Published online Nov 19, 2023. doi: 10.5498/wjp.v13.i11.816
Peer-review started: September 5, 2023
First decision: September 14, 2023
Revised: September 23, 2023
Accepted: October 11, 2023
Article in press: October 11, 2023
Published online: November 19, 2023
Processing time: 73 Days and 0.6 Hours
Standard management protocols are lacking and specific antidotes are unavailable for acute carbamazepine (CBZ) poisoning. The objective of this review is to provide currently available information on acute CBZ poisoning, including its management, by describing and summarizing various therapeutic methods for its treatment according to previously published studies. Several treatment methods for CBZ poisoning will be briefly introduced, their advantages and disadvantages will be analyzed and compared, and suggestions for the clinical treatment of CBZ poisoning will be provided. A literature search was performed in various English and Chinese databases. In addition, the reference lists of identified articles were screened for additional relevant studies, including non-indexed reports. Non-peer-reviewed sources were also included. In the present review, 154 articles met the inclusion criteria including case reports, case series, descriptive cohorts, pharmacokinetic studies, and in vitro studies. Data on 67 patients, including 4 fatalities, were reviewed. Based on the summary of cases reported in the included articles, the cure rate of CBZ poisoning after symptomatic treatment was 82% and the efficiency of hemoperfusion was 58.2%. Based on the literature review, CBZ is moderately dialyzable and the recommendation for CBZ poisoning is supportive management and gastric lavage. In severe cases, extracorporeal treatment is recommended, with hemodialysis as the first choice.
Core Tip: There is no effective Antidote and standard treatment management for carbamazepine (CBZ) poisoning. This paper describes and summarizes the detailed clinical evidence of the treatment and management of CBZ poisoning, including gastric lavage and activated carbon therapy, hemoplavage, hemodialysis, continuous renal replacement treatment and plasmapheresis. Finally, this paper also provides the lipid resuscitation therapy to provide help for clinical treatment. In general, this paper summarized the results of CBZ poisoning related research, and provided the best plan for CBZ poisoning treatment.
- Citation: Wang L, Wang Y, Zhang RY, Wang Y, Liang W, Li TG. Management of acute carbamazepine poisoning: A narrative review. World J Psychiatry 2023; 13(11): 816-830
- URL: https://www.wjgnet.com/2220-3206/full/v13/i11/816.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i11.816
Carbamazepine (CBZ) was discovered by Walter Schindler in 1953 and first utilized to treat partial tonic-clonic seizures in the United States in 1974[1]. Currently, CBZ is widely used for the treatment of epilepsy and peripheral neuralgia. Broad indications for the use and widespread availability of CBZ increase the risk of overdose, and CBZ intoxication accounts for a large proportion of life-threatening cases of anticonvulsant toxicity[2-4]. A systematic review of CBZ was published in 2014 by the Extracorporeal Treatments in Poisoning (EXTRIP) workgroup that provides recommendations for the use of extracorporeal treatments (ECTRs) in cases of CBZ poisoning[5]. Currently, standard management protocols are lacking and specific antidotes for acute CBZ poisoning are unavailable; thus, we reviewed and summarized the various therapeutic methods for the treatment of CBZ poisoning described in previous studies. Our goal was to provide currently available information on acute CBZ poisoning, including its management. We believe this paper will help clinicians effectively manage patients suffering from CBZ intoxication.
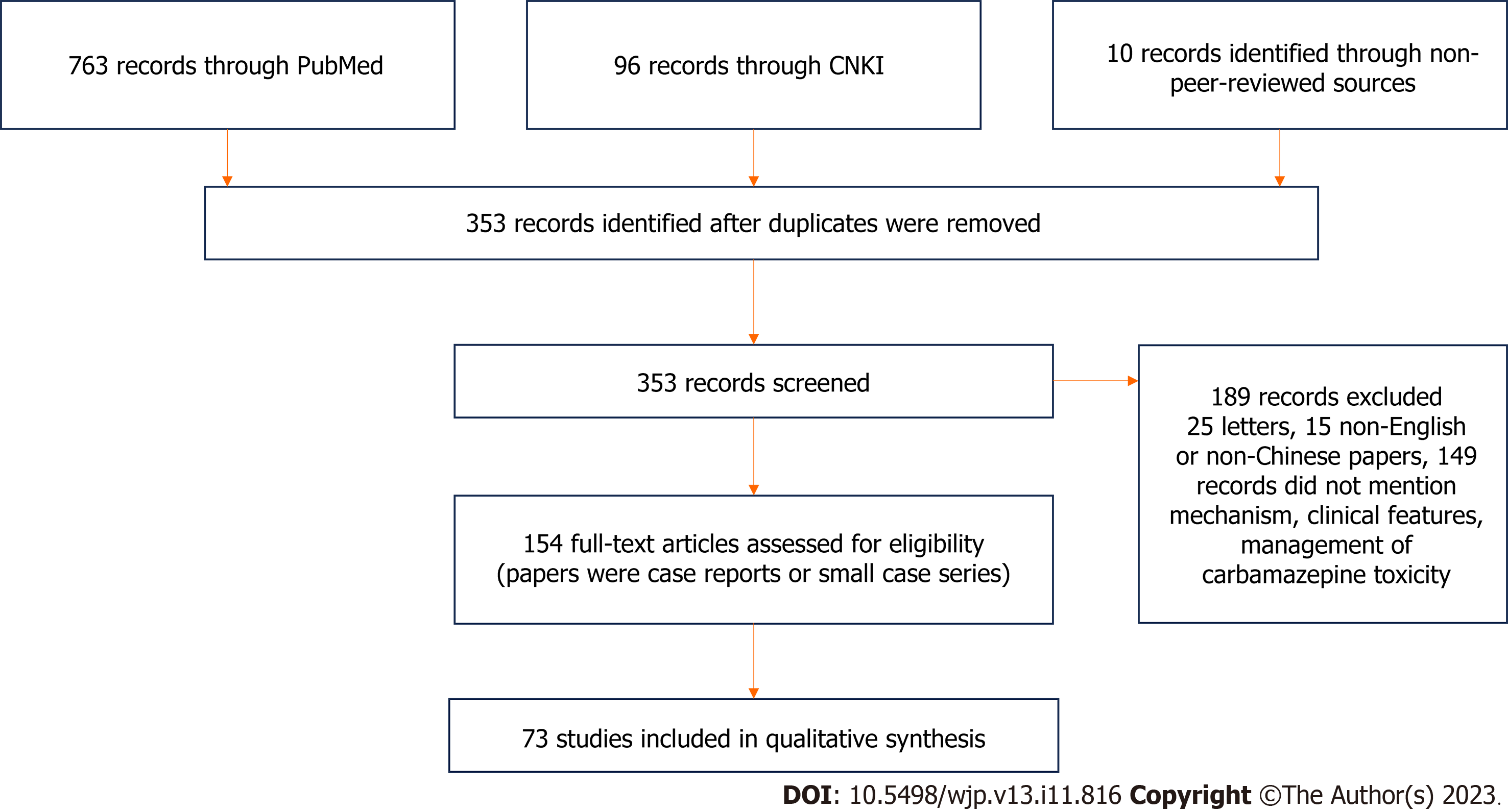
A literature search was performed in the following databases: PubMed, Embase, Chinese National Knowledge Infrastructure (CNKI), and Wanfang. The search strategy, in English and Chinese, used the terms “acute” and “carbamazepine” in combination with the keywords “poisoning”, “intoxication”, “toxication”, “toxicity”, “overdose”, and “overdosage”. These search terms identified 861 records. In addition, the reference lists of the identified articles were screened for additional relevant studies, and these additional searches added three records. Non-peer-reviewed sources were also searched, including books and Internet resources, which yielded 10 records. A total of 874 records were found, which were screened based on title, author, journal name, and publication date for repeatability and relevance. We excluded papers that were duplicates, non-English or non-Chinese papers, letters to editors, and those not referring specifically to the mechanisms of action, clinical features, and management of CBZ toxicity in humans. Finally, 154 articles were considered eligible for review. The majority of papers were case reports or small case series. The literature selection process is described in Figure 1.
CBZ is an iminostilbene derivative that is structurally similar to tricyclic antidepressants and is mainly used as a potent first-line agent to treat epilepsy, including simple partial, complex partial, and general tonic-clonic seizures[6,7]. The therapeutic effects of CBZ have also been proven for the treatment of neuropathic pain, especially prosopalgia, central diabetes insipidus, attention-deficit/hyperactivity disorder, and bipolar disorder. CBZ is thought to exert its anticonvulsant effects by blocking presynaptic voltage-gated sodium channels in the central nervous system (CNS), which further prevents the release of glutamate and similar neurotransmitters, ultimately inhibiting high-frequency epileptic foci.
CBZ has a molecular weight of 236 Da and high protein binding affinity (70%-80%) for both albumin and alpha-1-acid glycoprotein. This percentage does not markedly decline, even in cases of overdose[8]. CBZ is highly lipophilic, a property that allows it to be rapidly distributed after ingestion with a moderately large volume of distribution ranging between 0.8 L/kg and 1.4 L/kg. Thus, at therapeutic doses, the immediate-release tablets and controlled-release formulation of CBZ have peak serum concentrations at approximately 6-8 h and 12-24 h post-ingestion, respectively[9-11]. However, following overdose, peak concentrations may be delayed up to 106 h[12] due to the anticholinergic properties of CBZ, which are responsible for decreased gastrointestinal (GI) motility and delayed and prolonged absorption[10,13].
CBZ metabolism predominantly and extensively occurs in the liver by epoxidation and hydroxylation reactions through the cytochrome P450 system. Only 1%–3% of the drug is not metabolized and excreted through the kidney. CBZ-10,11-epoxide is the primary active metabolite and equally produces antiepileptic and toxic effects based on its capacity to form covalent protein conjugates[14,15]. CBZ-10,11-epoxide blood concentrations vary substantially between individuals, ranging from 15% to 55% of CBZ levels in adults and from 5% to 81% of CBZ levels in children[11]. CBZ metabolism is complicated because both normal metabolism and autoinduction of metabolism occur with chronic use.
There is wide interindividual variability in the pharmacokinetics of CBZ, as well as general adaptation[16]. CBZ is eliminated according to zero-order kinetics (i.e. a constant amount of drug is eliminated per unit time) in the case of elevated plasma drug levels, as seen in overdose; however, a delay in CBZ elimination occurs when no intervention is available[16]. The reported biological half-life of the initial dose of CBZ is 25-65 h, but is shortened to 12-17 h in chronically treated epileptic patients due to self-induction[17]. Nevertheless, the half-life is apparently much longer after excessive dosing and the use of controlled-release preparations[1,12], likely reflecting delayed or continuous absorption, impaired elimination, or a combination of both.
Toxicity from CBZ overdose was first described by Guntelberg in 1967[18]. CBZ intoxication increasingly accounts for a large proportion of life-threatening cases among anticonvulsant poisonings[2,3]. A total of 3734 toxic exposures to CBZ were reported in 2014 by the American Association of Poison Control Centers. Of these, 1880 were isolated ingestions. There were no deaths, but 62 cases were life-threatening or severely disabling due to the significant toxicity[19].
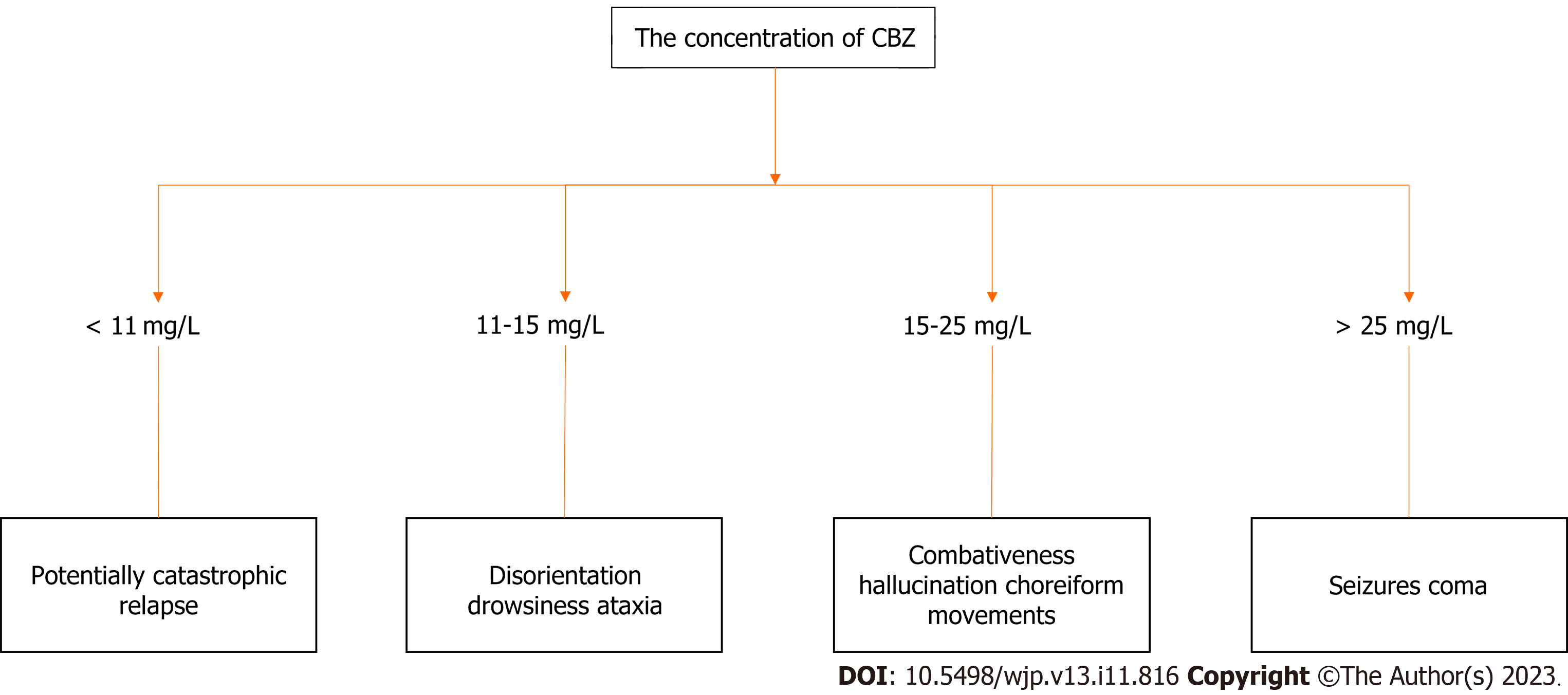
Although the toxic concentration of CBZ in patients is not completely consistent with clinical manifestations of toxicity, doctors often initially treat patients based on the patient’s clinical status. However, serum CBZ concentration is a practical method used to confirm clinical exposure, and severe intoxication occurs at serum levels > 20 mg/L [therapeutic index range: 4-12 mg/L (17-51 μmol/L)][20]. Serum CBZ levels > 40 mg/L are generally predictive of severe toxicity[21], which potentially occurs at a lower concentration[22], especially in children[23-25], and tends to be associated with an increased risk of seizure, coma, respiratory depression, and cardiotoxicity[26,27]. In addition, the pharmacologically active metabolite of CBZ, CBZ-10,11-epoxide, contributes to the risk of toxicity[28,29]. Based on the circulating level of CBZ in blood, the effects of CBZ on the CNS can be classified into four stages: (1) Seizures and coma at levels > 25 mg/L; (2) Combativeness, hallucinations, and choreiform movements at levels of 15-25 mg/L; (3) Disorientation, drowsiness, and ataxia at levels of 11-15 mg/L; and (4) Potentially catastrophic relapse at levels < 11 mg/L[30]. The duration and severity of these stages are variable and depend upon the magnitude of the overdose[31]. The detailed clinical symptoms corresponding to the toxic dose are described in Figure 2.
Toxicity usually occurs within 1-3 h after ingestion, but may be prolonged due to the delayed and erratic absorption of CBZ[32,33]. A wide diversity of clinical manifestations such as neurologic, respiratory, and cardiac findings can be seen in CBZ poisoning[22,34]. CBZ intoxication primarily manifests as CNS symptoms including nystagmus, movement disorders, disturbance of consciousness, altered mental status ranging from drowsiness to coma, and convulsions[35-39]. Seizures are not uncommon, occurring in 11%-18% of overdose cases[40,41]. Respiratory depression, a common mani-festation of severe overdose, may delineate the severity of toxicity in the respiratory system and may be complicated by concomitant aspiration. Myocardial depression, sinus tachycardia, hemodynamic instability hypotension, and cardiac conduction disturbances are characteristics of CBZ cardiotoxicity. Some rare electrocardiogram (ECG) abnormalities may also occur such as atrioventricular block, QRS interval prolongation, QTc (corrected QT time in ECG) prolongation (> 420 ms), bundle branch block, Brugada-type patterns, and premature ventricular contractions[42,43]. Death, though very uncommon (approximately 2% of cases), generally results from cardiovascular toxicity[44,45]. An overall mortality rate of 13% was documented and reported in a cohort study of 427 patients with CBZ intoxication, with an average CBZ intake in lethal cases of 23.6 g[22]. Refractory cardiovascular toxicity has also been reported[46,47].
In clinical practice, doctors initially treat patients based on their clinical symptoms, and supportive care plays a key role at this stage. For example, seizures are treated with benzodiazepines[48], hypotension is treated with vasopressors, and endotracheal intubation is used for patients with respiratory insufficiency. GI decontamination techniques such as gastric lavage (GL) and single-dose activated charcoal (AC) (SDAC) can be applied if patients are administered care within 1-2 h after ingestion without contraindications[49]. However, CBZ metabolism and distribution are complicated, and specific life-saving antidotes for CBZ poisoning are unavailable. Several modalities have been proposed to enhance CBZ clearance in patients with severe or life-threatening ingestion, including multiple-dose AC (MDAC), ECTRs, and lipid resuscitation therapy (LRT)[50].
Application of appropriate supportive care is adequate to deal with the vast majority of CBZ intoxication cases. Supportive care may involve airway protection with endotracheal intubation, treatment of seizures with benzo-diazepines, and correction of hypotension with fluid challenges and vasopressors, if needed. The use of hypertonic sodium bicarbonate is indicated if ECG findings (QRS duration exceeding 100 msec) show that the sodium channel is blocked.
Serum CBZ concentrations in an acute overdose should be monitored serially (typically every 4-6 h) until a definitive downward trend is observed and the patient is improving clinically[51,52]. Continuous monitoring of serum CBZ concentrations in patients is necessary, especially in those with severe poisoning. These patients are likely to deteriorate after the initial improvement of clinical symptoms or develop a delayed toxic reaction due to a rebound effect (i.e. sudden rise in serum drug concentration following intermittent treatment due to drug redistribution from tissue to serum[53]) or sustained increase in serum concentration.
Patients with significant CNS depression may lose protective airway reflexes and should be intubated, particularly due to their lower seizure threshold. Short-acting neuromuscular blocking agents (e.g., succinylcholine) are preferable so subsequent seizure activity is not masked. Induction agents with gamma-amino butyric acid agonist activity (e.g., midazolam) may be preferable, depending on the patient’s hemodynamic status.
Hypotension is initially treated with isotonic crystalloid. Caution should be exercised in patients at risk of volume overload, such as those with underlying heart disease or CBZ-induced myocardial dysfunction. Direct-acting vaso-pressors are used if intravenous fluids fail to correct the hypotension.
Sodium channel blockade may cause QRS interval prolongation, which, in other poisonings, has been shown to predispose patients to ventricular arrhythmias[54,55]. QRS prolongation due to CBZ toxicity is treated with sodium bicarbonate. Currently, there are no clearly established treatment thresholds based on QRS duration or dosing guidelines or evidence regarding the preferred method of administering sodium bicarbonate with respect to boluses, infusions, or treatment duration[56]. The proposed methods of administration are for patients with QRS intervals longer than 110 ms, particularly those with hypotension, and include boluses of a 1 mEq per 1 mL (8.4%) solution at a quantity of 1-2 mEq/kg to obtain a serum pH of 7.45-7.55 and an infusion of 150 mEq mixed with 1 L of 5% dextrose in water at a rate to maintain that pH[54]. Continuous cardiac monitoring of patients with a 12-lead ECG is simultaneously needed. Volume overload, hypokalemia, hypernatremia, and metabolic alkalosis may result from prolonged bicarbonate infusions, and clinical and laboratory parameters must be closely followed to avoid these complications.
GL has been routinely used to manage cases of poisoning for more than 200 years. However, GL carries significant risks, which, given its questionable clinical benefit, usually outweigh arguments in favor of its use; thus, it is not recommended for routine GI decontamination[57]. GL works by eliminating poison remaining in the GI tract and should be considered based on the time elapsed from oral ingestion to admission to the emergency department. In cases of suspected CBZ intoxication, prior to the determination of the current plasma concentration of CBZ, it is imperative to remove undigested tablets through GL to further reduce GI absorption. This one simple measure may be particularly helpful for CBZ intoxication given its anticholinergic effects on the GI tract that would delay gastric emptying[58]. However, there is less conclusive evidence showing that GL has clear effects on CBZ poisoning[59,60]. As noted in a position statement related to GL use, GL should not be considered unless a potentially life-threatening amount of a poison is ingested by the patient, and this approach should be initiated within 60 min of ingestion[61]. Nevertheless, the clinical benefit has not been confirmed in controlled studies. Moreover, GL is not appropriate for all patients, and not all patients can benefit from GL. GL is contraindicated in patients who have impaired consciousness, recent surgery, no protection of the airway, risk of GI bleeding and perforation, and potentially increased risk and severity of aspiration[57]. The major complications of GL include perforation of the esophagus and stomach, pulmonary aspiration, and aspiration pneumonia[62].
AC therapy is still the most frequently employed method of GI decontamination for acute drug overdoses in developed countries. With a highly developed internal pore structure, the tremendous surface area of AC permits the adsorption of drugs and toxins in the GI tract within minutes of contact, which reduces their systemic absorption and subsequent toxicity and enhances their elimination[63]. AC therapy involves SDAC and MDAC, which indicates scheduled administration of more than two small doses of AC.
MDAC should only be considered if a patient has ingested a life-threatening amount of CBZ. MDAC therapy is effective in poisoning due to three distinct mechanisms: (1) Reducing ongoing absorption of extended or delayed-release formulations remaining in the gut[49]; (2) Interfering with enterohepatic and enterogastric circulation of drugs[64-66]; and (3) Promoting the diffusion of drugs from circulation into the gut lumen and trapping them there to be discharged later in the excrement (this process is sometimes called “GI dialysis”)[67].
The “routine” use of MDAC has not been shown to reduce morbidity and mortality in poisoned patients in controlled clinical trials. However, evidence from animal and volunteer studies, as well as poisoning cases, indicates that MDAC therapy significantly enhances total body clearance and shortens the half-life of CBZ[33,68-71]. Mittag et al[19] recently reported a case of CBZ overdose in which the patient was treated with AC (1 g/kg body weight); his CBZ concentration dropped from the initial value of 41.5 mg/L to approximately 12 mg/L after 24 h. A randomized crossover study in 5 fasted volunteers performed by Neuvonen and Elonen[65] showed that the elimination of CBZ could be significantly enhanced by MDAC. In a study by Boldy et al[70] involving 15 patients with CBZ poisoning, researchers gave patients MDAC therapy and found that the mean half-life decreased to 8.6 ± 2.4 h and the mean total body clearance increased to 113 ± 44 mL/min. By comparison, the mean elimination half-life in another two groups of patients managed with supportive treatments only was approximately 19 h[8,72]; similar conclusions have been reached in other studies[73,74].
MDAC enhances the clearance of CBZ in the body and has been approved by the latest joint statement issued by the American Academy of Clinical Toxicology and the European Association of Poisons Centres and Clinical Toxicologists[64]. The statement recommends that MDAC be considered only if a patient has ingested a life-threatening amount of CBZ. In addition, several conditions are considered contradictions including an unprotected airway, the presence of intestinal obstruction, and a GI tract not anatomically intact. Attention should be paid to prevent complications such as constipation, ileus, and aspiration pneumonia in an unprotected airway, although these occurrences are rare[75].
The efficacy of AC is inversely related to the time elapsed after the ingestion of toxic substances[42,76]; thus, administration of charcoal as soon as possible yields the best results. However, the considerably delayed absorption of drugs is often noted in cases of acute poisoning due to inhibition of gastric emptying and gut motility and the limited solubility of drugs, among other reasons[21,22]. Therefore, AC administration is apparently indicated if delayed or prolonged absorption is anticipated, even after a delay of 24-48 h[75].
There is no established optimal dosage regimen for AC; however, available data indicate a dose-response relationship: An AC: Drug ratio of 10:1 or higher is considered optimal for AC adsorption[77-79]. However, the efficacy of AC is influenced by many variables, making it difficult to administer the optimum dose[63]. Consequently, in practice, a fixed dose of 1 g/kg is generally recommended with the maximum amount not exceeding 50-70 g. A flexible dose of 0.25-1.0 g/kg is used for MDAC, and the dosing interval varies based on laboratory (e.g., increasing blood concentrations) or clinical evidence for continuing drug absorption and ranges from 1 h to 4 h. Vomiting is an important factor to consider when substantial doses of AC are administered repeatedly. Continuous nasogastric infusion of AC or frequent administration of AC with smaller doses, at least following a large initial dose (50-100 g), may contribute to reduced vomiting[80,81]. In some cases, the combined use of antiemetics, such as metoclopramide or ondansetron, may have desirable effects.
ECTR, also known as blood purification treatment, is a medical technology that removes poison from the blood by moving the blood from the patient into a purification device. ECTRs are classified based on their mechanism: Hemodialysis (HD) and peritoneal dialysis act by diffusion; hemoperfusion (HP) acts by adsorption; hemofiltration acts by convection; and therapeutic plasma exchange acts by centrifugation[82,83]. Each technique has a potentially different role in eliminating poison from the body. Whatever modality is used, optimizing ECTR characteristics to achieve maximum removal of poison is crucial; for example, higher flow rates of blood and dialysate, filters with larger surface area, and longer duration of ECTR maximize poison removal[84].
According to the EXTRIP workgroup on the use of ECTR in CBZ poisoning, ECTRs are recommended for patients with significant or potentially significant clinical poisoning because they are preferable interventions for rapidly and substantially removing CBZ[5]. Even when considering its weaknesses, such as high risks, high costs, and relative uncertainty, ECTR is generally considered by the workgroup to be worth implementing in patients with severe CBZ poisoning (as manifested by signs such as multiple refractory seizures, hemodynamic instability requiring vasopressors, or life-threatening dysrhythmias). ECTR can prevent the worsening of CBZ poisoning symptoms such as severe hypotension, as well as comatose complications associated with chronic shortness of breath such as ventilator-associated pneumonia and pulmonary emboli[5]. In addition, ECTR is expected by most participants in the workgroup to be a potentially cost-saving technique by minimizing the mechanical ventilation period and residence time in the intensive care unit (ICU), ultimately reducing ICU-related costs[5]. Whether and when to initiate ECTR should be primarily based on clinical symptoms and signs, rather than serum CBZ concentrations, although more severe toxicity apparently occurs when CBZ concentrations are > 40 mg/L[21,85]. The optimal duration of extracorporeal removal should be adjusted to the patient’s clinical condition and CBZ levels. Furthermore, the continuous and regular monitoring of CBZ serum concentrations after ECTR is essential, as concentrations may rebound[86]. If concentrations rebound to threatening levels or if there is a recurrence of toxic symptoms, another session of ECTR may be required. ECTR is recommended for patients with significant or potentially significant clinical poisoning. Intermittent HD is the preferred modality for ECTR in CBZ poisoning. Intermittent HP and continuous renal replacement modalities are alternatives if intermittent HD is not available.
HP: HP refers to anticoagulated blood that circulates through a cartridge containing adsorbent particles (AC particles or resin beads) onto which the poison can be adsorbed to achieve the goal of blood purification[87,88]. This modality is suitable for removing poisons with medium or large molecular weight, high lipid solubility, and high protein binding. CBZ is a highly lipid-soluble drug that can be rapidly distributed in tissues, and its plasma protein binding is as high as 70%-80%. Therefore, HP is historically identified as the preferred modality for extracorporeal removal in cases of severe CBZ poisoning[89-92], although the EXTRIP workgroup published a contrary opinion. Despite limitations such as early saturation of the cartridges, the successful removal of CBZ through either resin or charcoal HP has been reported with varying clearance efficiencies ranging from 25% to 55%[93-97]. In a recent study conducted by Yang et al[98], the authors concluded that the early initiation of HP during treatment of CBZ poisoning would significantly lower the serum concentration and minimize the CBZ detection time, leading to symptom relief and shortening of the overall treatment period. However, this procedure is often difficult to perform and carries risks such as thrombocytopenia, leukopenia, coagulopathy, and hypocalcemia, which are thought to be associated with cell adsorption and continuous replenishment of the AC[99,100]. Therefore, the frequent monitoring of platelet counts or application of new biocompatible polymer membranes is indispensable for decreasing these complications[101].
HD: HD takes advantage of the principle of diffusion, allowing poison to pass through a semipermeable membrane from a higher- to lower-level side to remove the poison in the blood. This modality is suitable for poisons with high water solubility, low molecular weight (< 500 D), medium molecular weight, and low protein binding. Although intermittent HP is historically preferred for the removal of CBZ, HD should be considered based on its theoretical superiority in clearing protein-bound poisons such as CBZ, and the EXTRIP workgroup recommends intermittent HD over HP. Indeed, several factors prove that HD is superior to HP. First, its clearance efficiency is superior. Although a previous study showed that HP has higher clearance than HD, the comparison was based on older, less efficient cuprophane dialyzers[5]. Recent data have shown that high-efficiency HD is almost as effective as charcoal HP and results in rapid clinical improvement[13,102-104] when equipped with a highly permeable high-flux membrane with increased dialysate flow, which produces comparable efficacy by allowing the removal of larger molecules and highly protein-bound poisons[105-109]. In addition, there is a risk of hemolysis. Blood flow circulating in cartridges during HP remains limited to 300-350 mL/min; however, the use of brand new catheters during HD enables blood flow up to 400 mL/min[110]. Furthermore, there are high amounts of free CBZ molecules and its metabolites in blood at increased concentrations, which are good candidates for removal by HD[111,112]. Second, the manipulability of HD is superior; HD is more available than HP, and HD is the preferred technique for dialysis for patients with acute kidney injury and end-stage kidney disease worldwide. Early initiation of ECTR is critical, considering that clinical improvements are likely associated with how quickly poisons can be cleared from the body. In addition, physicians and nurses are more familiar and experienced with HD; thus, this technique is more likely to be implemented with fewer delays and uncertainties. Third, outcomes of HD are superior; complications are less likely to occur with HD than with HP[113]. Finally, HD has a favorable cost compared with HP. A recent investigation of availability, time to initiation, and cost of ECTR in acute settings worldwide confirmed the lower cost of HD compared with other ECTRs (median cost ratio of HP:HD = 1.7)[114]. The high spending associated with HP is largely due to multiple replacements of expensive charcoal cartridges because of its easy saturation, which would decrease poison clearance[12,91]. Moreover, monitoring and treating complications of HP contribute to its high expenses.
Continuous renal replacement treatment: Continuous renal replacement treatment (CRRT), also known as continuous blood purification, is a treatment involving the continuous, slow removal of water and solutes. CRRT includes continuous venovenous HD, continuous venovenous hemodiafiltration (CVVHDF), and continuous venovenous hemofiltration[115]. These continuous therapies are usually performed in the ICU to facilitate management of hemodynamically unstable patients who cannot withstand high-efficiency intermittent treatments. Therefore, lower dialysate rates during CRRT (up to 10 L/h) than the dialysate flow rates used in intermittent HD (500–800 mL/min) are routinely offered, leading to markedly lower clearance[5]. However, CRRTs play a better role in mitigating rebound effects than intermittent techniques, regardless of the drug clearance rate. Numerous studies are available documenting the use of CRRTs in patients with CBZ intoxication; however, techniques and outcomes vary substantially. Thus, the benefits of these techniques to eliminate CBZ from the body are unable to be determined[89,116-119]. Consequently, continuous techniques are regarded as low-priority modalities by the EXTRIP workgroup and are only advocated when intermittent treatments are unavailable (because of technical or personnel reasons in critical care settings). Nevertheless, some authors approve of the joint use of intermittent procedures and CRRTs in patients with poisoning to avoid “rebound phenomenon”.
Recently, the addition of albumin to the dialysate appeared to improve CBZ clearance compared with conventional CRRT in some studies[120-124]. In a study conducted by Choi et al[122], the applied CVVHDF showed a 24% increase in CBZ clearance when using dialysate with the addition of 20% albumin. Another in vitro study using modeled continuous HD performed in 2009 also confirmed that CBZ clearance was significantly enhanced with 2.5% or 5% albumin-supplemented dialysate compared with control (albumin-free) dialysate[123]. This technique could be explained by basic principles of thermodynamic protein-binding affinity and solute movement along a concentration gradient. The albumin added to the dialysate works as a “sink” to bind CBZ that moves across the dialyzer membrane along the concentration gradient formed between the blood side and dialysate side[124]. However, extremely high costs have limited their widespread use. In addition, whether these procedures have superiority to either HP or HD remains unclear and further investigation is needed.
LRT is a relatively new method to improve the clinical manifestations of toxicity from overdose of certain drugs through administration of intravenous lipid emulsions (ILEs). ILE was conventionally used for parenteral nutrition and first applied in 1998 by Weinberg and colleagues[125] as a treatment option in toxicology. The first human case of ILE clinical use was reported by Rosenblatt et al[126] in 2006 to treat local anesthetic (bupivacaine) systemic toxicity. Since then, ILEs have been widely used in clinical toxicology as a life-saving treatment for local anesthetic-induced cardiotoxicity[127]. With further research, the field has expanded progressively with successful clinical translation and expansion of use to treat other types of non-local anesthetic lipophilic drug overdoses[128-132].
The ILE mechanisms of action are not entirely clear but can be partially explained by dynamic scavenging/partitioning and direct cardiovascular effects[133,134]. In 1998, Weinberg and colleagues originally proposed a mechanistic hypothesis, namely, that ILE provides a novel compartment into which lipophilic drugs circulating in the blood are able to partition, preventing them from affecting other organs. This theory was widely accepted and referred to as “the lipid sink”. However, with improved understanding of this treatment, a “lipid shuttle” or a capture/release mechanism has been suggested in which ILEs play a role in accelerating the redistribution of drugs and moving drugs around, as opposed to acting like a “lipid sink” that catches and segregates drugs[135]. In addition, ILEs exert direct effects on myocardial tissue that improve cardiac output; however, the relevant potential mechanisms are not fully understood[136,137]. Based on these mechanisms of action, ILEs have been used to manage toxicity from non-local anesthetic lipophilic drugs.
CBZ is a highly lipophilic molecule and can induce cardiotoxicity in cases of significant overdose. ILEs have been administered to treat CBZ intoxication in several cases with varying results[116,138-141]. ILE treatment was used in two case reports for CBZ-induced cardiotoxicity and showed beneficial effects[138,139]. In a retrospective study investigating the clinical effects of lipid emulsion for the treatment of childhood CBZ poisoning, researchers enrolled 48 patients and divided them into Group A (lipid emulsion + HP) and Group B (HP); a statistically significant difference was not observed in the blood concentration of CBZ between groups (P > 0. 05). After 24, 48, and 72 h of treatment, the CBZ blood concentration in Group A was significantly lower than that in Group B (P < 0.05), and the researchers concluded that the lipid emulsion could be used for the treatment of CBZ poisoning in children to reduce the blood CBZ concentration as quickly as possible[142]. Although the sole effect of ILE in CBZ clearance has not been proven, ILE is still considered to be a potentially beneficial alternative to ECTR methods in CBZ intoxication. Whether and when to initiate LRT is discretionary and depends on the clinical judgment of the treating physician. Blood clotting and fat deposition commonly occur in extracorporeal circuits when combined with LRT[140,141], and thus should be monitored during therapy.
We conducted a retrospective review of selected literature. The case reports contained detailed data describing patient status and treatments, and an aggregate description was made (Table 1). After discussion and analyses of CBZ poisoning cases, we established recommendations for the treatment of CBZ poisoning (Table 2); 67 patients were included and no randomized controlled trials were identified. The median CBZ peak concentration was 39.0 mg/L and the median age was 42.3 years. Notably, all patients had varying degrees of consciousness impairment, which were due to individual differences. In addition, some patients experienced respiratory depression, dysrhythmias, hypotension, seizures, or a combination of these symptoms. Among the patients, 32 showed varying degrees of respiratory depression. Overall, 4 cases were described as deaths caused by CBZ poisoning rather than the effect of the treatment[43,44,105]. GL is a simple and convenient treatment, but only 6 patients received GL because the treatment is prohibited in patients with impaired consciousness, recent surgery, no airway protection, risk of GI bleeding and perforation, or potential for increased risk and severity of aspiration[16,19,21,23,26,28]. In these situations, considering the patient’s economic conditions and affordability, MDAC was chosen instead of GL. For decades, MDAC has been used as a universal antidote for the majority of poisons because of its ability to prevent absorption of the most toxic agents from the GI tract and enhance the elimination of some agents already absorbed[143,144]. The optimum dose of MDAC is difficult to precisely determine for an individual patient. Optimum dosage depends on many variables such as the volume and pH of gastric and intestinal fluid, and the presence of other agents or food absorbed by AC[145-148]. Notably, repeated administration is still effective against substances that stay longer in the stomach, which is another advantage of MDAC. The recommended dose for adults is typically 50 g; however, the amount administered to children is determined based on body weight (0.5-1 g/kg). Reported complications and adverse effects of MDAC include diarrhea, constipation, vomiting, pulmonary aspiration, and intestinal obstruction. Consequently, MDAC should be used with caution in the following situations: unprotected airway; presence of intestinal obstruction; or GI tract not anatomically intact. Therefore, intestinal motility in patients receiving MDAC should be continuously monitored as necessary to maintain electrolyte and water balance.
| Patient demographics | Values |
| Median age (yr) | 25 (1.5-85) |
| Male sex | 49.3 |
| Poisoning exposure | |
| Mean peak carbamazepine concentration in mg/L | 39.0 (17.7-93.8) |
| Clinical symptoms and signs | |
| Respiratory depression | 47.8 |
| Decreased consciousness | 100 |
| Seizure | 20.9 |
| Hypotension | 14.9 |
| Dysrhythmias | 19.4 |
| Treatment measure1 | |
| Supportive care | 9.0 |
| Gastric lavage | 29.9 |
| Multiple-dose activated charcoal | 58.2 |
| Hemoperfusion | 22.4 |
| Hemodialysis | 22.4 |
| Continuous renal replacement treatment | 12.0 |
| Plasma exchange and plasmapheresis | 4.5 |
| Lipid resuscitation therapy | 4.5 |
| Outcome | |
| Recovery | 82.0 |
| Sequelae | 12.0 |
| Fatalities | 6.0 |
| General statement |
| The primary treatment for CBZ poisoning is GL |
| A mainstay of treatment is multiple-dose activated charcoal |
| ECTR is suggested in cases of severe CBZ poisoning |
| Intermittent hemodialysis is the preferred ECTR for CBZ poisoning |
| Lipid resuscitation therapy is an effective adjunctive treatment to ECTR |
| Supportive care |
| Severe central nervous system depression requires endotracheal intubation |
| To avoid masking subsequent seizures, short-acting neuromuscular blockers are recommended |
| Isotonic crystalloid to correct hypotension should be considered first; when isotonic crystals do not work, direct-acting vasopressors can be applied |
| QRS prolongation is treated with sodium bicarbonate |
| Management precautions |
| GL is contraindicated when patients have impaired consciousness, recent surgery, no protection of airway, risk of gastrointestinal bleeding and perforation, and the potential for increased risk and severity of aspiration |
| Multiple-dose activated charcoal should be used with caution in cases of an unprotected airway, presence of intestinal obstruction, or gastrointestinal tract not anatomically intact |
HP was the most common treatment for the patients in our review, accounting for 58.2% of cases. HP was considered the most timely and effective treatment for patients with excessive CBZ toxicity and life-threatening symptoms because it can remove mid-to-large-sized toxin molecules bound to proteins. However, the earlier enthusiasm for HP has decreased and HD is currently the treatment of choice for poisoned patients. HP presents some challenges compared with HD. For example, HP is associated with more complications than HD (namely, hypocalcemia, leucopenia, thrombocytopenia[147,148]), and the rate of complications is greater during HP than HD. Furthermore, HP is more expensive and results in the early saturation of columns. Notably, the same percentage of patients received HD and CRRT therapy (22.4%). HD and CRRT are commonly used to provide renal support to critically ill patients with acute kidney injury, particularly those who are hemodynamically unstable. Although initially developed as an arteriovenous therapy, most CRRT is now performed using pump-driven venovenous extracorporeal circuits. However, initiation of CRRT requires vascular access, which is generally established through placement of a large-bore double lumen catheter in an internal jugular, femoral, or subclavian vein. Furthermore, hypotension during CRRT is common, occurring in some series in more than one-third of patients, but is most often unrelated to the CRRT procedure. Increased hemodynamic instability due to ultrafiltration may be the most likely treatment-related factor contributing to hypotension[149]. Therefore, we conclude that in the absence of accurate, scientific data indicating effectiveness and risk, a sound recommendation for the use of CRRT cannot be made. Again, the treating physician must weigh the theoretic benefit against the potential for complications in each clinical scenario. HD is indicated for the therapeutic management of acute poisoning or drug overdose and is the most commonly favored extracorporeal technique in poisoning situations due to its availability, cost, and safety profile. With the development of science and technology, the advent of high-efficiency, high-flux dialyzers has rendered other techniques, such as HP, almost obsolete. Furthermore, HD enables the correction of acid-base and electrolyte abnormalities.
Among patients, plasma exchange and plasmapheresis as well as lipid emulsion were the least frequent (both 4.5%). Plasma exchange and plasmapheresis refer to the extracorporeal technique preformed in an apheresis device where the patient’s plasma is separated from whole blood and removed, and the cellular blood components are returned to the patient together with a replacement fluid[150]. Therapeutic procedures commonly include therapeutic plasma exchange and red blood cell exchange with or without depletion, in addition to more specialized procedures, such as low-density lipoprotein (LDL) apheresis and extracorporeal photopheresis, which was widely used in China to treat patients with coronavirus disease 2019[151]. However, because performing extracorporeal photopheresis is complex and requires precision, the method is rarely used in drug intoxication cases. The mechanism of action in LDL apheresis creates an expanded, intravascular lipid phase in which equilibria are established that drive the offending drug from target tissues into the newly formed “lipid sink”. Based on this theory, LDL has been considered a candidate for generic reversal of toxicity caused by overdose of any lipophilic drug. However, the use of LDL apheresis requires caution, especially in patients with a history of hypersensitivity to lipid emulsion or ingredients (e.g., eggs, soy), severe sepsis, severe liver disease, acute pancreatitis, and acute myocardial infarction. Therefore, we suggest that LDL apheresis be considered under circumstances of refractory hemodynamic instability.
Herein, we presented recommendations for therapies in CBZ poisoning. Risk assessment of selected case reports showed that in most poisoned patients, general supportive measures usually suffice, including airway management and protection, ventilatory support, fluid resuscitation, correction of electrolyte and acid-base disorders, and management of poison-related hypo/hyperthermia. Primary GI decontamination with AC and routine GL are widely used. Aggressive ECTR is required for rapid and substantial removal of CBZ and its metabolites when clinical symptoms and signs are serious or deteriorated. Choice of ECTR modality should be based on clearance efficiency, availability, cost, and complications. Among the various ECTRs available, HD provides the best expected removal for CBZ poisoning with the lowest incidence of complications and should therefore be the preferred modality in most cases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chudzik A, Poland; Keeler J, United Kingdom S-Editor: Lin C L-Editor: A P-Editor: Chen YX
| 1. | Güntelberg E. Carbamazepine Tegretol poisoning. A review and case report. Ugeskr Laeger. 1967;129:161-163. [PubMed] |
| 2. | Günaydın YK, Akıllı NB, Dündar ZD, Köylü R, Sert ET, Çekmen B, Akıncı E, Cander B. Antiepileptic drug poisoning: Three-year experience. Toxicol Rep. 2015;2:56-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Saraçoğlu TM, Hocaoğlu N, Yanturalı S. Retrospective analysis of anticonvulsant exposures admitted to department of emergency medicine in dokuzeylül university hospital. J Bas Clin Hea Sci. 2017;1:11-17. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Bialer M. Chemical properties of antiepileptic drugs (AEDs). Adv Drug Deliv Rev. 2012;64:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Ghannoum M, Yates C, Galvao TF, Sowinski KM, Vo TH, Coogan A, Gosselin S, Lavergne V, Nolin TD, Hoffman RS; EXTRIP workgroup. Extracorporeal treatment for carbamazepine poisoning: systematic review and recommendations from the EXTRIP workgroup. Clin Toxicol (Phila). 2014;52:993-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Holland KD. Efficacy, pharmacology, and adverse effects of antiepileptic drugs. Neurol Clin. 2001;19:313-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Jones AL, Proudfoot AT. Features and management of poisoning with modern drugs used to treat epilepsy. QJM. 1998;91:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Vree TB, Janssen TJ, Hekster YA, Termond EF, van de Dries AC, Wijnands WJ. Clinical pharmacokinetics of carbamazepine and its epoxy and hydroxy metabolites in humans after an overdose. Ther Drug Monit. 1986;8:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Winnicka RI, Topaciński B, Szymczak WM, Szymańska B. Carbamazepine poisoning: elimination kinetics and quantitative relationship with carbamazepine 10,11-epoxide. J Toxicol Clin Toxicol. 2002;40:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Bertilsson L, Tomson T. Clinical pharmacokinetics and pharmacological effects of carbamazepine and carbamazepine-10,11-epoxide. An update. Clin Pharmacokinet. 1986;11:177-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 205] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Cameron RJ, Hungerford P, Dawson AH. Efficacy of charcoal hemoperfusion in massive carbamazepine poisoning. J Toxicol Clin Toxicol. 2002;40:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Sikma MA, van den Broek MP, Meulenbelt J. Increased unbound drug fraction in acute carbamazepine intoxication: suitability and effectiveness of high-flux haemodialysis. Intensive Care Med. 2012;38:916-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Smollin CG, Petrie MS, Kearney T. Carbamazepine and carbamazepine-10,11-epoxide clearance measurements during continuous venovenous hemofiltration in a massive overdose. Clin Toxicol (Phila). 2016;54:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Bu HZ, Kang P, Deese AJ, Zhao P, Pool WF. Human in vitro glutathionyl and protein adducts of carbamazepine-10,11-epoxide, a stable and pharmacologically active metabolite of carbamazepine. Drug Metab Dispos. 2005;33:1920-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Chauhan B, Patanvadiya A, Dash GK. Carbamazepine Toxicity-Induced Spindle Coma: A Novel Case Report. Clin Neuropharmacol. 2017;40:100-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Rockoff S, Baselt RC. Severe Carbamazepine Poisoning. Clin Toxicol Sci. 1981;18:935-939. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Lurie Y, Bentur Y, Levy Y, Baum E, Krivoy N. Limited efficacy of gastrointestinal decontamination in severe slow-release carbamazepine overdose. Ann Pharmacother. 2007;41:1539-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Gummin DD, Mowry JB, Beuhler MC, Spyker DA, Bronstein AC, Rivers LJ, Pham NPT, Weber J. 2020 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59:1282-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 206] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 19. | Mittag N, Meister S, Berg AM, Walther UI. A Case Report of a Carbamazepine Overdose With Focus on Pharmacokinetic Aspects. Pharmacopsychiatry. 2016;49:76-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Hojer J, Malmlund HO, Berg A. Clinical features in 28 consecutive cases of laboratory confirmed massive poisoning with carbamazepine alone. J Toxicol Clin Toxicol. 1993;31:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Schmidt S, Schmitz-Buhl M. Signs and symptoms of carbamazepine overdose. J Neurol. 1995;242:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Perez A, Wiley JF. Pediatric carbamazepine suspension overdose-clinical manifestations and toxicokinetics. Pediatr Emerg Care. 2005;21:252-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Takia L, Kc S, Randhawa M, Angurana SK, Nallasamy K, Bansal A, Jayashree M. Clinical Features, Intensive Care Needs, and Outcome of Carbamazepine Poisoning in Children. Indian J Pediatr. 2022;89:1022-1024. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Stremski ES, Brady WB, Prasad K, Hennes HA. Pediatric carbamazepine intoxication. Ann Emerg Med. 1995;25:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Spiller HA, Carlisle RD. Status epilepticus after massive carbamazepine overdose. J Toxicol Clin Toxicol. 2002;40:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Bridge TA, Norton RL, Robertson WO. Pediatric carbamazepine overdoses. Pediatr Emerg Care. 1994;10:260-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 27. | Russell JL, Spiller HA, Baker DD. Markedly Elevated Carbamazepine-10,11-epoxide/Carbamazepine Ratio in a Fatal Carbamazepine Ingestion. Case Rep Med. 2015;2015:369707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Fisher RS, Cysyk B. A fatal overdose of carbamazepine: case report and review of literature. J Toxicol Clin Toxicol. 1988;26:477-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Unei H, Ikeda H, Murakami T, Tanigawa K, Kihira K. Detoxication treatment for carbamazepine and lithium overdose. Yakugaku Zasshi. 2008;128:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Weaver DF, Camfield P, Fraser A. Massive carbamazepine overdose: clinical and pharmacologic observations in five episodes. Neurology. 1988;38:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Graudins A, Peden G, Dowsett RP. Massive overdose with controlled-release carbamazepine resulting in delayed peak serum concentrations and life-threatening toxicity. Emerg Med (Fremantle). 2002;14:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Patel VH, Schindlbeck MA, Bryant SM. Delayed elevation in carbamazepine concentrations after overdose: a retrospective poison center study. Am J Ther. 2013;20:602-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Fricke-Galindo I, LLerena A, Jung-Cook H, López-López M. Carbamazepine adverse drug reactions. Expert Rev Clin Pharmacol. 2018;11:705-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Kuz GM, Manssourian A. Carbamazepine-induced hyponatremia: assessment of risk factors. Ann Pharmacother. 2005;39:1943-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Jha A, Abhilash KP, Bandhyopadhyay R, Victor PJ. Hypoglycemia - a rare complication of carbamazepine overdose. Indian J Pharmacol. 2014;46:651-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Lifshitz M, Gavrilov V, Sofer S. Signs and symptoms of carbamazepine overdose in young children. Pediatr Emerg Care. 2000;16:26-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Xu W, Chen YL, Zhao Y, Wang LJ, Li JJ, Liu CF. A Clinical Study of Toxication Caused by Carbamazepine Abuse in Adolescents. Biomed Res Int. 2018;2018:3201203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Seymour JF. Carbamazepine overdose. Features of 33 cases. Drug Saf. 1993;8:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Hatice B, Dürdane A, Mehmet E. The Increased Frequency of Seizures Caused by Carbamazepine Overdose: A Case Report. Epilepsi Sci. 2012;18:21-24. [DOI] [Full Text] |
| 41. | Leslie PJ, Heyworth R, Prescott LF. Cardiac complications of carbamazepine intoxication: treatment by haemoperfusion. Br Med J (Clin Res Ed). 1983;286:1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Mégarbane B, Leprince P, Deye N, Guerrier G, Résière D, Bloch V, Baud FJ. Extracorporeal life support in a case of acute carbamazepine poisoning with life-threatening refractory myocardial failure. Intensive Care Med. 2006;32:1409-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Martín-Echevarría E, De Arriba G, Pereira-Juliá A, Albaya A. [Acute carbamazepine intoxication treated with hemoperfusion]. Rev Clin Esp. 2006;206:300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Hruby K, Lenz K, Druml W, Kleinberger G. [Experiences with acute carbamazepine poisoning]. Nervenarzt. 1982;53:414-418. [PubMed] |
| 45. | De Rubeis DA, Young GB. Continuous EEG monitoring in a patient with massive carbamazepine overdose. J Clin Neurophysiol. 2001;18:166-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Chyka PA, Seger D, Krenzelok EP, Vale JA; American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. Position paper: Single-dose activated charcoal. Clin Toxicol (Phila). 2005;43:61-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Goodman JW, Goldfarb DS. The role of continuous renal replacement therapy in the treatment of poisoning. Semin Dial. 2006;19:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Tintinalli EJ, Kelen GD. Emergency Medicine — A Comprehensive Study Guide, Companion Handbook. Stapczynski JS, editor. New York: McGraw Hill, 2000. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 49. | May DC. Acute carbamazepine intoxication: clinical spectrum and management. South Med J. 1984;77:24-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Liebelt EL. Targeted management strategies for cardiovascular toxicity from tricyclic antidepressant overdose: the pivotal role for alkalinization and sodium loading. Pediatr Emerg Care. 1998;14:293-298. [PubMed] |
| 51. | Cave G, Sleigh JW. ECG features of sodium channel blockade in rodent phenytoin toxicity and effect of hypertonic saline. Vet Hum Toxicol. 2003;45:254-255. [PubMed] |
| 52. | Gheshlaghi F, Yaraghi A, Soh EH, Ghoreishi A. Relationship of cardiovascular complications with level of consciousness in patients with acute carbamazepine intoxication. Med Arh. 2012;66:9-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 53. | Behnoush B, Bazmi E, Thaddosinejad F. Carbamazepine Poisoning and Effect of Multiple-Dose Activated Charcoal. Acta Medica Iranica. 2009;47:9-14. |
| 54. | Apfelbaum JD, Caravati EM, Kerns WP 2nd, Bossart PJ, Larsen G. Cardiovascular effects of carbamazepine toxicity. Ann Emerg Med. 1995;25:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Bruccoleri RE, Burns MM. A Literature Review of the Use of Sodium Bicarbonate for the Treatment of QRS Widening. J Med Toxicol. 2016;12:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 56. | Benson BE, Hoppu K, Troutman WG, Bedry R, Erdman A, Höjer J, Mégarbane B, Thanacoody R, Caravati EM; American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. Position paper update: gastric lavage for gastrointestinal decontamination. Clin Toxicol (Phila). 2013;51:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 57. | Durelli L, Massazza U, Cavallo R. Carbamazepine toxicity and poisoning. Incidence, clinical features and management. Med Toxicol Adverse Drug Exp. 1989;4:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Patsalos PN, Krishna S, Elyas AA, Lascelles PT. Carbamazepine and carbamazepine-10,11-epoxide pharmacokinetics in an overdose patient. Hum Toxicol. 1987;6:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Mochizuki K, Hamano Y, Miyama H, Arakawa K, Kobayashi T, Imamura H. Successful treatment of a case with concurrent ingestion of carbamazepine overdose and grapefruit juice. Acute Med Surg. 2016;3:36-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Vale JA. Position statement: gastric lavage. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1997;35:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 155] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 61. | Albertson TE, Owen KP, Sutter ME, Chan AL. Gastrointestinal decontamination in the acutely poisoned patient. Int J Emerg Med. 2011;4:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 62. | Gaudreault P. Activated Charcoal Revisited. Clini Pediat Emerg Med. 2005;6:76-80. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Position statement and practice guidelines on the use of multi-dose activated charcoal in the treatment of acute poisoning. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1999;37:731-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 178] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 64. | Levy RH, Pitlick WH, Troupin AS, Green JR, Neal JM. Pharmacokinetics of carbamazepine in normal man. Clin Pharmacol Ther. 1975;17:657-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 106] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Neuvonen PJ, Elonen E. Effect of activated charcoal on absorption and elimination of phenobarbitone, carbamazepine and phenylbutazone in man. Eur J Clin Pharmacol. 1980;17:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 132] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Levy G. Gastrointestinal clearance of drugs with activated charcoal. N Engl J Med. 1982;307:676-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 166] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 67. | Wason S, Baker RC, Carolan P, Seigel R, Druckenbrod RW. Carbamazepine overdose--the effects of multiple dose activated charcoal. J Toxicol Clin Toxicol. 1992;30:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Mise S, Jukić I, Tonkić A, Titlić M, Mise S. Multidose activated charcoal in the treatment of carbamazepine overdose with seizures: a case report. Arh Hig Rada Toksikol. 2005;56:333-338. [PubMed] |
| 69. | Sethna M, Solomon G, Cedarbaum J, Kutt H. Successful treatment of massive carbamazepine overdose. Epilepsia. 1989;30:71-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Boldy DA, Heath A, Ruddock S, Vale JA, Prescott LF. Activated charcoal for carbamazepine poisoning. Lancet. 1987;1:1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Hundt HK, Aucamp AK, Müller FO. Pharmacokinetic aspects of carbamazepine and its two major metabolites in plasma during overdosage. Hum Toxicol. 1983;2:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Montoya-Cabrera MA, Sauceda-García JM, Escalante-Galindo P, Flores-Alvarez E, Ruiz-Gómez A. Carbamazepine poisoning in adolescent suicide attempters. Effectiveness of multiple-dose activated charcoal in enhancing carbamazepine elimination. Arch Med Res. 1996;27:485-489. [PubMed] |
| 73. | Brahmi N, Kouraichi N, Thabet H, Amamou M. Influence of activated charcoal on the pharmacokinetics and the clinical features of carbamazepine poisoning. Am J Emerg Med. 2006;24:440-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Soderstrom J, Murray L, Little M, Daly FF. Toxicology case of the month: Carbamazepine overdose. Emerg Med J. 2006;23:869-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Gude AB, Hoegberg LC, Angelo HR, Christensen HR. Dose-dependent adsorptive capacity of activated charcoal for gastrointestinal decontamination of a simulated paracetamol overdose in human volunteers. Basic Clin Pharmacol Toxicol. 2010;106:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 76. | Jürgens G, Hoegberg LC, Graudal NA. The effect of activated charcoal on drug exposure in healthy volunteers: a meta-analysis. Clin Pharmacol Ther. 2009;85:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | Isik Y, Soyoral L, Karadas S, Emre H, Cegin MB, Goktas U. Effectivity of one session charcoal hemoperfusion treatment in severe carbamazepine poisoning. Iran Red Crescent Med J. 2013;15:749-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 78. | Olson KR. Activated charcoal for acute poisoning: one toxicologist's journey. J Med Toxicol. 2010;6:190-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 79. | Filippone GA, Fish SS, Lacouture PG, Scavone JM, Lovejoy FH Jr. Reversible adsorption (desorption) of aspirin from activated charcoal. Arch Intern Med. 1987;147:1390-1392. [PubMed] |
| 80. | Ohning BL, Reed MD, Blumer JL. Continuous nasogastric administration of activated charcoal for the treatment of theophylline intoxication. Pediatr Pharmacol (New York). 1986;5:241-245. [PubMed] |
| 81. | Ghannoum M, Hoffman RS, Gosselin S, Nolin TD, Lavergne V, Roberts DM. Use of extracorporeal treatments in the management of poisonings. Kidney Int. 2018;94:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 82. | Fischer S, Balslev E. Vascular protein deposits in temporal arteritis with special reference to failure of histological findings. APMIS. 1989;97:1125-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 83. | Yang R, Chen X, Li S, Wang X, Liu D. From Critical Care Nephrology To Critical Care Blood Purification. J Transl Int Med. 2021;9:4-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 84. | Payette A, Ghannoum M, Madore F, Albert M, Troyanov S, Bouchard J. Carbamazepine poisoning treated by multiple extracorporeal treatments. Clin Nephrol. 2015;83:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Shalkham AS, Kirrane BM, Hoffman RS, Goldfarb DS, Nelson LS. The availability and use of charcoal hemoperfusion in the treatment of poisoned patients. Am J Kidney Dis. 2006;48:239-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Zhao X, Ge S. The efficacy and safety of gabapentin vs. carbamazepine in patients with primary trigeminal neuralgia: A systematic review and meta-analysis. Front Neurol. 2023;14:1045640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 87. | Winchester JF, Harbord NB. Intoxications amenable to extracorporeal removal. Adv Chronic Kidney Dis. 2011;18:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | Vallianou N, Giannopoulou M, Trigkidis K, Bei E, Margellou E, Apostolou T. A case of severe carbamazepine overdose treated successfully with combined hemoperfusion and hemodialysis technique. Saudi J Kidney Dis Transpl. 2017;28:906-908. [PubMed] |
| 89. | Vree D, Dalen R, Geerts Y, Kolmer EWJVB. Clinical Pharmacokinetics of a Massive Overdose of Carbamazepine and Acute Renal Failure. Clin Drug Inves. 1997;14:66-75.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 90. | Kuhlmann U, Becker R, Ebel H, Gressner A, Lange H. Extracorporeal elimination of carbamazepine by haemoperfusion. Biomater Artif Cells Immobilization Biotechnol. 1992;20:1171-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 91. | Deshpande G, Meert KL, Valentini RP. Repeat charcoal hemoperfusion treatments in life threatening carbamazepine overdose. Pediatr Nephrol. 1999;13:775-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 92. | Ghannoum M, Bouchard J, Nolin TD, Ouellet G, Roberts DM. Hemoperfusion for the treatment of poisoning: technology, determinants of poison clearance, and application in clinical practice. Semin Dial. 2014;27:350-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 93. | Ghannoum M, Roberts DM, Hoffman RS, Ouellet G, Roy L, Decker BS, Bouchard J. A stepwise approach for the management of poisoning with extracorporeal treatments. Semin Dial. 2014;27:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | de Groot G, van Heijst AN, Maes RA. Charcoal hemoperfusion in the treatment of two cases of acute carbamazepine poisoning. J Toxicol Clin Toxicol. 1984;22:349-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 95. | Peces R, Azorín S, Peces C, Selgas R. [Extended hemoperfusion in the treatment of acute carbamazepine intoxication]. Nefrologia. 2010;30:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 96. | Nilsson C, Sterner G, Idvall J. Charcoal hemoperfusion for treatment of serious carbamazepine poisoning. Acta Med Scand. 1984;216:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Chan KM, Aguanno JJ, Jansen R, Dietzler DN. Charcoal hemoperfusion for treatment of carbamazepine poisoning. Clin Chem. 1981;27:1300-1302. [PubMed] |
| 98. | Yang X, Xin S, Zhang Y, Li T. Early hemoperfusion for emergency treatment of carbamazepine poisoning. Am J Emerg Med. 2018;36:926-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 99. | Gary NE, Byra WM, Eisinger RP. Carbamazepine poisoning: treatment by hemoperfusion. Nephron. 1981;27:202-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 100. | Milonovich LM, Headrick CL, Seikaly M, Morriss FC. Charcoal hemoperfusion via a continuous venovenous hemofiltration circuit to treat carbamazepine overdose. Crit Care Nurse. 2001;21:25-28. [PubMed] |
| 101. | Extracorporeal Treatment of Poisoning. In: Pocket Companion to Brenner and Rector's The Kidney. 8th ed. Clarkson MR, Magee CN, Brenner BM, editor. Extracorporeal Treatment of Poisoning. Amsterdam: Elsevier Inc, 2011: 789-810. |
| 102. | Kielstein JT, Schwarz A, Arnavaz A, Sehlberg O, Emrich HM, Fliser D. High-flux hemodialysis--an effective alternative to hemoperfusion in the treatment of carbamazepine intoxication. Clin Nephrol. 2002;57:484-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 103. | Schuerer DJ, Brophy PD, Maxvold NJ, Kudelka T, Bunchman TE. High-efficiency dialysis for carbamazepine overdose. J Toxicol Clin Toxicol. 2000;38:321-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Pilapil M, Petersen J. Efficacy of hemodialysis and charcoal hemoperfusion in carbamazepine overdose. Clin Toxicol (Phila). 2008;46:342-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 105. | Ozhasenekler A, Gökhan S, Güloğlu C, Orak M, Ustündağ M. Benefit of hemodialysis in carbamazepine intoxications with neurological complications. Eur Rev Med Pharmacol Sci. 2012;16 Suppl 1:43-47. [PubMed] |
| 106. | Murat Ö. Massive carbamazepine overdose: any role of hemodialysis? case report. Turkiye Klinikleri J Med. 2011;31:702-705. [DOI] [Full Text] |
| 107. | Azak A, Koçak G, Huddam B, Duranay M. Is conventional hemodialysis enough to manage carbamazepine intoxication? Blood Purif. 2012;33:225-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 108. | Ram Prabahar M, Raja Karthik K, Singh M, Singh RB, Singh S, Dhamodharan J. Successful treatment of carbamazepine poisoning with hemodialysis: a case report and review of the literature. Hemodial Int. 2011;15:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 109. | Rahman MH, Haqqie SS, McGoldrick MD. Acute hemolysis with acute renal failure in a patient with valproic acid poisoning treated with charcoal hemoperfusion. Hemodial Int. 2006;10:256-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 110. | Yaylacı S, Demir MV, Acar B, Sipahi S, Tamer A. Successful treatment of excessive dose of carbamazepine. Indian J Pharmacol. 2012;44:417-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 111. | Koh KH, Tan HH. High-flux haemodialysis treatment as treatment for carbamazepine intoxication. Med J Malaysia. 2006;61:109-111. [PubMed] |
| 112. | Shannon MW. Comparative efficacy of hemodialysis and hemoperfusion in severe theophylline intoxication. Acad Emerg Med. 1997;4:674-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 113. | Bouchard J, Lavergne V, Roberts DM, Cormier M, Morissette G, Ghannoum M. Availability and cost of extracorporeal treatments for poisonings and other emergency indications: a worldwide survey. Nephrol Dial Transplant. 2017;32:699-706. [PubMed] [DOI] [Full Text] |
| 114. | Ouellet G, Bouchard J, Ghannoum M, Decker BS. Available extracorporeal treatments for poisoning: overview and limitations. Semin Dial. 2014;27:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 115. | Harder JL, Heung M, Vilay AM, Mueller BA, Segal JH. Carbamazepine and the active epoxide metabolite are effectively cleared by hemodialysis followed by continuous venovenous hemodialysis in an acute overdose. Hemodial Int. 2011;15:412-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 116. | Shah S, Tomlin M, Sparkes D. Lack of effect of high-volume continuous veno-venous haemofiltration with dialysis in massive carbamazepine overdose. BMJ Case Rep. 2012;2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 117. | Goktas U, Kati I, Yuce HH. Management of a severe carbamazepine overdose with continuous venovenous hemodiafiltration. Am J Emerg Med. 2010;28:260.e1-260.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 118. | Yildiz TS, Toprak DG, Arisoy ES, Solak M, Toker K. Continuous venovenous hemodiafiltration to treat controlled-release carbamazepine overdose in a pediatric patient. Paediatr Anaesth. 2006;16:1176-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 119. | Askenazi DJ, Goldstein SL, Chang IF, Elenberg E, Feig DI. Management of a severe carbamazepine overdose using albumin-enhanced continuous venovenous hemodialysis. Pediatrics. 2004;113:406-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 120. | Churchwell MD, Pasko DA, Smoyer WE, Mueller BA. Enhanced clearance of highly protein-bound drugs by albumin-supplemented dialysate during modeled continuous hemodialysis. Nephrol Dial Transplant. 2009;24:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Narayan R, Rizzo M, Cole M. Successful treatment of severe carbamazepine toxicity with 5% albumin-enhanced continuous venovenous hemodialysis. J Artif Organs. 2014;17:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 122. | Choi JS, Kim CS, Bae EH, Ma SK, Kee SJ, Woo YJ, Kim SW. Enhanced clearance of carbamazepine using albumin-containing dialysate during CVVHDF. Intensive Care Med. 2013;39:159-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 123. | Chung YK, Chang KY, Park HS, Kim MH, Lee KM, Lim TS, Kim HW. Severe carbamazepine intoxication unresponsive to albumin-enhanced continuous venovenous hemodiafiltration with low dialysate flow. Hemodial Int. 2014;18:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 124. | Patzer J. Principles of bound solute dialysis. Ther Apher Dial. 2006;10:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 125. | Weinberg GL, VadeBoncouer T, Ramaraju GA, Garcia-Amaro MF, Cwik MJ. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anesthesiology. 1998;88:1071-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 399] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 126. | Rosenblatt MA, Abel M, Fischer GW, Itzkovich CJ, Eisenkraft JB. Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest. Anesthesiology. 2006;105:217-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 374] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 127. | Litz RJ, Popp M, Stehr SN, Koch T. Successful resuscitation of a patient with ropivacaine-induced asystole after axillary plexus block using lipid infusion. Anaesthesia. 2006;61:800-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 228] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 128. | Liang CW, Diamond SJ, Hagg DS. Lipid rescue of massive verapamil overdose: a case report. J Med Case Rep. 2011;5:399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 129. | Jovic-Stosic J, Gligic B, Putic V, Brajkovic G, Spasic R. Severe propranolol and ethanol overdose with wide complex tachycardia treated with intravenous lipid emulsion: a case report. Clin Toxicol (Phila). 2011;49:426-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 130. | Hendron D, Menagh G, Sandilands EA, Scullion D. Tricyclic antidepressant overdose in a toddler treated with intravenous lipid emulsion. Pediatrics. 2011;128:e1628-e1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 131. | Sirianni AJ, Osterhoudt KC, Calello DP, Muller AA, Waterhouse MR, Goodkin MB, Weinberg GL, Henretig FM. Use of lipid emulsion in the resuscitation of a patient with prolonged cardiovascular collapse after overdose of bupropion and lamotrigine. Ann Emerg Med. 2008;51:412-415, 415.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 193] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 132. | Finn SD, Uncles DR, Willers J, Sable N. Early treatment of a quetiapine and sertraline overdose with Intralipid. Anaesthesia. 2009;64:191-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 133. | Weinberg G, Di Gregorio G, Hiller D, Hewett A, Sirianni A. Reversal of haloperidol-induced cardiac arrest by using lipid emulsion. Ann Intern Med. 2009;150:737-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 134. | Jakkala-Saibaba R, Morgan PG, Morton GL. Treatment of cocaine overdose with lipid emulsion. Anaesthesia. 2011;66:1168-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 135. | Fettiplace MR, Weinberg G. Past, Present, and Future of Lipid Resuscitation Therapy. JPEN J Parenter Enteral Nutr. 2015;39:72S-83S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 136. | Fettiplace MR, Akpa BS, Ripper R, Zider B, Lang J, Rubinstein I, Weinberg G. Resuscitation with lipid emulsion: dose-dependent recovery from cardiac pharmacotoxicity requires a cardiotonic effect. Anesthesiology. 2014;120:915-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 137. | Fettiplace MR, Lis K, Ripper R, Kowal K, Pichurko A, Vitello D, Rubinstein I, Schwartz D, Akpa BS, Weinberg G. Multi-modal contributions to detoxification of acute pharmacotoxicity by a triglyceride micro-emulsion. J Control Release. 2015;198:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 138. | Avcil M, Ozluer K, Karaman M, Kapci B, Kantekin B. Treatment of Severe Carbamazepine Intoxication With Intravenous Lipid Emulsion Therapy. Pharmacol Clin Toxicol. 2015;3:1052-1055. |
| 139. | Hurley WT, Hanlon P. Lipid emulsion as an antidote at the Washington poison center: use in carbamazepine, flecanide, hydroxychloroquine, bupivacaine and bupropion. Clin Toxicol Sci. 2009;47:729-731. |
| 140. | Karaman K, Türkdoğan KA, Deniz AT, Çanakçı SE. Which is the best in carbamazepine overdose? Clin Case Rep. 2017;5:1612-1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 141. | Robben JH, Dijkman MA. Lipid Therapy for Intoxications. Vet Clin North Am Small Anim Pract. 2017;47:435-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 142. | Lee HM, Archer JR, Dargan PI, Wood DM. What are the adverse effects associated with the combined use of intravenous lipid emulsion and extracorporeal membrane oxygenation in the poisoned patient? Clin Toxicol (Phila). 2015;53:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 143. | Lapus RM. Activated charcoal for pediatric poisonings: the universal antidote? Curr Opin Pediatr. 2007;19:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 144. | Olkkola KT, Neuvonen PJ. Do gastric contents modify antidotal efficacy of oral activated charcoal? Br J Clin Pharmacol. 1984;18:663-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 145. | Neuvonen PJ, Olkkola KT, Alanen T. Effect of ethanol and pH on the adsorption of drugs to activated charcoal: studies in vitro and in man. Acta Pharmacol Toxicol (Copenh). 1984;54:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 146. | ANDERSEN AH. Experimental studies on the pharmacology of activated charcoal; the effect of pH on the adsorption by charcoal from aqueous solutions. Acta Pharmacol Toxicol (Copenh). 1947;3:119-218. [PubMed] |
| 147. | ANDERSEN AH. Experimental studies on the pharmacology of activated charcoal; adsorption power of charcoal in aqueous solutions. Acta Pharmacol Toxicol (Copenh). 1946;2:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 148. | Gil HW, Kim SJ, Yang JO, Lee EY, Hong SY. Clinical outcome of hemoperfusion in poisoned patients. Blood Purif. 2010;30:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 149. | Sigwalt F, Bouteleux A, Dambricourt F, Asselborn T, Moriceau F, Rimmelé T. Clinical Complications of Continuous Renal Replacement Therapy. Contrib Nephrol. 2018;194:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 150. | Fernández-Zarzoso M, Gómez-Seguí I, de la Rubia J. Therapeutic plasma exchange: Review of current indications. Transfus Apher Sci. 2019;58:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 151. | Qin Z, Wang X, Wang W. Malnutrition and Venous Thromboembolism are Specific Issues in Elderly Patients with Coronavirus Disease 2019. J Transl Int Med. 2021;9:223-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |