Published online Nov 19, 2023. doi: 10.5498/wjp.v13.i11.929
Peer-review started: August 19, 2023
First decision: September 19, 2023
Revised: October 6, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 19, 2023
Processing time: 89 Days and 15.2 Hours
Observational studies have suggested that depression is associated with sarcopenia. However, the causal relationship between depression and sarcopenia remains unclear.
To investigate the causal relationship between depression and sarcopenia.
We performed a Mendelian randomization (MR) analysis to identify the bidirectional relationship between depression and sarcopenia-related traits. Summary-level data and independent variants used as instrumental variables came from large genome-wide association studies of depression (414055 cases and 892299 controls), of appendicular lean mass (ALM, 450243 participants), and of hand grip strength (exposure: 360000 participants; outcome: 334925 participants).
We identified a negative association of depression with lower ALM [odds ratio (OR): 0.932, 95% confidence interval (95%CI): 0.889-0.979, P = 0.005]. In the reverse MR analysis, we also observed an inverse association of hand grip strength with depression (OR: 0.200, 95%CI: 0.108-0.370, P < 0.001). Similar results were obtained in sensitivity analyses.
Depression was causally related to decreased muscle mass, and declined muscle strength might lead to a higher risk of depression.
Core Tip: In this Mendelian randomization study, we established a bidirectional relationship between depression and reduced muscle mass, specifically lower appendicular lean mass and hand grip strength. Our findings highlight a potential bidirectional relationship between depression and sarcopenia with implications for both mental and physical health.
- Citation: Wang DK, Li YH, Guo XM. Depression and sarcopenia-related traits: A Mendelian randomization study. World J Psychiatry 2023; 13(11): 929-936
- URL: https://www.wjgnet.com/2220-3206/full/v13/i11/929.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i11.929
Sarcopenia is a complex geriatric disorder marked by a gradual and progressive reduction of skeletal muscle mass, decrease in skeletal muscle strength, and deterioration in physical performance[1]. In addition to elevating the risks of disability, sarcopenia also relates closely to a wide range of adverse consequences, such as falls, hospitalization due to fall-related injuries, and even mortality[2,3]. As the population ages, the prevalence of sarcopenia increases, making it an important public health issue and a global health burden. In addition, depression is common among the elderly, with an average 12-mo occurrence rate of approximately 6%. This is related to negative health consequences, including increased mortality and reduced quality of life[4,5]. Thus, depression and sarcopenia are important concerns for the elderly population, and it is crucial to establish a clear understanding of their relationship.
Numerous observational studies have suggested that depression and sarcopenia are common comorbidities, but there is no direct evidence of causality[6-8]. In a clinical trial investigating sarcopenia as a therapeutic target, the management of sarcopenia was found to be associated with a notable reduction in depressive symptoms[9]. Some studies have suggested that skeletal muscle may influence psychiatric illnesses through neurotrophic factors[10]. However, those studies were unable to provide convincing evidence to elucidate a null association for the effect of depression on sarcopenia.
Mendelian randomization (MR) represents a compelling genetic epidemiological approach that utilizes genetic variants associated with exposures, which can effectively avoid the potential methodological limitations of observational studies, including reverse causation bias. We conducted this bidirectional MR study to examine the causal relationship between depression and sarcopenia.
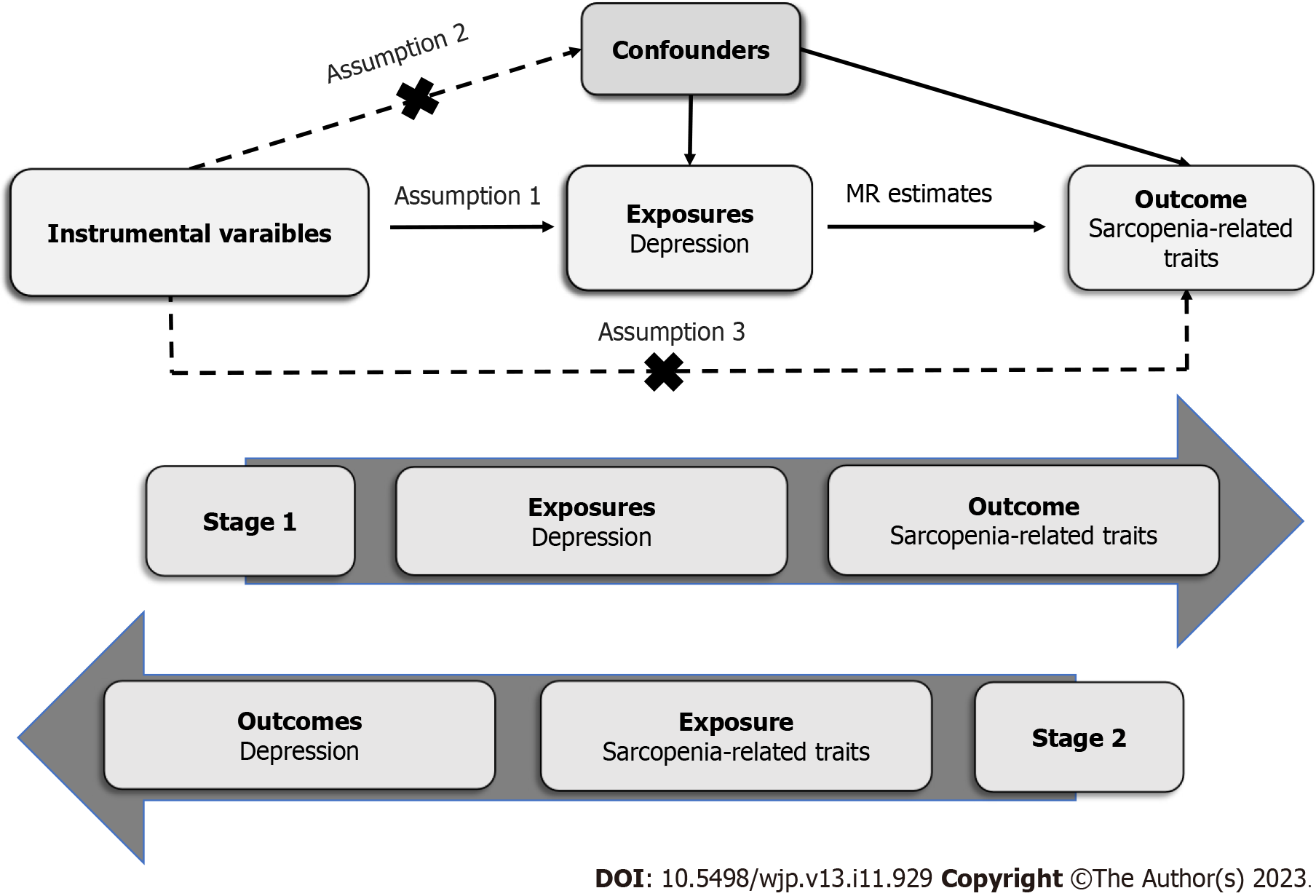
The diagram of this MR study is displayed in the Figure 1. The genetic variations selected as instrumental variables (IVs) were based on three predominant assumptions: (1) Selected IVs are strongly associated with exposures; (2) There is no observed association between the IVs and potential confounding factors; and (3) The IVs affect outcomes only through exposures without any other pathways[11].
The IVs for the MR analyses were derived from several different genome-wide association studies (GWASs). Single nucleotide polymorphisms (SNPs) were chosen as IVs when the SNPs for exposures reached genome-wide significance (P < 5.0 × 10-8). All IVs were clumped for independence (linkage disequilibrium r2 < 0.1; region size, 3000 kb) according to the European data from the 1000 Genomes Project. If the SNPs for exposures were not available in the outcome datasets, proxy SNPs (linkage disequilibrium r2 > 0.8) were adopted online (ldlink.nci.nih.gov/). Palindromic SNPs were excluded in the analyses when harmonizing the directions of SNP effects on exposures and outcomes. We also calculated the F-statistics to assess the instrument strength. IVs with F-statistics < 10 were considered to have a weak instrument bias.
Genetic IVs associated with depression were obtained from a GWAS meta-analysis that included 414055 cases and 892299 controls from the UK Biobank, 23andMe_307k, and PGC_139k[12]. In the UK Biobank cohort, the depression phenotype, referred to as “broad depression,” was determined based on self-reported responses to a web-based questionnaire. In the cohort from 23andMe_307k, the depression phenotype was determined based on self-reported information regarding clinical diagnosis or treatment for depression. In PGC_139k, the depression phenotype was clinically diagnosed. In total, this GWAS identified 102 independent SNPs located at 101 Loci that were associated with depression at a level of genome-wide significance, which led to an 8.9% variance in depression.
Appendicular lean mass (ALM) and hand grip strength were selected as a measure of muscle mass and strength. Unlike whole body lean mass, ALM is primarily affected by skeletal muscle and is recommended by the European Working Group on Sarcopenia in Older People due to its high predictive power for sarcopenia-related health outcomes[1]. The summary statistic for ALM was obtained from a GWAS of the UK Biobank (n = 450243)[13]. That GWAS had measured ALM by bioelectrical impedance analysis (BIA) for fat-free mass at the arms and legs. The summary statistic for hand grip strength was obtained from the UK Biobank (nealelab.is/uk-biobank) and included approximately 360000 participants from Europe. Right-hand grip strength (n = 359729) and left-hand grip strength (n = 359704) were meticulously assessed using a calibrated Jamar J00105 hydraulic hand dynamometer adjusted to accommodate hand size variations. We derived 139 independent genetic IVs associated with hand grip from a GWAS from the UK Biobank, which included 334925 individuals[14]. These variants accounted for 1.7% of the variability in grip strength. In this study, relative grip strength was applied, as it may show a better correlation with physical capability than absolute hand grip strength.
Each study incorporated in the GWAS used in the present study was approved by local research ethics committees or Institutional Review Boards, and all participants had given their informed consent.
In the main analyses, the random-effects inverse-variance weighted (IVW) approach was applied to evaluate a bidirectional relationship between depression and sarcopenia-related traits[15]. In addition, several sensitivity analyses were also conducted to identify potential pleiotropy. Cochran’s Q test was used to assess the heterogeneity among various IVs. The weighted median method was used to include only valid IVs, allowing less than 50% of the genetic variants to be invalid IVs[16]. The MR-Egger method was conducted to detect and correct for any pleiotropic bias[17]. Furthermore, we used the MR Pleiotropy Residual Sum and Outlier (MR-PRESSO) method to conduct a global test of heterogeneity and to identify horizontal pleiotropy. Any identified SNPs with pleiotropic effects were excluded, and a repeated IVW analysis was subsequently performed to ensure the robustness of the results[18].
All tests were two-sided, and the Bonferroni-corrected significance threshold was set to P ≤ 0.01 (correcting for 5 outcomes) to account for multiple comparisons. The P values ranging from 0.05 to 0.01 were considered to be suggestive of a potential association between exposures and outcomes. All analyses were conducted using TwoSampleMR and MR-PRESSO packages in R software (Version 4.1.3).
The main characteristics of all SNPs adopted in the MR analyses are shown in Table 1. The F-statistics for all IVs were higher than the threshold of 10, indicating the absence of weak instrument bias in the present study. The summary information of SNPs for the three traits is displayed in Supplementary Tables 1 and 2. The results of Cochran’s Q tests and MR-Egger regression are shown in Table 2.
| Trait | Data source | Sample size, cases/controls | Ancestry |
| Depression | UK Biobank1 | 127552/233763 | European |
| 23andMe_307K2 | 75607/231747 | ||
| PGC_139K3 | 43204/95680 | ||
| Replication | 474574/1032579 | ||
| Appendicular lean mass | UK Biobank1 | 450243 | European |
| Hand grip strength, exposures | UK Biobank1 | Approximately 360000 | European |
| Hand grip strength, outcomes | UK Biobank1 | 334925 | European |
| Parameter | Cochran's Q | P for Cochran's Q | Intercept | P for intercept |
| Outcome | ||||
| Appendicular lean mass | 911.568 | < 0.001 | -0.002 | 0.602 |
| Left hand grip strength | 409.92 | 0.004 | 0.002 | 0.486 |
| Right hand grip strength | 379.511 | < 0.001 | 0.002 | 0.243 |
| Exposure | ||||
| Appendicular lean mass | 1401.776 | < 0.001 | 0.001 | 0.953 |
| Hand grip strength | 306.463 | < 0.001 | 0.003 | 0.887 |
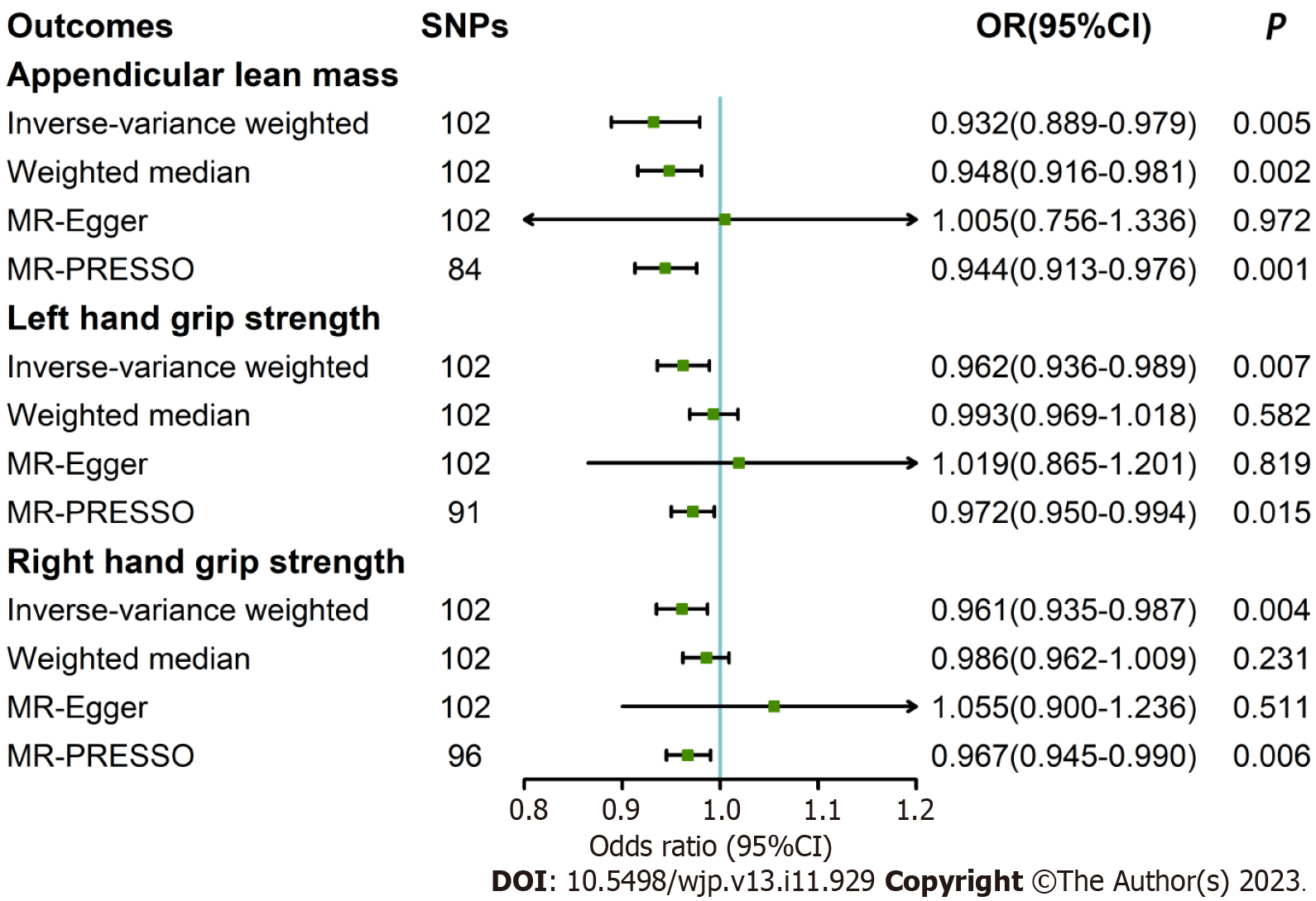
In the random-effect IVW estimates, genetically determined depression was causally associated with lower ALM [odds ratio (OR): 0.932, 95% confidence interval (95%CI): 0.889–0.979, P = 0.005; Figure 2]. This association was robust in the weighted median. The MR-PRESSO analysis identified 18 potential SNP outliers. After removing the outliers, the result was similar. There was significant association of depression with left hand grip and right hand grip (OR: 0.962, 95%CI: 0.936–0.989, P = 0.007; OR: 0.961, 95%CI: 0.935–0.987, P = 0.004). However, these results were not confirmed in the sensitivity analyses. Several potential SNP outliers were identified in the MR-PRESSO tests. After removing the outliers, the results remained significant.
A significant association was observed between decreased ALM and depression (OR: 0.969, 95%CI: 0.947–0.992, P = 0.047; Figure 3). In the sensitivity analyses, weighted median and MR-Egger tests revealed similar effects but with broader confidence intervals. Nine SNPs were detected in the MR-PRESSO test. After removing outliers, the result indicated a suggestive association (OR: 0.978, 95%CI: 0.957–0.999, P = 0.044). In addition, genetically determined hand grip strength was causally associated with depression by the IVW method (OR: 0.200, 95%CI: 0.108–0.370, P < 0.001). The weighted median method yielded similar results despite the MR-Egger test revealing a null association. In the MR-PRESSO test, three outliers were identified and removed, and the result remained significant.
We performed this MR study to explore the bidirectional causal association between depression and sarcopenia. In the forward MR analyses, depression was associated with decreased ALM. Results from IVW suggested that decreased ALM was associated with depression and that depression was associated with lower hand grip strength. However, these results could not be repeated in sensitivity analyses. In the reverse MR analyses, lower hand grip strength was associated with a higher risk of depression. Overall, we reported a significant bidirectional association between depression and sarcopenia.
Previous observational studies, mainly cross-sectional in design, suggested that there was a positive association between depression and sarcopenia[8,19-22]. Two meta-analyses included also drew the conclusion that the prevalence of depression in patients with sarcopenia is higher than in the general population[6,7]. Despite considering many common covariates, these studies were still unable to provide evidence of causality between the two conditions. A recent longitudinal study comprising 115601 older adults reported that higher hand grip strength was associated with a lower risk of depression among older adults[23]. Similar results were also drawn from a 7-year prospective cohort study conducted in China[24]. Although this topic has received widespread attention, few studies have investigated the effect of depression on sarcopenia. Overall, our MR study provided evidence that depression and sarcopenia are possibly connected in a bidirectional manner, whereas previous studies primarily focused on investigating the effect of depression on sarcopenia in a unidirectional manner.
The exact mechanism between depression and sarcopenia remains inconclusive. However, there are several potential connections between the two conditions. First, in the original GWAS of hand grip strength, expression quantitative trait loci analyses revealed multiple genes associated with neurodevelopmental disorders or brain function[14]. The results of a meta-analysis demonstrated a significant enrichment of gene expression of brain-related transcripts[14]. In another study, researchers used twin data from China to explore the genetic overlap between depression and grip strength[25]. They observed potential genetic correlations, SNPs, genes, and pathways common to both conditions, which indicates a shared genetic basis[25]. Second, some studies have suggested that brain-derived neurotrophic factor secreted by skeletal muscle may play a role in depression and anxiety[10]. Brain-derived neurotrophic factor drives hippocampal neu
One of the key strengths of this analysis was the utilization of well-powered GWAS data for depression and sarcopenia-related traits. The implementation of a bidirectional MR design allowed for a comprehensive evaluation of the mutually causal relationship. Nevertheless, there are several limitations that need to be addressed. First, sample overlap between the exposure and outcome populations might potentially influence the study results. There was also a study, however, supporting the applicability of a single large dataset from large biobanks in two-sample MR studies[31]. Second, ALM was not directly measured by the BIA equipment but was estimated based on whole-body electrical conductivity. The estimates of BIA can be influenced by factors such as age, ethnicity, hydration status, and other related discrepancies. All of these factors may subsequently influence the MR results. Third, in the original GWAS the authors reported that they used the broad definition of depression in the cohort of the UK Biobank. This reporting mechanism differed from the self-declared clinical depression phenotype of the 23andMe_307k cohort and the clinically obtained phenotype of the PGC_139k cohort. This difference may have an impact on the results of the MR analysis. Fourth, the population in our study was restricted to Europe, which limits the generalizability of our findings to non-European populations. Finally, potential directional pleiotropy may contribute to bias in the estimation of causal inference even though MR-Egger regression and MR-PRESSO methods were applied.
We provided evidence of a bidirectional association between depression and sarcopenia. Depression was causally related to decreased muscle mass. Meanwhile, declined muscle strength might lead to a higher risk of depression. Our study highlighted the importance of assessing sarcopenia and depression among older adults to understand and address the interplay between physical and mental health.
Sarcopenia is a complex geriatric disorder marked by a gradual and progressive reduction of skeletal muscle mass, decrease in skeletal muscle strength, and deterioration in physical performance. Depression is also common among the elderly. Observational studies have suggested that depression is associated with sarcopenia.
The causal relationship between depression and sarcopenia remains unclear.
To investigate the causal relationship between depression and sarcopenia.
We performed a Mendelian randomization (MR) analysis to identify the bidirectional relationship between depression and sarcopenia-related traits. Summary-level data and independent variants were used as instrumental variables that came from large genome-wide association studies of depression (414055 cases and892299 controls), of appendicular lean mass (ALM, 450243 participants), and of hand grip strength (exposures: 360000 participants; outcomes: 334925 participants).
We identified a negative association of depression with lower ALM. In the reverse MR analysis, we also observed an inverse association of hand grip strength with depression. Similar results were obtained in the sensitivity analyses.
Depression was causally related to decreased muscle mass. Declined muscle strength might lead to a higher risk of depression.
Our findings highlighted a potential bidirectional relationship between depression and sarcopenia with implications for both mental and physical health.
The authors thank the participants in the UK Biobank for their contribution to the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chakrabarti S, India; Gazouli M, Greece S-Editor: Lin C L-Editor: A P-Editor: Guo X
| 1. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6646] [Cited by in RCA: 7759] [Article Influence: 1293.2] [Reference Citation Analysis (1)] |
| 2. | Senior HE, Henwood TR, Beller EM, Mitchell GK, Keogh JW. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas. 2015;82:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | Maggio M, Lauretani F. Prevalence, incidence, and clinical impact of cognitive-motoric risk syndrome in Europe, USA, and Japan: facts and numbers update 2019. J Cachexia Sarcopenia Muscle. 2019;10:953-955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | McCarron RM, Shapiro B, Rawles J, Luo J. Depression. Ann Intern Med. 2021;174:ITC65-ITC80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 276] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 5. | Rodda J, Walker Z, Carter J. Depression in older adults. BMJ. 2011;343:d5219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 265] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 6. | Li Z, Tong X, Ma Y, Bao T, Yue J. Prevalence of depression in patients with sarcopenia and correlation between the two diseases: systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13:128-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 7. | Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing. 2017;46:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 8. | Zhu Y, Guo X, Zhang X, Shi X, Yang Y, Zhang Q. Sex differences in the relationship of serum creatinine to cystatin C ratio and depressive symptoms among middle-aged and older adults in China. J Affect Disord. 2022;319:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Sparrow D, Gottlieb DJ, Demolles D, Fielding RA. Increases in muscle strength and balance using a resistance training program administered via a telecommunications system in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Pasco JA, Williams LJ, Jacka FN, Stupka N, Brennan-Olsen SL, Holloway KL, Berk M. Sarcopenia and the Common Mental Disorders: a Potential Regulatory Role of Skeletal Muscle on Brain Function? Curr Osteoporos Rep. 2015;13:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. 2017;26:2333-2355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 1080] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 12. | Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, Coleman JRI, Hagenaars SP, Ward J, Wigmore EM, Alloza C, Shen X, Barbu MC, Xu EY, Whalley HC, Marioni RE, Porteous DJ, Davies G, Deary IJ, Hemani G, Berger K, Teismann H, Rawal R, Arolt V, Baune BT, Dannlowski U, Domschke K, Tian C, Hinds DA; 23andMe Research Team; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, Trzaskowski M, Byrne EM, Ripke S, Smith DJ, Sullivan PF, Wray NR, Breen G, Lewis CM, McIntosh AM. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1774] [Cited by in RCA: 1616] [Article Influence: 269.3] [Reference Citation Analysis (0)] |
| 13. | Pei YF, Liu YZ, Yang XL, Zhang H, Feng GJ, Wei XT, Zhang L. The genetic architecture of appendicular lean mass characterized by association analysis in the UK Biobank study. Commun Biol. 2020;3:608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 14. | Tikkanen E, Gustafsson S, Amar D, Shcherbina A, Waggott D, Ashley EA, Ingelsson E. Biological Insights Into Muscular Strength: Genetic Findings in the UK Biobank. Sci Rep. 2018;8:6451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology. 2017;28:30-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 1283] [Article Influence: 183.3] [Reference Citation Analysis (0)] |
| 16. | Burgess S, Zuber V, Gkatzionis A, Foley CN. Modal-based estimation via heterogeneity-penalized weighting: model averaging for consistent and efficient estimation in Mendelian randomization when a plurality of candidate instruments are valid. Int J Epidemiol. 2018;47:1242-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 2877] [Article Influence: 359.6] [Reference Citation Analysis (0)] |
| 18. | Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 5356] [Article Influence: 765.1] [Reference Citation Analysis (0)] |
| 19. | Kim J, Kang S, Hong H, Kang H, Kim JH, Woo SK. Muscle Strength Moderates the Relationship between Nutritional Health Risk and Depression in Korean Older Adults. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Ulugerger Avci G, Suzan V, Bektan Kanat B, Unal D, Emiroglu Gedik T, Doventas A, Suna Erdincler D, Yavuzer H. Depressive symptoms are associated with sarcopenia and malnutrition in older adults. Psychogeriatrics. 2023;23:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Delibaş DH, Eşkut N, İlhan B, Erdoğan E, Top Kartı D, Yılmaz Küsbeci Ö, Bahat G. Clarifying the relationship between sarcopenia and depression in geriatric outpatients. Aging Male. 2021;24:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Cabanas-Sánchez V, Esteban-Cornejo I, Parra-Soto S, Petermann-Rocha F, Gray SR, Rodríguez-Artalejo F, Ho FK, Pell JP, Martínez-Gómez D, Celis-Morales C. Muscle strength and incidence of depression and anxiety: findings from the UK Biobank prospective cohort study. J Cachexia Sarcopenia Muscle. 2022;13:1983-1994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 23. | López-Bueno R, Calatayud J, Andersen LL, Casaña J, Koyanagi A, Del Pozo Cruz B, Smith L. Dose-response association of handgrip strength and risk of depression: a longitudinal study of 115 601 older adults from 24 countries. Br J Psychiatry. 2023;222:135-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 24. | Bao M, Chao J, Sheng M, Cai R, Zhang N, Chen H. Longitudinal association between muscle strength and depression in middle-aged and older adults: A 7-year prospective cohort study in China. J Affect Disord. 2022;301:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Zhang T, Ji L, Luo J, Wang W, Tian X, Duan H, Xu C, Zhang D. A genetic correlation and bivariate genome-wide association study of grip strength and depression. PLoS One. 2022;17:e0278392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 961] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 27. | Brown PJ, Rutherford BR, Yaffe K, Tandler JM, Ray JL, Pott E, Chung S, Roose SP. The Depressed Frail Phenotype: The Clinical Manifestation of Increased Biological Aging. Am J Geriatr Psychiatry. 2016;24:1084-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, Palla SL, Ambrosius WT, Tracy RP, Pahor M. Sarcopenia, obesity, and inflammation--results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 29. | Mac Giollabhui N, Ng TH, Ellman LM, Alloy LB. The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol Psychiatry. 2021;26:3302-3314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 195] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 30. | Budui SL, Rossi AP, Zamboni M. The pathogenetic bases of sarcopenia. Clin Cases Miner Bone Metab. 2015;12:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Minelli C, Del Greco M F, van der Plaat DA, Bowden J, Sheehan NA, Thompson J. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int J Epidemiol. 2021;50:1651-1659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 214] [Article Influence: 53.5] [Reference Citation Analysis (0)] |