Published online Nov 19, 2023. doi: 10.5498/wjp.v13.i11.937
Peer-review started: August 23, 2023
First decision: September 28, 2023
Revised: October 11, 2023
Accepted: October 27, 2023
Article in press: October 27, 2023
Published online: November 19, 2023
Processing time: 86 Days and 2.6 Hours
Schizophrenia is a psychiatric disorder characterized by chronic or recurrent symptoms. Lurasidone was licensed in China in 2019 for the treatment of adult schizophrenia in adults with a maximum dose of 80 mg/d. However, post-market surveillance (PMS) with an adequate sample size is required for further validation of the drug’s safety profile and effectiveness.
To conduct PMS in real-world clinical settings and evaluate the safety and effectiveness of lurasidone in the Chinese population.
A prospective, multicenter, open-label, 12-wk surveillance was conducted in mainland China. All patients with schizophrenia from 10 sites who had begun medication with lurasidone between September 2019 and August 2022 were eligible for enrollment. Safety assessments included adverse events (AEs), adverse drug reactions (ADRs), extrapyramidal symptoms (EPS), akathisia, use of EPS drugs, weight gain, and laboratory values as metabolic parameters and the QTc interval. The effectiveness was assessed using the brief psychiatric rating scale (BPRS) from baseline to the end of treatment.
A total of 965 patients were enrolled in the full analysis set and 894 in the safety set in this interim analysis. The average daily dose was 61.7 ± 19.08 mg (mean ± SD) during the treatment. AEs and ADRs were experienced by 101 patients (11.3%) and 78 patients (8.7%), respectively, which were mostly mild. EPS occurred in 25 individuals with a 2.8% incidence, including akathisia in 20 individuals (2.2%). Moreover, 59 patients received drugs for treating EPS during the treatment, with an incidence of 6.6% which dropped to 5.4% at the end of the treatment. The average weight change was 0.20 ± 2.36 kg (P = 0.01687) with 0.8% of patients showing a weight gain of ≥ 7% at week 12 compared with that at the baseline. The mean values of metabolic parameters and the QTc interval at baseline and week 12 were within normal ranges. The mean changes in total BPRS scores were -8.9 ± 9.76 (n = 959), -13.5 ± 12.29 (n = 959), and -16.8 ± 13.97 (n = 959) after 2/4, 6/8, and 12 wk, respectively (P < 0.001 for each visit compared with the baseline) using the last-observation-carried-forward method.
The interim analysis of the PMS of adult patients with schizophrenia demonstrate the safety and effectiveness of lurasidone in the Chinese population. No new safety or efficacy concerns were identified.
Core Tip: We conducted the first post-marketing surveillance of the actual use of lurasidone in the treatment of patients with schizophrenia in real-world clinical practice since the drug was licensed in mainland China in 2019, and evaluated the safety profile and effectiveness of lurasidone in Chinese population. Here, we report an interim analysis based on 965 patients who received the medication between 2019 and 2022. This study hold significance as it contributes additional safety and effectiveness data on lurasidone beyond what was gathered in pre-marketing trials. Furthermore, it provides valuable reference information for clinical decision-making of schizophrenia treatment.
- Citation: Wei YM, Wang XJ, Yang XD, Wang CS, Wang LL, Xu XY, Zhao GJ, Li B, Zhu DM, Wu Q, Shen YF. Safety and effectiveness of lurasidone in the treatment of Chinese schizophrenia patients: An interim analysis of post-marketing surveillance. World J Psychiatry 2023; 13(11): 937-948
- URL: https://www.wjgnet.com/2220-3206/full/v13/i11/937.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i11.937
Schizophrenia is a psychiatric disorder marked by chronic or recurrent symptoms, often resulting in an impairment of social and occupational functioning. The characteristics of schizophrenia typically include incoherent or illogical thoughts, delusions, hallucinations, unusual behaviors, and cognitive impairment[1]. Its global prevalence is approximately 1%, with an incidence of about 1.5 per 10000[2]. It is a highly disabling and costly condition, ranking among the top 10 burdensome diseases[3].
The goal of schizophrenia treatment is to reduce acute symptoms and prevent recurrence while maximizing the patient's quality of life[4]. Lurasidone is a second-generation antipsychotic drug with a high affinity for dopamine D2, serotonin 5-HT2A, and 5-HT7 receptors and a low affinity for H1 and 5-HT2C receptors. It is considered to be associated with relatively less side effects[5-7].
Lurasidone was licensed in mainland China in 2019 for the treatment of schizophrenia in adults, with a maximum dose of 80 mg/d. A series of clinical studies have been conducted to verify its efficacy and safety[8,9] and numerous clinical experiences have been accumulated for Chinese patients with schizophrenia since the launch. However, a post-market surveillance (PMS) for the drug with an adequate sample size is also required by the Chinese government. This study thus aimed to conduct a surveillance of the actual use of lurasidone among Chinese patients with schizophrenia in real-world clinical practice and evaluate the safety profile and effectiveness of the drug in the Chinese population. Here, we report an interim analysis based on 965 patients who received lurasidone between 2019 and 2022.
This study represents an interim analysis of a 12-wk, prospective, observational, multi-center, open-label PMS conducted in China. Patients with schizophrenia from 10 sites across China who had begun treatment with Latuda® between September 2019 and August 2022 were enrolled. The patients were required to provide informed consent before participating. An electronic data capture system was used for the collection of diagnostic and treatment information such as details on drug use, adverse events (AEs), and other related parameters including but not limited to laboratory examinations. All patients who met the inclusion requirement were included in the full analysis set (FAS), whereas the safety set (SS) consisted of patients who had received at least one treatment and had data on safety indicators recorded after the treatment. The study protocol was approved by the ethics committees of Shanghai Mental Health Center (the leading site) and other sites.
The administered dose of lurasidone was decided by the treating physicians, typically initiating at 40 mg/d with no requirement for titration. Depending on the treatment response and tolerability, the dose could be increased to a maximum daily dose of 80 mg/d, according to the approved prescribing information in China (a dose of 160 mg/d is approved overseas). Because this study was non-interventional, concomitant therapy deemed essential by the physician was permitted and reported on the electronic Case Report Form, including but not limited to antipsychotic drugs, mood stabilizers, antidepressants, anxiolytics, and antiepileptics.
Safety analyses were based on the SS and evaluated by the frequency and severity of AEs that occurred during the trial. AEs were coded according to the International Council for Harmonisation International Dictionary of Medical Terms (MEDDRA 24.0, Medical Dictionary for Regulatory Activities). Assessments were made at visit 1 (week 0), visit 2 (weeks 2-4), visit 3 (weeks 6-8), and visit 4 (week 12) and were reported by the treating physicians. The assessment included the incidence of total AEs, extrapyramidal symptoms (EPS), akathisia, use of EPS drugs, and mean changes in weight from baseline. Adverse drug reactions (ADRs) were defined as AEs for which the participating physicians could not rule out lurasidone as the cause.
The assessment of effectiveness was based on FAS and assessed by the change in the overall brief psychiatric rating scale (BPRS) score relative to the baseline at weeks 2-4/ weeks 6-8, and week 12. The BPRS is a scale that was created to assess clinical changes in patients with schizophrenia, with 18 items designed to represent discrete symptom areas. Items are rated on a 7-point Likert scale, from 1 = "not present" to 7 = "extremely severe", with the total scores ranging from 18 to 126 (calculated by summation of the item scores). The BPRS evaluates five areas: Anxiety-depression, anergia, thought disturbance, activation, and hostility-suspiciousness. Scores for the 5-factor model were also calculated[10].
The data were in the form of descriptive statistics as the study was non-interventional and were analyzed using R software version 4.1.0 (The R Foundation for Statistical Computing, Vienna, Austria). Categorical variables are presented as frequencies and proportions and shown as n (%). Continuous variables are presented as mean ± SD and compared using paired t-tests, with P < 0.05 indicating statistical significance. Effectiveness endpoint data were analyzed using the last-observation-carried forward (LOCF) approach for the missing values.
The statistical methods of this study were reviewed by a member of the Biostatistical Service from the Shanghai Medical Insight Technology Co., Ltd.
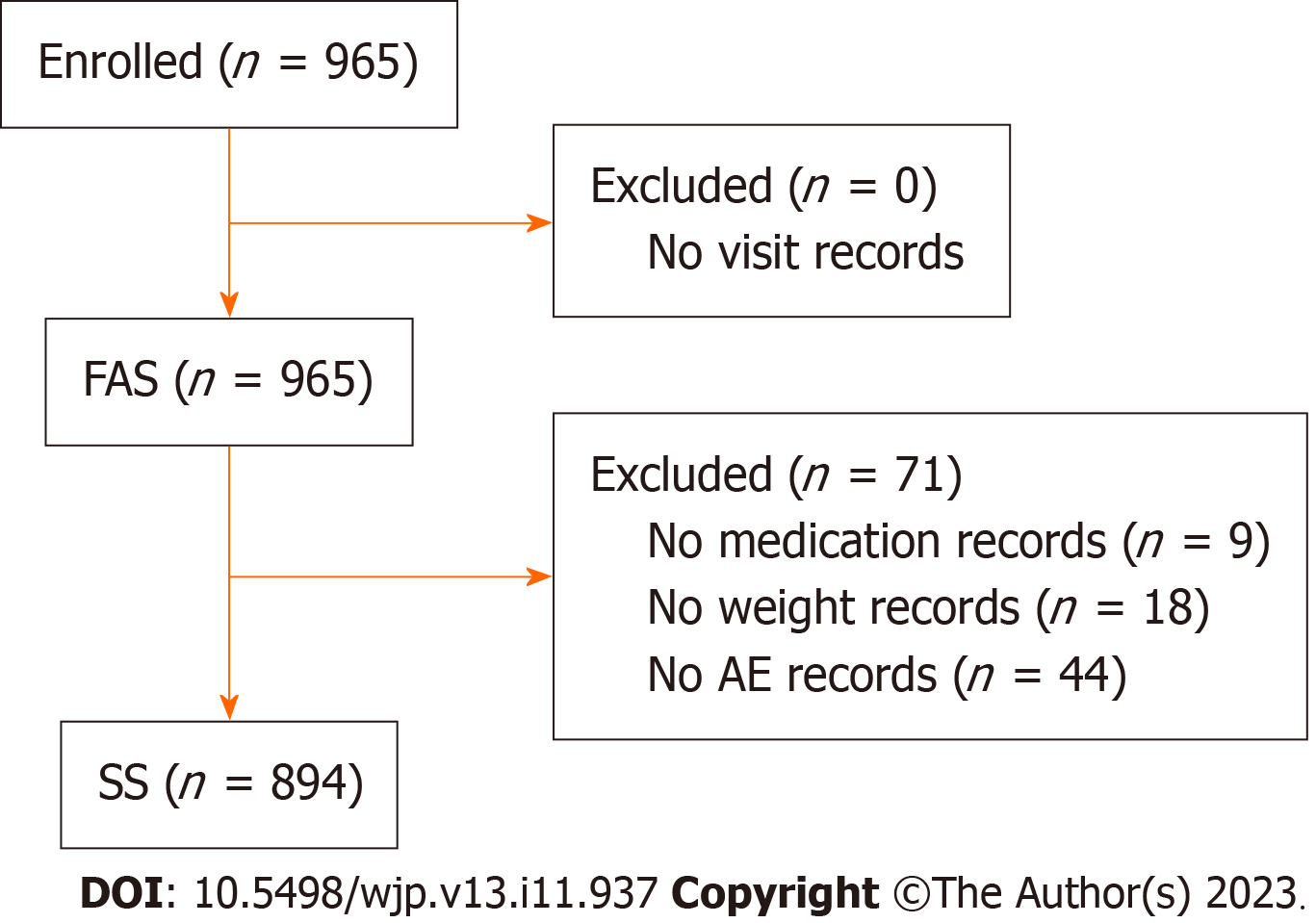
A total of 965 participants were enrolled in the FAS. The SS comprised 894 participants after excluding 71 patients owing to a lack of AE documentation (Figure 1).
The baseline demographics showed that 39.2% of the patients were male and 60.8% were female. The average age was 35.4 ± 14.27 years and 7.5% of the patients were aged < 18 years. The average height was 166.1 ± 7.97 cm and average body weight was 66.0 ± 13.86 kg. Overall, 55.3% of the patients had obtained antipsychotic medication within 1 mo prior to the baseline (Table 1).
| Background factor | Category | n (%) |
| Sex | Male | 378 (39.2%) |
| Female | 587 (60.8%) | |
| Age | mean ± SD | 35.4 ± 14.27 |
| < 18 yr | 72 (7.5%) | |
| 18 yr | 892 (92.5%) | |
| Height (cm) | mean ± SD | 166.1 ± 7.97 |
| Weight (kg) | mean ± SD | 66.0 ± 13.86 |
| Months since onset of first episode | mean ± SD | 92.6 ± 113.95 |
| Months since onset of current episode | mean ± SD | 5.8 ± 55.52 |
| Baseline BPRS score | mean ± SD | 43.6 ± 15.28 |
| Comorbidity of mental disorder besides schizophrenia | Yes | 958 (99.3%) |
| Comorbidity besides mental disorder | Yes | 848 (88.1%) |
| Antipsychotic medication within 1 mo prior to baseline | Yes | 532 (55.3%) |
In the FSA comprising 965 patients, the mean exposure level of lurasidone was 5547.5 ± 3477.04 mg with 88.4 ± 40.98 exposure days and an average daily dose of 61.7 ± 19.08 mg during treatment. A total of 57.3% of the 949 participants in the FAS received concomitant medication during the surveillance, comprising drug (n = 520, 54.8%) and non-drug (n = 156, 16.4%) therapies. The number and proportion of patients in each treatment category are detailed in Table 2.
| Category | n | % |
| All | 544 | 57.3 |
| Pharmacological therapy | 520 | 54.8 |
| Oral antipsychotic drugs | 434 | 45.7 |
| Injectable antipsychotics | 12 | 1.3 |
| Sedative-hypnotics/EPS drugs | 112 | 11.8 |
| Mood stabilizers/antiepileptic drugs | 39 | 4 |
| Antidepressants/anxiolytics | 61 | 6.4 |
| Other categories | 105 | 11.0 |
| Non-pharmacological therapy | 156 | 16.4 |
| ECT | 12 | 1.3 |
| rTMS | 14 | 1.5 |
| Other categories | 142 | 15 |
Overall, 101 (11.3%) and 78 (8.7%) of the 894 patients in the SS cohort developed AEs and ADRs, respectively. Most of these were mild AEs (n = 87, 9.7%) and ADRs (n = 65, 7.3%). Fourteen (1.6%) and 12 (1.3%) patients developed AEs and ADRs, respectively, prompting discontinuation, whereas the presence of SAEs was only reported in one patient (Table 3). According to the System Organ Class (SOC) classification, the most common SOC AEs were investigations and nervous system disorders, and the most frequent AEs were akathisia (n = 22, 2.5%), blood prolactin increased (n = 21, 2.3%), and nausea (n = 9, 1.0%) (Supplementary Table 1).
| Category | n | % |
| Any AE | 101 | 11.3% |
| Any ADR | 78 | 8.7% |
| AE leading to discontinuation | 14 | 1.6% |
| ADR leading to discontinuation | 12 | 1.3% |
| EPS AE | 29 | 3.1% |
| EPS ADR | 25 | 2.7% |
| EPS AE leading to discontinuation | 3 | 0.3% |
| EPS ADR leading to discontinuation | 3 | 0.3% |
| Serious AE | 1 | 0.1% |
| Serious ADR | 1 | 0.1% |
EPS: EPS ADRs occurred in 25 patients (2.8%); of these, 2.2% experienced akathisia, 0.8% acute dystonia, and 0.2% parkinsonism, as demonstrated by the subgroup analysis. No reports of tardive dyskinesia or other EPS ADRs occurred during treatment (Table 4).
| Category | n | % |
| All | 25 | 2.8% |
| Acute dystonia | 7 | 0.8% |
| Akathisia | 20 | 2.2% |
| Parkinsonism | 2 | 0.2% |
| Tardive dystonia | 0 | 0.0% |
| Other EPS ADR | 0 | 0.0% |
Use of EPS drugs: In the SS, 59 of 894 participants used EPS drugs to reduce the incidence and severity of extrapyramidal side effects during the treatment period. The incidence was 6.6%, which dropped to 5.4% at the end of the treatment. The most frequent EPS drug used was Benzhexol, which was used by 46 patients (5.1%).
Weight gain: The mean body weights of the patients at baseline and week 12 were 66.21 ± 14.01 kg and 66.21 ± 13.70 kg, respectively, resulting in a mean weight change of 0.20 ± 2.36 kg. This weight change demonstrated statistical significance in the intra-group comparison (P = 0.0168). Furthermore, 7 (0.8%) patients were found to have gained ≥ 7% of their weight at visit 4 (Table 5).
| Total | |
| Baseline (mean ± SD) | 66.21 ± 14.01 |
| 12 weeks (mean ± SD) | 66.21 ± 13.70 |
| Change in body weight (mean ± SD) | 0.20 ± 2.361 |
| Weight gain from baseline ≥ 7%, n (%) | 7 (0.8%) |
Laboratory values: The mean values of glutamic-pyruvic transaminase (GPT), glutamic oxaloacetic transaminase, serum creatinine, fasting blood glucose, total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and QTc interval were within normal ranges at baseline and had not significantly changed when measured at the week 12 visit, except for GPT (P = 0.0189) (Table 6).
| Measure | n | Mean | SD |
| GPT (U/L) | |||
| Baseline | 376 | 25.14 | 21.548 |
| Week 12 | 109 | 27.33 | 20.624 |
| Mean change | 93 | 5.381 | 21.705 |
| GOT (U/L) | |||
| Baseline | 363 | 22.19 | 12.715 |
| Week 12 | 108 | 24.39 | 12.565 |
| Mean change | 92 | 0.96 | 16.625 |
| Serum creatinine (μmol/L) | |||
| Baseline | 370 | 65.86 | 16.383 |
| Week 12 | 106 | 69.26 | 14.695 |
| Mean change | 90 | 1.21 | 10.854 |
| Fasting blood glucose (mmol/L) | |||
| Baseline | 368 | 5.478 | 1.536 |
| Week 12 | 101 | 5.451 | 1.5486 |
| Mean change | 84 | -0.162 | 1.4467 |
| Total cholesterol (mmol/L) | |||
| Baseline | 363 | 4.501 | 1.0389 |
| Week 12 | 101 | 4.551 | 1.0888 |
| Mean change | 83 | -0.079 | 1.1075 |
| Triglycerides (mmol/L) | |||
| Baseline | 362 | 1.589 | 1.3002 |
| Week 12 | 101 | 1.655 | 0.8413 |
| Mean change | 82 | -0.207 | 1.3234 |
| HDL cholesterol (mmol/L) | |||
| Baseline | 359 | 1.287 | 0.365 |
| Week 12 | 99 | 1.308 | 0.3959 |
| Mean change | 82 | 0.073 | 0.415 |
| LDL cholesterol (mmol/L) | |||
| Baseline | 344 | 2.694 | 0.8467 |
| Week 12 | 90 | 2.746 | 0.7907 |
| Mean change | 73 | -0.086 | 0.7312 |
| QTc interval (ms) | |||
| Baseline | 280 | 415.6 | 93.41 |
| Week 12 | 71 | 402 | 29.32 |
| Mean change | 57 | -0.2 | 35.76 |
The changes observed in the total BPRS score indicated a significant improvement in the severity of schizophrenia throughout the course of the treatment. The total BPRS score stood at 43.6 ± 15.28 (n = 959) at baseline. Over the course of treatment, it progressively decreased to 34.7 ± 12.22 (n = 959), 30.1 ± 10.68 (n = 959), and 26.8 ± 9.58 (n = 959), respectively, at 2/4, 6/8, and 12 wk, with overall mean changes of -8.9 ± 9.76, -13.5 ± 12.29, and -16.8 ± 13.97, respectively (P < 0.001 for each visit compared with the baseline) based on the LOCF method. The changes in the 5-factor model scores exhibited a similar trend as the total BPRS score, with highly significant reductions in the score observed on each visit (P < 0.001) (Table 7).
| BPRS (mean ± SD) | Baseline (n = 959) | 2/4 wk (n = 959) | 6/8 wk (n = 959) | 12 wk ± 14 d (n = 959) |
| Total score | 43.6 ± 15.28 | 34.7 ± 12.22 | 30.1 ± 10.68 | 26.8 ± 9.58 |
| Change in total score | - | -8.9 ± 9.761 | -13.5 ± 12.291 | -16.8 ± 13.971 |
| Anxiety-depression | 9.3 ± 4.00 | 7.6 ± 3.20 | 6.7 ± 2.75 | 6.0 ± 2.45 |
| Change in anxiety-depression score | - | -1.6 ± 2.401 | -2.6 ± 2.941 | -3.3 ± 3.421 |
| Anergia | 8.9 ± 3.55 | 7.5 ± 3.01 | 6.7 ± 2.75 | 6.2 ± 2.47 |
| Change in anergia score | - | -1.4 ± 2.281 | -2.2 ± 2.691 | -2.8 ± 3.001 |
| Thought disturbance | 10.7 ± 4.40 | 8.3 ± 3.49 | 7.1 ± 3.07 | 6.2 ± 2.79 |
| Change in thought disturbance score | - | -2.4 ± 3.031 | -3.7 ± 3.771 | -4.5 ± 4.151 |
| Activation | 5.9 ± 3.10 | 4.7 ± 2.35 | 4.2 ± 1.86 | 3.8 ± 1.58 |
| Change in activation score | - | -1.2 ± 2.011 | -1.7 ± 2.411 | -2.1 ± 2.701 |
| Hostility-suspiciousness | 8.8 ± 3.93 | 6.5 ± 2.85 | 5.4 ± 2.47 | 4.6 ± 2.17 |
| Change in hostility-suspiciousness score | - | -2.3 ± 2.811 | -3.4 ± 3.421 | -4.1 ± 3.771 |
Lurasidone is a novel benzisothiazole antipsychotic drug that was authorized by the FDA in 2010 for the treatment of adult patients with schizophrenia[5] and has been licensed in many other countries, including China in 2019. Numerous pre-marketing trials have been conducted, which have demonstrated the efficacy and safety of lurasidone[8,11-13]. However, pre-marketing research has limitations. These include a small sample size, difficulties in detecting AEs with low incidence but high severity during the trial phase, and relatively strict participant inclusion and exclusion criteria, preventing the participation of certain patients with specific diseases in the trials. Thus, safety issues may not be fully identified and the use of concomitant medication tends to be controlled, which is inconsistent with real-world clinical practice. The use of PMS is thus important to further evaluate the safety and effectiveness of drugs. This study represents the initial PMS of lurasidone conducted in a Chinese population. The interim analysis confirmed that lurasidone was effective, generally safe, and well-tolerated as a treatment option for schizophrenia in adult Chinese patients.
Common adverse reactions of lurasidone include akathisia, EPS, somnolence, and nausea[14,15]. The most frequent treatment-emergent AEs (TEAEs) among Asian participants during a 6-wk study were found to be headache, cons-tipation, akathisia, and nausea, with the latter two being more common in the 80 mg/d group compared with that in the 40 mg/d group[16]. During 26 wk of open-label extension treatment with lurasidone, the most frequently observed TEAEs were EPS, insomnia, akathisia, nasopharyngitis, schizophrenia, anxiety, nausea, and headache[17]. In the current PMS, the most frequently reported AEs included akathisia, elevated blood prolactin levels, and nausea, with the majority of the cases characterized as mild in severity. The pattern of AEs/ADRs identified in this interim analysis closely resembled the AE/ADR profile with the use of lurasidone 40 mg/d in previous trials for the treatment of schizophrenia[13]. The most frequently employed measures in response to EPS ADRs involve reduction of the antipsychotic drug, transitioning to antipsychotic drugs with a lower propensity to induce EPS, or utilization of specific medications designed for EPS treatment. In this study, the proportion of EPS drugs used during the treatment was 6.6%, which was higher than the incidence of EPS ADR (2.8%). The primary reasons for this could be attributed to two factors: (1) EPS treatment induced by antipsychotic drugs other than lurasidone in participants who were administered concomitant antipsychotics (47%); and (2) the utilization of prophylactic medications aimed at preventing patients from developing EPS during the course of treatment. The latter strategy is mainly aimed at patients who are susceptible to EPS (patients in the early stages of treatment and young males), to reduce their discomfort and improve compliance.
Lurasidone exhibits a strong affinity for dopamine D2, 5-HT7, and serotonin 5-HT2A receptors. This favorable D2/5HT2A balance not only ensures the drug’s antipsychotic effectiveness but also helps mitigate the EPS resulting from D2 blockade[18]. Thus, as with other atypical agents, lurasidone has antipsychotic effects with limited EPS risk[14,19,20]. The incidence of EPS-related events (excluding akathisia and restlessness) was found to be 14.7% for lurasidone and 5.1% for placebo in short-term clinical trials[15]. In two long-term trials, the proportions of participants reporting EPS AEs in the lurasidone group during 12 mo of treatment were 12.9% and 11.9%, respectively[21,22].
Akathisia is one of the most frequent ADRs observed in multiple trials conducted in different regions. In a pooled analysis of European data from three randomized controlled trials (RCTs), the incidence of akathisia was 11.3%, representing 8.1% in the 40–80 mg/d group and 15.8% in the 120–160 mg group[23]. In the Asian population, the proportion of patients that experienced akathisia was found to be 7.3% and 10.4% in the 40 and 80 mg/d groups, respectively, in another RCT that included mostly patients from East Asia[16]. In Chinese patients, the incidence of akathisia was reported to be 7.2% and 13.6% in patients treated separately with lurasidone and risperidone, respectively, by a pre-marketing RCT[8]. In the current PMS, the incidence of EPS ADRs was 2.8%; of these, 2.2% was contributed by akathisia, which is lower than the rates reported in previous studies. It is speculated that this inconsistency may result from three factors. First, the dosage of the drug. The development of akathisia was found to be dose-dependent in a previous trial[13]. In the current surveillance study, the average daily dose of lurasidone was 61.7 ± 19.08 mg, which is relatively low and, importantly, unlike pre-marketing clinical studies where fixed doses are commonly used, the treatment dosage in this PMS could be adjusted by the physicians based on the patient’s tolerance, which may also help in reducing the occurrence of ADRs. Second, the concomitant medication. Drugs for treating EPS were allowed in the present study owing to its observational design of real-world clinical practice, and these medications may have led to reduced EPS and akathisia side effects. Last, sample size and monitoring requirements. Compared with pre-marketing RCTs, PMS studies typically entail larger sample sizes and involve less stringent monitoring protocols, which may occasionally result in a relatively lower proportion of AEs/ADRs reported by the participating physicians. However, a direct comparison of the data between these studies is generally not appropriate owing to differences in eligibility criteria and study methods.
Weight gain has been demonstrated to be a risk factor for treatment non-adherence in patients with schizophrenia[24,25] as it results in a worse quality of life, social exclusion, and increased stigma[26]. Moreover, a higher risk of developing metabolic syndrome (MetS) is linked to increased body weight[27,28]. A network meta-analysis of 100 RCTs comprising 25952 patients with schizophrenia found evidence of weight gain associated with the use of nine antipsychotics[29]. The reason why antipsychotic drugs induce weight gain remains unclear but might partly be ascribed to 5-HT2C- and H1- receptor affinity, which leads to hyperphagia and increased food intake[30,31]. However, lurasidone has only weak affinity for 5-HT2C receptors and poor or no affinity for histamine H1[32]. Thus, the risk of weight gain might be relatively low from a mechanistic perspective, as confirmed by multiple clinical trials[33,34]. For short-term outcomes, 15 antipsychotic medications were evaluated for their effectiveness and tolerability by a meta-analysis of 212 trials and it was found that lurasidone, compared with placebo, is one of the only three drugs that did not lead to increased weight gain[33]. In terms of long-term outcomes, a pooled analysis of six studies showed that the mean weight change at month 12 was significantly greater in patients treated with risperidone and quetiapine extended-release (quetiapine XR) than those with lurasidone (+ 2.6 kg, + 1.2 kg vs − 0.4 kg)[34]. Previous trials did not find a dose-response relationship between the drugs and weight gain [11-13,16], and Wu et al[35] observed that the dose-response curve plateaued at 60 mg/d in a dose-response meta-analysis of 97 studies. In the current PMS, although the body weights of the patients at week 0 and week 12 were remarkably close (66.21 ± 14.010 kg vs 66.21 ± 13.696 kg), the number of participants with weight data recorded at these two visits varied (888 vs 801). Therefore, we used paired t-tests to analyze the mean change in weight, which was found to be 0.20 ± 2.356 kg at week 12 compared with the baseline paired population, showing a significant difference (P = 0.0168). However, this change remained minimal, and only 0.8% of patients showed a weight gain of ≥ 7% from baseline to week 12. Overall, in line with prior studies, there was a low chance of clinically significant weight gain in patients treated with lurasidone according to the results of the current PMS.
MetS is a collection of clinical and laboratory findings that are associated with an increased risk of cardiovascular disease[36]. The causes of MetS in patients with schizophrenia are not entirely elucidated, with antipsychotic drug side effects being a considered contributing factor[37]. Second-generation antipsychotics appear to be more likely to cause metabolic disturbances than first-generation antipsychotics, probably because of their affinity for the 5HT2C- and H1- receptors as well as the M3 receptors that regulate the release of insulin, and thus maintain glucose homeostasis[37,38]. Previous studies have found that lurasidone is associated with a relatively low incidence of MetS[39,40]. The odds of developing MetS during the study period were significantly lower in patients receiving lurasidone than those treated with olanzapine and quetiapine XR in short-term treatment, and risperidone in long-term treatment, as demonstrated by a pooled analysis[41]. Despite the lack of a control group, the metabolic parameters of the study participants recorded at each visit in the current PMS were predominantly within the normal range, and a high proportion of those who were normal at baseline continued to remain normal during further follow-up visits (data not shown). This supports previous findings that lurasidone treatment has limited risk for adverse metabolic effects.
Hyperprolactinemia may be developed in patients with schizophrenia with or without antipsychotic medication[42]. Elevated prolactin levels induced by antipsychotic drugs are mainly associated with D2 receptor affinity[43]. Several short-term studies have found no evidence of clinically significant changes in prolactin levels in patients treated with lurasidone[11-13]. A multicenter RCT conducted in mainland China showed that compared with the risperidone-treated group, the incidence of increased prolactin was remarkably lower in the lurasidone-treated group (3.1% vs 14.1%)[8]. In the current PMS, the incidence of increased prolactin was 2.3%, verifying that lurasidone has limited effects on prolactin levels, consistent with prior studies.
Lurasidone is a full antagonist of the dopamine D2 receptor, with a receptor occupancy ratio of the latter after taking lurasidone 40 mg/d of 60%–80%, which is a prerequisite for its antipsychotic effectiveness[6]. Its targeting of the 5-HT7 receptors can potentially enhance cognition by improving emotional regulation and sensory processing[6]. For acute phase treatment, a post hoc analysis of five studies in patients with acute schizophrenia found that lurasidone can reduce agitation early and sustainably, as measured by the PANSS-EC score[44]. Another meta-analysis of eight short-term RCTs confirmed that lurasidone was superior to placebo irrespective of positive or negative symptoms[45]. For maintenance treatment, an extension trial of 6 mo following a 6-wk acute-phase treatment of lurasidone showed further reduction and stabilization of the total PANSS score after receiving a flexible dose of lurasidone[46]. In terms of mood disorder, a pooled analysis of four studies verified significant improvement in depressive symptoms by lurasidone with greater decreases in MADRS score[47]. Overall, previous studies have supported the effectiveness of lurasidone in the treatment of schizophrenia and the interim analysis of the current PMS confirmed this conclusion, as supported by the continuous decrease in the total BPRS and the 5-factor model scores across all four visits during the surveillance period.
This study has some limitations. It was a single-armed, observational study with no control group, and the use of concomitant therapy, such as antipsychotic and EPS drugs, was allowed, which may represent confounders to the analysis and interpretation of the results. The sample sizes at each visit varied significantly and several participants did not provide their complete information, especially at visits 2 and 3. This may have an impact on the average of the measured data values and may not reflect the actual changes in the patients’ condition. Although we used paired t-tests to partially address this issue, bias remains inevitable. The study period of this interim analysis was only 12 wk, and thus some AEs/ADRs that require a relatively long time to manifest may have not been observed and recorded during this period. It is worth mentioning that the study will be followed by a 12-month extension period, which we believe will provide further data support for clinical applications.
This preliminary data analysis from the PMS of lurasidone in adult patients with schizophrenia demonstrated its safety and effectiveness in a Chinese population, without emergence of any novel safety or efficacy issues. The study is ongoing and will facilitate continue to provide additional data and insights into the characteristics of lurasidone and its practical application in clinical settings.
Schizophrenia is a psychiatric disorder characterized by chronic or recurrent symptoms. Lurasidone was licensed in China in 2019 for the treatment of adult schizophrenia in adults.
To further validate the safety profile and effectiveness of lurasidone.
To evaluate the safety and effectiveness of lurasidone in the Chinese population.
We conducted a prospective, multicenter, open-label, 12-wk surveillance in mainland China, and reported the interim analysis based on 965 patients who received the medication between 2019 and 2022.
Mean changes in total brief psychiatric rating scale scores were -8.9 ± 9.76 (n = 959), -13.5±12.29 (n = 959), and -16.8 ± 13.97 (n = 959) after 2/4, 6/8, and 12 wk, respectively (P < 0.001 for each visit compared with the baseline) using the last-observation-carried-forward method. adverse events and adverse drug reactions were experienced by 101 (11.3%) and 78 patients (8.7%), respectively, which were mostly mild.
The interim analysis of the post-market surveillance demonstrate the safety and effectiveness of lurasidone in the Chinese population.
This study contributes additional safety and effectiveness data on lurasidone, surpassing those obtained in pre-marketing trials. It offers essential guidance for clinical decision-making in the treatment of schizophrenia.
We would like to acknowledge all the physicians and patients who participated in this surveillance study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khosravi M, Iran; Stoyanov D, Bulgaria S-Editor: Lin C L-Editor: A P-Editor: Xu ZH
| 1. | Marder SR, Cannon TD. Schizophrenia. N Engl J Med. 2019;381:1753-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 319] [Article Influence: 53.2] [Reference Citation Analysis (1)] |
| 2. | McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 1455] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 3. | Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, McGrath JJ, Whiteford HA. Global Epidemiology and Burden of Schizophrenia: Findings From the Global Burden of Disease Study 2016. Schizophr Bull. 2018;44:1195-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 958] [Article Influence: 136.9] [Reference Citation Analysis (0)] |
| 4. | Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M. Guidelines for the Pharmacotherapy of Schizophrenia in Adults. Can J Psychiatry. 2017;62:604-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 5. | Corponi F, Fabbri C, Bitter I, Montgomery S, Vieta E, Kasper S, Pallanti S, Serretti A. Novel antipsychotics specificity profile: A clinically oriented review of lurasidone, brexpiprazole, cariprazine and lumateperone. Eur Neuropsychopharmacol. 2019;29:971-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 6. | Jaeschke RR, Sowa-Kućma M, Pańczyszyn-Trzewik P, Misztak P, Styczeń K, Datka W. Lurasidone: The 2016 update on the pharmacology, efficacy and safety profile. Pharmacol Rep. 2016;68:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Macaluso M, Kazanchi H, Preskorn SH. How the pharmacokinetics and receptor-binding profile of lurasidone affect the clinical utility and safety of the drug in the treatment of schizophrenia. Expert Opin Drug Metab Toxicol. 2015;11:1317-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Feng Y, Shi J, Wang L, Zhang X, Tan Y, Zhao J, Ning Y, Xie S, Liu X, Liu Q, Li K, Wang X, Li L, Xu X, Deng W, Luo X, Wang G. Randomized, double-blind, 6-week non-inferiority study of lurasidone and risperidone for the treatment of schizophrenia. Psychiatry Clin Neurosci. 2020;74:336-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Hu C, Wang Y, Song R, Yu C, Luo X, Jia J. Single- and Multiple-Dose Pharmacokinetics, Safety and Tolerability of Lurasidone in Healthy Chinese Subjects. Clin Drug Investig. 2017;37:861-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Velakoulis D, Walterfang M. Clinical Assessment of Mental Status. In: Neurology and Clinical Neuroscience. Schapira AHV, Byrne E; DiMauro S, Frackowiak RS, Johnson RT, Mizuno Y, Samuels MA, Silberstein SD, Wszolek ZK editors. Philadelphia: Mosby, 2007: 2-21. |
| 11. | Loebel A, Cucchiaro J, Sarma K, Xu L, Hsu C, Kalali AH, Pikalov A, Potkin SG. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res. 2013;145:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Meltzer HY, Cucchiaro J, Silva R, Ogasa M, Phillips D, Xu J, Kalali AH, Schweizer E, Pikalov A, Loebel A. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168:957-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 13. | Nasrallah HA, Silva R, Phillips D, Cucchiaro J, Hsu J, Xu J, Loebel A. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res. 2013;47:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Caccia S, Pasina L, Nobili A. Critical appraisal of lurasidone in the management of schizophrenia. Neuropsychiatr Dis Treat. 2012;8:155-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2011;65:189-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Higuchi T, Ishigooka J, Iyo M, Yeh CB, Ebenezer EG, Liang KY, Lee JS, Lee SY, Lin SK, Yoon BH, Nakamura M, Hagi K, Sato T. Lurasidone in the treatment of schizophrenia: Results of a double-blind, placebo-controlled trial in Asian patients. Asia Pac Psychiatry. 2019;11:e12352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Higuchi T, Ishigooka J, Iyo M, Hagi K. Safety and effectiveness of lurasidone for the treatment of schizophrenia in Asian patients: Results of a 26-week open-label extension study. Asia Pac Psychiatry. 2020;12:e12377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Meyer JM, Loebel AD, Schweizer E. Lurasidone: a new drug in development for schizophrenia. Expert Opin Investig Drugs. 2009;18:1715-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Luft B, Taylor D. A review of atypical antipsychotic drugs versus conventional medication in schizophrenia. Expert Opin Pharmacother. 2006;7:1739-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21:106S-115S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 484] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 21. | Citrome L, Cucchiaro J, Sarma K, Phillips D, Silva R, Tsuchiya S, Loebel A. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol. 2012;27:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Loebel A, Cucchiaro J, Xu J, Sarma K, Pikalov A, Kane JM. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, noninferiority study. Schizophr Res. 2013;147:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Calisti F, Cattaneo A, Calabrese M, Mao Y, Tocco M, Pikalov A, Goldman R. Efficacy and safety of lurasidone in schizophrenia: pooled analysis of European results from double-blind, placebo-controlled 6-week studies. Int Clin Psychopharmacol. 2022;37:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Velligan DI, Weiden PJ, Sajatovic M, Scott J, Carpenter D, Ross R, Docherty JP; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70 Suppl 4:1-46; quiz 47. [PubMed] |
| 25. | Weiden PJ, Mackell JA, McDonnell DD. Obesity as a risk factor for antipsychotic noncompliance. Schizophr Res. 2004;66:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 297] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 26. | Kolotkin RL, Corey-Lisle PK, Crosby RD, Swanson JM, Tuomari AV, L'italien GJ, Mitchell JE. Impact of obesity on health-related quality of life in schizophrenia and bipolar disorder. Obesity (Silver Spring). 2008;16:749-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207:928-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 437] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 28. | Nguyen NT, Nguyen XM, Wooldridge JB, Slone JA, Lane JS. Association of obesity with risk of coronary heart disease: findings from the National Health and Nutrition Examination Survey, 1999-2006. Surg Obes Relat Dis. 2010;6:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, Beck K, Natesan S, Efthimiou O, Cipriani A, Howes OD. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:64-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 30. | Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. 2015;2:452-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 31. | Reynolds GP, Kirk SL. Metabolic side effects of antipsychotic drug treatment--pharmacological mechanisms. Pharmacol Ther. 2010;125:169-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 299] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 32. | Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, Matsumoto K, Nishikawa H, Ueda Y, Toma S, Oki H, Tanno N, Saji I, Ito A, Ohno Y, Nakamura M. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther. 2010;334:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 334] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 33. | Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1686] [Cited by in RCA: 1762] [Article Influence: 146.8] [Reference Citation Analysis (0)] |
| 34. | Meyer JM, Mao Y, Pikalov A, Cucchiaro J, Loebel A. Weight change during long-term treatment with lurasidone: pooled analysis of studies in patients with schizophrenia. Int Clin Psychopharmacol. 2015;30:342-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Wu H, Siafis S, Hamza T, Schneider-Thoma J, Davis JM, Salanti G, Leucht S. Antipsychotic-Induced Weight Gain: Dose-Response Meta-Analysis of Randomized Controlled Trials. Schizophr Bull. 2022;48:643-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 36. | DE Hert M, Schreurs V, Vancampfort D, VAN Winkel R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 436] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 37. | Ventriglio A, Gentile A, Stella E, Bellomo A. Metabolic issues in patients affected by schizophrenia: clinical characteristics and medical management. Front Neurosci. 2015;9:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Papanastasiou E. The prevalence and mechanisms of metabolic syndrome in schizophrenia: a review. Ther Adv Psychopharmacol. 2013;3:33-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 39. | De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 751] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 40. | Loebel A, Citrome L. Lurasidone: a novel antipsychotic agent for the treatment of schizophrenia and bipolar depression. BJPsych Bull. 2015;39:237-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Tocco M, Newcomer JW, Mao Y, Pikalov A, Loebel A. Lurasidone and risk for metabolic syndrome: results from short- and long-term clinical studies in patients with schizophrenia. CNS Spectr. 2020;1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Riecher-Rössler A, Rybakowski JK, Pflueger MO, Beyrau R, Kahn RS, Malik P, Fleischhacker WW; EUFEST Study Group. Hyperprolactinemia in antipsychotic-naive patients with first-episode psychosis. Psychol Med. 2013;43:2571-2582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 43. | Fitzgerald P, Dinan TG. Prolactin and dopamine: what is the connection? A review article. J Psychopharmacol. 2008;22:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 266] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 44. | Allen MH, Citrome L, Pikalov A, Hsu J, Loebel A. Efficacy of lurasidone in the treatment of agitation: A post hoc analysis of five short-term studies in acutely ill patients with schizophrenia. Gen Hosp Psychiatry. 2017;47:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 45. | Zheng W, Cai DB, Yang XH, Li L, Zhang QE, Ng CH, Ungvari GS, Li XB, Ning YP, Xiang YT. Short-term efficacy and tolerability of lurasidone in the treatment of acute schizophrenia: A meta-analysis of randomized controlled trials. J Psychiatr Res. 2018;103:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Stahl SM, Cucchiaro J, Simonelli D, Hsu J, Pikalov A, Loebel A. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry. 2013;74:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Nasrallah HA, Cucchiaro JB, Mao Y, Pikalov AA, Loebel AD. Lurasidone for the treatment of depressive symptoms in schizophrenia: analysis of 4 pooled, 6-week, placebo-controlled studies. CNS Spectr. 2015;20:140-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |