Published online May 19, 2023. doi: 10.5498/wjp.v13.i5.203
Peer-review started: December 7, 2022
First decision: February 20, 2023
Revised: March 2, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: May 19, 2023
Processing time: 163 Days and 2.6 Hours
Functional near-infrared spectroscopy (fNIRS) is commonly used to study human brain function by measuring the hemodynamic signals originating from cortical activation and provides a new noninvasive detection method for identifying dementia.
To investigate the fNIRS imaging technique and its clinical application in differential diagnosis of subtype dementias including frontotemporal lobe dementia, Lewy body dementia, Parkinson’s disease dementia (PDD) and Alzheimer’s disease (AD).
Four patients with different types of dementia were examined with fNIRS during two tasks and a resting state. We adopted the verbal fluency task, working memory task and resting state task. Each patient was compared on the same task. We conducted and analyzed the fNIRS data using a general linear model and Pearson’s correlation analysis.
Compared with other types of dementias, fNIRS showed the left frontotemporal and prefrontal lobes to be poorly activated during the verbal fluency task in frontotemporal dementia. In Lewy body dementia, severe asymmetry of prefrontal lobes appeared during both verbal fluency and working memory tasks, and the patient had low functional connectivity during a resting state. In PDD, the patient’s prefrontal cortex showed lower excitability than the temporal lobe during the verbal fluency task, while the prefrontal cortex showed higher excitability during the working memory task. The patient with AD showed poor prefrontal and temporal activation during the working memory task, and more activation of frontopolar instead of the dorsolateral prefrontal cortex.
Different hemodynamic characteristics of four types of dementia (as seen by fNIRS imaging) provides evidence that fNIRS can serve as a potential tool for the diagnosis between dementia subtypes.
Core Tip: Four types of dementia showed different patterns of activation when examined by functional near-infrared spectroscopy (fNIRS) during two tasks and a resting state. The patient with frontotemporal dementia showed the lowest activation during the verbal fluency task and the patient with Alzheimer’s disease showed the lowest activation during the working memory task. During the resting state, functional connectivity was poor in the patients with Lewy bodies dementia and Parkinson’s disease dementia. fNIRS imaging in dementia patients may be able to differentiate between types of dementia, and may be useful in diagnosis for these patients.
- Citation: Mei X, Zou CJ, Hu J, Liu XL, Zheng CY, Zhou DS. Functional near-infrared spectroscopy in elderly patients with four types of dementia. World J Psychiatry 2023; 13(5): 203-214
- URL: https://www.wjgnet.com/2220-3206/full/v13/i5/203.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i5.203
Dementia is a disease commonly studied in psychiatry and neurology. Clinical diagnosis is based on symptoms and is supplemented by neuropsychological scales and laboratory examinations. Brain imaging technology is an important tool for exploring brain diseases, and has undergone a period of rapid development in the past 20 years. To improve the accuracy of diagnosis between types of dementia, different technologies have been developed, including computed tomography (CT), positron emission tomography (PET) and magnetic resonance imaging (MRI) to examine brain structure, electroencephalography (EEG) to examine electrical physiology[1]. In addition to functional MRI (fMRI), EEG, and event-related potentials, functional near-infrared spectroscopy (fNIRS) to examine functional connectivity between regions has become an important supplement to existing functional imaging technologies[2,3].
fNIRS imaging evaluates human brain activity by measuring the oxygen level in the prefrontal and temporal cortices[4]. While participants perform different tasks, the fNIRS system provides continuous and real-time oxygen change display of oxy-hemoglobin (oxy-Hb) and deoxygenated hemoglobin values[5]. In aspects of specificity and temporal resolution, fNIRS assessment of cognitive function eliminates many shortcomings of fMRI[6]. While PET and MRI studies have generated insights into the pathological changes in brain oxygenation and activity associated with mild cognitive impairment (MCI) and dementia, these methods have some limitations involving the injection of radioactive compounds and motion artifacts[7].
fNIRS imaging systems possess high temporal and spatial resolutions, which are critical to withstand interference (both electromagnetic and from head motion). The temporal resolutions of fNIRS (11 Hz in this study) and EEG are in the order of seconds and milliseconds, respectively. The spatial resolution of fNIRS (3 cm in this study) was higher than that of EEG[8,9]. Because EEG recording is a surface potential change of skull, the accuracy of spatial localization is not high. fNIRS probe acquires the cortical activity directly and has a centimeter level of resolution[10]. fNIRS is used to monitor hemodynamic changes evoked by neural activity by taking advantage of the fact that biological tissues are relatively transparent to near-infrared light 700-1000 nm[11]. Recent studies on fNIRS detection of dementia focused on MCI and Alzheimer’s disease (AD)[12,13]. It was shown that resting-state fNIRS recordings from prefrontal regions can provide a potential methodology for detecting MCI and its progression[14]. The sensitivity and specificity increase as the cognitive impairment worsens[15].
Amnestic MCI is more predictive of AD than nonamnestic MCI, and nonamnestic MCI is more predictive of other types of dementia including Lewy bodies dementia (LBD) and frontotemporal dementia (FTD)[16,17]. To measure cortical activation in patients with behavioral variant of the FTD (bvFTD), fNIRS was used while performing the verbal fluency task[18]. The flexible of fNIRS makes the possibility of measurement of the neurology of gait in cognitive dysfunction or dementia during dual-task gait assessment[19]. Similar studies focused on old people with risk of dementia, such as those with subjective memory complaints, were reported to be examined by fNIRS indual-task gait[20].
The use of optical techniques, specifically fNIRS, to study brain hemodynamics and to assess prefrontal cortex’s activity of older adults for detection of certain types of seizures and cortical spreading deactivation in cognitive tasks is also important. fNIRS can be useful to investigate the altered prefrontal mechanisms of neurological and neuropsychiatric diseases and discover neuroimaging biomarkers for different neurodegenerative disorders[21-24]. The reliability of fNIRS in estimating global cerebral function was supported by previous studies[25]. fNIRS measurements are reproducible and can be reliable used in single subjects for neuroscientific research and clinical applications[26]. It could be a critical tool to investigate frontal lobe oxygenation in patients with different types of dementia and age-related decline of neurovascular coupling responses[27,29].
In this study, using fNIRS, we examined brain functional patterns in patients with four types of dementia: FTD, LBD, Parkinson’s disease dementia (PDD), and AD. Because of neurovascular coupling, different types of dementia may cause different hemodynamic alterations. Here, we report four subjects in which fNIRS was used to examine the brain function of patients with dementia (during two tasks and a resting state).
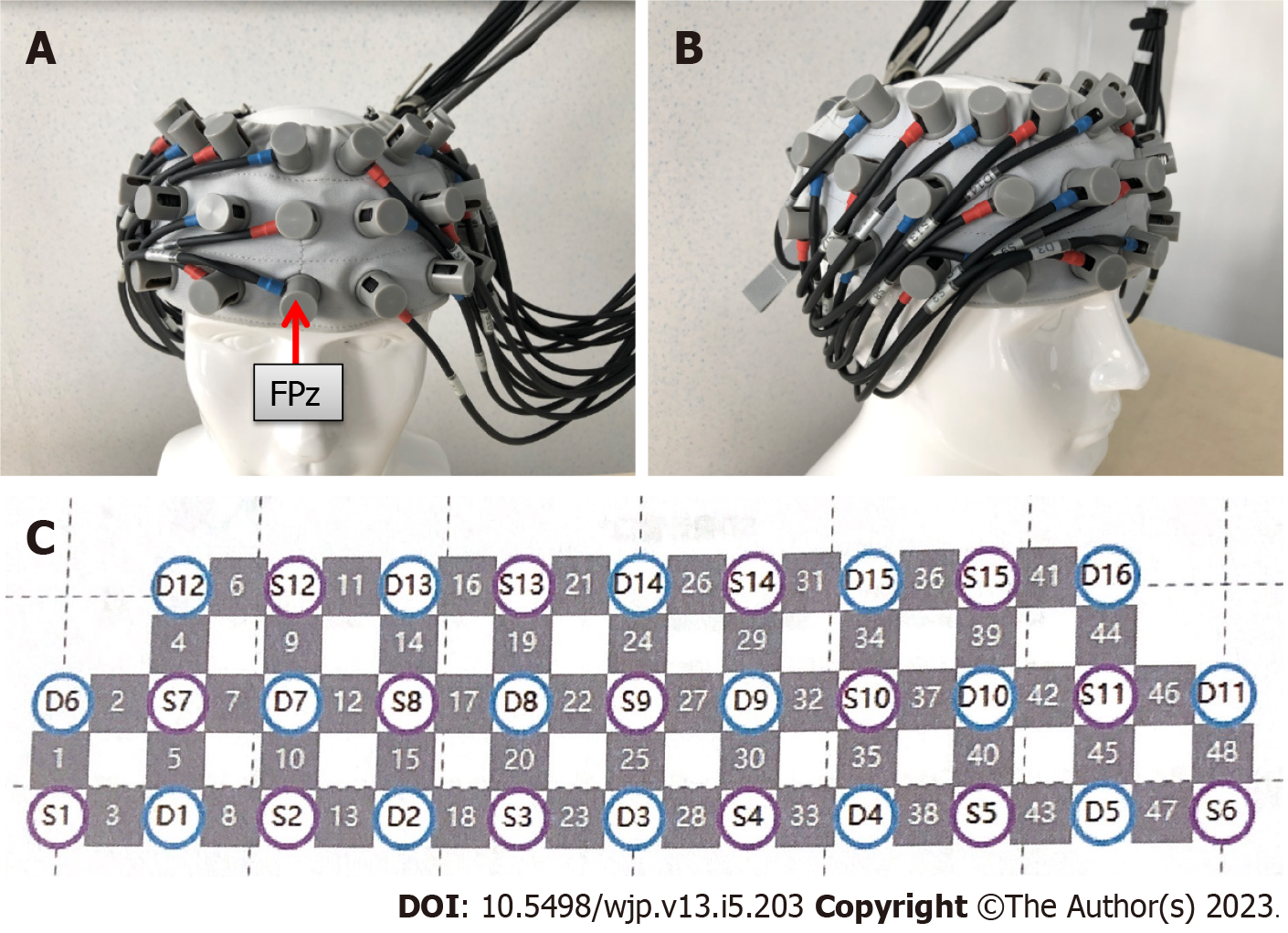
Patients with FTD, LBD, PDD, and AD signed an informed consent form and underwent routine fNIRS examinations at a brain function testing center in the hospital. A multi-channel continuous-wave fNIRS imaging system (Nirscan, Danyang Huichuang Medical Equipment Co. Ltd, China) was employed to measure signals from the frontal and bilateral temporal cortices as described in our previous study[8]. This light-emitting diode-based fNIRS system contained 24 light sources and 24 detectors. The distance between detectors was 3 cm, as shown in Figure 1. The center detector of the middle probe set row was placed at FPz, according to the 10/20 international system. The sampling rate was 11 Hz and the wavelengths used were 780, 808, and 850 nm.
The verbal fluency task consisted of three steps: (1) Participants repeatedly counted from 1 to 5 for 30 s to obtain the baseline value of cognitive performance; (2) during the task period, participants were instructed to generate as many words as possible, they continuously named different words beginning with a specific letters for 20 s (trials for three letters totaled 60 s); and (3) participants repeated step 1 for 70 s to return to baseline. The task took less than three minutes total. Other matters needing attention were described in previous studies on verbal fluency task of fNIRS[30-33].
The memory task contained two components on a tablet computer: A working memory search task and a non-working memory search task. In the working memory search task, three graphic icons appeared. Participants were instructed to remember the colors, shapes, and order in which they appeared; subsequently, they were asked to identify the icons in the summary diagram. In the non-working memory search task, participants only needed to select the icons when prompted. The two sets of tasks were alternated and repeated four times. Working memory performance was used to reflect the cognitive function in many studies[34-36].
The resting-state signal was recorded continuously for at least 10 min, during which participants were required to sit still and close their eyes without falling asleep. Functional connectivity was calculated on a scale from 0 to 1, where 0.3 represents an average level of functional connection strength (Supple
The participants were guided to the experimental room, and seated on a wooden stool with a wooden table. During the experimental preparation phase, the participants were asked to wear an electrode cap. The experimenter repeatedly adjusted the electrode cap to maximize the signal channel gain. When the experiments begin, participants performed the task by listening to the instruction (verbal fluency task) or using an ipad (working memory task), as our previous work[8].
The NirSpark software package (Danyang Huichuang Medical Equipment Co. Ltd, China) was used to analyze the fNIRS data, as previously described[37-39]. Physiological noises (including respiration, cardiac activity, and low-frequency signal drift) were corrected by a band-pass filter with cutoff frequencies of 0.01-0.20 Hz. The cubic spline interpolation method was adopted to eliminate motion artifacts. The modified Beer-Labert law was used to convert the optical density into changes in oxy-Hb and deoxy-Hb concentrations. A general linear model was used to calculate brain activation strength. Functional connectivity was calculated by conducting Pearson’s correlation analysis between the time series of every pair of measurement channels.
Clinical manifestation: A 57-year-old man was diagnosed with FTD. Two years prior, the patient began to show impaired judgment without warning and was defrauded of 700000 yuan. He reported insomnia, anxiety, and depression. His mental state continued to deteriorate, and he was initially diagnosed with “recurrent depressive disorder”. He also exhibited memory problems such as being unable to recall whether he had eaten or not, and he was often unable to complete tasks assigned to him by his family. For the past 2 years, the patient had been treated with systematic antidepressants (such as sertraline, doxetine, mirtazapine) and modified electroconvulsive therapy. His depression partially eased, but his memory problems persisted. One month previously, the patient again showed nervousness and fidgeting. The patient also experienced hallucinations, slow walking, and involuntary limb shaking. He had been admitted to the neurology department, who had ruled out Parkinson’s disease before he was admitted to our department for further treatment.
Examinations: The patient’s psychiatric examination at admission showed clear consciousness, accurate orientation, lack of cooperation, less autonomous language, decreased language expression ability, poor vocabulary, stereotypes and imitation speech, stable mood, no obvious manifestations of emotional depression, and partial decline in memory intelligence. Physical examination revealed that the patient’s right lower limb twitches could be relieved after massage. A brain MRI showed cystic foci in the left medial temporal lobe and senile brain changes. His Mini-Mental State Examination (MMSE) score was 22/30. Considering to his education level of high school, the severity of dementia was moderate. The patient was diagnosed as FTD accompanied by mild depression.
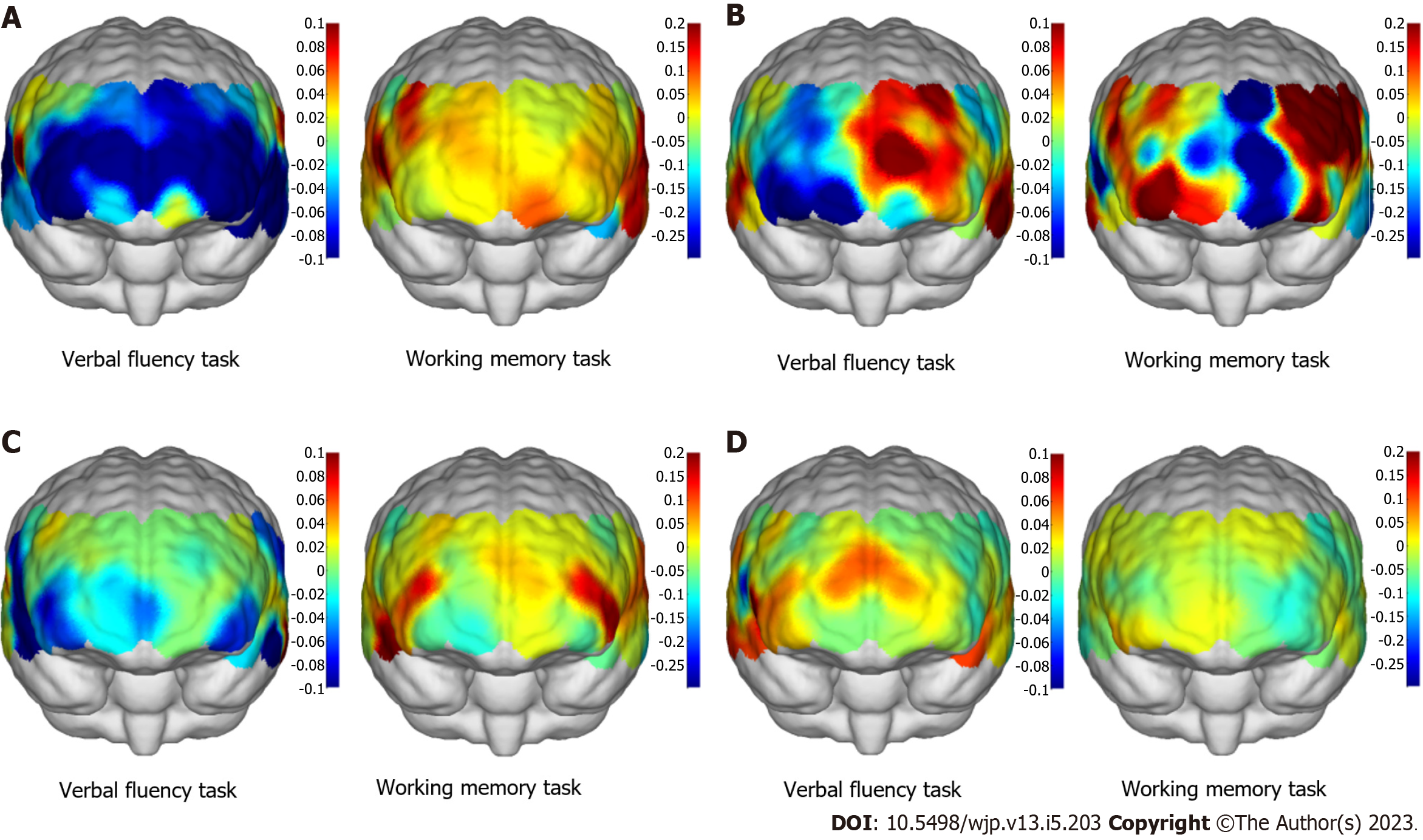
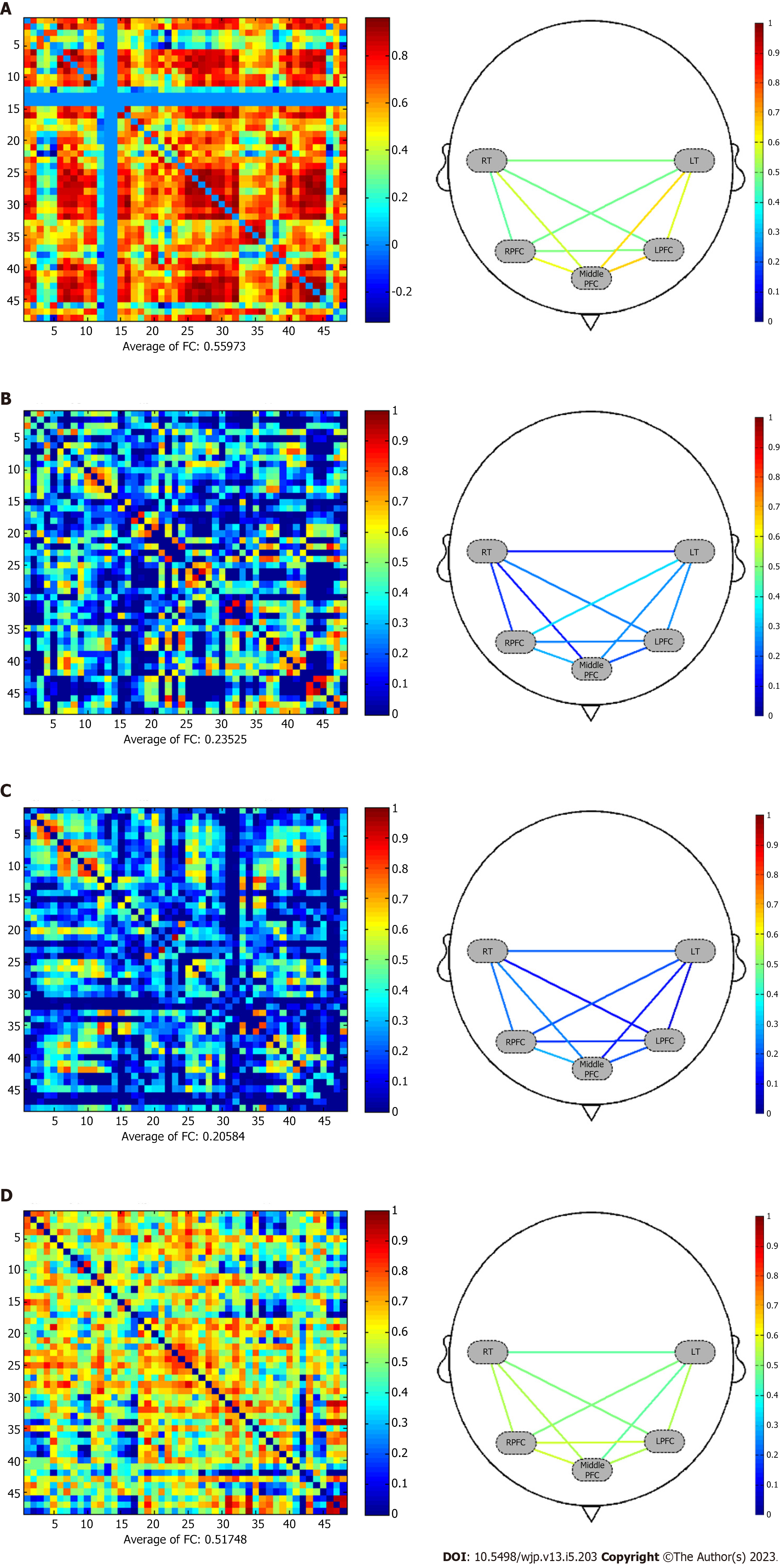
fNIRS results: In Figure 2A, fNIRS showed global lower activation in frontotemporal lobe when the patient performed the verbal fluency task. This was consisted with his clinical manifestation of poor verbal function. During the working memory task, the patient’s prefrontal lobe activation was lower than that of the temporal lobe, and the frontal lobe activation was also low. Frontalpolar and temporopolar area showed most activation than other regions. The overall pattern was different from other three subtypes of dementia. During a resting state, the average strength functional connection of all channels was considered high at 0.559 as shown in Figure 3A.
Clinical manifestation: A 74-year-old man was diagnosed with LBD. He had been experiencing progressive memory loss for three years, as well as symptoms such as hand shaking and bradykinesia; the neurology department considered him to have Parkinson’s disease. Over three years, the patient’s memory waxed and waned, while symptoms such as slow walking persisted. The patient occasionally experienced hallucinations such as seeing vivid images of dead people. In the last six months, his memory problem had significantly worsened; sometimes, he could not recall a family member’s name. His speech was not very fluent, and he had difficulty communicating with others. His sleep was poor. His ability to perform daily living had significantly declined and he needed someone to take care of him.
Examinations: The patient was admitted to our hospital for further treatment, and his psychiatric examination at admission showed clear consciousness, loss of sense of smell, unsteady gait, varying degrees of limb tremor, and increased muscle tone. He had slow thinking, aphasia, impaired memory, impaired common sense, reduced calculation ability, and visual hallucinations. A brain MRI showed localized atrophy in the temporal, frontal and parietal lobes. His MMSE score was 6/30. The degree of dementia was severe. The patient was diagnosed as primary neurodegenerative disease LBD.
fNIRS results: The fNIRS showed severe asymmetry in both hemispheres during both the verbal fluency and working memory tasks, particularly that the left frontal lobe showed more activation than the right lobe as shown in Figure 2B. In Figure 3B, the patient had low functional connection strength of 0.235 during a resting state.
Clinical manifestation: A 79-year-old man was diagnosed with PDD. Two years prior, the patient began to develop symptoms such as hand tremor and slow gait, and was subsequently diagnosed with “Parkinson’s disease” by the neurology department. He was given symptomatic treatment of dopamine (0.0625 g tid) and pramipexole (0.25 mg bid) in oral form since then, and symptoms such as hand tremors and slow gait improved. About six months ago, his family reported development of memory problems. The patient forgot things immediately after being told, and forgot where he put things. His ability to perform daily tasks slowly declined, sometimes requiring family members to help him dress. Two months prior, the patient’s mental state worsened due to sleep disorders and frequent nightmares. On occasion, he wakes in the middle of the night and begin to choke his wife. The patient was admitted for further treatment, and reported a family history of AD (in his sister).
Examinations: Upon mental examination, the patient’s consciousness was clear. His recent memory decline was evidenced by his report of no memory of events after he went to bed. He gave simple answers to questions and demonstrated limited vocabulary and emotional instability, particularly irritability. His risk-taking behavior had increased, particularly in dangerous movements that may lead to falls or rejecting advice from others. CT showed multiple ischemic foci in both the frontal and parietal lobes and periventricular white matter. His MMSE score was 13/30. The degree of dementia was severe. The patient was diagnosed as typical PDD accompanied by sleep disorders.
fNIRS results: fNIRS showed that the patient’s prefrontal cortex had lower excitability than the temporal lobe during the verbal fluency task, while the patient’s prefrontal cortex had higher excitability during the working memory task. As shown in Figure 2C, the dorsolateral prefrontal cortex (DLPFC) was activated strongly (symmetrical pattern; in red color region) during the working memory task. During a resting state in Figure 3C, the average strength functional connection of all channels was 0.206, lower than the normal level.
Clinical manifestation: A 73-year-old woman was diagnosed with dementia secondary to AD. About one year prior, the patient began to experience short-term memory loss, such as forgetting what she had said and done and failing to find the objects she had just placed. The ability to perform daily tasks declined slowly, requiring partial assistance from family members. The patient had a history of recurrent depressive disorder for approximately 10 years and long-term antidepressant treatment was moderately effective.
Examinations: The mental examination on admission found memory decline, computing power decline, orientation impairment, comprehension and expression ability decline, language vocabulary reduction, and emotional stability. A brain MRI showed reduced hippocampal volume and internal olfactory cortex volume bilaterally (MTA-score > 2). Her MMSE score was 14/30. The degree of dementia was moderate to severe due to her education level of 1 year. The patient was diagnosed as typical AD accompanied with mild depression.
fNIRS results: fNIRS revealed poor prefrontal and temporal activation during the working memory task (Figure 2D). The patient had high functional connection strength of 0.518 during a resting state (Figure 3D). The contrast of the two demented subjects (PDD vs AD) was characterized by a nearly symmetrical pattern in both task contrasts: prefrontal lobe was more activated in verbal fluency task in the AD than in PDD, while the frontotemporal lobe was more activated in working memory task in the PDD than in AD (Figure 2C and D). Regarding to the working memory task, the activation of brain in AD was the weakest of four dementias.
The results of this study indicate that cortical activation measured with fNIRS while performing a verbal fluency and working memory task differs in patients suffering from four types of neurodegenerative dementia including FTD, LBD, PDD, and AD. Furthermore, this activation differs between the four types of neurodegenerative dementia, a result shown for the first time using fNIRS in antidiastole of dementia subtypes.
Patients with FTD show low function or atrophy of the frontotemporal lobe that may be accompanied by low levels of oxy-Hb low function of the frontotemporal lobe[40]. Although patients with FTD and those with psychiatric disorders behave similarly, differences can exist in hypoperfusion and hypometabolism of the frontotemporal lobe regions[41]. A reduction in cortical activation during verbal fluency task performance in FTD patient compared to other three dementias has been shown in this study. FTD disorders include behavioral variant FTD (bvFTD), nonfluent/agrammatic variant primary progressive aphasia (nfvPPA), and semantic variant PPA (svPPA)[42]. PPA patients showed differential linguistic features of verbal fluency from bvFTD[43]. In this FTD case, the patient showed the poor verbal function. The fNIRS pattern showed low activation during the verbal fluency task. He had been treated with antidepressants. Currently, antidepressants are routinely used in the treatment of dementia to supplement serotonin availability[44]. There was no effect on vasculature at the relevant therapeutic dose for this patient, so it is unlikely that this affected blood flow in this patient. Furthermore, depressive pseudo dementia can also manifest as cognitive decline, but the brain functions, as well as neurovascular coupling, are not as poor in these patients compared to patients with dementia[45]. Thus, fNIRS can distinguish between depressive pseudo-dementia and dementia.
The AD pattern is weaker and more similar to the healthy pattern, whereas the bvFTD pattern is qualitatively different, namely more frontopolar and without frontoparietal compensation activation[18]. Our results showed the AD patient have lower and slower activation in the bilateral PFC and left parietal cortex during working memory maintenance. This was consisted with previous study on moderate to severe AD[2]. aMCI patients, as early stage AD, were reported a larger reduction in frontal deoxy-Hb during the memory task[46].
Regarding to the functional connection reflected by fNIRS, connections between different brain regions, as well as synergies between them, work together to provide comprehensive cognitive functions. As shown in Figure 3, the total scores of MMSE of patient with PDD and AD were similar in our study, but the functional connection strength is much different. The MMSE scale included six cognitive domains of orientation, immediately recall, attention, delayed recall, language and executive, and visual function[47]. Although the total MMSE scores of PDD and AD patients are close, they have different sub-scores in different cognitive domains, which reflect different brain area functions. In this aspect, the functional connectivity reflected by fNIRS can distinguish these differences, and enhanced the diagnostic accuracy as an auxiliary method.
The blood supply to the brain can reflect local changes in functional activity. In dementia patients, brain activity demonstrates a gradual increase in oxygenated hemoglobin and decrease in deoxygenated hemoglobin[48]. The working mode is believed to require networks across the entire brain; that is, no complex function is performed by a single brain area[6]. Imaging of the resting-state networks can also reveal information, such as the correlations between neural activities and the efficiency of transmission[49]. This is especially significant for patients with a low degree of cooperation[50]. Regarding to prefrontal cortex, primarily DLPFC, activation has a positive correlation with working memory load and performance until the working memory load exceeds the capacity[51].
fNIRS technology is widely used in the detection of neuropsychiatric disorders and brain functions individuals abusing different types of drugs[52-54]. Changes to oxy-Hb concentration of the bilateral prefrontal cortex in a schizophrenia group were reported to be significantly lower than those in a healthy group[55]. fNIRS has also been used to accurately distinguish patients with major depression from those with bipolar disorder or schizophrenia who have depressive symptoms[56]. When fNIRS technology was utilized to explore functional connectivity and network changes in patients with attention-deficit/hyperactivity disorder (ADHD), the development pattern of brain networks in children with ADHD was different from that of healthy children[57]. fNIRS also has applications in the field of sleep research to study brain activation during dreaming[49,57].
Limitations and future directions: one limitation is that a single patient for each group may introduce individual differences, including sex, age, and education. A larger sample size would help to eliminate the impact of such individual differences. Our results show that further study is needed to examine the diagnostic utility of fNIRS in dementia. Although our fNIRS recording technique was a multichannel flexible tool to detect the brain function in patients with different type of dementia, it focused on the frontal and temporal lobe of the brain, not the global brain region. This can be improved by using whole brain detection of fNIRS in the future.
Although subtypes of dementia may have similar clinical symptoms, they have different objective indicators; some that are observable during a resting state and some that are observable during the task state. Since fNIRS can detect changes in both states, it may be a useful tool for differential diagnosis. This study visualized four different types of dementia (FTD, LBD, PDD, and AD) using fNIRS, and found differences of brain activation during the task condition and functional connectivity during the resting state for all four types. The patient with FTD showed the lowest activation during the verbal fluency task and the patient with AD showed the lowest activation during the working memory task. During the resting state, functional connectivity was poor in the patients with LBD and PDD. These differences could be used as biomarkers to distinguish the different subtypes of dementia. In the future, as a non-invasive tool, multichannel fNIRS technology can provide high spatial and temporal resolution signals to continuously assess regional cerebral oxygenation. The sensitivity of fNIRS increased its use as a wide-spread clinical tool for the robust assessment of brain function.
In addition to functional magnetic resonance imaging, electroencephalography, and event-related potentials, functional near-infrared spectroscopy (fNIRS) to examine functional connectivity between regions has become an important supplement to existing functional imaging technologies.
fNIRS technology will be widely used in the detection of neuropsychiatric disorders.
fNIRS could be a potential tool for the diagnosis between dementia subtypes.
We tested four types of dementia by using fNIRS in the verbal fluency task, working memory task and resting state task.
fNIRS examinations were adopted to test the fNIRS paramters. The results shown that different types of dementia have different fNIRS patterns.
fNIRS can be used as a potential method to diagnose dementia and cognitive decline.
We want to study whether the fNIRS can be a potential tool for the diagnosis between dementia subtypes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Masaru T, Hungary; Ni YC, Taiwan S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
| 1. | Bonifacio G, Zamboni G. Brain imaging in dementia. Postgrad Med J. 2016;92:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Li R, Rui G, Chen W, Li S, Schulz PE, Zhang Y. Early Detection of Alzheimer's Disease Using Non-invasive Near-Infrared Spectroscopy. Front Aging Neurosci. 2018;10:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Pinti P, Tachtsidis I, Hamilton A, Hirsch J, Aichelburg C, Gilbert S, Burgess PW. The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann N Y Acad Sci. 2020;1464:5-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 587] [Article Influence: 117.4] [Reference Citation Analysis (0)] |
| 4. | Pereira T, Castro MA, Villafaina S, Carvalho Santos A, Fuentes-García JP. Dynamics of the Prefrontal Cortex during Chess-Based Problem-Solving Tasks in Competition-Experienced Chess Players: An fNIR Study. Sensors (Basel). 2020;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Scholkmann F, Kleiser S, Metz AJ, Zimmermann R, Mata Pavia J, Wolf U, Wolf M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage. 2014;85 Pt 1:6-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 1133] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 6. | Sato H, Yahata N, Funane T, Takizawa R, Katura T, Atsumori H, Nishimura Y, Kinoshita A, Kiguchi M, Koizumi H, Fukuda M, Kasai K. A NIRS-fMRI investigation of prefrontal cortex activity during a working memory task. Neuroimage. 2013;83:158-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 7. | Davison CM, O'Brien JT. A comparison of FDG-PET and blood flow SPECT in the diagnosis of neurodegenerative dementias: a systematic review. Int J Geriatr Psychiatry. 2014;29:551-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Chen WL, Wagner J, Heugel N, Sugar J, Lee YW, Conant L, Malloy M, Heffernan J, Quirk B, Zinos A, Beardsley SA, Prost R, Whelan HT. Functional Near-Infrared Spectroscopy and Its Clinical Application in the Field of Neuroscience: Advances and Future Directions. Front Neurosci. 2020;14:724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 9. | Villringer A, Chance B. Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci. 1997;20:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1113] [Cited by in RCA: 882] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 10. | Yeung MK, Chan AS. Functional near-infrared spectroscopy reveals decreased resting oxygenation levels and task-related oxygenation changes in mild cognitive impairment and dementia: A systematic review. J Psychiatr Res. 2020;124:58-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 11. | Keles HO, Karakulak EZ, Hanoglu L, Omurtag A. Screening for Alzheimer's disease using prefrontal resting-state functional near-infrared spectroscopy. Front Hum Neurosci. 2022;16:1061668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Zhang S, Zhu T, Tian Y, Jiang W, Li D, Wang D. Early screening model for mild cognitive impairment based on resting-state functional connectivity: a functional near-infrared spectroscopy study. Neurophotonics. 2022;9:045010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Baik JS, Ko MH, Ko SH, Min JH, Choi JK, Baek JY, Kim TH, Jung NY, Jo Y, Lee TY, Shin YI. Assessment of Functional Near-infrared Spectroscopy by Comparing Prefrontal Cortex Activity: A Cognitive Impairment Screening Tool. Alzheimer Dis Assoc Disord. 2022;36:266-268. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Ferman TJ, Smith GE, Kantarci K, Boeve BF, Pankratz VS, Dickson DW, Graff-Radford NR, Wszolek Z, Van Gerpen J, Uitti R, Pedraza O, Murray ME, Aakre J, Parisi J, Knopman DS, Petersen RC. Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology. 2013;81:2032-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 15. | Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord. 2006;22:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 226] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Metzger FG, Schopp B, Haeussinger FB, Dehnen K, Synofzik M, Fallgatter AJ, Ehlis AC. Brain activation in frontotemporal and Alzheimer's dementia: a functional near-infrared spectroscopy study. Alzheimers Res Ther. 2016;8:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Sui SX, Hendy AM, Teo WP, Moran JT, Nuzum ND, Pasco JA. A Review of the Measurement of the Neurology of Gait in Cognitive Dysfunction or Dementia, Focusing on the Application of fNIRS during Dual-Task Gait Assessment. Brain Sci. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 18. | Teo WP, Rantalainen T, Nuzum N, Valente L, Macpherson H. Altered prefrontal cortex responses in older adults with subjective memory complaints and dementia during dual-task gait: An fNIRS study. Eur J Neurosci. 2021;53:1324-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Battaglia S. Neurobiological advances of learned fear in humans. Adv Clin Exp Med. 2022;31:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 20. | Battaglia S, Orsolini S, Borgomaneri S, Barbieri R, Diciotti S, di Pellegrino G. Characterizing cardiac autonomic dynamics of fear learning in humans. Psychophysiology. 2022;59:e14122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 21. | Tanaka M, Szabó Á, Spekker E, Polyák H, Tóth F, Vécsei L. Mitochondrial Impairment: A Common Motif in Neuropsychiatric Presentation? The Link to the Tryptophan-Kynurenine Metabolic System. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 22. | Balogh L, Tanaka M, Török N, Vécsei L, Taguchi S. Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Chincarini M, Dalla Costa E, Qiu L, Spinelli L, Cannas S, Palestrini C, Canali E, Minero M, Cozzi B, Ferri N, Ancora D, De Pasquale F, Vignola G, Torricelli A. Reliability of fNIRS for noninvasive monitoring of brain function and emotion in sheep. Sci Rep. 2020;10:14726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Wyser DG, Kanzler CM, Salzmann L, Lambercy O, Wolf M, Scholkmann F, Gassert R. Characterizing reproducibility of cerebral hemodynamic responses when applying short-channel regression in functional near-infrared spectroscopy. Neurophotonics. 2022;9:015004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Csipo T, Mukli P, Lipecz A, Tarantini S, Bahadli D, Abdulhussein O, Owens C, Kiss T, Balasubramanian P, Nyúl-Tóth Á, Hand RA, Yabluchanska V, Sorond FA, Csiszar A, Ungvari Z, Yabluchanskiy A. Assessment of age-related decline of neurovascular coupling responses by functional near-infrared spectroscopy (fNIRS) in humans. Geroscience. 2019;41:495-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 26. | Udina C, Avtzi S, Durduran T, Holtzer R, Rosso AL, Castellano-Tejedor C, Perez LM, Soto-Bagaria L, Inzitari M. Functional Near-Infrared Spectroscopy to Study Cerebral Hemodynamics in Older Adults During Cognitive and Motor Tasks: A Review. Front Aging Neurosci. 2019;11:367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Tang TB, Chan YL. Functional Connectivity Analysis on Mild Alzheimer's Disease, Mild Cognitive Impairment and Normal Aging using fNIRS. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Liu X, Cheng F, Hu S, Wang B, Hu C, Zhu Z, Zhuang W, Mei X, Li X, Zhou Q, Zhang W, Tang Y, Zhou D. Cortical activation and functional connectivity during the verbal fluency task for adolescent-onset depression: A multi-channel NIRS study. J Psychiatr Res. 2022;147:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 29. | Husain SF, Tang TB, Tam WW, Tran BX, Ho CS, Ho RC. Cortical haemodynamic response during the verbal fluency task in patients with bipolar disorder and borderline personality disorder: a preliminary functional near-infrared spectroscopy study. BMC Psychiatry. 2021;21:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Xiang Y, Li Y, Shu C, Liu Z, Wang H, Wang G. Prefrontal Cortex Activation During Verbal Fluency Task and Tower of London Task in Schizophrenia and Major Depressive Disorder. Front Psychiatry. 2021;12:709875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Huang J, Zhang J, Zhang T, Wang P, Zheng Z. Increased Prefrontal Activation During Verbal Fluency Task After Repetitive Transcranial Magnetic Stimulation Treatment in Depression: A Functional Near-Infrared Spectroscopy Study. Front Psychiatry. 2022;13:876136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Lang X, Wen D, Li Q, Yin Q, Wang M, Xu Y. fNIRS Evaluation of Frontal and Temporal Cortex Activation by Verbal Fluency Task and High-Level Cognition Task for Detecting Anxiety and Depression. Front Psychiatry. 2021;12:690121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Han YMY, Chan MC, Chan MMY, Yeung MK, Chan AS. Effects of working memory load on frontal connectivity in children with autism spectrum disorder: a fNIRS study. Sci Rep. 2022;12:1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Vural Keleş Ö, Yıldırım E. Depression affects working memory performance: A Functional Near Infrared Spectroscopy (fNIRS) Study. Psychiatry Res Neuroimaging. 2023;329:111581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 35. | Baker JM, Bruno JL, Gundran A, Hosseini SMH, Reiss AL. fNIRS measurement of cortical activation and functional connectivity during a visuospatial working memory task. PLoS One. 2018;13:e0201486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Zhang N, Yuan X, Li Q, Wang Z, Gu X, Zang J, Ge R, Liu H, Fan Z, Bu L. The effects of age on brain cortical activation and functional connectivity during video game-based finger-to-thumb opposition movement: A functional near-infrared spectroscopy study. Neurosci Lett. 2021;746:135668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Wang Z, Liao M, Li Q, Zhang Y, Liu H, Fan Z, Bu L. Effects of three different rehabilitation games' interaction on brain activation using functional near-infrared spectroscopy. Physiol Meas. 2020;41:125005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Li Q, Feng J, Guo J, Wang Z, Li P, Liu H, Fan Z. Effects of the multisensory rehabilitation product for home-based hand training after stroke on cortical activation by using NIRS methods. Neurosci Lett. 2020;717:134682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 39. | Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet. 2015;386:1672-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 676] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 40. | Le Ber I, Guedj E, Gabelle A, Verpillat P, Volteau M, Thomas-Anterion C, Decousus M, Hannequin D, Véra P, Lacomblez L, Camuzat A, Didic M, Puel M, Lotterie JA, Golfier V, Bernard AM, Vercelletto M, Magne C, Sellal F, Namer I, Michel BF, Pasquier J, Salachas F, Bochet J; French research network on FTD/FTD-MND, Brice A, Habert MO, Dubois B. Demographic, neurological and behavioural characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain. 2006;129:3051-3065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Olney NT, Spina S, Miller BL. Frontotemporal Dementia. Neurol Clin. 2017;35:339-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 270] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 42. | van den Berg E, Dijkzeul JCM, Poos JM, Eikelboom WS, van Hemmen J, Franzen S, de Jong FJ, Dopper EGP, Vonk JMJ, Papma JM, Satoer D, Jiskoot LC, Seelaar H. Differential linguistic features of verbal fluency in behavioral variant frontotemporal dementia and primary progressive aphasia. Appl Neuropsychol Adult. 2022;1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Banerjee S, Hellier J, Dewey M, Romeo R, Ballard C, Baldwin R, Bentham P, Fox C, Holmes C, Katona C, Knapp M, Lawton C, Lindesay J, Livingston G, McCrae N, Moniz-Cook E, Murray J, Nurock S, Orrell M, O'Brien J, Poppe M, Thomas A, Walwyn R, Wilson K, Burns A. Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trial. Lancet. 2011;378:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 44. | Brodaty H, Connors MH. Pseudodementia, pseudo-pseudodementia, and pseudodepression. Alzheimers Dement (Amst). 2020;12:e12027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Vermeij A, Kessels RPC, Heskamp L, Simons EMF, Dautzenberg PLJ, Claassen JAHR. Prefrontal activation may predict working-memory training gain in normal aging and mild cognitive impairment. Brain Imaging Behav. 2017;11:141-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 46. | Li H, Jia J, Yang Z. Mini-Mental State Examination in Elderly Chinese: A Population-Based Normative Study. J Alzheimers Dis. 2016;53:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 353] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 47. | Roy CS, Sherrington CS. On the Regulation of the Blood-supply of the Brain. J Physiol. 1890;11:85-158.17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1046] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 48. | Hu Z, Liu G, Dong Q, Niu H. Applications of Resting-State fNIRS in the Developing Brain: A Review From the Connectome Perspective. Front Neurosci. 2020;14:476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 49. | Bu L, Wang D, Huo C, Xu G, Li Z, Li J. Effects of poor sleep quality on brain functional connectivity revealed by wavelet-based coherence analysis using NIRS methods in elderly subjects. Neurosci Lett. 2018;668:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Altamura M, Elvevåg B, Blasi G, Bertolino A, Callicott JH, Weinberger DR, Mattay VS, Goldberg TE. Dissociating the effects of Sternberg working memory demands in prefrontal cortex. Psychiatry Res. 2007;154:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Li R, Hosseini H, Saggar M, Balters SC, Reiss AL. Current opinions on the present and future use of functional near-infrared spectroscopy in psychiatry. Neurophotonics 2023; 10: 013505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 52. | Yeung MK, Lin J. Probing depression, schizophrenia, and other psychiatric disorders using fNIRS and the verbal fluency test: A systematic review and meta-analysis. J Psychiatr Res. 2021;140:416-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 53. | Gu X, Yang B, Gao S, Yan LF, Xu D, Wang W. Prefrontal fNIRS-based clinical data analysis of brain functions in individuals abusing different types of drugs. J Biomed Semantics. 2021;12:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Quaresima V, Giosuè P, Roncone R, Casacchia M, Ferrari M. Exploring prefrontal cortex oxygenation in schizophrenia by functional near-infrared spectroscopy. Adv Exp Med Biol. 2006;578:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 55. | Takizawa R, Fukuda M, Kawasaki S, Kasai K, Mimura M, Pu S, Noda T, Niwa S, Okazaki Y; Joint Project for Psychiatric Application of Near-Infrared Spectroscopy (JPSY-NIRS) Group. Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage. 2014;85 Pt 1:498-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 56. | Wang M, Hu Z, Liu L, Li H, Qian Q, Niu H. Disrupted functional brain connectivity networks in children with attention-deficit/hyperactivity disorder: evidence from resting-state functional near-infrared spectroscopy. Neurophotonics. 2020;7:015012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 57. | Oniz A, Inanc G, Taslica S, Guducu C, Ozgoren M. Sleep Is a Refreshing Process: An fNIRS Study. Front Hum Neurosci. 2019;13:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |