Published online Nov 19, 2023. doi: 10.5498/wjp.v13.i11.949
Peer-review started: September 13, 2023
First decision: September 28, 2023
Revised: October 7, 2023
Accepted: October 28, 2023
Article in press: October 28, 2023
Published online: November 19, 2023
Processing time: 65 Days and 0.7 Hours
Traditional treatments for major depressive disorder (MDD), including medication and therapy, often fail and have undesirable side effects. Electroconvulsive therapy (ECT) uses electrical currents to induce brief seizures in the brain, resulting in rapid and potent antidepressant effects. However, owing to misconceptions and controversies, ECT is not as widely used as it could and often faces stigmatization.
To evaluate the efficacy and safety of ECT compared to those of medication and/or therapy in patients with severe MDD.
This prospective cohort study included 220 individuals with severe MDD who were divided into the ECT and non-ECT groups. The patients in the ECT group underwent bilateral ECT three times a wk until they either achieved remission or reached a maximum of 12 sessions. The non-ECT group received medication and/or therapy according to clinical guidelines for MDD. The primary outcome was the variation in the hamilton depression rating scale (HDRS) score from treatment/ECT initiation to week 12. In addition, patients’ quality of life, cognitive abilities, and biomarkers were measured throughout the study.
Although both groups showed significant improvements in their HDRS scores over time, the improvement was more pronounced in the ECT group than in the non-ECT group. Additionally, the ECT group exhibited a more substantial improvement in the quality of life and cognitive function than those of the non-ECT group. Compared with the non-ECT group, the ECT group exhibited evi-dently lower variations in the brain-derived neurotrophic factor (BDNF) and cytokine interleukin-6 (IL-6) levels. The side effects were generally mild and comparable between the two groups. ECT is safer and more potent than medication and/or therapy in mitigating depressive symptoms, enhancing well-being, and bolstering cognitive capabilities in individuals with severe MDD. ECT may also affect the levels of BDNF and IL-6, which are indicators of neuroplasticity and inflammation, respectively.
ECT has emerged as a potentially advantageous therapeutic approach for patients with MDD who are unresponsive to alternative treatments.
Core Tip: Electroconvulsive therapy (ECT) is more efficient and safer in treating severe major depressive disorder (MDD) compared to medication and/or therapy. Here, ECT led to more pronounced improvements in depressive symptoms, quality of life, and cognitive function than non-ECT treatments. Additionally, ECT affected biomarkers related to neuroplasticity (brain-derived neurotrophic factor) and inflammation (interleukin-6). These findings highlight the potential of ECT as a therapeutic approach for individuals with severe MDD who do not respond to conventional treatments. By providing evidence of its beneficial outcomes, this study aimed to address the stigmatization surrounding ECT and facilitate its broader adoption in clinical practice.
- Citation: Han KY, Wang CM, Du CB, Qiao J, Wang YL, Lv LZ. Treatment outcomes and cognitive function following electroconvulsive therapy in patients with severe depression. World J Psychiatry 2023; 13(11): 949-957
- URL: https://www.wjgnet.com/2220-3206/full/v13/i11/949.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i11.949
Major depressive disorder (MDD) is a prevalent mental health condition with a profound impact on many individuals worldwide. The World Health Organization reported that MDD contributes significantly to the global health burden[1]. This condition is characterized by a persistent sense of sadness, diminished interest in daily activities, and a reduction in function that lasts for at least 2 wk. MDD can negatively affect physical, psychological, social, and occupational aspects of life, leading to increased morbidity, mortality, and suicide risk[2].
Conventional treatments for MDD include pharmacotherapy and psychotherapy, which aim to restore mood balance, reduce distress, and enhance coping strategies[3]. Pharmacotherapy involves the use of antidepressants, such as serotonin, dopamine, and norepinephrine, which act by targeting neurotransmitter systems[4]. Psychotherapy comprises a variety of psychological interventions that address the cognitive, behavioral, interpersonal, and emotional factors that contribute to depression[5]. Widely practiced and documented psychotherapy techniques include cognitive-behavioral therapy (CBT), interpersonal therapy (IPT), and supportive therapy[6]. However, conventional therapies are often insufficient and associated with adverse effects. Approximately 50% of patients achieve remission after a typical course of antidepressants, and approximately 30% remain resistant to multiple types of treatment[7]. Furthermore, antidepressants can cause adverse reactions, including nausea, weight gain, sexual issues, insomnia, and agitation[8]. Psychotherapy can also be ineffective or inaccessible to some patients because of a lack of availability, affordability, or motivation. Moreover, conventional therapies may take several wks or months before significant improvements are evident[9].
Electroconvulsive therapy (ECT) is a medical procedure performed under general anesthesia, using electrical currents to induce brief seizures in the brain. ECT has been used since 1938, primarily for treating schizophrenia, and was pioneered by Cerletti and Bini[10]. ECT has been widely used to treat many psychiatric disorders, particularly severe or treatment-resistant MDD[11]. It is a highly potent and safe remedy for acute or suicidal MDD, with remission rates ranging from 60% to 90%. ECT has been shown to improve the overall well-being and mental processing abilities of patients with severe MDD[11] and can produce rapid and robust antidepressant effects, with improvements observed within 1-2 wk[12].
However, ECT remains underutilized and stigmatized owing to misconceptions and controversies. Many patients and even some healthcare professionals have negative attitudes and beliefs concerning ECT, finding it cruel, dangerous, or outdated or believing that its effects can be substituted by long-term psychotherapy or hospitalization[13]. Some of these misconceptions are based on the historical use of ECT, which involves the delivery of large amounts of electrical charge, inadequate anesthesia, and frequent sessions, resulting in severe side effects, such as memory loss, brain damage, or death. However, modern ECT is a safe, effective, and regulated treatment that involves minimizing the amount of electrical charge delivered, administering adequate anesthesia, and designing patient-centered personalized sessions, resulting in minimal side effects, including transient headaches, nausea, and confusion[14]. Modern ECT does not cause brain damage or permanent memory loss and would not affect the personality or intelligence of patients[15].
The primary objective of the present study was to compare the efficacy and safety of ECT with those of pharmacotherapy and/or psychotherapy in patients with severe MDD. We hypothesized that ECT would be superior to pharmacotherapy and/or psychotherapy in terms of improving the hamilton depression rating scale (HDRS) scores, a validated clinician-rated outcome measure for MDD. We also hypothesized that ECT would be superior to pharmacotherapy and/or psychotherapy in improving the quality of life and cognitive function in patients with severe MDD. Brain-derived neurotrophic factor (BDNF), cytokine interleukin-6 (IL-6), and cortisol levels were also measured as biomarkers of neuroplasticity and inflammation.
This prospective cohort analysis was conducted at The First Hospital of Hebei Medical University in China between January 2021 and June 2022. The research methodology was approved by the ethics boards of all involved institutions and strictly adhered to the Declaration of Helsinki and Standards for Reporting Observational Research. All participants provided written informed consent before participating in the study.
The study recruited individuals aged ≥ 18 years who had been diagnosed with MDD according to the Diagnostic and Statistical Manual of Mental Disorders guidelines, had an HDRS score > 24 points, were willing to participate in the study, and attended subsequent visits. The exclusion criteria included a history of allergic reaction to ECT or anesthesia, prior neurological issues, brain trauma, seizure-related disorders, substance misuse or dependency in the past 6 mo, receiving any medication that may influence the seizure threshold or cognitive abilities in the preceding month (such as anticonvulsants, benzodiazepines, or antipsychotics), pregnancy or breastfeeding, diagnosis of any other mental or physical ailment that might affect the study results, or inability to provide blood samples for biomarker evaluation.
Eligible participants were allocated to the ECT and non-ECT groups depending on their treatment approach. In the ECT group, individuals underwent ECT as routine treatment for MDD. In the non-ECT group, individuals underwent drug therapy and/or psychotherapy (without ECT) as a routine MDD treatment. The treatment group was chosen at the discretion of the attending psychiatric team based on their clinical assessment and inclination. Participants were paired across groups based on age, sex, and HDRS scores at baseline.
Participants in the ECT group underwent bilateral ECT using an apparatus specifically designed to deliver brief pulses (Thymatron System IV; Somatics LLC, Lake Bluff, IL, United States) according to a standardized protocol[16]. The protocol commenced with an initial assessment, including physical checkups, electrocardiography (ECG), blood testing, and cognitive evaluation. ECT was performed under general anesthesia using propofol and succinylcholine. Stimulus power was estimated using titration technique and fine-tuned according to the seizure duration and quality. ECT was administered thrice a wk until remission was achieved or a maximum of 12 sessions were completed. Vital signs, oxygen levels, ECG findings, and side effects were recorded during and after each ECT session.
The non-ECT group was treated with medication and/or psychotherapy according to the clinical guidelines for the treatment of MDD[17]. Medications included antidepressants (selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, tricyclic antidepressants, or monoamine oxidase inhibitors), mood balancers (lithium or valproate), and supplementary agents (thyroid hormones or antipsychotics). Psychotherapy included CBT, IPT, or supportive therapy. Medication type, dosage, length of course, and the use or absence of psychotherapy were determined by the attending psychiatrists based on patient feedback and tolerance.
Both groups were advised not to use other treatments or medications that could influence their mood or cognitive function during the study period. They were also encouraged to maintain their regular lifestyle and environment and report any physical changes or side effects to the research team.
The main metric of interest was the change in the HDRS score between the initial assessment and 12-wk mark. The HDRS consists of 17 clinician-evaluated items that measure the intensity of depressive signs[18]. Scores on the HDRS range from 0 to 52 points, with higher scores indicating more pronounced depression. A score of ≤ 7 points on the HDRS was considered to signify remission, a reduction of ≥ 50% from the initial score was considered a response, and a reduction of < 50% was classified as non-response.
Additional outcomes of interest included changes in quality of life, cognitive capabilities, and specific biomarkers between the baseline and 12-wk assessments. The World Health Organization Quality of Life-BREF (WHOQOL-BREF) questionnaire[19], a 26-item tool, was used to measure participants’ quality of life and covered the domains of physical well-being, mental health, interpersonal relations, and the surrounding environment. The WHOQOL-BREF scores range from 0 to 100 points, with higher scores indicating higher quality of life. The Montreal Cognitive Assessment (MoCA)[20], a concise evaluation instrument, was used to gauge cognitive function, covering the areas of attention span, memory retention, linguistic skills, executive decision-making, and spatial cognition. The MoCA scores range from 0 to 30 points, with higher scores indicating greater cognitive ability. Blood samples collected at the ECT initiation and 12-wk mark were analyzed to measure BDNF, IL-6, and cortisol levels. BDNF is crucial for neuronal survival, differentiation, and adaptability. IL-6, a cytokine that promotes inflammation, is involved in immune reactions and mood and cognitive regulation. Cortisol, a hormone associated with stress, influences the hypothalamic (pituitary) axis and neuroendocrine mechanisms. Any adverse reactions were documented during each visit and categorized as mild, moderate, or intense based on their severity and association with the study interventions.
The required sample size was determined based on the primary outcome, HDRS, with an anticipated clinically meaningful difference of 5 points between the groups, a standard deviation of 10 points, a significance level of 0.05, and a statistical power of 0.8. Based on these criteria, we calculated that at least 100 individuals were required in each group to discern meaningful differences. Anticipating a 10% dropout rate, a prudent sample of 110 individuals was recruited from each group, totaling 220 participants.
All statistical analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY, United States). Data normality was rigorously verified using histograms and Kolmogorov-Smirnov tests. Comparative analyses of the groups’ baseline attributes were conducted using independent t-tests or Mann-Whitney U tests for continuous data and chi-square or Fisher's exact tests for categorical data. Changes in HDRS scores and secondary outcomes from baseline to 12 wk were assessed between the groups using analysis of covariance. Differences in means and 95% confidence intervals (CIs) were calculated using descriptive statistics. Adverse events between the groups were analyzed using chi-square or Fisher's exact tests. Statistical significance was set at P < 0.05.
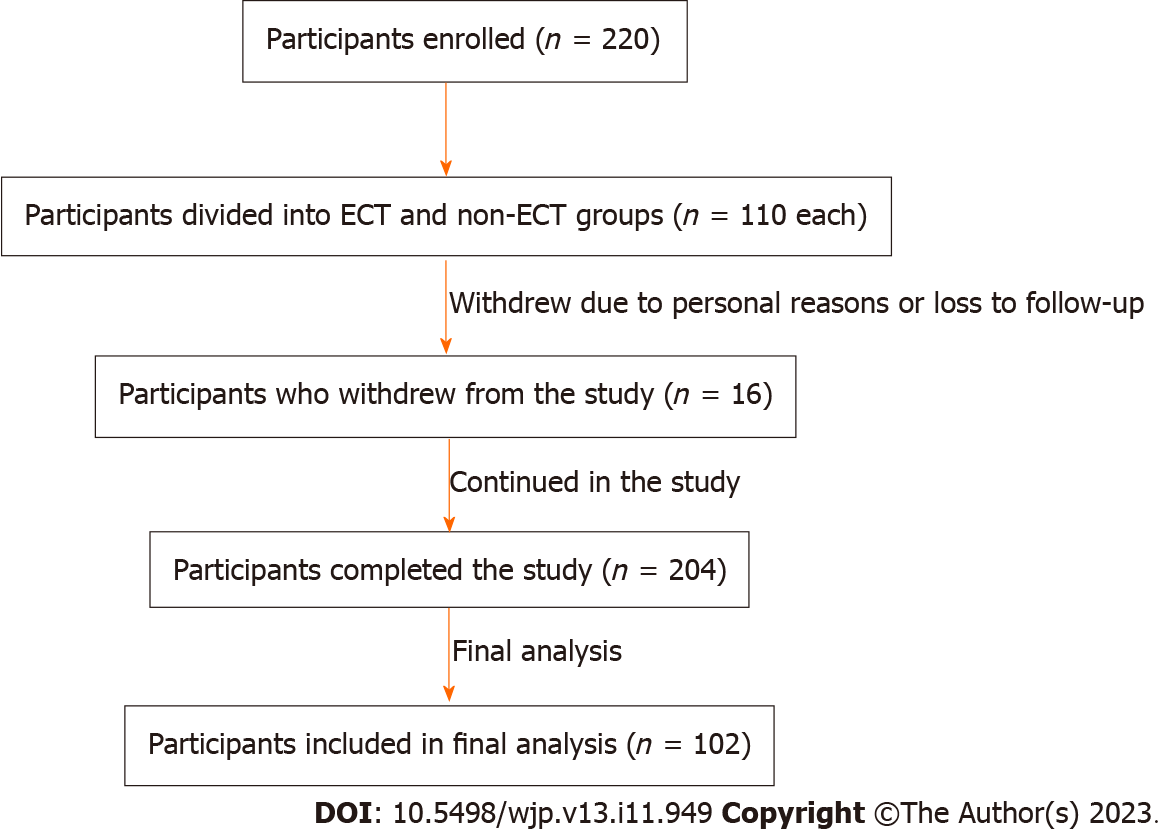
A total of 220 participants were recruited, of whom 110 were allocated to the ECT group and 110 to the non-ECT group. The participants’ progress throughout the study is shown in Figure 1. Eight participants withdrew from each group due to personal choices or were lost to follow-up, culminating in 204 participants (102 in each cohort) included in the final analysis.
Table 1 presents the baseline attributes of the participants. No significant baseline differences were observed between the groups in terms of age, sex, MDD duration, MDD type, HDRS score, WHOQOL-BREF score, MoCA score, BDNF level, IL-6 level, or cortisol level (all P > 0.05).
| Variable | ECT group (n = 102) | Non-ECT group (n = 102) | P value |
| Age (yr) | 46.3 ± 11.2 | 45.8 ± 10.9 | 0.69 |
| Sex (female/male) | 58/44 | 60/42 | 0.81 |
| Duration of MDD (mo) | 24.6 ±12.8 | 25.4 ± 13.1 | 0.07 |
| Type of MDD (unipolar/bipolar) | 82/20 | 84/18 | 0.76 |
| HDRS score | 28.4 ± 3.6 | 28.2 ± 3.7 | 0.72 |
| WHOQOL-BREF score | 42.6 ± 8.9 | 43.1 ± 9.2 | 0.66 |
| MoCA score | 24.8 ± 3.2 | 25.1 ± 3.4 | 0.54 |
| BDNF level (ng/mL) | 18.6 ± 5.4 | 18.9 ± 5.7 | 0.62 |
| IL-6 level (pg/mL) | 3.4 ± 1.2 | 3.5 ± 1.3 | 0.58 |
| Cortisol level (ng/mL) | 12.7 ± 4.1 | 12.9 ± 4.3 | 0.71 |
Table 2 displays the changes in HDRS scores from the initial assessment to the 12-wk mark. Both groups showed notable improvements in HDRS scores over time (P < 0.001 for both cohorts). The enhancement was significantly greater in the ECT than in the non-ECT group (P < 0.001). The average change in HDRS score between the cohorts was -5.4 (95%CI: -7.8 to -3.0), suggesting a substantial improvement in the ECT group.
| Outcome | ECT group (n = 102) | Non-ECT group (n = 102) | P value |
| HDRS score | -19.6 ± 6.4 (-69) | -14.2 ± 7.2 (-50) | < 0.001 |
| WHOQOL-BREF score | +15.4 ± 8.7 (+37) | +9.2 ± 7.9 (+21) | < 0.001 |
| MoCA score | +2.6 ± 1.4 (+10) | +1.0 ± 1.2 (+4) | < 0.001 |
| BDNF level (ng/mL) | -12.8 ± 4.6 (-69) | -18.6 ± 5.8 (-98) | < 0.001 |
| IL-6 level (pg/mL) | +2.2 ± 0.9 (+65) | +3.4 ± 1.2 (+97) | < 0.001 |
| Cortisol level (ng/mL) | -0.2 ± 3.8 (-2) | -0.1 ± 4.1 (-1) | 0.92 |
Recovery by 12 wk was significantly higher in the ECT than in the non-ECT group (68% vs 42%, P < 0.001). Similarly, the rate of positive response by the 12th wk was also significantly higher in the ECT cohort than in the non-ECT cohort (86% vs 64%, P < 0.001). Conversely, the rate of no response by 12 wk was significantly lower in the ECT than in the non-ECT group (14% vs 36%, P < 0.001).
Table 2 presents the variations in the quality of life, cognitive ability, and biomarker levels from the initial assessment to the 12-wk mark. Both participant groups exhibited notable enhancements in the WHOQOL-BREF and MoCA scores over time (P < 0.001 for both metrics and groups). However, the ECT group displayed significantly greater enhancement than the non-ECT group for both metrics (P < 0.05). The average difference between the groups was 6.2 points (95%CI: 3.4 to 9.0) for the WHOQOL-BREF and 1.6 points (95%CI: 0.8 to 2.4) for the MoCA.
Both groups showed a significant decrease in BDNF levels and a significant increase in IL-6 levels over time (P < 0.001 for both metrics and groups). However, these changes were significantly smaller in the ECT than in the non-ECT group for both metrics (P < 0.05). The average differences between the groups were -5.8 units (95%CI: -9.2 to -2.4) for BDNF and -1.2 units (95%CI: -2.1 to -0.3) for IL-6. None of the groups exhibited any notable changes in cortisol levels over the study duration (P > 0.05) or at the 12-wk mark (P > 0.05).
Table 3 shows that there were no significant differences in the occurrence of adverse effects between the two groups (P = 0.62). Commonly reported adverse effects included headache, nausea, dizziness, and memory issues, all of which were generally mild and short-lived. Moreover, throughout the study duration, no severe adverse effects were documented.
| Adverse event | ECT group (n = 102) | Non-ECT group (n = 102) | P value |
| Headache | 32 (31) | 28 (27) | 0.54 |
| Nausea | 24 (24) | 22 (22) | 0.76 |
| Dizziness | 18 (18) | 16 (16) | 0.72 |
| Memory impairment | 14 (14) | 12 (12) | 0.69 |
| Insomnia | 10 (10) | 14 (14) | 0.39 |
| Weight gain | 8 (8) | 10 (10) | 0.63 |
| Sexual dysfunction | 6 (6) | 8 (8) | 0.57 |
| Agitation | 4 (4) | 6 (6) | 0.51 |
| Confusion | 2 (2) | 2 (2) | > 0.99 |
| Seizure prolongation1 | 2 (2) | N/A2 | N/A2 |
This study demonstrated that ECT is safer and more effective than pharmacotherapy and/or psychotherapy in alleviating depressive symptoms, improving well-being, and boosting cognitive ability in individuals with severe MDD. ECT also appears to regulate the levels of BDNF and IL-6, which are biomarkers for neuroplasticity and inflammation, respectively.
These findings concur with those of previous studies highlighting the advantages of ECT over other treatments for patients with severe MDD. A meta-analysis of 16 randomized controlled trials (RCTs) involving 1391 patients with MDD found that ECT was more effective than pharmacotherapy or placebo in inducing remission and response[21]. Another meta-analysis of nine RCTs involving 619 patients with treatment-resistant depression found that ECT outperformed pharmacotherapy and placebo ECT in reducing the signs of depression[22]. Furthermore, a systematic review of 24 studies involving 1708 patients with MDD found that ECT produced greater improvements in patient well-being and cognitive abilities than other treatments[23].
Although the precise mechanism underlying the action of ECT is unclear, it is likely related to its effects on the brain structure and function. ECT may induce neurogenesis, synaptogenesis, angiogenesis, and neurotrophic factor expression in multiple brain regions, particularly in the hippocampus, amygdala, and prefrontal cortex[24], which are involved in mood regulation, memory formation, and executive functions. ECT may also modulate neurotransmitter systems, including serotonin and dopamine, which are implicated in depression and cognition[25] and may normalize the hypothalamo-pituitary-adrenocortical axis activity and reduce the inflammatory response, both of which are associated with stress and depression[26,27].
The merits of the present study include its prospective cohort design, large sample size, use of a standardized protocol for ECT administration, use of validated and objective outcome measures, and long follow-up duration. The potential limitations of this study include its confinement to a single nation, absence of randomization and blinding of participants, potential variability in the prescribed pharmacotherapy and psychotherapy given to participants in the non-ECT group, and lack of assessment of other outcomes, such as cost-effectiveness or patient satisfaction.
In conclusion, this study demonstrated that ECT is safer and more effective than pharmacotherapy and/or psychotherapy in enhancing signs of depression, overall well-being, and cognitive abilities in individuals with severe MDD, and in modulating the levels of BDNF and IL-6, which are biomarkers of neuroplasticity and inflammation, respectively. ECT may be a valuable treatment option for patients with MDD refractory to other treatments.
Major depressive disorder (MDD) is a prevalent mental health condition characterized by persistent feelings of sadness and loss of interest. Traditional treatments for MDD, such as medications and therapy, often have limited effectiveness and can lead to undesirable side effects. Electroconvulsive therapy (ECT) is an alternative treatment that uses electrical currents to induce controlled brain seizures, resulting in rapid and potent antidepressant effects. However, ECT is associated with misconceptions, controversies, and stigmatization, which hinder its wider acceptance and utilization.
The main theme of this study is to evaluate the efficacy and safety of ECT compared to medication and/or therapy in patients with severe MDD. The key problems that need to be addressed are the limited effectiveness and undesirable side effects of traditional treatments for MDD, which often lead to treatment failure. Despite its rapid and potent antidepressant effects, ECT faces misconceptions, controversies, and stigmatization, resulting in its underutilization.
The main objective of this study was to evaluate the efficacy and safety of ECT compared to medication and/or therapy in individuals with severe MDD. The specific goals accomplished were as follows: (1) Variation in the hamilton depression rating scale (HDRS) scores between the ECT and non-ECT groups over a 12-wk period; (2) improvements in the quality of life and cognitive function between the ECT and non-ECT groups were compared; (3) variations in the levels of brain-derived neurotrophic factor (BDNF) and interleukin-6 (IL-6), potential biomarkers of neuroplasticity and inflammation, respectively, between the ECT and non-ECT groups; and (4) evaluation of the safety and tolerability of ECT compared to medication and/or therapy. The achievement of these objectives has significant implications for future research. First, it provided evidence supporting the superior efficacy and safety of the ECT as a treatment option for individuals with severe MDD who do not respond to traditional therapy. This challenges the prevailing misconceptions and stigmatization surrounding ECT and encourages further investigation and acceptance of this modality. Second, the identification of biomarkers influenced by ECT opens avenues for future studies to explore the underlying mechanisms of its antidepressant effects and potentially identify predictors of treatment response. Overall, this study contributes to optimizing therapeutic approaches for severe MDD and guides future research toward better understanding and improved treatment outcomes for affected individuals.
A prospective cohort design was used to achieve the research objectives. The study included 220 individuals with severe MDD who were divided into the ECT and non-ECT groups. In the ECT group, bilateral ECT was administered three times a wk until remission was achieved or a maximum of 12 sessions were reached. The non-ECT group received medication and/or therapy according to clinical guidelines for MDD. The HDRS score was used as the primary outcome measure, with variations in scores assessed from the beginning to 12 wk. In addition to the HDRS score, this study measured the patients’ quality of life, cognitive abilities, and biomarkers throughout the study. The biomarkers of interest were BDNF and IL-6, which are indicators of neuroplasticity and inflammation, respectively. The collected data were analyzed using appropriate statistical methods, such as t-tests or analysis of variance, to compare the outcomes between the ECT and non-ECT groups. The study also assessed the safety and tolerability of ECT compared to medication and/or therapy by monitoring and documenting any side effects experienced by the participants. The novelty of this study lies in its comprehensive evaluation of the efficacy, safety, and biomarker influences of ECT compared to conventional treatments for severe MDD. By incorporating multiple outcome measures and biomarker assessments, this study provides a holistic understanding of the effects of ECT on depressive symptoms, well-being, cognitive function, neuroplasticity, and inflammation. This prospective cohort design allows the observation of treatment outcomes over time and contributes to a growing body of evidence supporting the potential advantages of ECT as a therapeutic approach for treatment-resistant MDD.
The results of this study indicated that ECT was more effective and safer than medication and/or therapy for severe MDDs. Both the ECT and non-ECT groups showed significant improvements in the HDRS scores over the 12-wk period. However, the ECT group demonstrated a more pronounced improvement in depressive symptoms than that of the non-ECT group. Moreover, the ECT group exhibited greater improvements in quality of life and cognitive function. The study also revealed that the ECT group had lower variations in the levels of BDNF and IL-6 compared with the non-ECT group. This suggests that ECT influences neuroplasticity and inflammation, potentially contributing to its therapeutic effects. These findings highlight the superior efficacy and safety of ECT as a treatment option for severe MDD, particularly in cases where traditional therapies fail. This study emphasizes the potential advantages of ECT in mitigating depressive symptoms, improving well-being, and enhancing cognitive capabilities. Furthermore, the exploration of biomarkers provides insights into the mechanisms underlying the effects of ECT and opens avenues for future research on personalized treatment approaches. Despite these positive outcomes, further investigations are required to address certain unresolved questions. Future studies should focus on the long-term effects, potential mechanisms of action, and comparisons between ECT and emerging treatment modalities. Further research is necessary to optimize therapeutic approaches, address stigmatization, and improve outcomes in patients with treatment-resistant MDD.
The conclusions of this study indicate that ECT is a potentially advantageous therapeutic approach for individuals with severe MDD who do not respond to traditional treatments. This study found that ECT is safer and more potent than medication and/or therapy in mitigating depressive symptoms, enhancing well-being, and bolstering cognitive capabilities. This study also demonstrated that ECT may influence the levels of BDNF and the cytokine IL-6, indicators of neuroplasticity and inflammation, respectively. This study provides new evidence for the efficacy and safety of ECT as a treatment option for severe MDD and challenges the misconceptions and stigma surrounding this alternative therapy. These findings have implications for the development of new therapeutic approaches for MDD and underscore the need for further research in this area.
Future studies in this field should focus on several aspects. First, further investigations are needed to examine the long-term effects of ECT on depressive symptoms, quality of life, and cognitive function in individuals with MDD. Longitudinal studies can provide valuable insights into the durability of the effects of ECT and its potential as maintenance therapy. Additionally, future research should explore the underlying mechanisms of ECT, particularly its influence on neuroplasticity and inflammatory markers such as BDNF and IL-6. Understanding these mechanisms could guide the development of targeted interventions and improve treatment outcomes. Moreover, studies should be conducted to directly compare ECT with other emerging treatments, such as transcranial magnetic stimulation or ketamine infusion therapy, to determine the most effective and personalized treatment options for different subgroups of patients with MDD. These research perspectives can contribute to advancing the field and optimizing therapeutic approaches for patients with severe MDD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Patsalos O, United Kingdom; Uzzan S, Israel S-Editor: Qu XL L-Editor: A P-Editor: Qu XL
| 1. | World Health Organization. Depression. July 19, 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/depression. |
| 2. | American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). 2013. |
| 3. | Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP Jr, Weiss PM, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A. 2003;100:14293-14296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 510] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 4. | Craighead WE, Johnson BN, Carey S, Dunlop BW. Psychosocial treatments for major depressive disorder. In: Nathan PE, Gorman JM, eds. A Guide to Treatments That Work. 2015;381-408. |
| 5. | van Hees ML, Rotter T, Ellermann T, Evers SM. The effectiveness of individual interpersonal psychotherapy as a treatment for major depressive disorder in adult outpatients: a systematic review. BMC Psychiatry. 2013;13:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Hollon SD, DeRubeis RJ, Fawcett J, Amsterdam JD, Shelton RC, Zajecka J, Young PR, Gallop R. Effect of cognitive therapy with antidepressant medications vs antidepressants alone on the rate of recovery in major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71:1157-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Thase ME, Connolly KR. Unipolar depression in adults: Choosing treatment for resistant depression. July 19, 2023. Available from: https://www.uptodate.com/cont-ents/unipolar-depression-in-adults-choosing-treatment-for-resistant-depression. |
| 8. | Armstrong C. APA releases guideline on treatment of patients with major depressive disorder. Am Fam Physician 2011; 83: 1219-1227. |
| 9. | Mayo Clinic. Treatment-resistant depression. July 19, 2023. Available from: https://www.mayoclinic.org/diseases-conditions/depression/in-depth/treatment-resistant-depression/art-20044324. |
| 10. | Cerletti U, Bini L. Electroshock (*)(†). Int Rev Psychiatry. 2018;30:153-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Kellner CH, Greenberg RM, Murrough JW, Bryson EO, Briggs MC, Pasculli RM. ECT in treatment-resistant depression. Am J Psychiatry. 2012;169:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 1069] [Article Influence: 48.6] [Reference Citation Analysis (1)] |
| 13. | Sackeim HA, Prudic J, Fuller R, Keilp J, Lavori PW, Olfson M. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology. 2007;32:244-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 365] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 14. | Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, Fitzsimons L, Moody BJ, Clark J. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 487] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 15. | Kellner CH, Husain MM, Knapp RG, McCall WV, Petrides G, Rudorfer MV, Young RC, Sampson S, McClintock SM, Mueller M, Prudic J, Greenberg RM, Weiner RD, Bailine SH, Rosenquist PB, Raza A, Kaliora S, Latoussakis V, Tobias KG, Briggs MC, Liebman LS, Geduldig ET, Teklehaimanot AA, Dooley M, Lisanby SH; CORE/PRIDE Work Group. A Novel Strategy for Continuation ECT in Geriatric Depression: Phase 2 of the PRIDE Study. Am J Psychiatry. 2016;173:1110-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 16. | Read J, Bentall R. The effectiveness of electroconvulsive therapy: a literature review. Epidemiol Psichiatr Soc. 2010;19:333-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | American Psychiatric Association. DSM-5-TR™ update. July 19, 2023. Available from: https://www.psychiatry.org/getmedia/34c43e15-2618-4d2b-9f67-6bef5c40f75a/APA-DSM5TR-Update-September-2022.pdf. |
| 18. | Anxiety and Depression Association of America. Clinical practice review for major depressive disorder. July 19, 2023. Available from: https://adaa.org/resources-professionals/practice-guidelines-mdd. |
| 19. | HAMILTON M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21041] [Cited by in RCA: 22852] [Article Influence: 351.6] [Reference Citation Analysis (0)] |
| 20. | . Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28:551-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4140] [Cited by in RCA: 4470] [Article Influence: 165.6] [Reference Citation Analysis (0)] |
| 21. | Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11622] [Cited by in RCA: 15928] [Article Influence: 796.4] [Reference Citation Analysis (0)] |
| 22. | Rhee TG, Shim SR, Forester BP, Nierenberg AA, McIntyre RS, Papakostas GI, Krystal JH, Sanacora G, Wilkinson ST. Efficacy and Safety of Ketamine vs Electroconvulsive Therapy Among Patients With Major Depressive Episode: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2022;79:1162-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 23. | Li XM, Shi ZM, Wang PJ, Hu H. Effects of ketamine in electroconvulsive therapy for major depressive disorder: meta-analysis of randomised controlled trials. Gen Psychiatr. 2020;33:e100117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Menon V, Varadharajan N, Faheem A, Andrade C. Ketamine vs Electroconvulsive Therapy for Major Depressive Episode: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2023;80:639-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 25. | Sheng JA, Bales NJ, Myers SA, Bautista AI, Roueinfar M, Hale TM, Handa RJ. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front Behav Neurosci. 2020;14:601939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 26. | Abbott CC, Jones T, Lemke NT, Gallegos P, McClintock SM, Mayer AR, Bustillo J, Calhoun VD. Hippocampal structural and functional changes associated with electroconvulsive therapy response. Transl Psychiatry. 2014;4:e483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 27. | Rybakowski JK, Suwalska A, Lojko D, Rymaszewska J, Kiejna A. Types of depression more frequent in bipolar than in unipolar affective illness: results of the Polish DEP-BI study. Psychopathology. 2007;40:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |