Published online Feb 19, 2023. doi: 10.5498/wjp.v13.i2.75
Peer-review started: September 30, 2022
First decision: December 1, 2022
Revised: December 9, 2022
Accepted: January 16, 2023
Article in press: January 16, 2023
Published online: February 19, 2023
Processing time: 140 Days and 6.2 Hours
Major depressive disorder (MDD) is the most frequent reason of disabled people in the world, as reported by the World Health Organization. However, the diagnosis of MDD is mainly based on clinical symptoms.
The clinical, genetic, and molecular characteristics of two Chinese families with MDD are described in this study. There were variable ages of onset and severity in depression among the families. Both Chinese families had a very low pre-valence of MDD. The mitochondrial genomes of these pedigrees were sequenced and indicated a homoplasmic T3394C (Y30H) mutation, with the polymorphism located at a highly conserved tyrosine at position 30 of ND1. The analysis also revealed unique sets of mitochondrial DNA (mtDNA) polymorphisms orig-inating from haplogroups M9a3 and M9a.
This finding of the T3394C mutation in two unrelated depressed patients provides strong evidence that this mutation may have a part in the etiology of MDD. However, In these two Chinese families having the T3394C mutation, no functional mtDNA mutation was observed. Therefore, T3394C mutations are related with MDD, and the phenotypic manifestation of these mutations may be affected by changes in nuclear genes or environmental factors.
Core Tip: We characterized two Chinese families with suspected maternal transmission of major depressive disorder at the clinical, genetic, and molecular levels in the present study. Molecular investigation revealed that the T3394C mutation in the ND1 gene was present in these Chinese families.
- Citation: Jing P, Mei X, Zhang YY, Zheng FJ, Luo XM, Liu LJ, Yu HH, Zhang XB. Major depressive disorder is correlated with the mitochondrial ND1 T3394C mutation in two Han Chinese families: Two case reports. World J Psychiatry 2023; 13(2): 75-83
- URL: https://www.wjgnet.com/2220-3206/full/v13/i2/75.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i2.75
Major depressive disorder (MDD) is a frequent dangerous human disease, which severely impairs the normal life and work of patients and causes a heavy burden on their family and society. In 2008, the World Health Organization rated it as the third leading cause of worldwide disability, and by 2030, it is expected to lead the list[1]. Despite its high incidence and prevalence, clinical symptoms are the primary basis for a diagnosis of MDD, and there is little evidence at the molecular level[2]. In addition, current drug therapy for MDD is not effective, with only 27% of patients in remission after the first treatment and 67% in remission after four complete treatments[3]. These considerations imply that the currently available antidepressants that target the monoaminergic system are insufficient for therapeutic use. In evaluating new therapeutic approaches, specific biomarkers should be identified that objectively determine the pathology involved in MDD, and relevant molecular targets might be revealed[4].
Mitochondrial DNA (mtDNA) is a 16.6 kb circular molecule that is maternally transferred and found within the mitochondrion[5]. Mitochondria can be considered "power generators" because they transform oxygen, energy substrates (proteins, carbohydrates, and lipids), and other substances into adenosine triphosphate[6]. In major psychiatric diseases, significant changes in the mitochondrial count, shape and electron transport activity in neurons are accompanied by an increase in mitochondrial DNA polymorphism, deletion and mutation, suggesting that defects in the mitochondria might be a primary cause of MDD[7,8]. Munakata et al[9] sequenced the whole mitochondrial genome of white blood cells from a family in which a proband was suspected of maternal inheritance of borderline personality disorder, MDD, suicide and other psychiatric disorders. The patient was identified as having both MDD and epilepsy. Comparing the proband sequence to the standard human mtDNA sequence, the authors identified 34 base changes. From the mtDNA sequence, it is possible to detect comprehensive subhaplogroups, new mutations, and common single nucleotide polymorphisms. It is also possible to determine private mutations in mtDNA through resequencing, especially if they are homoplasmic, which contributes to the variance between individuals in mtDNA composition[10].
This study aimed to characterize clinical, genetic, and molecular characteristics of two Chinese families with possible maternally transmitted MDD. The T3394C mutation in the ND1 gene in these Chinese families was identified through molecular analysis. We employed polymerase chain reaction (PCR) amplification of fragments covering the full mitochondrial genome and subsequent DNA sequence analysis to shed insight on the significance of the mitochondrial haplotype in the phenotypic expression of the T3394C mutation in two Chinese families.
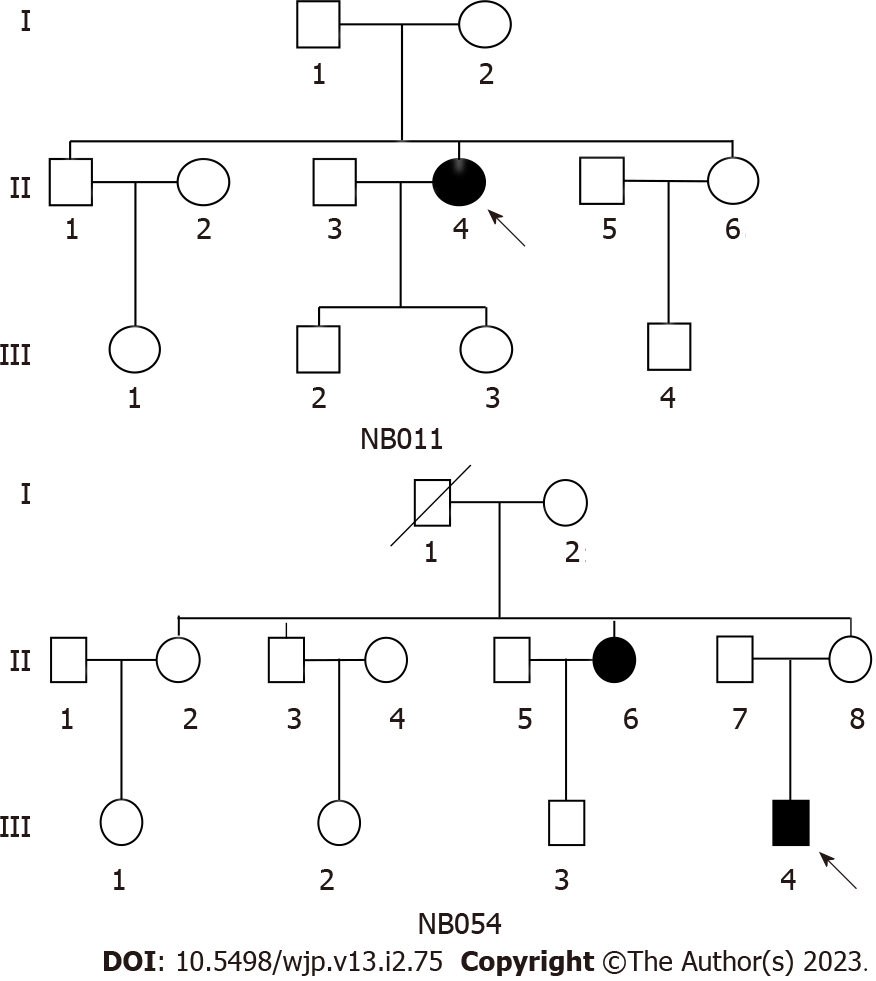
We ascertained two Han Chinese families (Figure 1) through the Psychiatric Clinic of Ningbo Kangning Hospital, Zhejiang. The Ningbo Kangning Hospital Ethics Committee approved the protocol, and obtaining clinical assessments and collecting blood samples from all family members required informed consent. In-depth interviews were conducted with members of these pedigrees to discover family and personal medicinal records of major depressive disorder and other medical abnormalities. To check for mtDNA alterations, we collected 167 DNA samples from healthy Chinese people who served as controls.
In family NB011, as shown in Table 1, the proband (II-4) complained of depression and visited the Psychiatric Clinic at Ningbo Kangning Hospital at the age of 62. She struggled with MDD approximately 12 years prior. The Hamilton depression rating scale (HDRS) showed a score of 24 and no history of suicide attempt. She exhibited the classic clinical features of MDD. The psychiatric examination found no other abnormalities. In addition, no further significant medical background was discovered. The family was from East China’s Zhejiang Province. None of the remaining seven matrilineal relatives displayed MDD.
| Subject | Gender | Age at testing (yr) | Age at onset (yr) | First episode | History of suicide attempt | HDRS | Level of depression | mtDNA haplogroup |
| NB011-Ⅱ-4 | F | 62 | 50 | N | N | 21 | Moderate | M9a3 |
| NB054-Ⅲ-4 | M | 20 | 12 | N | Y | 39 | Severe | M9a |
In the NB054 pedigree, the proband (III-4) visited the Psychiatric Clinic at Ningbo Kangning Hospital upon reaching the age of 20. He struggled with MDD 8 years prior. The HDRS showed a score of 39 and a history of suicide attempt. Thus, he had classic symptoms of clinical features of MDD. no further significant medical background was discovered. The family was also from Zhejiang Province in Eastern China. Clinical testing and additional research into II-6's family history confirmed that she had MDD. Other matrilineal relatives did not show signs of MDD.
In addition, there was no indication that any member of these families had an alternative recognized etiology for MDD.
Comprehensive family medical histories of these individuals showed no other clinical abnormalities existed in these people's families, such as diabetes, hearing loss, or vision problems.
The diagnosis of MDD for the probands was developed by utilizing structured clinical interviews[11] and was confirmed by a separate diagnostic examination conducted by a licensed psychiatrist. Depressive symptom intensity in patients with MDD was measured by the HDRS[12]. The probands with MDD got a minimum HDRS score of 17 on 17 items. The severity of MDD was determined by HDRS as follows: normal < 7; mild = 7-17; moderate = 17-24; severe > 24. The probands of MDD were not included if they had any of the following conditions according to DSM-IV: (1) Abuse of alcohol or drugs within the previous six months; (2) bipolar disorder; and (3) post-traumatic stress disorder or a history of an eating disorder within the previous month of study enrollment.
Mitochondrial genome mutational analysis: Utilizing Puregene DNA Isolation Kits (Gentra Systems), genomic DNA was extracted from the subjects' entire blood. As previously stated, L- and H-strands oligonucleotide primer sets were used to amplify the whole mitochondrial genomes of the two probands by PCR in 24 overlapping fragments[13]. Each purified fragment was examined using direct sequencing on an ABI 3100 automated DNA sequencer utilizing a Big Dye Terminator Cycle sequencing reaction kit. Consensus Cambridge sequence was matched to these sequence findings (GenBank accession number: NC 012920)[14]. The Seqweb program GAP (GCG) was used for DNA and protein sequence alignments. Genomic DNA from Chinese controls were used as templates, we amplified segments spanning the required sites by PCR and sequenced the resulting PCR products to determine the allele frequency of the T3394C mutation in the ND1 gene.
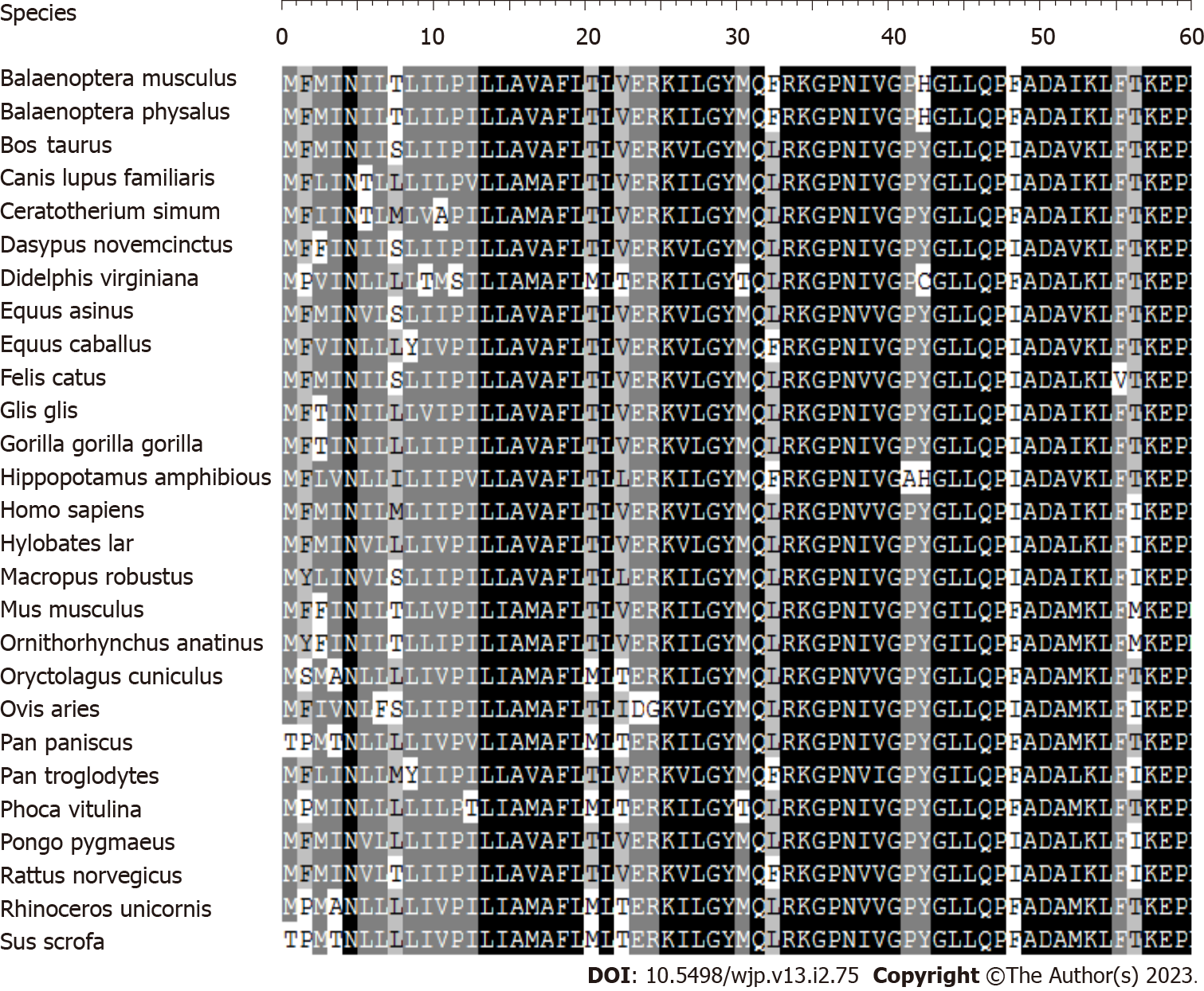
Phylogenetic analysis and haplogroup analyses: Homo sapiens[14], mouse[15], bovine[16], and Xenopus laevis[17] mitochondrial DNA sequences were utilized in the interspecific study. By comparing the four animals, the conservation was evaluated. The complete mtDNA sequences of the two Chinese probands with the T3394C mutation were allocated to the Asian mitochondrial haplogroups as per the nomenclature of mitochondrial haplogroups[18,19].
Mitochondrial DNA analysis: The mitochondrial genome of these probands for mutations was analyzed to determine the molecular basis of MDD. PCR was used to amplify the whole genome fragments of mitochondria that were then sequenced from the samples of these probands. Substances with MDD have been shown to carry a T-to-C transition at position 3394 (T3394C) in the ND1 gene, which causes the amino acid tyrosine to be replaced by histidine (Y30H) at position 30[20]. Figure 2 shows that the tyrosine at ND1 position 30 is significantly preserved across 27 different species. Leber's hereditary optic neuropathy (LHON) has been linked to this mutation in 3 Chinese families[21] and 1 Finnish family[22], as well as metabolic diseases[23], and deafness[24] in 1 Chinese family. From a sample of 167 unrelated Chinese control people, we know that one (20-year-old man) carries the T3394C mutation based on allele frequency analysis.
Besides the identical T3394C mutation (Table 2), these individuals displayed unique mtDNA polymorphisms. Other nucleotide variations in these mitochondrial genomes include 12 documented variations in the D-loop, three recognized variations in the 12S rRNA gene, 1 recognized variation in the 16S rRNA gene, 14 recognized silent variants, and 11 (2 novel/9 known) nonsense mutations in the polypeptide-encoding genes. These nonsense mutations are T4216C (T304H) in the ND1 gene; G4491A (V8I) in the ND2 gene; A8701G (T59A), TG8728T (T68G), A8860G (T112A) and A9136G (I204V) in the A6 gene; A10398G (T114A) in the ND3 gene; A14417G (V86A) in the ND6 gene; and C14766T (T7I) and A15326G (T194A) in the Cytb gene. There were 31 mutations that were carried by both probands. Phylogenetic analysis of these RNA and polypeptide variations and sequences from other taxa, such as mice[15], cattle[16], and Xenopus laevis[17], was used to assess these variants further. Nevertheless, with the exception of T3394C, none of these variations shown evolutionary conservation. The mtDNA sequence variants of two Chinese relatives were used to identify their haplogroup affiliation of each mtDNA using the nomenclature of mitochondrial haplogroups[18,19]. The mtDNA of pedigrees NB011 and NB054 correspond, respectively, to the Eastern Asian haplogroups M9a3 and M9a.
| Gene | Positon | Replacement | Conservationa (H/B/M/X) | CRSb | NB011 | NB054 | Previously reportedc |
| D-loop | 73 | A-G | A | G | G | Y | |
| 146 | T-C | T | C | C | Y | ||
| 153 | A-G | A | G | G | Y | ||
| 263 | A-G | A | G | G | Y | ||
| 309 | C-CCT | C | CCT | Y | |||
| 310 | T-C | T | TC | C | Y | ||
| 489 | T-C | T | C | C | Y | ||
| 16223 | C-T | C | T | T | Y | ||
| 16234 | C-T | C | T | T | Y | ||
| 16291 | C-T | C | T | Y | |||
| 16316 | A-G | A | G | G | Y | ||
| 16362 | T-C | T | C | C | Y | ||
| 12S rRNA | 750 | A-G | A/A/A/- | A | G | G | Y |
| 1041 | A-G | A/T/T/T | A | G | G | Y | |
| 1438 | A-G | A/A/A/G | A | G | G | Y | |
| 16S rRNA | 2706 | A-G | A/G/A/A | A | G | G | Y |
| ND1 | 3394 | T-C (Tyr30His) | Y/Y/Y/Y | T | C | C | Y |
| 4216 | T-C (Tyr304His) | Y/H/H/H | T | C | Y | ||
| ND2 | 4491 | G-A (Val8Ile) | V/I/I/V | G | A | A | Y |
| 4769 | A-G | A | G | G | Y | ||
| CO1 | 7028 | C-T | C | T | T | Y | |
| 7142 | T-C | T | C | Y | |||
| CO2 | 7861 | T-C | T | C | Y | ||
| ATP6 | 8701 | A-G (Thr59Ala) | T/S/L/Q | A | G | G | Y |
| 8728 | TG-T (Trp68Gly) | W/W/W/W | TG | T | N | ||
| 8860 | A-G (Thr112Ala) | T/A/A/T | A | G | G | Y | |
| 9136 | A-G (Ile204Val) | I/F/F/I | A | G | N | ||
| CO3 | 9242 | A-G | A | G | Y | ||
| 9540 | T-C | T | C | C | Y | ||
| ND3 | 10398 | A-G (Thr114Ala) | T/T/T/A | A | G | G | Y |
| 10400 | C-T | C | T | T | Y | ||
| ND4 | 10873 | T-C | T | C | C | Y | |
| 11719 | G-A | G | A | A | Y | ||
| ND5 | 12705 | C-T | C | T | T | Y | |
| ND6 | 14308 | T-C | T | C | C | Y | |
| 14417 | A-G (Val86Ala) | V/K/W/S | A | G | Y | ||
| CYTB | 14766 | C-T (Thr7Ile) | T/S/T/S | C | T | T | Y |
| 14783 | T-C | T | C | C | Y | ||
| 15043 | G-A | G | A | A | Y | ||
| 15301 | G-A | G | A | A | Y | ||
| 15326 | A-G (Thr194Ala) | T/M/I/I | A | G | G | Y |
The described patients are all ultimately diagnosed with MDD.
In this investigation, the clinical, genetic, and molecular characteristics of two Chinese families with MDD were determined. MDD had a distinct clinical phenotype and solely existed in the maternal lineage in the two families, prompting us to speculate that mtDNA may be the molecular foundation of MDD. Complete mitochondrial genome sequence study of the two pedigrees revealed different mtDNA polymorphisms except for the same T3394C (Y30H) mutation in the ND1 gene. The ND1 gene is the central component of the 45 subunits of complex I, and the T3394C mutation caused a shift from tyrosine to histidine (Y30H) at position 30[25,26]. Actually, the hydroxyl group on Y30 of ND1 interacts electrostatically with the side chain of E4 and the carbonyl group on M1 of NDUFA1[26,27]. Consequently, the T3394C mutation may disturb the interactions among ND1 and NDUFA1, consequently affecting the structure and function of complex I[28]. Leber's optic neuropathy[21,22], metabolism disorders[23] and deafness[24] are only some of the additional clinical problems linked to the T3394C mutation.
Although both families had typical clinical manifestations of major depressive disorder, there were differences in penetrance, age at onset, and severity. The penetrance of the two families was 8.3% and 14.3%, respectively. This relatively low penetrance of MDD in two Chinese families having the T3394C mutation and its presence in one out of 167 controls suggests that, similar to previous mutations[29,30], the T3394C mutation alone is inadequate to induce the clinical manifestation. Therefore, the T3394C mutation requires modifying variables, such as nuclear histories, additional external variables, and mitochondrial haplotypes for phenotypic expression. Particularly, it has been demonstrated that mitochondrial haplotypes alter the penetrance and expressivity of MDD linked with primary mtDNA mutations[31]. The mtDNA of pedigrees NB011 and NB054 correspond, respectively, to the Eastern Asian haplogroups M9a3 and M9a.
The homogenous mutation T3394C was found in MDD families with different genetic backgrounds. The minimal penetrance of MDD among families with this mutation suggests that the T3394C mutation is a molecular base for the pathogenesis of MDD, but the mutation alone is not sufficient to cause phenotypic expression of MDD. This suggests that other mediators played a synergistic role in the pathophysiology of these families. Furthermore, the whole mitochondrial genome of the two families did not carry other highly conserved and functional mutation sites, indicating that polymorphic sites related to mitochondrial haplomorphism may not have a significant impact in the pathophysiology of two MDD families with the T3394C mutation. Moreover, mtDNA epigenetics may be involved in the occurrence of MDD, but research on the role of mtDNA epigenetics in diseases and therapeutic targets is insufficient. Therefore, nuclear-modified genes or external variables have a function in the phenotypic expression of MDD related T3394C mutations in these Chinese individuals. In conclusion, the ND1 T3394C mutation may be a mitochondrial gene mutation site associated with MDD.
This observation of the T3394C mutation in two genetically unrelated individuals who suffer from depression strongly indicates that the current mutation might contribute to developing MDD. However, in these two Chinese families having the T3394C mutation, no functional mtDNA mutation was found. Therefore, the phenotypic manifestation of T3394C mutations linked to MDD may be affected by nuclear changed genes or environmental factors.
We thank the participants for their contributions to this study. We thank the Genetics Institute of Zhejiang University for its help in mitochondrial sequencing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosak L, Czech Republic; Kaur M, United States S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Malhi GS, Outhred T, Morris G, Boyce PM, Bryant R, Fitzgerald PB, Hopwood MJ, Lyndon B, Mulder R, Murray G, Porter RJ, Singh AB, Fritz K. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Med J Aust. 2018;208:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Goetzl EJ, Wolkowitz OM, Srihari VH, Reus VI, Goetzl L, Kapogiannis D, Heninger GR, Mellon SH. Abnormal levels of mitochondrial proteins in plasma neuronal extracellular vesicles in major depressive disorder. Mol Psychiatry. 2021;26:7355-7362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 3. | Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Psychiatr Serv. 2009;60:1439-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 432] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 4. | Schatzberg AF. Can Target Engagement Studies Miss Their Targets and Mislead Drug Development? Am J Psychiatry. 2021;178:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Sequeira A, Rollins B, Magnan C, van Oven M, Baldi P, Myers RM, Barchas JD, Schatzberg AF, Watson SJ, Akil H, Bunney WE, Vawter MP. Mitochondrial mutations in subjects with psychiatric disorders. PLoS One. 2015;10:e0127280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Johannsen DL, Ravussin E. The role of mitochondria in health and disease. Curr Opin Pharmacol. 2009;9:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Clay HB, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29:311-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 297] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 8. | Sequeira A, Martin MV, Rollins B, Moon EA, Bunney WE, Macciardi F, Lupoli S, Smith EN, Kelsoe J, Magnan CN, van Oven M, Baldi P, Wallace DC, Vawter MP. Mitochondrial mutations and polymorphisms in psychiatric disorders. Front Genet. 2012;3:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Munakata K, Fujii K, Nanko S, Kunugi H, Kato T. Sequence and functional analyses of mtDNA in a maternally inherited family with bipolar disorder and depression. Mutat Res. 2007;617:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Shao L, Martin MV, Watson SJ, Schatzberg A, Akil H, Myers RM, Jones EG, Bunney WE, Vawter MP. Mitochondrial involvement in psychiatric disorders. Ann Med. 2008;40:281-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 11. | First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders. Washington, DC: American Psychiatric Pub Inc, 1997. |
| 12. | Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21041] [Cited by in RCA: 22852] [Article Influence: 351.6] [Reference Citation Analysis (0)] |
| 13. | Rieder MJ, Taylor SL, Tobe VO, Nickerson DA. Automating the identification of DNA variations using quality-based fluorescence re-sequencing: analysis of the human mitochondrial genome. Nucleic Acids Res. 1998;26:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 410] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 14. | Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet. 1999;23:147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2374] [Cited by in RCA: 2373] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 15. | Bibb MJ, Van Etten RA, Wright CT, Walberg MW, Clayton DA. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981;26:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1197] [Cited by in RCA: 1254] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 16. | Gadaleta G, Pepe G, De Candia G, Quagliariello C, Sbisà E, Saccone C. The complete nucleotide sequence of the Rattus norvegicus mitochondrial genome: cryptic signals revealed by comparative analysis between vertebrates. J Mol Evol. 1989;28:497-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 390] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Roe BA, Ma DP, Wilson RK, Wong JF. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985;260:9759-9774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 467] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 18. | Kong QP, Bandelt HJ, Sun C, Yao YG, Salas A, Achilli A, Wang CY, Zhong L, Zhu CL, Wu SF, Torroni A, Zhang YP. Updating the East Asian mtDNA phylogeny: a prerequisite for the identification of pathogenic mutations. Hum Mol Genet. 2006;15:2076-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 319] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | Tanaka M, Cabrera VM, González AM, Larruga JM, Takeyasu T, Fuku N, Guo LJ, Hirose R, Fujita Y, Kurata M, Shinoda K, Umetsu K, Yamada Y, Oshida Y, Sato Y, Hattori N, Mizuno Y, Arai Y, Hirose N, Ohta S, Ogawa O, Tanaka Y, Kawamori R, Shamoto-Nagai M, Maruyama W, Shimokata H, Suzuki R, Shimodaira H. Mitochondrial genome variation in eastern Asia and the peopling of Japan. Genome Res. 2004;14:1832-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 380] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 20. | Fearnley IM, Walker JE. Conservation of sequences of subunits of mitochondrial complex I and their relationships with other proteins. Biochim Biophys Acta. 1992;1140:105-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 243] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Liang M, Guan M, Zhao F, Zhou X, Yuan M, Tong Y, Yang L, Wei QP, Sun YH, Lu F, Qu J, Guan MX. Leber's hereditary optic neuropathy is associated with mitochondrial ND1 T3394C mutation. Biochem Biophys Res Commun. 2009;383:286-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Puomila A, Hämäläinen P, Kivioja S, Savontaus ML, Koivumäki S, Huoponen K, Nikoskelainen E. Epidemiology and penetrance of Leber hereditary optic neuropathy in Finland. Eur J Hum Genet. 2007;15:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Saxena R, de Bakker PI, Singer K, Mootha V, Burtt N, Hirschhorn JN, Gaudet D, Isomaa B, Daly MJ, Groop L, Ardlie KG, Altshuler D. Comprehensive association testing of common mitochondrial DNA variation in metabolic disease. Am J Hum Genet. 2006;79:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Chen J, Yuan H, Lu J, Liu X, Wang G, Zhu Y, Cheng J, Wang X, Han B, Yang L, Yang S, Yang A, Sun Q, Kang D, Zhang X, Dai P, Zhai S, Han D, Young WY, Guan MX. Mutations at position 7445 in the precursor of mitochondrial tRNA(Ser(UCN)) gene in three maternal Chinese pedigrees with sensorineural hearing loss. Mitochondrion. 2008;8:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Scheffler IE. Mitochondrial disease associated with complex I (NADH-CoQ oxidoreductase) deficiency. J Inherit Metab Dis. 2015;38:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Zhu J, Vinothkumar KR, Hirst J. Structure of mammalian respiratory complex I. Nature. 2016;536:354-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 438] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 27. | Zickermann V, Wirth C, Nasiri H, Siegmund K, Schwalbe H, Hunte C, Brandt U. Structural biology. Mechanistic insight from the crystal structure of mitochondrial complex I. Science. 2015;347:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 330] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 28. | Ji Y, Zhang J, Yu J, Wang Y, Lu Y, Liang M, Li Q, Jin X, Wei Y, Meng F, Gao Y, Cang X, Tong Y, Liu X, Zhang M, Jiang P, Zhu T, Mo JQ, Huang T, Guan MX. Contribution of mitochondrial ND1 3394T>C mutation to the phenotypic manifestation of Leber's hereditary optic neuropathy. Hum Mol Genet. 2019;28:1515-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Qu J, Zhou X, Zhang J, Zhao F, Sun YH, Tong Y, Wei QP, Cai W, Yang L, West CE, Guan MX. Extremely low penetrance of Leber's hereditary optic neuropathy in 8 Han Chinese families carrying the ND4 G11778A mutation. Ophthalmology. 2009;116:558-564.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Zhou X, Wei Q, Yang L, Tong Y, Zhao F, Lu C, Qian Y, Sun Y, Lu F, Qu J, Guan MX. Leber's hereditary optic neuropathy is associated with the mitochondrial ND4 G11696A mutation in five Chinese families. Biochem Biophys Res Commun. 2006;340:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Rollins B, Martin MV, Sequeira PA, Moon EA, Morgan LZ, Watson SJ, Schatzberg A, Akil H, Myers RM, Jones EG, Wallace DC, Bunney WE, Vawter MP. Mitochondrial variants in schizophrenia, bipolar disorder, and major depressive disorder. PLoS One. 2009;4:e4913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |