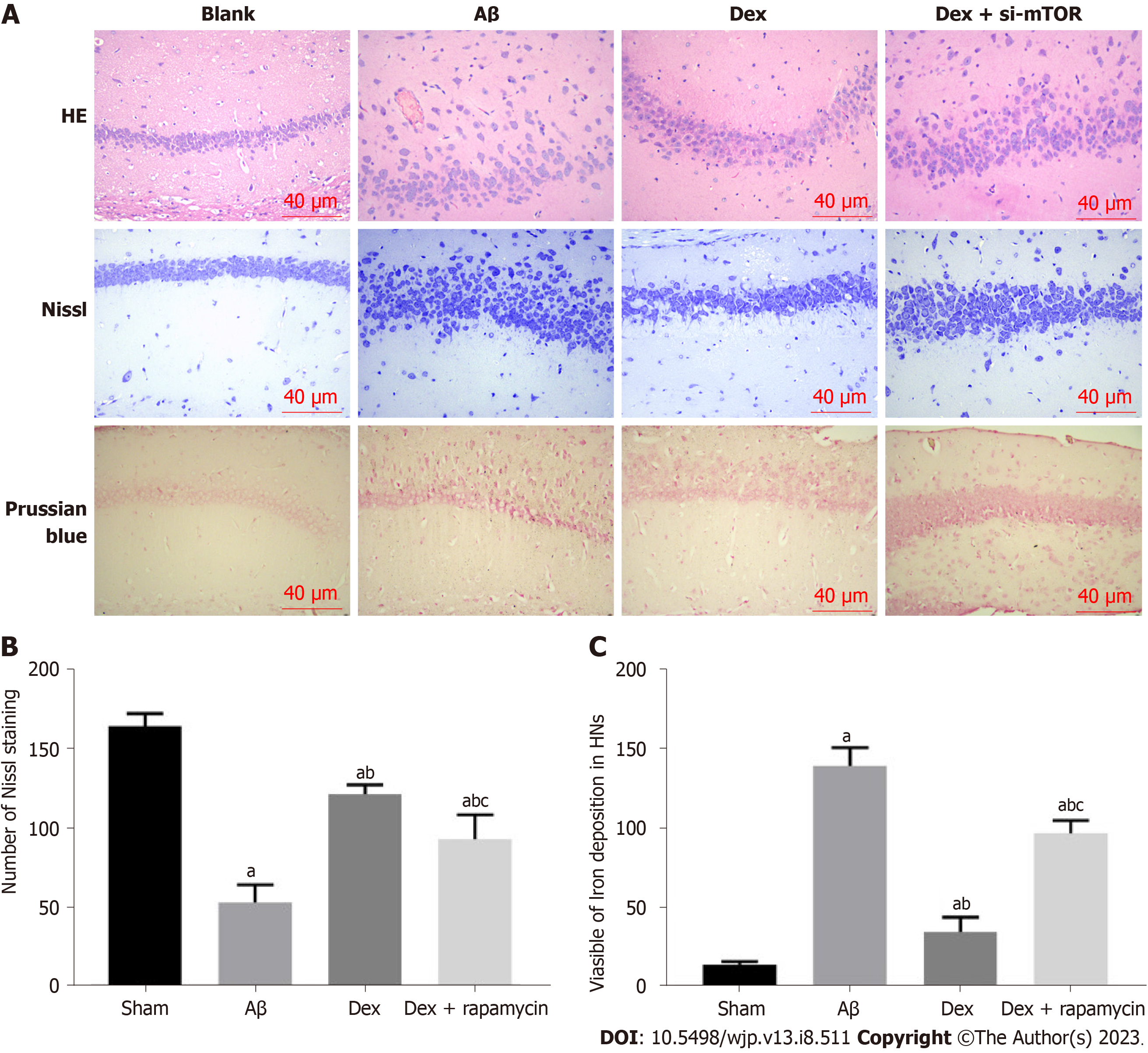
The mice were anesthetized with chloral hydrate after the MWM test. The right auricle was then cut open and the left ventricle was perfused with 4% paraformaldehyde solution. After sacrifice on ice by cervical dislocation, the mouse hippocampus tissues on both sides were quickly removed, some of which was stored in liquid nitrogen for later use. The remaining portion was fixed in 4% paraformaldehyde, and after gradient dehydration with ethanol, it was embedded in paraffin and sliced into sections (4 μm). The pathological changes of HNs were observed under a light microscope after conventional hematoxylin-eosin (HE) staining.
Part of the hippocampus tissue was frozen in liquid nitrogen to prepare frozen sections (5 μm), which were degreased with a gradient concentration of ethanol solution and then washed three times with ddH2O for 30 s each time. Cresyl violet (Nissl) dye solution (0.1%) was added dropwise and left at 56 °C for 1 h. After dyeing, the slices were washed with ddH2O for a further three times. Next, color separation was carried out for 2 min with acid alcohol differentiation solution (0.5%), followed by immersion in 100% ethanol for 30 s and xylene permeation for 1 min. After mounting with neutral gum, each slice was observed microscopically (400 ×) with five visual fields randomly selected, and the number of nerve cells was recorded (n = 5).
Paraffin slices were routinely dewaxed and rinsed with distilled water for 1 min. Perls staining solution, which was homogeneously formulated by mixing reagent A1 with reagent A2 in equal amounts, was used immediately after preparation. The sections were washed again with distilled water after 30 min of Perls staining. They were then nucleated with the nuclear fast red stain kit (reagent B) for 10 min and rinsed with distilled water, followed by conventional dehydration, vitrification, neutral-gum mounting, and air drying. The Prussian blue deposition in hippocampal tissue was observed under a light microscope. Five visual fields in each slice were randomly photographed, and the percentage of Prussian blue staining (PBS) positive area in the whole area was measured by image analysis.
Mouse HT22 HNs were immersed in HT22 dedicated culture medium and cultivated in a 37 °C, 5% CO2, and 95% humidified cell incubator, and the well-grown cells were selected for the following experiment. All neuron cell lines used were within 20 passages. Grouping: (1) Blank control group: HT22 cells was cultured with HT22-dedicated medium without any other treatment; (2) Aβ model group: HT22 cells were treated with Aβ at a final concentration of 20 μmol/L and cultured for 24 h; (3) Dex group: Following 2 h of treatment with Dex at a final concentration of 5 μmol/L, HT22 cells were cultured with Aβ at a final concentration of 20 μmol/L for 24 h; and (4) Dex + mTOR siRNA (si-mTOR) group: After transfection with si-mTOR using Lipofectamine 2000, HT22 cells were treated with 5 μmol/L Dex for 2 h and then cultured with 20 μmol/L Aβ for 24 h.
HT22 cells (1 × 105/mL) were seeded with 100 μL of cell suspension per well on a 96-well culture plate and cultured for 24 h. After different treatments, the CCK-8 reagent was added at 10 μL/well for 1 h at 37 °C, after which the absorbance value at 450 nm was measured by a microplate reader to assess cell viability.
Intracellular Fe2+ levels were measured with Mito-FerroOrange. After different cell treatments, the supernatant was discarded. Following three rinses with HBSS, 2 mL of cells were added to every 2 μL of Mito-FerroOrange working solution and incubated at 37 °C for 30 min. The cells were observed by confocal fluorescence microscopy and the fluo
Cell lipid peroxidation (LPO) was detected by BODIPY-C11 labeled fluorescent staining. 5 μM BODIPY-C11 was added to the cells and incubated in the dark for 20-30 min. After staining, the cells were collected by centrifugation and the LPO level was calculated by measuring the fluorescence intensity. In addition, HT22 cells were stained with 10 μM DCFH-DA fluorescent dye for 30 min, and were then collected for fluorescence intensity measurement, which reflected the reactive oxygen species (ROS) level. According to the manufacturer’s instructions, the level of MDA, the final product of LPO, was determined using the MDA colorimetric assay kit. The absorbance of reactants was calculated and determined according to the standard curve to evaluate the MDA concentration.
Following the standard protocol, HT22 cells in each group were lysed in RIPA buffer to extract the protein supernatant. In addition, the protein supernatants were isolated from the hippocampus following cryopreservation with liquid nitrogen and homogenized. The protein concentration was then quantitatively detected with a BCA assay kit. Following dilution and boiling, the protein samples were separated by 6%-10% polyacrylamide gel electrophoresis, after which semi-dry electroblotting was performed to transfer the protein bands to a PVDF membrane. The membrane was blocked in 5% defatted milk for 1 h, incubated with specific primary Abs at 4 °C overnight, washed with TBST twice, and incubated with HRP-labeled anti-p-mTOR, TFR1, SLC7A11 and GPX4 as well as the GAPDH specific secondary Ab (internal reference) for 1 h at ambient temperature. After dropwise addition of the ECL substrate to the protein bands, the grayscale values of the target protein bands were measured using the ECL detection system and the relative expression was calculated by the ratio of the grayscale values of the target protein to the reference protein.
Statistical processing and mapping of the data were performed using GraphPad Prism 7.0. Continuous variables that conformed to a normal distribution were described as the mean ± standard deviation (mean ± SD); statistical inter-group and multi-group differences, indicated by P < 0.05, were identified using the t-test and one-way variance analysis, respectively.
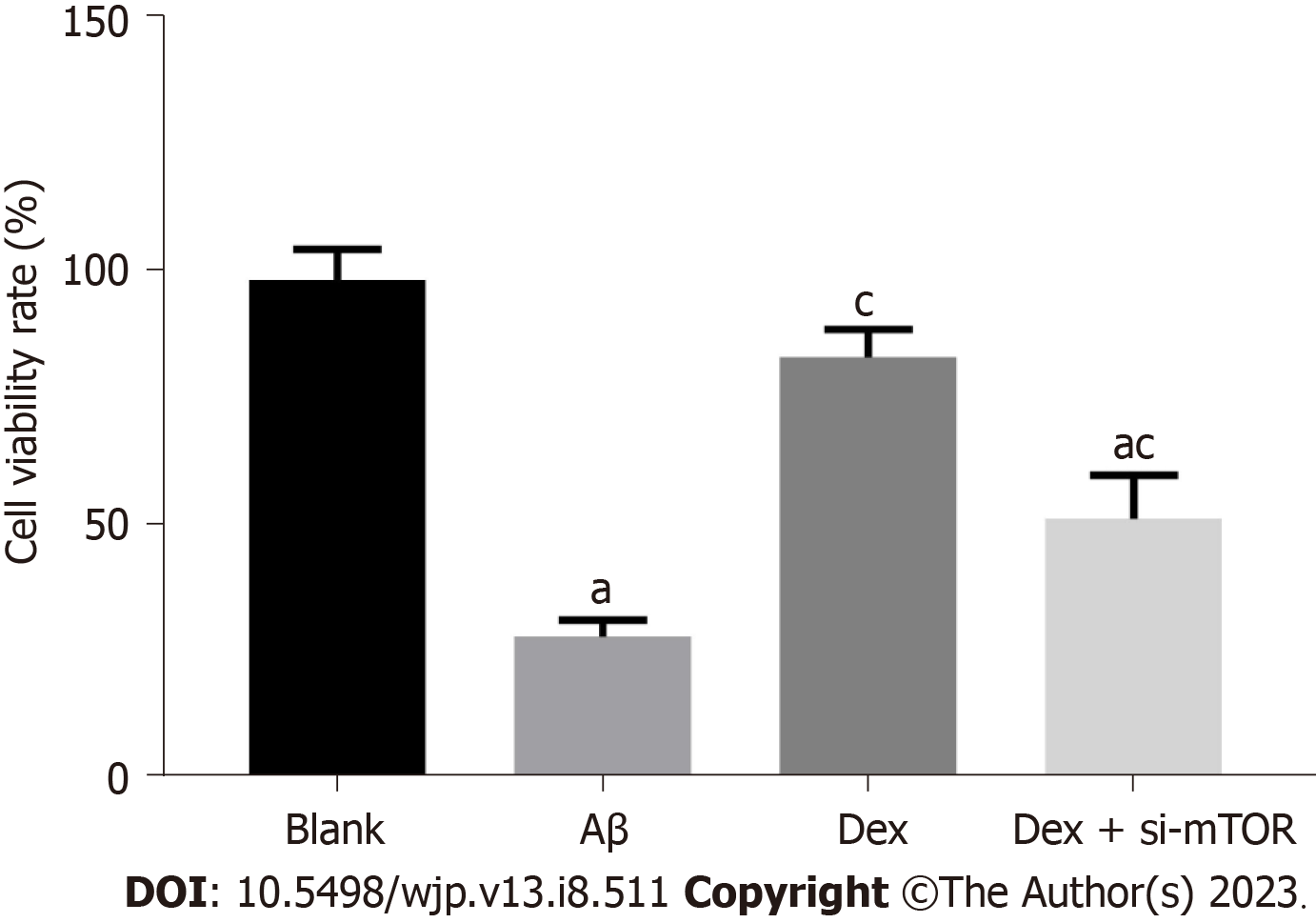
In order to confirm whether Dex can alleviate the cytotoxicity of mouse HNs, we first measured the impact of Dex on the viability of Aβ-treated mouse HNs. As shown by the CCK-8 assay (Figure 1), Aβ-treated HNs showed notably weakened cell viability compared with HNs from blank control mice (P < 0.05), while Dex treatment significantly restored the viability of Aβ-treated HNs (P < 0.05); in addition, the viability of Dex + si-mTOR treated cells was significantly lower than that of Dex-treated cells (P < 0.05). These findings suggest that Dex can alleviate the cytotoxicity of Aβ-treated mouse HNs, while inhibiting mTOR can block the effect of Dex treatment on cytotoxicity.
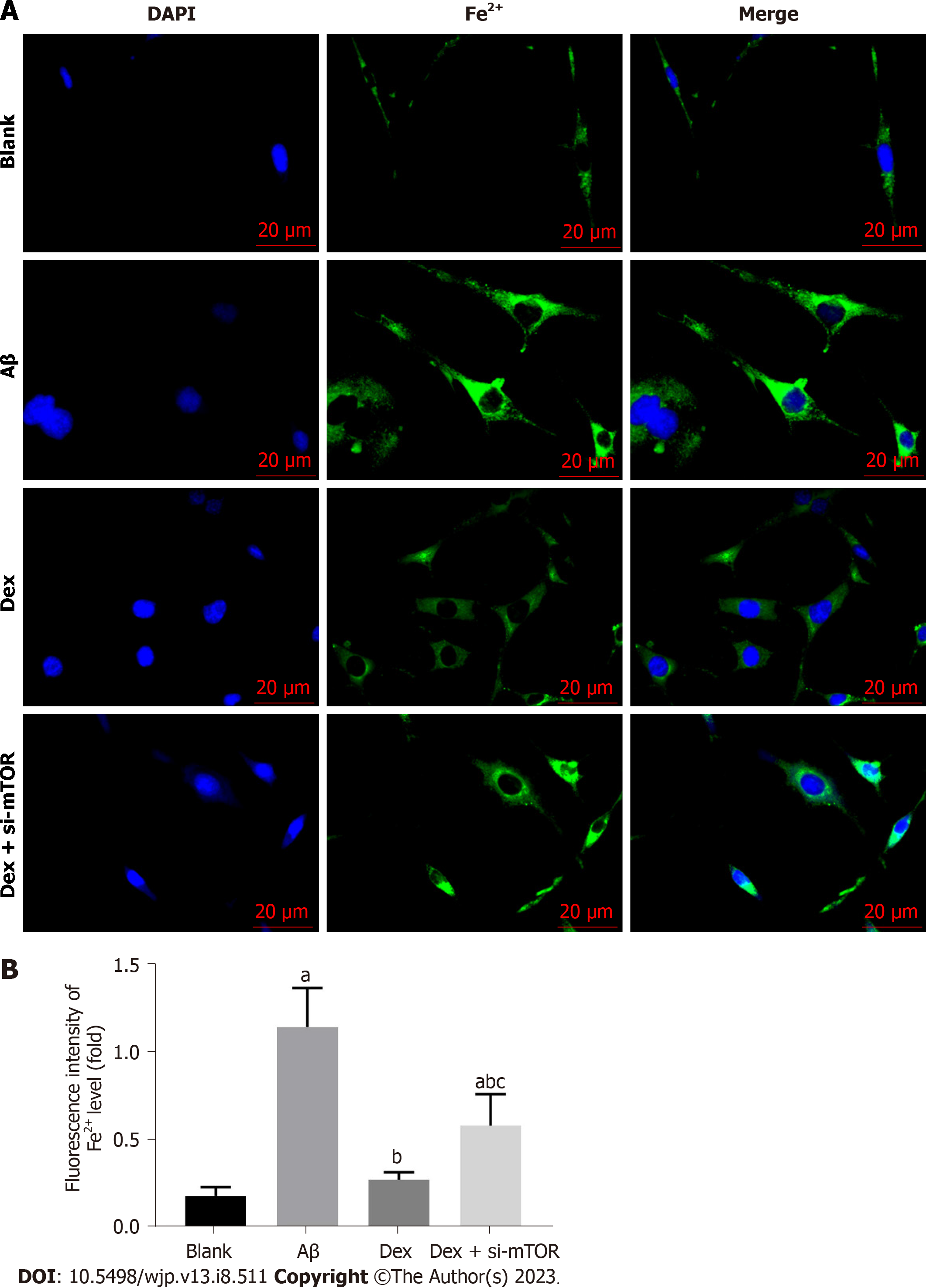
To further examine whether ferroptosis was responsible for Aβ-induced inhibition of cell proliferation, we first measured the impact of Dex on Fe2+ levels in Aβ-treated mouse HNs. As shown in Figure 2, Aβ-treated mouse HNs showed markedly elevated Fe2+ levels (P < 0.05), which were significantly reduced by Dex treatment (P < 0.05); moreover, Dex and si-mTOR co-treatment led to statistically higher Fe2+ levels in Aβ-treated HNs compared with Dex treatment alone (P < 0.05). These results show that Dex can inhibit the Aβ-induced increase of Fe2+ level in mouse HNs, and this inhibition is achieved by activating mTOR.
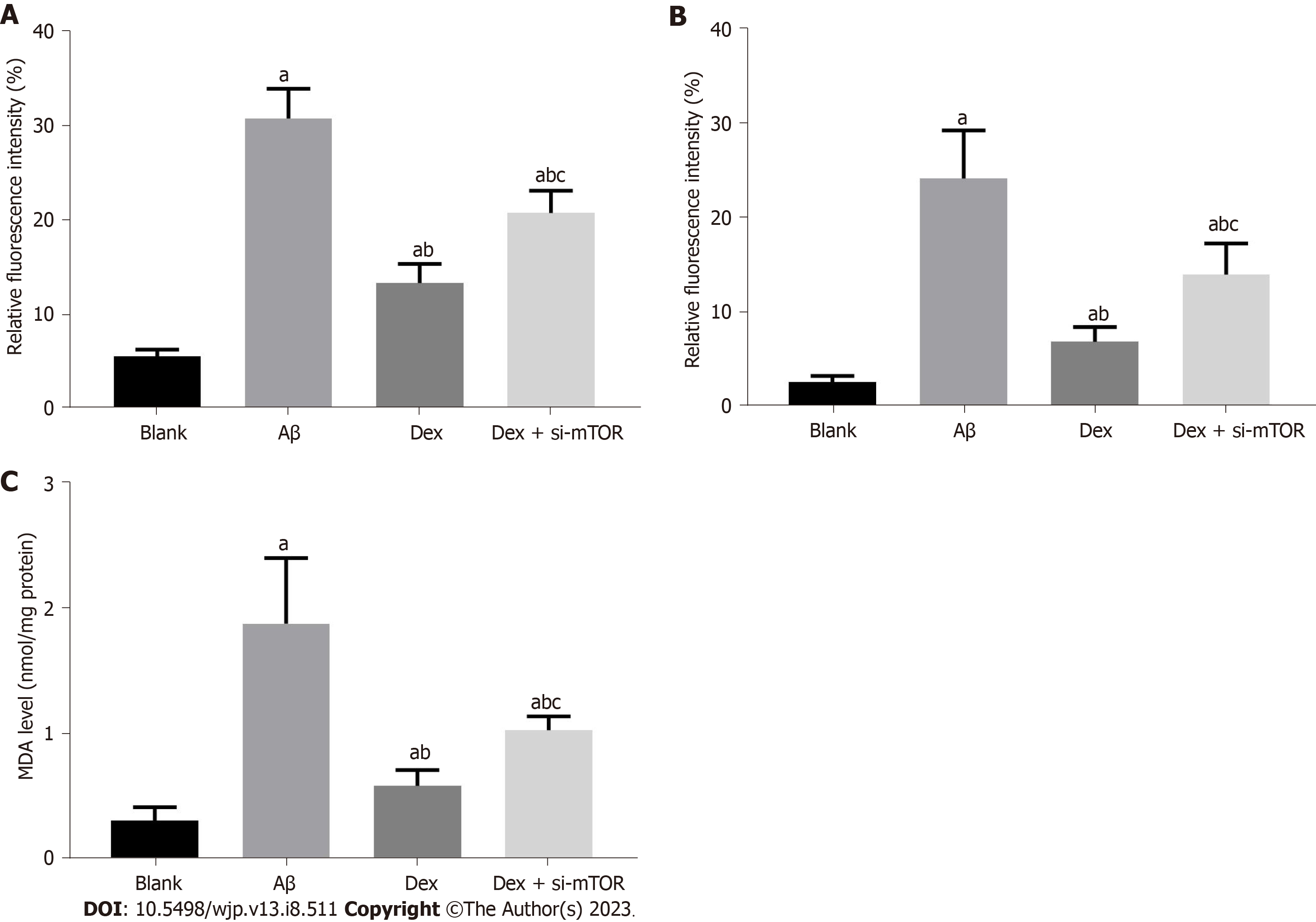
The increase of intracellular OS level in mouse HNs is also an important feature of ferroptosis. Significantly elevated ROS, LPO and MDA levels were observed in Aβ-treated mouse HNs (P < 0.05), which were reduced after Dex treatment (P < 0.05); in addition, Dex and si-mTOR co-treated cells showed higher ROS, LPO and MDA levels than those treated with Dex alone (P < 0.05), as shown in Figure 3. These results suggest that Dex treatment can alleviate the increase in intracellular OS level induced by Aβ in mouse HNs, and inhibiting mTOR can block this process.
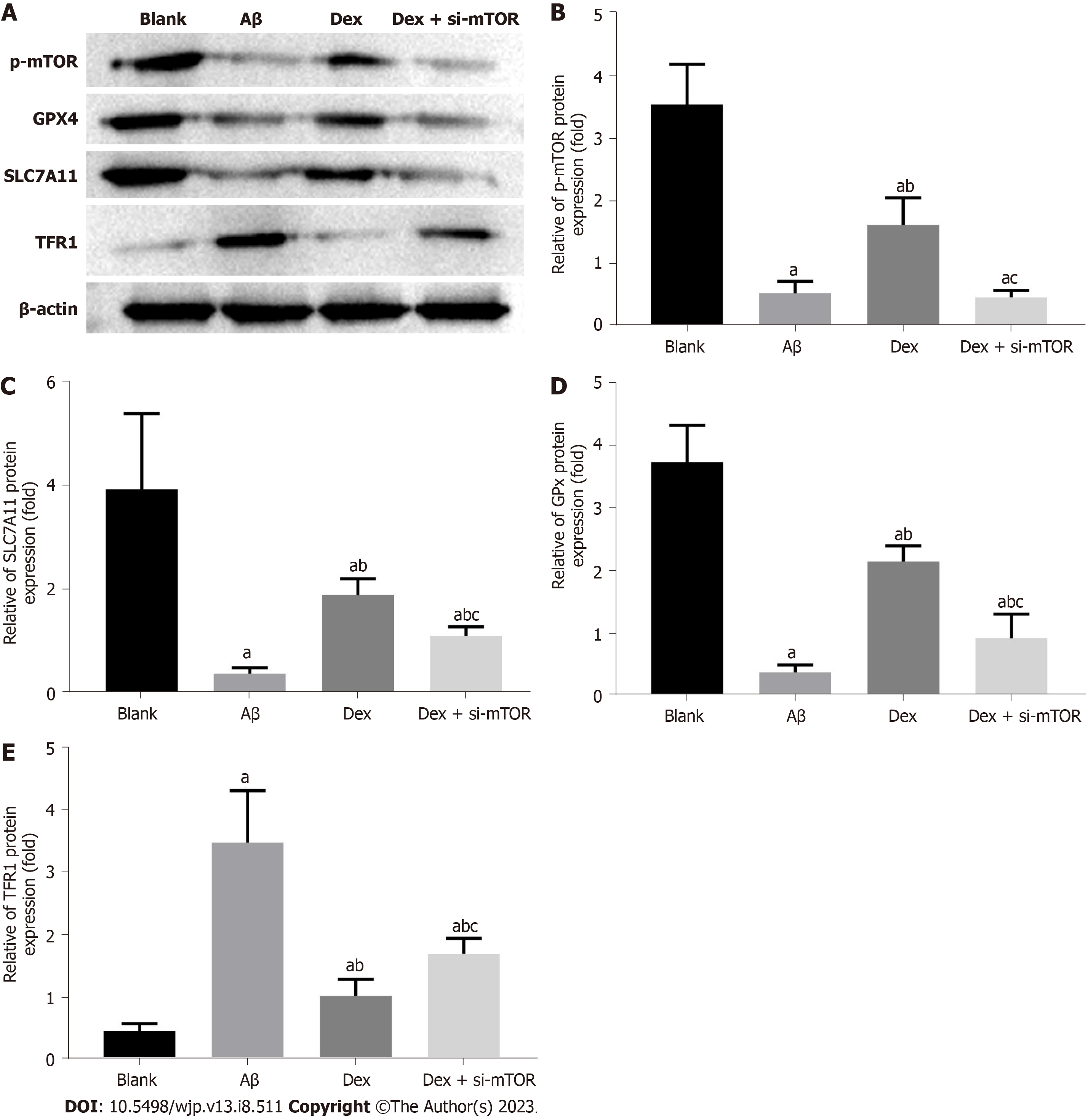
In order to confirm whether the antioxidant protection and iron metabolism effects of Dex are related to ferroptosis in mouse HNs, we performed western blot to determine the expression levels of the mTOR signal molecular protein p-mTOR and ferroptosis-associated marker proteins TFR1, SLC7A11, and GPX4 in Aβ-treated mouse HNs. The results showed a marked reduction in p-mTOR, SLC7A11, and GPX4 protein expression in mouse HNs induced by Aβ, and an obvious increase in TFR1 protein expression (P < 0.05). Compared with the Aβ group, Dex-treated cells showed enhanced p-mTOR, SLC7A11 and GPX4 levels, and decreased TFR1 (P < 0.05). Simultaneous treatment with Dex and si-mTOR further reduced p-mTOR, SLC7A11 and GPX4 protein levels, and promoted TFR1 protein expression, and these changes were statistically significant (P < 0.05), as shown in Figure 4. Therefore, Dex can significantly activate mTOR signaling and reduce Aβ-induced ferroptosis in mouse HNs by modulating the mTOR-TFR1 axis.
According to HE staining results, the HNs in Sham mice were evenly distributed, with abundant cytoplasm, clear nuclei, and normal and intact cell morphology and structure; in the Aβ group, the HNs were sparsely arranged and disordered, with less cytoplasm, unclear cell morphology and atrophy; the HNs in the Dex group showed less neuronal atrophy, with uniform cell distribution, regular arrangement, and deep cytoplasmic staining; while HNs in the Dex + RAPA (mTOR inhibitor) group showed more atrophy, more irregular arrangement and less clear cell morphology than the Dex group. Nissl staining results demonstrated that Nissl bodies in the hippocampus of the sham group were closely arranged and in large numbers; a notably reduced number of Nissl bodies was observed in the hippocampus of the Aβ model group; following Dex treatment, the number of Nissl bodies in the hippocampus of the Aβ model mice increased significantly; while the Nissl body count in the hippocampus of the Dex + RAPA group decreased markedly compared with the Dex group (Figure 5). These findings indicate that Dex can mitigate Aβ-induced hippocampal tissue damage and increase the number of HNs in AD mice, and this process is realized through activation of mTOR signaling.
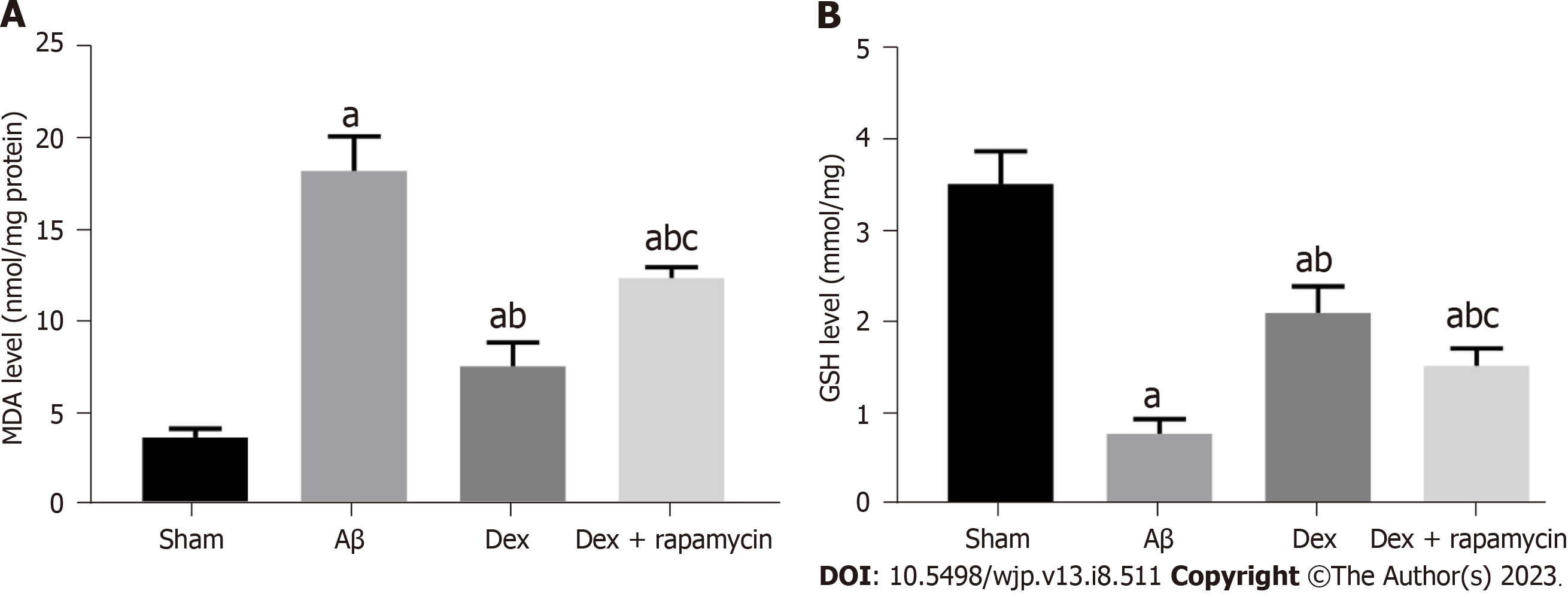
To confirm whether the antioxidant protection of Dex is related to its regulation of iron metabolism, we further evaluated the influence of Dex treatment on OS and Fe2+ level in the mouse hippocampus. MDA in the hippocampus of AD model mice induced by Aβ was significantly increased compared with Sham mice, while glutathione (GSH) was markedly reduced (P < 0.05). The Dex group showed reduced MDA and elevated GSH compared with the Aβ group (P < 0.05); moreover, co-treatment with Dex and RAPA in AD mice showed enhanced MDA and decreased GSH in the hippo
The MWM test was used to evaluate the learning and memory ability of mice in each group. AD mice in the Aβ group showed markedly prolonged escape latency, and a statistically significant reduced number of platform crossings and target quadrant residence time (P < 0.05). The escape latency, number of platform crossings and target quadrant residence time of AD mice in the Dex group and Dex + RAPA group were significantly improved compared with the Aβ group (P < 0.05), with improvement in cognitive dysfunction in the Dex group as compared with the Dex + RAPA group (P < 0.05), as shown in Table 1. These results suggest that Dex treatment improves the learning and memory ability of Aβ-treated AD mice, while RAPA exerts negative effects on this improvement.
| Groups | Sham (n = 10) | Aβ (n = 10) | Dex (n = 10) | Dex + rapamycin (n = 10) |
| Escape latency (s) | 5.38 ± 0.53 | 42.20 ± 3.89a | 13.81 ± 1.64a,b | 22.75 ± 3.06a,b,c |
| Number of platform crossings (times) | 7.26 ± 0.82 | 1.38 ± 0.35a | 5.90 ± 0.61a,b | 3.24 ± 0.43a,b,c |
| Target quadrant residence time (s) | 38.46 ± 5.27 | 7.92 ± 1.69a | 30.40 ± 4.26a,b | 22.52 ± 2.78a,b,c |
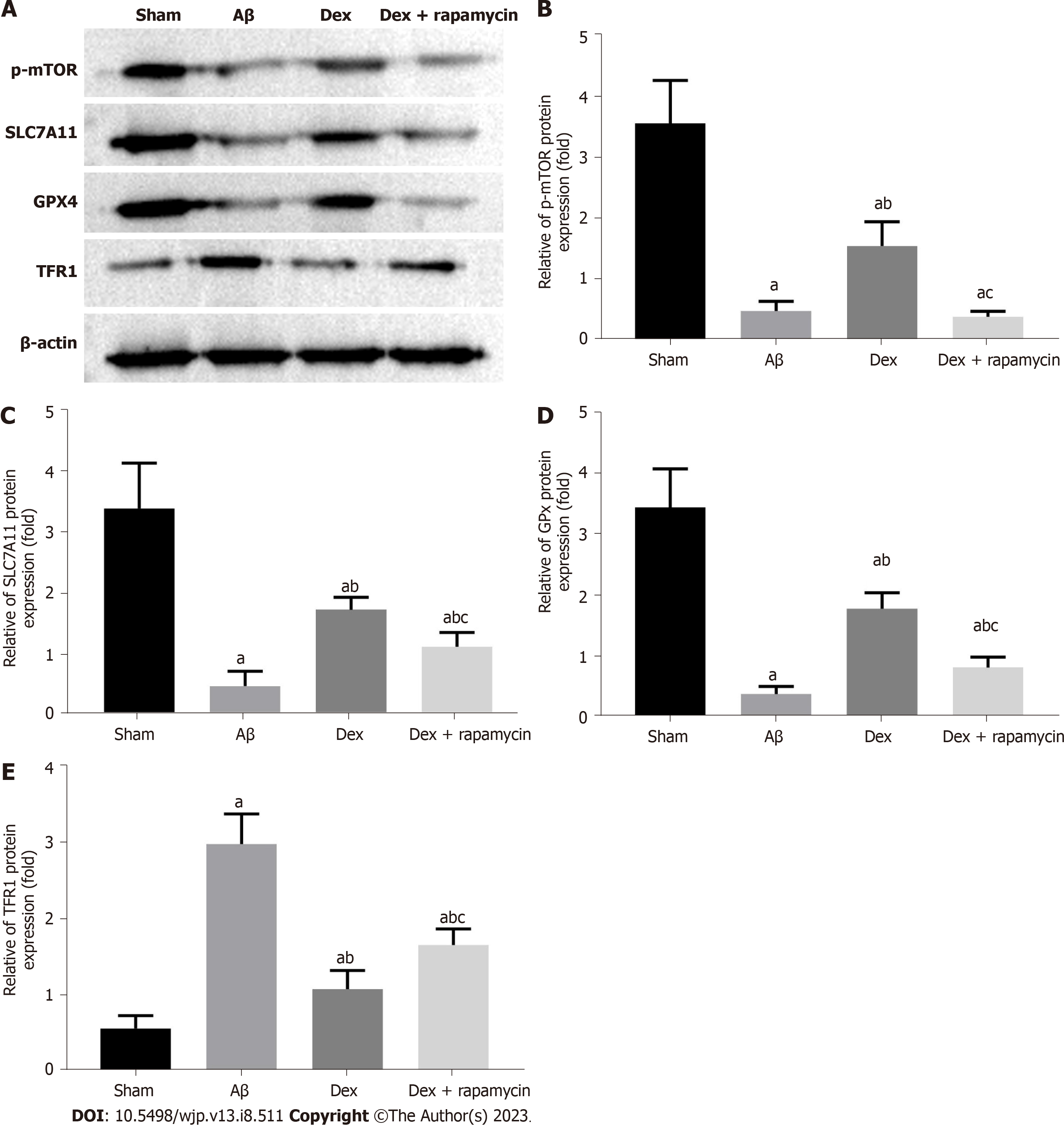
From the western blot analysis (Figure 7), it was found that the Aβ group had markedly reduced mTOR, SLC7A11 and GPX4 protein levels and elevated TFR1 protein expression in the hippocampus compared with the sham group (P < 0.05); Dex treatment enhanced mTOR, SLC7A11 and GPX4 protein expression in AD mice, and decreased TFR1 protein expression (P < 0.05); furthermore, the injection of RAPA effectively blocked the regulation of Dex on the expression of mTOR signaling molecules and ferroptosis markers TFR1, SLC7A11 and GPX4 in AD mouse hippocampus (P < 0.05). Therefore, Dex can mitigate ferroptosis in AD mouse hippocampus by activating the mTOR-TFR1 axis.
AD is the most common progressive NDD, and is mainly caused by synaptic loss of neurons in the cerebral cortex and hippocampus, and the death of brain neurons[15]. In recent years, ferroptosis has been found[16-18] to be the underlying mechanism of neuron loss in many NDDs, and the “ferroptosis hypothesis” has been proposed for AD. GPX4 mRNA and protein levels are reported to be aberrantly down-regulated in the brains of patients and mice with AD[19,20]. GPX4 relies on reduced GSH to catalyze LPO reduction, which is considered the key regulator of ferroptosis, while the inhibition of GPX4 can lead to LPO accumulation, a hallmark feature of ferroptosis that is considered an early event in the pathological process of AD[21]. During the process of iron imbalance, free Fe2+ released by ferritin degradation reacts with lipids through the Fenton reaction, and eventually forms lipid peroxy radicals and hydroperoxides, leading to LPO accumulation, the prime reason for ferroptosis[22]. The present study demonstrated that there were significant iron deposits and increased ROS and lipid oxidation levels in Aβ-treated mouse HNs and AD mouse hippocampus tissues, and Aβ treatment promoted the expression of iron transfer-related protein TFR1 and decreased the expression levels of ferroptosis regulation associated proteins GPX4 and SLC7A11. Furthermore, in vitro and in vitro studies demonstrated the occurrence of ferroptosis during AD. Thus, the above research suggests the important role of ferroptosis in AD.
Moreover, in the in vivo and in vitro experiments, it was found that Dex effectively reduced ferroptosis in HNs of AD mice. Previous studies have confirmed the neuroprotective effects of Dex, as it can inhibit the neuroinflammatory reaction and reduce OS, and has an anti-inflammatory and antioxidant role[23]. It has also been shown to prevent early cognitive dysfunction in senile mice after surgery[24]. Sun et al[25] also reported that Dex reduced Aβ1-induced HN apoptosis and OS responses in mice by modulation of the miR-129/YAP1/JAG1 axis, thus alleviating cognitive impairment and improving cognitive function in AD mice. In the present study, the number of Nissl bodies in the hippocampus of Aβ-treated AD mice decreased significantly compared with sham mice in the presence of neuronal atrophy, while intraperitoneal injection of Dex led to a marked elevation in Nissl body number in AD mouse hippocampus and an obvious reduction in neuronal atrophy. Moreover, the MWM test of mouse learning and memory ability in each group showed that the escape latency of AD mice in the Dex group was significantly shortened compared with the Aβ group, with a statistically significant higher number of platform crossings and target quadrant residence time, suggesting significantly reduced cognitive dysfunction in AD mice after Dex treatment. Considering the aforementioned inhibitory effect of Dex on ferroptosis of HNs in mice, it can be concluded that Dex can extend the survival of neurons by inhibiting HN fer
The mTOR axis has been reported to play a regulatory role in cell death and apoptosis in various diseases such as aging, NDDs, and brain injury[26]. Increasing attention has recently been paid to the mechanism of mTOR mediated ferroptosis. It has long been reported that mTOR plays a regulatory role in upstream targets of iron metabolism, and that the mTOR-TFR1 axis is essential in balancing iron homeostasis by regulating TFR1 expression[27]. Our experimental results revealed that inhibition of the mTOR pathway significantly promoted Fe2+, OS and TFR1 levels in mouse HNs, and lowered GPX4 and SLC7A11 expression, indicating that the ability of Dex to protect HNs from Aβ-induced ferroptosis and mitigate cognitive dysfunction in AD mice is weakened following inhibition of mTOR expression. It is suggested that Dex can reduce ferroptosis of HNs and ease cognitive dysfunction in AD mice, possibly by activating the mTOR axis.
Both in vitro and in vivo experiments in this study confirmed that Dex can inhibit Aβ-induced ferroptosis of mouse HNs, and effectively reduce HN loss and cognitive dysfunction in AD mice. Furthermore, Dex improved learning and memory in AD mice by modulating the mTOR-TFR1 axis to reduce ferroptosis.
The main pathological feature of Alzheimer’s disease (AD) is the formation of amyloid β-protein (Aβ) plaques in the brain, leading to loss of neuronal synapses, ultimately affecting the patient’s learning and memory abilities. Research has shown that iron death caused by iron overload in nerve cells is an important factor leading to neurodegenerative diseases, and plays a crucial role in the progression of AD. Recently, reports have demonstrated the high selectivity of dexmedetomidine (Dex), an α adrenergic receptor agonist, that can exert neuroprotective effects by inhibiting the release of inflammatory factors, thereby improving cognitive dysfunction in elderly rats.
Currently, there are still no effective drugs to delay the progression of AD, and iron death which may play a crucial role in the progression of this disease is attracting increasing attention from researchers.
To investigate the effect of Dex on iron death in the hippocampus of AD mice by both in vitro and in vivo research.
Construction and utilization of an AD model using Aβ. The mouse hippocampal neuronal cell line HT22 was induced, and the levels of cell proliferation activity and intracellular Fe2+ were measured by the cell-counting kit-8 assay and immunofluorescence. The contents of malondialdehyde and glutathione were measured by the TBA method and microplate method, respectively. In the AD mouse model Aβ was injected, and the pathological damage in the mouse hippocampus was detected by hematoxylin and eosin staining and Nissl staining. The Morris water maze was used to evaluate the learning and memory abilities of mice in each group, and protein immunoblotting was used to evaluate the expression levels of p-mammalian target of rapamycin (p-mTOR) and iron death-related proteins transferrin receptor 1 (TFR1), SLC7A11, and glutathione peroxidase 4.
Dex significantly improved lipid peroxidation and iron influx in mouse hippocampal neurons (HNs) both in vivo and in vitro, and inhibition of the mTOR signaling pathway blocked this process, demonstrating that Dex can inhibit the damage caused by iron death in mouse HNs by activating mTOR-TFR1 signaling regulation, thereby improving cognitive dys
Dex can significantly activate the mTOR-TFR1 signaling pathway and inhibit iron death in mouse HNs, thereby improving the learning and memory abilities of mice.
From in vitro and in vivo molecular experimental research, it is possible to analyze the effect of Dex on iron death in AD related nerve cells.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mechili EA, Albania; Wutich A, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 1907] [Article Influence: 317.8] [Reference Citation Analysis (1)] |
| 2. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 11743] [Article Influence: 903.3] [Reference Citation Analysis (1)] |
| 3. | Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13:1045-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 1306] [Article Influence: 118.7] [Reference Citation Analysis (0)] |
| 4. | Zhang P, Chen L, Zhao Q, Du X, Bi M, Li Y, Jiao Q, Jiang H. Ferroptosis was more initial in cell death caused by iron overload and its underlying mechanism in Parkinson's disease. Free Radic Biol Med. 2020;152:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 5. | Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA, Lei P. Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther. 2021;6:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 382] [Cited by in RCA: 830] [Article Influence: 207.5] [Reference Citation Analysis (0)] |
| 6. | Liu Y, Wang Y, Liu J, Kang R, Tang D. Interplay between MTOR and GPX4 signaling modulates autophagy-dependent ferroptotic cancer cell death. Cancer Gene Ther. 2021;28:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 7. | Han D, Jiang L, Gu X, Huang S, Pang J, Wu Y, Yin J, Wang J. SIRT3 deficiency is resistant to autophagy-dependent ferroptosis by inhibiting the AMPK/mTOR pathway and promoting GPX4 levels. J Cell Physiol. 2020;235:8839-8851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 8. | Baba Y, Higa JK, Shimada BK, Horiuchi KM, Suhara T, Kobayashi M, Woo JD, Aoyagi H, Marh KS, Kitaoka H, Matsui T. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2018;314:H659-H668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 9. | Shan Y, Sun S, Yang F, Shang N, Liu H. Dexmedetomidine protects the developing rat brain against the neurotoxicity wrought by sevoflurane: role of autophagy and Drp1-Bax signaling. Drug Des Devel Ther. 2018;12:3617-3624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Zhao L, Zhai M, Yang X, Guo H, Cao Y, Wang D, Li P, Liu C. Dexmedetomidine attenuates neuronal injury after spinal cord ischaemia-reperfusion injury by targeting the CNPY2-endoplasmic reticulum stress signalling. J Cell Mol Med. 2019;23:8173-8183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Wang C, Yuan W, Hu A, Lin J, Xia Z, Yang CF, Li Y, Zhang Z. Dexmedetomidine alleviated sepsisinduced myocardial ferroptosis and septic heart injury. Mol Med Rep. 2020;22:175-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 12. | Gao G, Xie Z, Li EW, Yuan Y, Fu Y, Wang P, Zhang X, Qiao Y, Xu J, Hölscher C, Wang H, Zhang Z. Dehydroabietic acid improves nonalcoholic fatty liver disease through activating the Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis. J Nat Med. 2021;75:540-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 13. | Liu MJ, Zhao XC, Gong HS, You YQ, Li JY. Dexmedetomidine prevents hemorrhagic brain injury by reducing damage induced by ferroptosis in mice. Neurosci Lett. 2022;788:136842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 14. | Zhou SY, Cui GZ, Yan XL, Wang X, Qu Y, Guo ZN, Jin H. Mechanism of Ferroptosis and Its Relationships With Other Types of Programmed Cell Death: Insights for Potential Interventions After Intracerebral Hemorrhage. Front Neurosci. 2020;14:589042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Ren C, Li D, Zhou Q, Hu X. Mitochondria-targeted TPP-MoS(2) with dual enzyme activity provides efficient neuroprotection through M1/M2 microglial polarization in an Alzheimer's disease model. Biomaterials. 2020;232:119752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 16. | Hou L, Huang R, Sun F, Zhang L, Wang Q. NADPH oxidase regulates paraquat and maneb-induced dopaminergic neurodegeneration through ferroptosis. Toxicology. 2019;417:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 17. | Ayton S, Wang Y, Diouf I, Schneider JA, Brockman J, Morris MC, Bush AI. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol Psychiatry. 2020;25:2932-2941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 264] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 18. | Tian R, Abarientos A, Hong J, Hashemi SH, Yan R, Dräger N, Leng K, Nalls MA, Singleton AB, Xu K, Faghri F, Kampmann M. Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis. Nat Neurosci. 2021;24:1020-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 229] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 19. | Bao WD, Pang P, Zhou XT, Hu F, Xiong W, Chen K, Wang J, Wang F, Xie D, Hu YZ, Han ZT, Zhang HH, Wang WX, Nelson PT, Chen JG, Lu Y, Man HY, Liu D, Zhu LQ. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer's disease. Cell Death Differ. 2021;28:1548-1562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 447] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 20. | da Rocha TJ, Silva Alves M, Guisso CC, de Andrade FM, Camozzato A, de Oliveira AA, Fiegenbaum M. Association of GPX1 and GPX4 polymorphisms with episodic memory and Alzheimer's disease. Neurosci Lett. 2018;666:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Sultana R, Perluigi M, Butterfield DA. Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic Biol Med. 2013;62:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 351] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 22. | Liang D, Minikes AM, Jiang X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell. 2022;82:2215-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 674] [Article Influence: 224.7] [Reference Citation Analysis (0)] |
| 23. | Gao J, Sun Z, Xiao Z, Du Q, Niu X, Wang G, Chang YW, Sun Y, Sun W, Lin A, Bresnahan JC, Maze M, Beattie MS, Pan JZ. Dexmedetomidine modulates neuroinflammation and improves outcome via alpha2-adrenergic receptor signaling after rat spinal cord injury. Br J Anaesth. 2019;123:827-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Qian XL, Zhang W, Liu MZ, Zhou YB, Zhang JM, Han L, Peng YM, Jiang JH, Wang QD. Dexmedetomidine improves early postoperative cognitive dysfunction in aged mice. Eur J Pharmacol. 2015;746:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Sun W, Zhao J, Li C. Dexmedetomidine Provides Protection Against Hippocampal Neuron Apoptosis and Cognitive Impairment in Mice with Alzheimer's Disease by Mediating the miR-129/YAP1/JAG1 Axis. Mol Neurobiol. 2020;57:5044-5055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 26. | Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;169:361-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1487] [Article Influence: 185.9] [Reference Citation Analysis (0)] |
| 27. | Guiney SJ, Adlard PA, Bush AI, Finkelstein DI, Ayton S. Ferroptosis and cell death mechanisms in Parkinson's disease. Neurochem Int. 2017;104:34-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 266] [Article Influence: 33.3] [Reference Citation Analysis (0)] |