Published online Oct 19, 2023. doi: 10.5498/wjp.v13.i10.714
Peer-review started: August 16, 2023
First decision: August 31, 2023
Revised: September 8, 2023
Accepted: September 22, 2023
Article in press: September 22, 2023
Published online: October 19, 2023
Processing time: 56 Days and 19.3 Hours
Cognitive dysfunction in epileptic patients is a high-incidence complication. Its mechanism is related to nervous system damage during seizures, but there is no effective diagnostic biomarker. Neuronal pentraxin 2 (NPTX2) is thought to play a vital role in neurotransmission and the maintenance of synaptic plasticity. This study explored how serum NPTX2 and electroencephalogram (EEG) slow wave/
To determine if serum NPTX2 could serve as a potential biomarker for diagnosing cognitive impairment in epilepsy patients.
The participants of this study, conducted from January 2020 to December 2021, comprised 74 epilepsy patients with normal cognitive function (normal group), 37 epilepsy patients with cognitive dysfunction [epilepsy patients with cognitive dysfunction (ECD) group] and 30 healthy people (control group). The mini-mental state examination (MMSE) scale was used to evaluate cognitive function. We determined serum NPTX2 levels using an enzyme-linked immunosorbent kit and calculated the signal value of EEG regions according to the EEG recording. Pearson correlation coefficient was used to analyze the correlation between serum NPTX2 and the MMSE score.
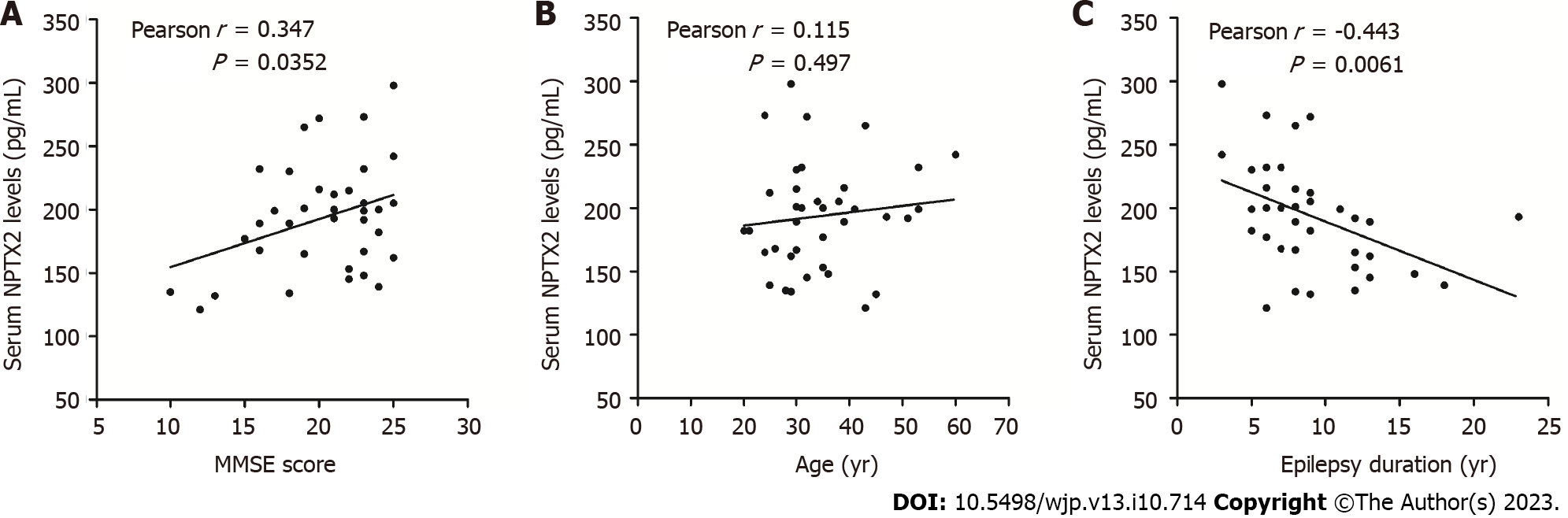
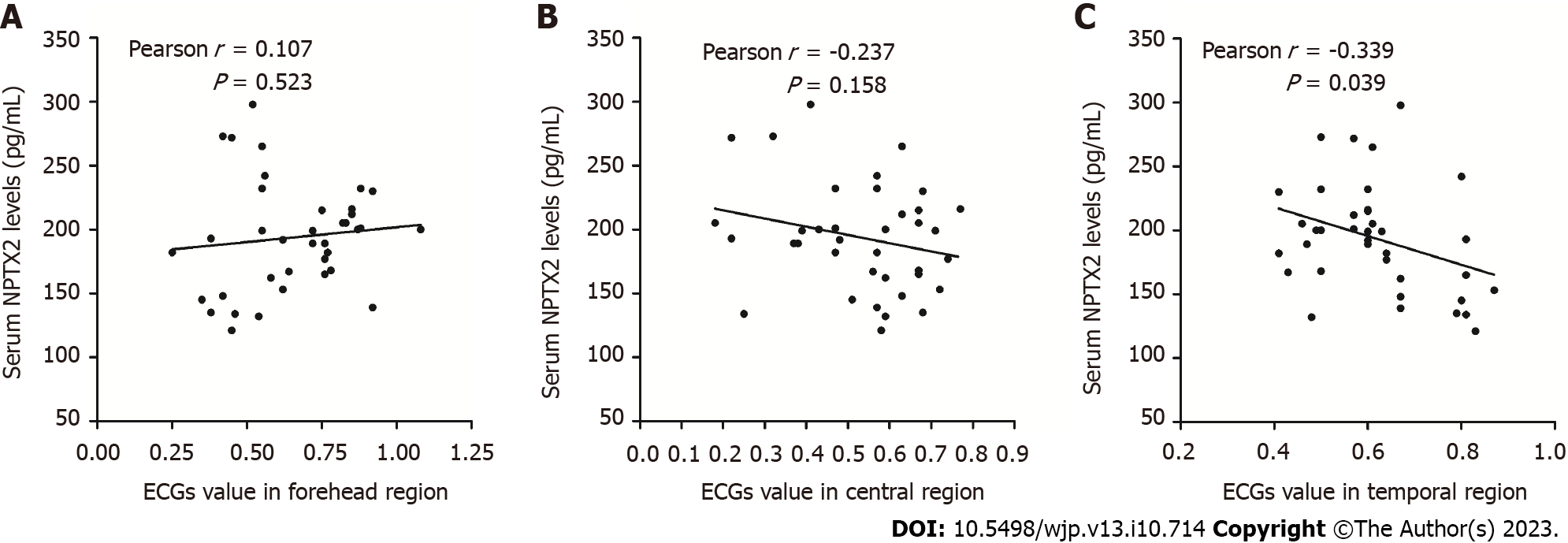
The serum NPTX2 level in the control group, normal group and ECD group were 240.00 ± 35.06 pg/mL, 235.80 ± 38.01 pg/mL and 193.80 ± 42.72 pg/mL, respectively. The MMSE score was lowest in the ECD group among the three, while no significant difference was observed between the control and normal groups. In epilepsy patients with cognitive dysfunction, NPTX2 level had a positive correlation with the MMSE score (r = 0.367, P = 0.0253) and a negative correlation with epilepsy duration (r = −0.443, P = 0.0061) and the EEG slow wave/fast wave frequency ratio value in the temporal region (r = −0.339, P = 0.039).
Serum NPTX2 was found to be related to cognitive dysfunction and the EEG slow wave/fast wave frequency ratio in patients with epilepsy. It is thus a potential biomarker for the diagnosis of cognitive impairment in patients with epilepsy.
Core Tip: Here, we found serum neuronal pentraxin 2 (NPTX2) levels were found to be significantly higher in the normal group than in the cognitive dysfunction group. Additionally, NPTX2 levels showed a positive correlation with cognitive function scores and a negative correlation with epilepsy duration and electroencephalogram (EEG) slow wave/fast wave frequency ratio values in the temporal region. Serum NPTX2 level and the EEG slow wave/fast wave frequency ratio value had good sensitivity and specificity for evaluating cognitive dysfunction. These findings suggest that serum NPTX2 could be a valuable biomarker for diagnosing cognitive impairment in patients with epilepsy, providing important insights for clinical practice.
- Citation: Huang XF, Xu MX, Chen YF, Lin YQ, Lin YX, Wang F. Serum neuronal pentraxin 2 is related to cognitive dysfunction and electroencephalogram slow wave/fast wave frequency ratio in epilepsy. World J Psychiatry 2023; 13(10): 714-723
- URL: https://www.wjgnet.com/2220-3206/full/v13/i10/714.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i10.714
Epilepsy is a common neurological disorder characterized by abnormal synchronous firing of neurons in the brain[1]. The causes of epilepsy are sometimes known and sometimes unknown (idiopathic epilepsy). Epidemiological studies have shown that approximately 70% of adults with epilepsy have cognitive dysfunction[2,3]. However, there are no effective cognitive assessment criteria for patients with epilepsy. Cognitive dysfunction in epilepsy patients occurs on multiple levels, including executive ability, attention, language, and memory ability. Various epilepsy-related factors are closely related to cognitive dysfunction, including epilepsy course, lesion location, underlying neuropathology, and antiepileptic seizure drugs[2]. Cognitive impairment is common in epileptic patients and is characterized by impairment in neurological function. Due to the lack of early diagnostic methods for cognitive impairment in epilepsy patients, unavoidable neurological damage is present in epilepsy patients[3,4].
Neuronal pentraxin 2 (NPTX2), also named “neuronal activity-regulated pentraxin”, is first find in in 1995[5]. NPTX2 is being found to take part in neurotransmission, the maintenance of synaptic plasticity, and the formation of excitatory synapses at presynaptic and postsynaptic sites[6,7]. NPTX2 is also being found to take part in Parkinson’s disease[8,9], ischemic diseases[10,11], and Alzheimer’s disease[12,13]. A recent study reported that NPTX2 was a novel biomarker for Alzheimer’s disease[14]. In addition, researchers have explored the role of NPTX2 in vascular dementia. A recent study reported significantly higher serum levels of NPTX2 in patients with vascular dementia than in healthy controls. Fur
This study evaluated NPTX2 serum levels in epilepsy patients, and future study their relationship with the patients’ cognitive function.
This study enrolled 111 patients diagnosed with epilepsy at the First Affiliated Hospital of Fujian Medical University between January 2020 and December 2021. In addition, 30 healthy volunteers were recruited as a control group. The inclusion criteria were as follows: (1) Clinically confirmed epilepsy [electroencephalogram (EEG) with or without epileptiform discharge]; (2) No seizures 24 h before enrollment; (3) Age between 18 and 60 years; (4) Clear consciousness and cooperative during the examinations; (5) Normal vision, hearing, and speech functions; (6) An education level of at least primary school and an ability to understand the scale content sufficiently to answer the questions; (7) Signed an informed consent form; and (8) Asymptomatic epilepsy (head computed tomography or magnetic resonance imaging does not show intracranial lesions). The exclusion criteria were as follows: (1) Liver dysfunction (alanine transaminase or aspartate aminotransferase > 50 U/L) or renal impairment (serum creatinine > 135 µmol/L); (2) In the acute stage of the disease course; (3) A long-term history of alcoholism or psychoactive substance abuse or recent use of drugs that could affect cognitive function such as antidepressants, antipsychotics, baclofen, and benzodiazepines; or (4) Uncooperative behavior. The study was conducted in accordance with the Declaration of Helsinki (revised in 2013) and was approved by the ethics board of the First Affiliated Hospital of Fujian Medical University (No. [2019]274). Informed consent was obtained from all participants.
The mini-mental state examination (MMSE) scale was used to evaluate cognitive function in all participants. The MMSE scale consists of 30 questions related to cognitive function; each correct answer receives one point. An MMSE scale score of less than 27 indicates cognitive dysfunction in persons with at least a junior high school education[16].
Fasting venous blood was drawn from all participants. The peripheral blood was centrifuged at room temperature to obtain serum, which was then frozen in liquid nitrogen, awaiting further tests. Serum NPTX2 was detected using a Human Neuronal pentraxin-2 (NPTX2) enzyme-linked immunosorbent assay kit (CSB-EL016030HU; CUSABIO, Houston, TX, United States).
All patients with epilepsy were subjected to an EEG test. The test was conducted in a quiet room, and the patients were told to relax and stay awake with closed eyes. The patients were also subjected to induction tests such as opening their eyes and hyperventilation. The EEG detection parameter settings were as follows: Filter channel 0.5–30 Hz, time constant 0.3, paper feed speed 3 cm/s, gain 100 µV = l cm, and scalp resistance of each electrode not exceeding 5,000 Ω. After selecting monopolar lead tracing for 1 min and once the EEG signal was stable, the EEG signal sampling without artifacts and representing EEG background activity was selected. Each patient took 8 s for one sampling unit, with 10 sampling units selected intermittently. The EEG slow wave/fast wave frequency ratio (EEGs value) was calculated using the fast Fourier transform method: EEGs value = (δ+θ)/(α1+α2+β1+β2). δ (1.0–3.9 Hz), θ (4.0–7.9 Hz), α1 (8.0–10.0 Hz), α2 (10.1–13.9 Hz), β1 (14.0–19.9 Hz), and β2 (20.0–30.0 Hz).
Data were recorded in an Excel sheet and were analyzed using SPSS 25.0 (IBM, Corp., Armonk, NY, United States). Count data were expressed as percentages, while continuous data were expressed as mean ± SD. The Kolmogorov–Smirnov test was used to test whether the quantitative data were normally distributed. Normally distributed data were presented as (mean ± SD), and differences between groups were analyzed using unpaired Student's t-test. Non-normally distributed quantitative data were presented as the median (interquartile range), and differences between groups were analyzed using the Mann–Whitney U-test. The Pearson correlation coefficient was used to analyze the correlation between two variables of measurement data. Furthermore, the receiver operating characteristic curves were constructed, and the area under the curve (AUC) was calculated to assess the performance of NPTX2 serum levels and EEGs values in diagnosing cognitive dysfunction in patients with epilepsy. A P value < 0.05 was considered statistically significant.
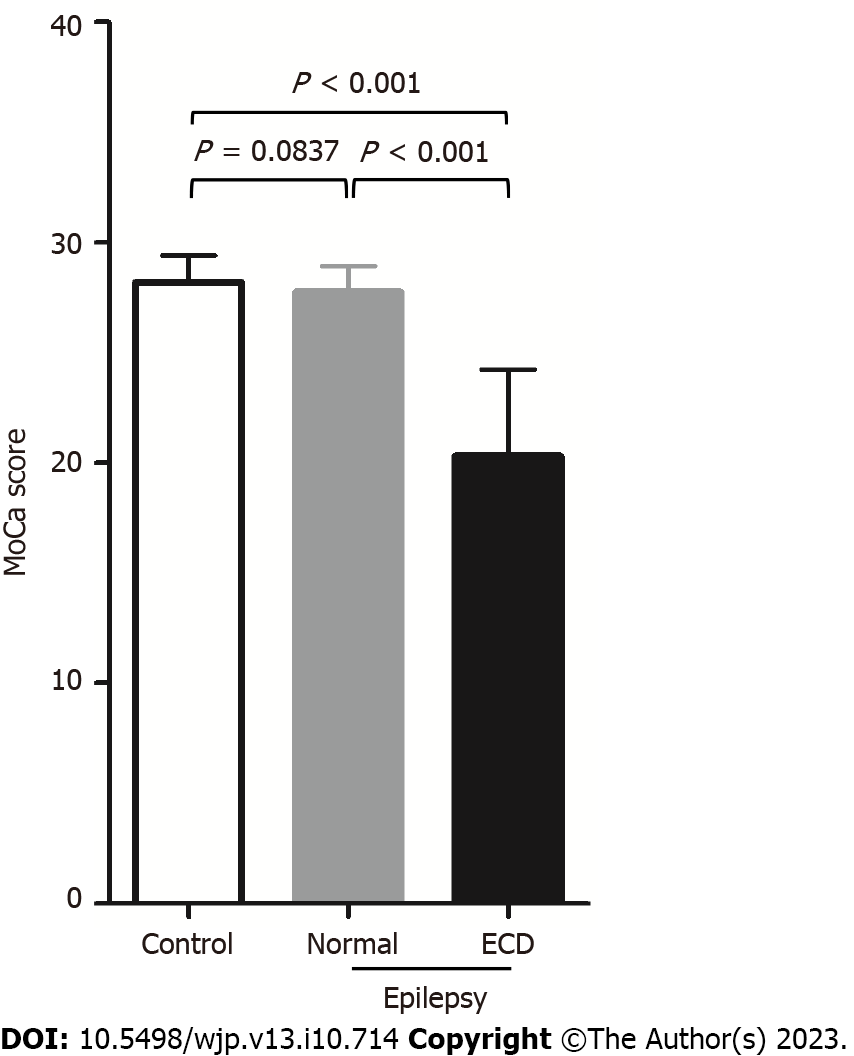
Cognitive function was assessed using the MMSE scale. The epilepsy patients with cognitive dysfunction (ECD) group recorded the lowest MMSE score among the three groups. Furthermore, no significant difference in MMSE scores was observed between the control and normal groups (P > 0.05, Figure 1). In addition, the age, gender, education and something related to epilepsy between normal group and ECD group are omparable (P > 0.05, Table 1).
| Variable | Control (n = 30) | Epilepsy | P value | ||
| Normal (n = 74) | ECD (n = 37) | P1 | P2 | ||
| Age (yr, mean ± SD) | 33.93 ± 9.39 | 35.27 ± 9.73 | 34.68 ± 9.50 | 0.523 | 0.760 |
| Gender, n (%) | |||||
| Male | 17 | 48 (64.86) | 20 (54.05) | 0.434 | 0.270 |
| Female | 13 | 26 (35.14) | 17 (45.95) | ||
| Education level, n (%) | |||||
| Junior/senior high school | 19 | 46 (62.16) | 20 (54.05) | 0.911 | 0.412 |
| University or above | 11 | 28 (27.84) | 17 (45.95) | ||
| Epilepsy onset age (yr, mean ± SD) | – | 26.15 ± 7.41 | 25.62 ± 10.52 | – | 0.760 |
| Epilepsy duration (yr, mean ± SD) | – | 9.12 ± 5.60 | 9.05 ± 4.10 | 0.948 | |
| Epilepsy type, n (%) | |||||
| Focal | – | 20 (27.03) | 9 (24.32) | – | 0.760 |
| Overall | – | 54 (72.97) | 28 (75.68) | ||
| Types of epilepsy drugs, n (%) | |||||
| 0–1 | – | 57 (77.03) | 26 (70.27) | – | 0.440 |
| 2–3 | – | 17 (22.97) | 11 (29.73) | ||
| Epilepsy treatment protocol, n (%) | |||||
| VPN | – | 39 (52.70) | 19 (51.35) | – | 0.890 |
| No-VPN | – | 35 (47.30) | 18 (48.65) | ||
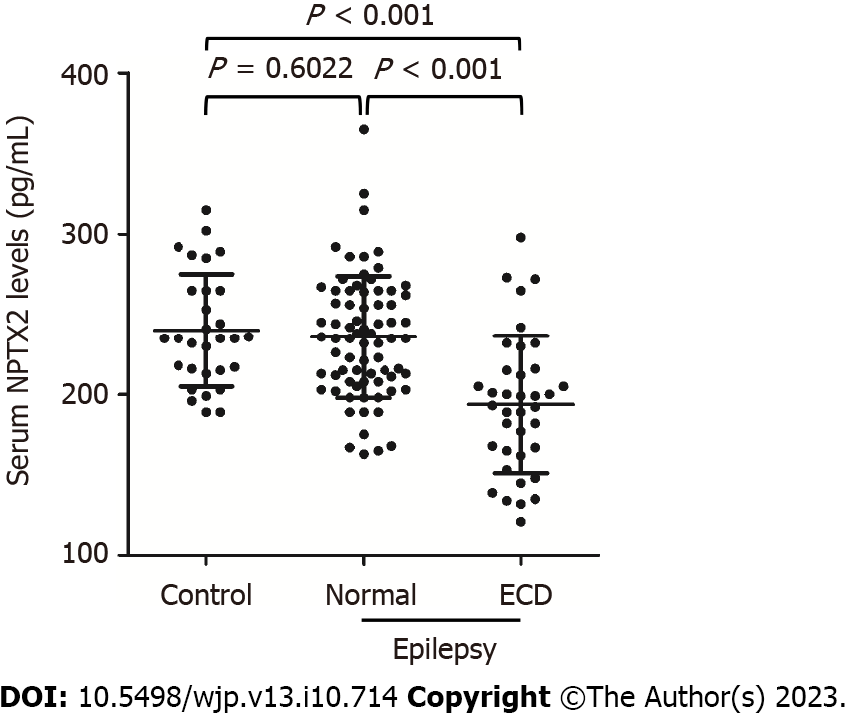
No statistically significant difference was observed in NPTX2 serum levels between the control and normal groups (P > 0.05). However, serum NPTX2 levels in normal group were significantly higher than that in the ECD group (P < 0.05, Figure 2). The serum level of NPTX2 was positively related to MMSE score (r = 0.367, P = 0.0253), not to age (r = 0.115, P = 0.497), and negatively related to epilepsy duration (r = -0.443, P = 0.0061, Figure 3) in the ECD group. In addition, no significant differences were found in gender, education level, epilepsy type, epilepsy drug types, or treatment protocol between the ECD and normal groups (Figure 4).
Patients in the normal and ECD groups showed different EEG slow wave/fast wave frequency ratios (EEG value). Patients in the normal group recorded lower EEGs values in the frontal, central, top, temporal, and occipital regions than patients in the ECD group. However, significant differences in EEG values were observed only in the frontal, central, and temporal regions (P < 0.05, Table 2).
| Area of ECG | Epilepsy | t | P | |
| Normal (n = 74) | ECD (n = 37) | |||
| Frontal region | 0.54 ± 0.20 | 0.65 ± 0.20 | 2.781 | 0.006 |
| Central region | 0.45 ± 0.18 | 0.53 ± 0.16 | 2.441 | 0.016 |
| Top region | 0.39 ± 0.21 | 0.45 ± 0.16 | 1.461 | 0.147 |
| Temporal region | 0.39 ± 0.17 | 0.62 ± 0.13 | 7.127 | < 0.001 |
| Occipital region | 0.28 ± 0.13 | 0.34 ± 0.19 | 1.827 | 0.070 |
The correlation analysis showed that NPTX2 serum levels in the group were not correlated with EEG values in the frontal or central region (P > 0.05). However, NPTX2 serum levels in the group were negatively correlated with the EEG values in the temporal region (P < 0.05, Figure 5).
The AUC value of NPTX2 for diagnosing cognitive impairment in epilepsy patients is 0.777, and the 95%confidence interval (95%CI) is 0.679-0.876 (Figure 6). When the cutoff NPTX2 serum level for distinguishing cognitive function in patients with epilepsy was 206.50 pg/mL, the sensitivity and the specificity was 91.89% and 85.14%, respectively (Figure 6).
Moreover, the AUC value of electrocardiogram (ECG) for diagnosing cognitive impairment in epilepsy patients is 0.815, and the 95%CI is 0.739–0.892 (Figure 6). When the cutoff EEG value for distinguishing cognitive function in patients with epilepsy in the temporal region was set at 0.455, the sensitivity and the specificity was 91.89% and 68.92%, respectively (Figure 6).
Epilepsy is a disease that damages the nervous system, causing damage to the patient's nervous system and subsequently leading to cognitive impairment[17,18]. Epilepsy patients with cognitive impairment are affected in various aspects of their lives. However, it can be confirmed that if diagnosed in the early stages of neu
In this study, we first found that the serum NPTX2 in epilepsy were strongly higher than that in healthy people, and is related to the cognitive function score of epilepsy patients. However, previous studies have shown that NPTX2 is associated with neurological damage, such as significantly accelerating the onset time of Parkinson's mice by upregulating NPTX2 blood levels, and NPTX2 has been identified as associated with neurological damage in Parkinson's patients. In addition, previous studies have also found that the level of NPTX2 in the hippocampus of mice decreases due to cognitive decline caused by neuropathic pain, and it is related to cognitive function in mice after cerebral ischemia[19].
In 1995, NPTX2, a secreted protein, was first discovered[20]. Subsequently, research on NPTX2 was reported, and its main function was mainly studied in synapses, indicating that NPTX2 plays an important role in the nervous system[21,22]. This function makes NPTX2 associated with the occurrence and development of many neurological diseases, such as stroke, Huntington's disease, and Amyotropic lateral sclerosis[23]. The development and damage of the nervous system are closely related to cognitive function, especially in the hippocampus, and has been reported to have significant expression levels in the cerebrospinal fluid of Alzheimer's disease patients, and it is related to the patient's cognitive function score[24]. In this study, we not only found that the levels of serum NPTX2 in ECD group were lowest, but also found that NPTX2 levels was strongly related to the the patient's cognitive function score. Therefore, these data indicate that NPTX2 is associated with nerve injury and cognitive impairment caused by nerve injury, making it a potential biomarker for diagnosing cognitive impairment in epilepsy patients.
EEG is widely used for diagnosis, identification, prognosis evaluation, and treatment efficacy assessment for neurological diseases, including epilepsy[25]. Quantitative EEG transforms the brain wave signals from the time domain in the ordinary EEG into the frequency domain[26]. Due to the inability to effectively control the onset time and pattern of epilepsy patients, it is very difficult for us to detect the EEG during the seizure period of epilepsy patients. Therefore, researchers usually study the EEG during the interval between seizures[27]. Therefore, we selected EEG in the background of the interseizure period. This study found lower EEG values in the frontal, central, top, temporal, and occipital regions of patients with epilepsy and normal cognitive function than in those with epilepsy and cognitive dysfunction. Importantly, this study also found that NPTX2 serum levels were negatively correlated with EEG values in the temporal region of patients with epilepsy and cognitive dysfunction. These findings suggest that NPTX2 serum levels and temporal region's EEG values is related to cognitive impairment in epilepsy patients.
This study has several limitations. First, this study was conducted in a single center, and had a low sample size. Second, we did not monitor NPTX2 serum levels dynamically. Third, several factors that could affect NPTX2 serum levels—such as smoking, alcohol use, and drug use history—were not considered. Finally, our patient follow-up period was short.
In this study, we found that NPTX2 levels in epilepsy patients were lower than those in the healthy population and were associated with cognitive function scores, seizure duration, and ECG values in epilepsy patients. All in all, these results indicate that serum NPTX2 levels are potential biomarkers for diagnosing cognitive dysfunction in epilepsy patients.
Cognitive dysfunction is a common complication in epileptic patients, but there is a lack of effective diagnostic biomarkers. This study investigated the relationship between serum levels of neuronal pentraxin 2 (NPTX2), an important molecule involved in neurotransmission and synaptic plasticity, and cognitive dysfunction in epilepsy patients. The study also explored the association between electroencephalogram (EEG) slow wave/fast wave frequency ratio and cognitive impairment. The aim was to determine if serum NPTX2 could serve as a potential biomarker for diagnosing cognitive impairment in epilepsy patients, addressing the need for reliable diagnostic tools in this population.
The high incidence of cognitive dysfunction in epileptic patients highlights the need for effective diagnostic biomarkers. Currently, there is a lack of reliable tools to identify cognitive impairment in this population. This study aimed to investigate the correlation between serum NPTX2 levels and EEG slow wave/fast wave frequency ratios with cognitive dysfunction in epilepsy patients. By exploring these potential biomarkers, the study aimed to contribute to the deve
The main objectives of this study were to investigate the relationship between serum levels of NPTX2 and EEG with cognitive dysfunction in epilepsy patients. The study aimed to determine if serum NPTX2 could serve as a potential biomarker for diagnosing cognitive impairment in patients with epilepsy. Additionally, the study aimed to assess the correlation between serum NPTX2 levels, EEG patterns, and cognitive function using the mini-mental state examination (MMSE) scale. The ultimate goal was to contribute to the development of effective diagnostic tools for identifying cognitive impairment in epilepsy patients.
The study enrolled three groups of participants: Normal group, 74 epilepsy patients without cognitive dysfunction; epilepsy patients with cognitive dysfunction group, 37 epilepsy patients with cognitive dysfunction; Control group, 30 healthy individuals. Cognitive function was evaluated using the MMSE scale. Serum levels of NPTX2 were measured using an enzyme-linked immunosorbent kit, and EEG recordings were used to calculate the slow wave/fast wave frequency ratio in different EEG regions. Statistical analyses were performed to compare variables among the groups and assess correlations between biomarkers and cognitive function. Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the diagnostic performance of serum NPTX2 and EEG patterns for cognitive dysfunction in epilepsy patients.
The study found no significant differences in age, gender, or education level among the three groups. There were also no significant differences in epilepsy-related factors between the normal group and the cognitive dysfunction group. Serum levels of NPTX2 were significantly higher in the normal group compared to the cognitive dysfunction group, while the control group showed no significant difference from the normal group. The cognitive dysfunction group had the lowest MMSE scores. The EEG slow wave/fast wave frequency ratio values were significantly higher in the cognitive dysfunction group compared to the normal group in various EEG regions. In epilepsy patients with cognitive dys
The study concluded that serum NPTX2 levels are associated with cognitive dysfunction and the EEG slow wave/fast wave frequency ratio in epilepsy patients. Serum NPTX2 shows potential as a diagnostic biomarker for cognitive impairment in epilepsy. The study found no significant differences in demographic and epilepsy-related factors between the normal and cognitive dysfunction groups. However, serum NPTX2 levels were significantly higher in the normal group compared to the cognitive dysfunction group. The EEG slow wave/fast wave frequency ratios were also higher in the cognitive dysfunction group. These findings suggest that serum NPTX2 and EEG patterns may serve as valuable indicators for diagnosing cognitive impairment in epilepsy patients.
The findings of this study highlight the potential of serum NPTX2 as a diagnostic biomarker for cognitive impairment in epilepsy patients. Further research is needed to validate and expand upon these results. Future studies could explore the underlying mechanisms linking NPTX2 levels and cognitive dysfunction, investigating the role of NPTX2 in neurotransmission and synaptic plasticity. Additionally, larger sample sizes and longitudinal studies could provide more robust evidence regarding the relationship between serum NPTX2, EEG patterns, and cognitive dysfunction. Ultimately, establishing reliable biomarkers could aid in early detection and intervention for cognitive impairments in epilepsy, improving patient outcomes and quality of life.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cicero N, Italy; Karakose T, Turkey S-Editor: Lin C L-Editor: A P-Editor: Wu RR
| 1. | Falco-Walter J. Epilepsy-Definition, Classification, Pathophysiology, and Epidemiology. Semin Neurol. 2020;40:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 2. | Helmstaedter C, Witt JA. Epilepsy and cognition - A bidirectional relationship? Seizure. 2017;49:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 3. | Kanner AM, Helmstaedter C, Sadat-Hossieny Z, Meador K. Cognitive disorders in epilepsy I: Clinical experience, real-world evidence and recommendations. Seizure. 2020;83:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Li KY, Huang LC, Chang YP, Yang YH. The effects of lacosamide on cognitive function and psychiatric profiles in patients with epilepsy. Epilepsy Behav. 2020;113:107580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Hsu YC, Perin MS. Human neuronal pentraxin II (NPTX2): conservation, genomic structure, and chromosomal localization. Genomics. 1995;28:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Chang S, Bok P, Tsai CY, Sun CP, Liu H, Deussing JM, Huang GJ. NPTX2 is a key component in the regulation of anxiety. Neuropsychopharmacology. 2018;43:1943-1953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Wang Z, Jin T, Le Q, Liu C, Wang X, Wang F, Ma L. Retrieval-Driven Hippocampal NPTX2 Plasticity Facilitates the Extinction of Cocaine-Associated Context Memory. Biol Psychiatry. 2020;87:979-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Lang Y, Li Y, Yu H, Lin L, Chen X, Wang S, Zhang H. HOTAIR drives autophagy in midbrain dopaminergic neurons in the substantia nigra compacta in a mouse model of Parkinson's disease by elevating NPTX2 via miR-221-3p binding. Aging (Albany NY). 2020;12:7660-7678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Moran LB, Hickey L, Michael GJ, Derkacs M, Christian LM, Kalaitzakis ME, Pearce RK, Graeber MB. Neuronal pentraxin II is highly upregulated in Parkinson's disease and a novel component of Lewy bodies. Acta Neuropathol. 2008;115:471-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Wang R, Man Y, Zhou M, Zhu Y, Wang L, Yang J. Neuropathic pain-induced cognitive dysfunction and down-regulation of neuronal pentraxin 2 in the cortex and hippocampus. Neuroreport. 2021;32:274-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Cai M, Zhu Y, Li Z, Josephs-Spaulding J, Zhou Y, Hu Y, Chen H, Liu Y, He W, Zhang J. Profiling the Gene Expression and DNA Methylation in the Mouse Brain after Ischemic Preconditioning. Neuroscience. 2019;406:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Xiao MF, Xu D, Craig MT, Pelkey KA, Chien CC, Shi Y, Zhang J, Resnick S, Pletnikova O, Salmon D, Brewer J, Edland S, Wegiel J, Tycko B, Savonenko A, Reeves RH, Troncoso JC, McBain CJ, Galasko D, Worley PF. NPTX2 and cognitive dysfunction in Alzheimer's Disease. Elife. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 13. | Belbin O, Xiao MF, Xu D, Carmona-Iragui M, Pegueroles J, Benejam B, Videla L, Fernández S, Barroeta I, Nuñez-Llaves R, Montal V, Vilaplana E, Altuna M, Clarimón J, Alcolea D, Blesa R, Lleó A, Worley PF, Fortea J. Cerebrospinal fluid profile of NPTX2 supports role of Alzheimer's disease-related inhibitory circuit dysfunction in adults with Down syndrome. Mol Neurodegener. 2020;15:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Libiger O, Shaw LM, Watson MH, Nairn AC, Umaña KL, Biarnes MC, Canet-Avilés RM, Jack CR Jr, Breton YA, Cortes L, Chelsky D, Spellman DS, Baker SA, Raghavan N, Potter WZ; Alzheimer's Disease Neuroimaging Initiative (ADNI); Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium, Longitudinal CSF Proteomics Project Team. Longitudinal CSF proteomics identifies NPTX2 as a prognostic biomarker of Alzheimer's disease. Alzheimers Dement. 2021;17:1976-1987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 15. | Shao K, Shan S, Ru W, Ma C. Association between serum NPTX2 and cognitive function in patients with vascular dementia. Brain Behav. 2020;10:e01779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Ahn SJ, Kim TJ, Cha KS, Jun JS, Byun JI, Shin YW, Sunwoo JS, Lee S, Yu KS, Jang IJ, Chu K, Lee SK, Jung KY. Effects of perampanel on cognition and quantitative electroencephalography in patients with epilepsy. Epilepsy Behav. 2021;115:107514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Zhu L, Chen L, Xu P, Lu D, Dai S, Zhong L, Han Y, Zhang M, Xiao B, Chang L, Wu Q. Genetic and molecular basis of epilepsy-related cognitive dysfunction. Epilepsy Behav. 2020;104:106848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Sen A, Capelli V, Husain M. Cognition and dementia in older patients with epilepsy. Brain. 2018;141:1592-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 195] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 19. | Lim B, Tsolaki M, Soosaipillai A, Brown M, Zilakaki M, Tagaraki F, Fotiou D, Koutsouraki E, Grosi E, Prassas I, Diamandis EP. Liquid biopsy of cerebrospinal fluid identifies neuronal pentraxin receptor (NPTXR) as a biomarker of progression of Alzheimer's disease. Clin Chem Lab Med. 2019;57:1875-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Lim B, Sando SB, Grøntvedt GR, Bråthen G, Diamandis EP. Cerebrospinal fluid neuronal pentraxin receptor as a biomarker of long-term progression of Alzheimer's disease: a 24-month follow-up study. Neurobiol Aging. 2020;93:97.e1-97.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Xiao MF, Roh SE, Zhou J, Chien CC, Lucey BP, Craig MT, Hayes LN, Coughlin JM, Leweke FM, Jia M, Xu D, Zhou W, Conover Talbot C Jr, Arnold DB, Staley M, Jiang C, Reti IM, Sawa A, Pelkey KA, McBain CJ, Savonenko A, Worley PF. A biomarker-authenticated model of schizophrenia implicating NPTX2 Loss of function. Sci Adv. 2021;7:eabf6935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Swanson A, Willette AA; Alzheimer’s Disease Neuroimaging Initiative. Neuronal Pentraxin 2 predicts medial temporal atrophy and memory decline across the Alzheimer's disease spectrum. Brain Behav Immun. 2016;58:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Feyissa AM, Tatum WO. Adult EEG. Handb Clin Neurol. 2019;160:103-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Benbadis SR, Beniczky S, Bertram E, MacIver S, Moshé SL. The role of EEG in patients with suspected epilepsy. Epileptic Disord. 2020;22:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 25. | Sansevere AJ, Hahn CD, Abend NS. Conventional and quantitative EEG in status epilepticus. Seizure. 2019;68:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Admiraal MM, Ramos LA, Delgado Olabarriaga S, Marquering HA, Horn J, van Rootselaar AF. Quantitative analysis of EEG reactivity for neurological prognostication after cardiac arrest. Clin Neurophysiol. 2021;132:2240-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Gao Y, Tang X, Wen Y, Qian D, Pan X, Zhang L. Effects of the hospital-community-family ternary linkage continuous nursing model on compliance, cognitive function, resilience, and quality of life for children with epilepsy: a retrospective study. Transl Pediatr. 2022;11:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |