Published online Sep 19, 2022. doi: 10.5498/wjp.v12.i9.1141
Peer-review started: January 30, 2022
First decision: April 18, 2022
Revised: April 29, 2022
Accepted: August 14, 2022
Article in press: August 14, 2022
Published online: September 19, 2022
Processing time: 232 Days and 16.9 Hours
Understanding neuropsychological mechanisms of mindfulness meditation (MM) has been a hot topic in recent years. This review was conducted with the goal of synthesizing empirical relationships via the genomics, circuits and networks between MM and mental disorders. We describe progress made in assessing the effects of MM on gene expression in immune cells, with particular focus on stress-related inflammatory markers and associated biological pathways. We then focus on key brain circuits associated with mindfulness practices and effects on symptoms of mental disorders, and expand our discussion to identify three key brain networks associated with mindfulness practices including default mode network, central executive network, and salience network. More research efforts need to be devoted into identifying underlying neuropsychological mechanisms of MM on how it alleviates the symptoms of mental disorders.
Core Tip: Recently, understanding neuropsychological mechanisms of mindfulness meditation (MM) has been a hot topic. We describe progress made in assessing the effects of MM on gene expression in inflammatory processes, with particular focus on stress-related inflammatory markers and associated biological pathways. We then discuss primary brain circuits related to MM and effects on symptoms of mental disorders, and three brain networks associated with MM including default mode network, central executive network, and salience network. More research examining MM effects and outcomes at the potential molecular mechanisms, critical genes and the network level is necessary.
- Citation: Gu YQ, Zhu Y. Underlying mechanisms of mindfulness meditation: Genomics, circuits, and networks. World J Psychiatry 2022; 12(9): 1141-1149
- URL: https://www.wjgnet.com/2220-3206/full/v12/i9/1141.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i9.1141
Mindfulness meditation (MM) refers to a conscious, non-judgmental way of concentrating on the present[1-3], which has originated from a systematically Buddhist notion 2550 years ago[4]. It is an instant and tranquil mental state with observing all mental contents (including virtually sensations, perceptions, cognitions and feelings) at any given moment[5,6]. MM was first introduced into the mainstream medical practices by Dr. Kabat-Zinn[7] of the Massachusetts Medical School in 1982. MM developing strategies include sustained attention training, somatic and non-judgmental awareness, emotion control, detaching from a self-centered view and acceptance of the “here-and-now”[8-10]. The great majority of MM research is about clinical practices[11], especially in mental disorders such as anxiety disorder, major depressive disorder, attention-deficit/hyperactivity disorder, obsessive-compulsive disorder, eating disorder and substance abuse[12-16].
In recent years, there has been a burgeoning interest in underlying mechanisms of MM, mainly due to increasing evidence of its positive effects on mental disorders and physical well-being. In parallel to research evaluating the effectiveness of these MM approaches, a second line of investigation focuses on unraveling the neurophysiological and psychological processes involved[17]. Recent functional and structural neuroimaging studies are beginning to provide evidence that diverse brain areas have been congruously found in both beginners undergoing temporary practice and experienced meditators[18,19]. These areas have been determined to specialize in some of these critical functions[20]. However, many of these neural areas or correlates are much more complicated and the so-called “networks or neural circuits” are likely to perform higher-level processes and multiple mental functions[21].
Understanding neuropsychological mechanisms of MM has been a hot topic in recent years. This review was conducted with the goal of synthesizing empirical relationships via the genomics, circuits and networks between MM and mental disorders. We describe progress made in assessing the effects of MM on gene expression in immune cells, with particular focus on stress-related inflammatory markers and associated biological pathways. We then discuss key brain circuits related to MM and effects on symptoms of mental disorders, and three brain networks associated with MM including default mode network (DMN), central executive network (CEN), and salience network. More research examining MM effects and outcomes at the potential molecular mechanisms, critical genes and the network level is necessary.
Genetic studies of MM showed that differential transcription occurs in genes involved in DNA damage response, oxidative stress, and inflammatory metabolism processes, in both short and long-term practitioners[22-24]. In most studies, these results were correlated with reduced stress and fatigue, improved immune response, and clinical symptoms. A few studies examined neurotrophins[25,26]. Trans-criptomic analyses were performed in both healthy and clinical populations combining diverse MM activities in several longitudinal and mixed design studies and obtained similar results[24,27-29].
Creswell and colleagues reported NF-κB-related gene expression in older adults responding to the Mindfulness-Based Stress Reduction (MBSR) intervention compared to a wait-list control group, who in contrast, showed the gene to be up-regulated[30,31]. Bakker et al[32] showed that genetic variation in muscarinic acetylcholine receptor M2 (CHRM2) and the μ1 opioid receptor (OPRM1) moderate the positive impact on the level of positive affect following mindfulness-based cognitive therapy (MBCT) with depressive symptoms, and proposed that variation in genetic factors in response to MBCT may be contingent on the association with the regulation of positive affect[32].
In the study by Dada et al[33], intraocular pressure in primary open angle glaucoma appeared significantly decreased after MM. Significant upregulation of the anti-inflammatory genes and downregulation of the proinflammatory genes were found in glaucoma patients who underwent a 3-wk MM course. These results indicate that MM has a direct impact on trabecular meshwork gene expression in ocular tissues. Similarly, the practice of MM was shown to improve immune function by normalizing stress-related serum biomarkers, and positively modifying gene expression[25]. Moreover, increased blood levels of brain-derived neurotrophic factor indicated a positive impact on retinal ganglion cells rescue from death in patients with primary open angle glaucoma[26].
Genome-wide approaches to gene activity have started to elucidate the effects of MM on gene modulation[34]. For example, utilizing microarray analysis of global mRNAs to study the methylome of peripheral blood mononuclear cells of 17 experienced meditators of one-day intensive MM practice, found 61 differentially methylated regions[35]. Similarly, studying the transcriptomic effects in six individuals after twice-daily transcendental MM practice revealed 200 genes differentially expressed[24]. Studies focusing on the impact of MM for treating hypertension, irritable bowel syndrome and inflammatory bowel disease showed that several genes related to fundamental pathways were differentially expressed[27,28].
Nevertheless, most previous studies were cross-sectional studies with small sample sizes[22,26,36,37]. The large-scale genomic study, by Chandran et al[38], analyzed the meditation-specific core network of advanced MM practice, rather than changes in the expression of a few individual genes. They observed that the up-regulated RNA coexpression networks are directly related to the immune response, including 68 genes differentially expressed after MM. Interestingly, these authors reported that the top 10 hub genes in the up-regulated module included many previously identified genes known to regulate the immune system and related to the type I interferon signaling pathway. They identified nine coexpression and protein–protein interaction networks associated with MM using a multistage approach. This suggests that MM, as a behavioral intervention, may be an effective component in treating diseases characterized by increased inflammatory responsiveness with a weakened immune system.
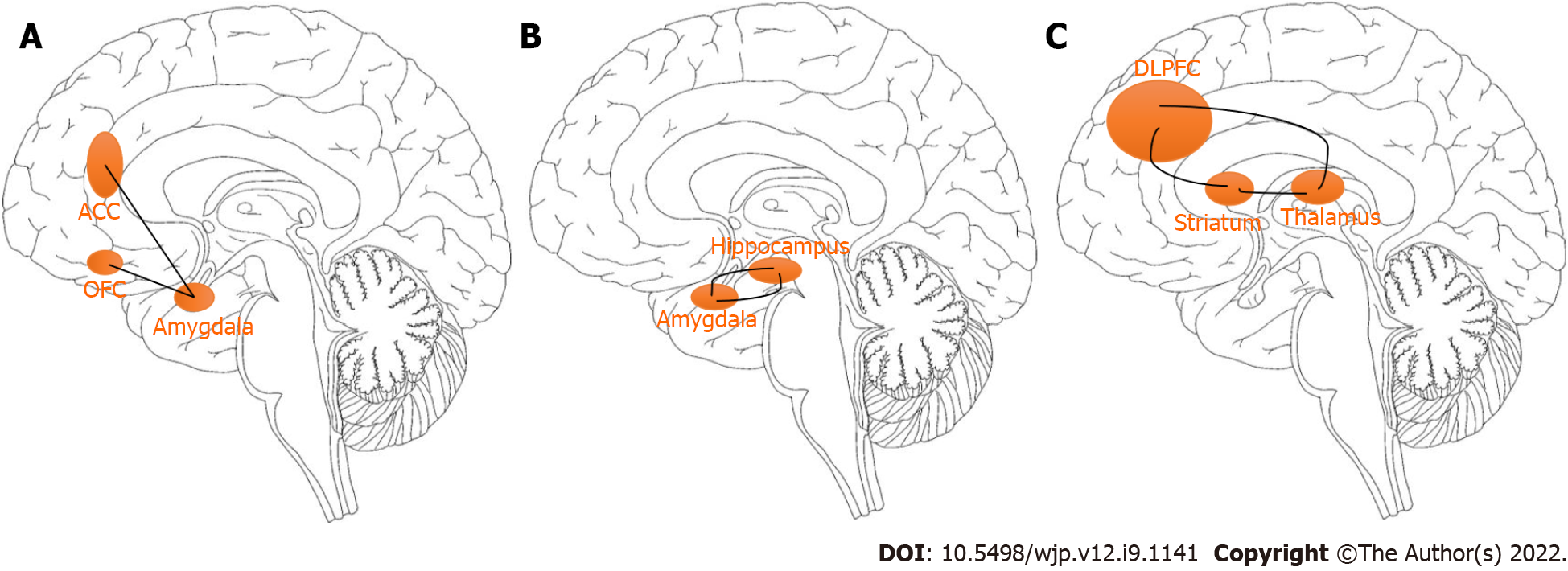
The connections between the amygdala and key areas of the prefrontal cortex, specifically the anterior cingulate cortex (ACC) and orbitofrontal cortex can regulate the feelings of fear (Figure 1A). Specifically, the overactivation of these circuits may lead to feelings of fear. King et al[39] examined the neurobiological effects of 16-week mindfulness-based exposure therapy (MBET) compared with present-centered group therapy in task-evoked functional connectivity of combat veterans with posttraumatic stress disorder (PTSD). The MBET group showed higher neural activation in the rostral ACC, dorsal medial prefrontal cortex (mPFC), and left amygdala that were significantly associated with improvement in PTSD symptoms. The interactive results of group and time showed that MBET increased responses of the left medial PFC related to fearful faces, and greater post-therapy effects on the fusiform/lingual gyrus and amygdala to angry faces, suggesting that MM practices may be related to greater involvement in threat cues of patients with PTSD. It also found that MBET was associated with increased activation of the lingual/fusiform gyrus and amygdala to angry faces. It was proved that mindfulness-based art therapy is associated with significant changes in cerebral blood flow, including the insula, amygdala, hippocampus, and caudate nucleus, which is associated with a period of reduced anxiety within 8 wk[40]. These brain structures are involved in MM tasks and emotional processing related to anxiety[41-43].
Hoge and colleagues provide some support that MM could mitigate the elevated response to acute stress observed in generalized anxiety disorder on the hypothalamic pituitary adrenal (HPA) axis, by measuring blood levels of cortisol and adrenocorticotropic hormone (ACTH) with treatment. Over the course of the treatment, participants in the MM group exhibited a reduction in their ACTH Area-Under-the-Curve concentrations[44]. Similarly, Pace et al[45] demonstrated that healthy participants who practiced more MM had a faster drop in cortisol after the Trier Social Stress Test than healthy participants who practiced MM less frequently[45]. The physiological reaction to a fearful stimulus involves activation of multiple systems, including the autonomic nervous system, respiratory system, and endocrine system[46,47]. Part of the characteristic of the fear response may be endocrine influence[48]. The HPA axis is responsible for endocrine output during the stress/fear response, and is regulated by the amygdala via reciprocal connections with the hypothalamus[49-51].
Activation of the autonomic system is regulated by connections between the amygdala, the locus coeruleus, and parabrachial nucleus and leads to an increase in heart rate, respiration rate and blood pressure that is necessary for a fight/flight reaction[52,53]. Several studies have consistently found an association between cardio-respiratory parameters and MM related to slow paced breathing[54]. Park and Park[55], and Stark et al[56] found an increase in the high frequency power paralleled during paced breathing of MM at 10 b/min as compared to spontaneous breathing. Generally, slow breathing techniques (such as MM exercises) enhance interactions between autonomic nerves, cerebral, and mental flexibility, linking parasympathetic and central nervous system activities with emotional control and well-being. Slow breathing techniques seem to promote a predominance of the parasympathetic autonomic system with respect to the sympathetic one, mediated by the vagal activity[57,58].
Sevinc et al[59] investigated potential neural correlates of MM intervention and in extinction learning (the context-dependent recall of extinction) using MBSR training. Group-by-time interactions found that MBET was associated with greater increases in the hippocampus and the supramarginal gyrus during extinction recall. Also during the early phase, the MBSR training group showed increased hippocampal connectivity to the supramarginal gyrus. Increased connectivity between the hippocampus and primary somatosensory cortex during retrieval of extinguished stimuli following MBSR training was also observed[60]. Furthermore, Sevinc et al[61] demonstrated an association between functional changes in the hippocampal connectivity and changes in anxiety following MM training. These findings provide a better understanding of the mechanisms through which MM training relieves anxiety. Anxiety can be triggered not only by an external stimulus but also internally through traumatic memories stored in the hippocampus (Figure 1B), which can activate the amygdala, causing the amygdala, in turn, to activate other brain regions and generate a fear response[46,62]. This is known as re-experiencing and is a central feature of PTSD[63].
King et al[64] studied the potential neural relevance of MBET among combat veterans who suffered from PTSD following deployment to Afghanistan and/or Iraq. MBET showed increased connectivity with the dorsolateral prefrontal cortex (DLPFC) and dorsal ACC following therapy by a group × time interaction; and posterior cingulate cortex (PCC)-DLPFC connectivity was related to improvement of avoidant and hyperarousal symptoms in PTSD. Worry refers to anxious misery, apprehensive expectation, catastrophic thinking, and obsessions (Figure 1C). It is hypothetically related to a cortico-striatal-thalamic-cortical loop originating in the DLPFC and projecting the striatal complex, than the thalamus, and ending in the DLPFC[65,66]. Overactivation of the DLPFC can result in symptoms such as worry or obsessions[67-69].
In identifying the neural mechanism of MM, most inferences have focused on the role of isolated brain areas in supporting the observed cognitive processes and concurrently enhancing behavioral outcomes; however, consisting of key areas that are temporally correlated with one another (a large-scale brain network) must be considered[70]. There are three key functional networks related to attention, cognitive control and interoceptive awareness: DMN, CEN, and salience network according to the former neuroimaging literature on MM[71].
The DMN is associated with task-irrelevant and mind-wandering thoughts[72,73]. Greater activations in core nodes of the PCC, mPFC, and bilateral parietal cortices, lead to introspective thought, including activities such as daydreaming or retrieving memories[74-77]. The CEN, with core nodes located in the bilateral parietal cortices and DLPFCs, is typically associated with increased activation during distractibility and goal-directed behavior[78-80]. The CEN is linked to decision making by converging external information with internal representations[75,81-83]. The salience network is responsible for changing and monitoring the states of the CEN and the DMN, and presumably accepts the distribution of attentional resources to support cognitive control[84].
Based on structural and functional neuroimaging studies, MM is related to the activities and connections in the three networks, each of which is responsible for different stages of MM in experienced practitioners[85-87]. The activity and connectivity of the DMN have been suggested as potential biomarkers for monitoring the effect of MM[88]. It describes that MM may improve DMN, CEN and salience network functions to target symptoms of anxiety disorders[9]. King et al[64] investigated potential neural correlates of MBET in patients with PTSD compared with an active control therapy. After MM training, the connection between the DMN and CEN increase, which may improve the ability to shifting of voluntary attention. There is increased connection between the DMN and the DLPFC areas in CEN before and after MBET.
Currently, few scientific studies have investigated the neural connections of MM at the level of critical genes and brain networks[89-93]. Notably, there has been a shift from isolated areas to large-scale networks, circuits or large-scale genetic changes[38,94,95]. Further research examining MM effects and outcomes at the potential molecular mechanisms, critical genes and the network level is necessary[96,97]. As the knowledge of brain function increases, we can better understand what the neural connections that affect clinical symptoms are. In turn, this will better characterize the specific deficiencies of any particular patient. We can predict that the development of neuroscience research on MM will help strengthen neuronal circuits that are damaged by mental disorders, and help develop personalized interventions for individuals’ unique defects and strengths.
Recently, understanding neuropsychological mechanisms of MM has been a hot topic[98-100]. We describe progress made in assessing the effects of MM on gene expression in inflammatory processes, with particular focus on stress-related inflammatory markers and associated biological pathways. We then discuss primary brain circuits related to MM and effects on symptoms of mental disorders, and expand our discussion to identify three brain networks associated with MM including the DMN, CEN, and salience network. More research examining MM effects and outcomes at the potential molecular mechanisms, critical genes and the network level is necessary.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Menendez-Menendez J, Spain; Tanabe S, Japan S-Editor: Fan JR L-Editor: Webster JR P-Editor: Fan JR
| 1. | Baer, RA. Mindfulness Training as a Clinical Intervention: A Conceptual and Empirical Review. Clin Psychol-Sci Pr. 2003;10:125-143. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1437] [Cited by in RCA: 1445] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 2. | Kabat-zinn J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness, 15th anniversary ed. New York, NY: Delta Trade Paperback/Bantam Dell, 2005. |
| 3. | Kabat-zinn J. Mindfulness-Based Interventions in Context: Past, Present, and Future. Clin Psychol-Sci Pr. 2003;10:144-156. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2119] [Cited by in RCA: 2208] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 4. | Keng SL, Smoski MJ, Robins CJ. Effects of mindfulness on psychological health: a review of empirical studies. Clin Psychol Rev. 2011;31:1041-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1480] [Cited by in RCA: 1067] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 5. | Grossman P. Mindfulness for Psychologists: Paying Kind Attention to the Perceptible. Mindfulness. 2010;1:87-97. [DOI] [Full Text] |
| 6. | Brown KW, Ryan RM, Creswell JD. Mindfulness: Theoretical Foundations and Evidence for its Salutary Effects. Psychol Inq. 2007;18:211-237. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1775] [Cited by in RCA: 1178] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 7. | Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4:33-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2296] [Cited by in RCA: 1838] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 8. | Frank JL, Jennings PA, Greenberg MT. Mindfulness-Based Interventions in School Settings: An Introduction to the Special Issue INTRODUCTION. Res Hum Dev. 2013;10:205-210. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How Does Mindfulness Meditation Work? Perspect Psychol Sci. 2011;6:537-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1475] [Cited by in RCA: 1284] [Article Influence: 128.4] [Reference Citation Analysis (0)] |
| 10. | Krisanaprakornkit T, Ngamjarus C, Witoonchart C, Piyavhatkul N. Meditation therapies for attention-deficit/hyperactivity disorder (ADHD). Cochrane Database Syst Rev. 2010;CD006507. [DOI] [Full Text] |
| 11. | Ivanovski B, Malhi GS. The psychological and neurophysiological concomitants of mindfulness forms of meditation. Acta Neuropsychiatr. 2007;19:76-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Dunne J. Mindfulness in Anorexia Nervosa: An Integrated Review of the Literature. J Am Psychiatr Nurses Assoc. 2018;24:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Gu Y, Xu G, Zhu Y. A Randomized Controlled Trial of Mindfulness-Based Cognitive Therapy for College Students With ADHD. J Atten Disord. 2018;22:388-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Key BL, Rowa K, Bieling P, McCabe R, Pawluk EJ. Mindfulness-based cognitive therapy as an augmentation treatment for obsessive-compulsive disorder. Clin Psychol Psychother. 2017;24:1109-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Priddy SE, Howard MO, Hanley AW, Riquino MR, Friberg-Felsted K, Garland EL. Mindfulness meditation in the treatment of substance use disorders and preventing future relapse: neurocognitive mechanisms and clinical implications. Subst Abuse Rehabil. 2018;9:103-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Williams JM, Crane C, Barnhofer T, Brennan K, Duggan DS, Fennell MJ, Hackmann A, Krusche A, Muse K, Von Rohr IR, Shah D, Crane RS, Eames C, Jones M, Radford S, Silverton S, Sun Y, Weatherley-Jones E, Whitaker CJ, Russell D, Russell IT. Mindfulness-based cognitive therapy for preventing relapse in recurrent depression: a randomized dismantling trial. J Consult Clin Psychol. 2014;82:275-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 17. | Malinowski P. Neural mechanisms of attentional control in mindfulness meditation. Front Neurosci. 2013;7:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 18. | Tang YY, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16:213-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1175] [Cited by in RCA: 1214] [Article Influence: 121.4] [Reference Citation Analysis (0)] |
| 19. | Tang YY, Lu Q, Feng H, Tang R, Posner MI. Short-term meditation increases blood flow in anterior cingulate cortex and insula. Front Psychol. 2015;6:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Zeidan F, Martucci KT, Kraft RA, McHaffie JG, Coghill RC. Neural correlates of mindfulness meditation-related anxiety relief. Soc Cogn Affect Neurosci. 2014;9:751-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Gu S, Pasqualetti F, Cieslak M, Telesford QK, Yu AB, Kahn AE, Medaglia JD, Vettel JM, Miller MB, Grafton ST, Bassett DS. Controllability of structural brain networks. Nat Commun. 2015;6:8414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 470] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 22. | Kaliman P, Alvarez-López MJ, Cosín-Tomás M, Rosenkranz MA, Lutz A, Davidson RJ. Rapid changes in histone deacetylases and inflammatory gene expression in expert meditators. Psychoneuroendocrinology. 2014;40:96-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Chaix R, Alvarez-López MJ, Fagny M, Lemee L, Regnault B, Davidson RJ, Lutz A, Kaliman P. Epigenetic clock analysis in long-term meditators. Psychoneuroendocrinology. 2017;85:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Wenuganen S, Walton KG, Katta S, Dalgard CL, Sukumar G, Starr J, Travis FT, Wallace RK, Morehead P, Lonsdorf NK, Srivastava M, Fagan J. Transcriptomics of Long-Term Meditation Practice: Evidence for Prevention or Reversal of Stress Effects Harmful to Health. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Dada T, Mittal D, Mohanty K, Faiq MA, Bhat MA, Yadav RK, Sihota R, Sidhu T, Velpandian T, Kalaivani M, Pandey RM, Gao Y, Sabel BA, Dada R. Mindfulness Meditation Reduces Intraocular Pressure, Lowers Stress Biomarkers and Modulates Gene Expression in Glaucoma: A Randomized Controlled Trial. J Glaucoma. 2018;27:1061-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 26. | Gagrani M, Faiq MA, Sidhu T, Dada R, Yadav RK, Sihota R, Kochhar KP, Verma R, Dada T. Meditation enhances brain oxygenation, upregulates BDNF and improves quality of life in patients with primary open angle glaucoma: A randomized controlled trial. Restor Neurol Neurosci. 2018;36:741-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Bhasin MK, Denninger JW, Huffman JC, Joseph MG, Niles H, Chad-Friedman E, Goldman R, Buczynski-Kelley B, Mahoney BA, Fricchione GL, Dusek JA, Benson H, Zusman RM, Libermann TA. Specific Transcriptome Changes Associated with Blood Pressure Reduction in Hypertensive Patients After Relaxation Response Training. J Altern Complement Med. 2018;24:486-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Kuo B, Bhasin M, Jacquart J, Scult MA, Slipp L, Riklin EI, Lepoutre V, Comosa N, Norton BA, Dassatti A, Rosenblum J, Thurler AH, Surjanhata BC, Hasheminejad NN, Kagan L, Slawsby E, Rao SR, Macklin EA, Fricchione GL, Benson H, Libermann TA, Korzenik J, Denninger JW. Genomic and clinical effects associated with a relaxation response mind-body intervention in patients with irritable bowel syndrome and inflammatory bowel disease. PLoS One. 2015;10:e0123861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Epel ES, Puterman E, Lin J, Blackburn EH, Lum PY, Beckmann ND, Zhu J, Lee E, Gilbert A, Rissman RA, Tanzi RE, Schadt EE. Meditation and vacation effects have an impact on disease-associated molecular phenotypes. Transl Psychiatry. 2016;6:e880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JM, Ma J, Breen EC, Cole SW. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun. 2012;26:1095-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 358] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 31. | Ho L, Bloom PA, Vega JG, Yemul S, Zhao W, Ward L, Savage E, Rooney R, Patel DH, Pasinetti GM. Biomarkers of Resilience in Stress Reduction for Caregivers of Alzheimer's Patients. Neuromolecular Med. 2016;18:177-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Bakker JM, Lieverse R, Menne-Lothmann C, Viechtbauer W, Pishva E, Kenis G, Geschwind N, Peeters F, van Os J, Wichers M. Therapygenetics in mindfulness-based cognitive therapy: do genes have an impact on therapy-induced change in real-life positive affective experiences? Transl Psychiatry. 2014;4:e384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Dada T, Bhai N, Midha N, Shakrawal J, Kumar M, Chaurasia P, Gupta S, Angmo D, Yadav R, Dada R, Sihota R. Effect of Mindfulness Meditation on Intraocular Pressure and Trabecular Meshwork Gene Expression: A Randomized Controlled Trial. Am J Ophthalmol. 2021;223:308-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Buric I, Farias M, Jong J, Mee C, Brazil IA. What Is the Molecular Signature of Mind-Body Interventions? Front Immunol. 2017;8:670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 35. | Chaix R, Fagny M, Cosin-Tomás M, Alvarez-López M, Lemee L, Regnault B, Davidson RJ, Lutz A, Kaliman P. Differential DNA methylation in experienced meditators after an intensive day of mindfulness-based practice: Implications for immune-related pathways. Brain Behav Immun. 2020;84:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | García-campayo J, Puebla-guedea M, Labarga A, Urdánoz A, Roldán M, Pulido L, de Morentin XM, Perdones-montero Á, Montero-marín J, Mendioroz M. Epigenetic Response to Mindfulness in Peripheral Blood Leukocytes Involves Genes Linked to Common Human Diseases. Mindfulness. 2018;9:1146-1159. [DOI] [Full Text] |
| 37. | Wang Y, Fan L, Zhu Y, Yang J, Wang C, Gu L, Zhong S, Huang Y, Xie X, Zhou H, Luo S, Wu X. Neurogenetic Mechanisms of Self-Compassionate Mindfulness: the Role of Oxytocin-Receptor Genes. Mindfulness. 2019;10:1792-1802. [DOI] [Full Text] |
| 38. | Chandran V, Bermúdez ML, Koka M, Chandran B, Pawale D, Vishnubhotla R, Alankar S, Maturi R, Subramaniam B, Sadhasivam S. Large-scale genomic study reveals robust activation of the immune system following advanced Inner Engineering meditation retreat. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 39. | King AP, Block SR, Sripada RK, Rauch SA, Porter KE, Favorite TK, Giardino N, Liberzon I. A Pilot Study of Mindfulness-Based Exposure Therapy in OEF/OIF Combat Veterans with PTSD: Altered Medial Frontal Cortex and Amygdala Responses in Social-Emotional Processing. Front Psychiatry. 2016;7:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Monti DA, Kash KM, Kunkel EJ, Brainard G, Wintering N, Moss AS, Rao H, Zhu S, Newberg AB. Changes in cerebral blood flow and anxiety associated with an 8-week mindfulness programme in women with breast cancer. Stress Health. 2012;28:397-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiatry. 2009;66:886-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 42. | Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67:380-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 43. | Wu B, Li X, Zhou J, Zhang M, Long Q. Altered Whole-Brain Functional Networks in Drug-Naïve, First-Episode Adolescents With Major Depression Disorder. J Magn Reson Imaging. 2020;52:1790-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Hoge EA, Bui E, Palitz SA, Schwarz NR, Owens ME, Johnston JM, Pollack MH, Simon NM. The effect of mindfulness meditation training on biological acute stress responses in generalized anxiety disorder. Psychiatry Res. 2018;262:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 45. | Pace TW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, Issa MJ, Raison CL. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34:87-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 334] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 46. | Stahl SM. Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications (5th ed). Cambridge, UK: Cambridge University Press, 2021. |
| 47. | Steimer T. The biology of fear- and anxiety-related behaviors. Dialogues Clin Neurosci. 2002;4:231-249. [PubMed] |
| 48. | Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2503] [Cited by in RCA: 2217] [Article Influence: 138.6] [Reference Citation Analysis (0)] |
| 49. | Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol. 2016;6:603-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 1161] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 50. | Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34:468-483. [PubMed] |
| 51. | Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1637] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 52. | Myers B, Scheimann JR, Franco-Villanueva A, Herman JP. Ascending mechanisms of stress integration: Implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neurosci Biobehav Rev. 2017;74:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 53. | Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol. 2008;6:254-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 337] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 54. | Zaccaro A, Piarulli A, Laurino M, Garbella E, Menicucci D, Neri B, Gemignani A. How Breath-Control Can Change Your Life: A Systematic Review on Psycho-Physiological Correlates of Slow Breathing. Front Hum Neurosci. 2018;12:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 303] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 55. | Park YJ, Park YB. Clinical utility of paced breathing as a concentration meditation practice. Complement Ther Med. 2012;20:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Stark R, Schienle A, Walter B, Vaitl D. Effects of paced respiration on heart period and heart period variability. Psychophysiology. 2000;37:302-309. [PubMed] |
| 57. | Streeter CC, Gerbarg PL, Saper RB, Ciraulo DA, Brown RP. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. 2012;78:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 269] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 58. | Brown RP, Gerbarg PL, Muench F. Breathing practices for treatment of psychiatric and stress-related medical conditions. Psychiatr Clin North Am. 2013;36:121-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 59. | Sevinc G, Hölzel BK, Greenberg J, Gard T, Brunsch V, Hashmi JA, Vangel M, Orr SP, Milad MR, Lazar SW. Strengthened Hippocampal Circuits Underlie Enhanced Retrieval of Extinguished Fear Memories Following Mindfulness Training. Biol Psychiatry. 2019;86:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 60. | Goldfarb EV, Sinha R. Fighting the Return of Fear: Roles of Mindfulness-Based Stress Reduction and the Hippocampus. Biol Psychiatry. 2019;86:652-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Sevinc G, Greenberg J, Hölzel BK, Gard T, Calahan T, Brunsch V, Hashmi JA, Vangel M, Orr SP, Milad MR, Lazar SW. Hippocampal circuits underlie improvements in self-reported anxiety following mindfulness training. Brain Behav. 2020;10:e01766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1305] [Cited by in RCA: 1354] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 63. | Sherin JE, Nemeroff CB. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin Neurosci. 2011;13:263-278. [PubMed] |
| 64. | King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, Angstadt M, Kessler D, Welsh R, Liberzon I. Altered default mode network (DMN) resting state functional connectivity following a mindfulness-based exposure therapy for posttraumatic stress disorder (PTSD) in combat veterans of Afghanistan and IRAQ. Depress Anxiety. 2016;33:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 65. | Peters SK, Dunlop K, Downar J. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front Syst Neurosci. 2016;10:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 401] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 66. | Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 593] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 67. | Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive-compulsive disorder in children and adults. Dev Psychopathol. 2008;20:1251-1283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 68. | Li H, Hu X, Gao Y, Cao L, Zhang L, Bu X, Lu L, Wang Y, Tang S, Li B, Yang Y, Biswal BB, Gong Q, Huang X. Neural primacy of the dorsolateral prefrontal cortex in patients with obsessive-compulsive disorder. Neuroimage Clin. 2020;28:102432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, van den Heuvel OA, Simpson HB. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 410] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 70. | Marchand WR. Neural mechanisms of mindfulness and meditation: Evidence from neuroimaging studies. World J Radiol. 2014;6:471-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 107] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (5)] |
| 71. | Doll A, Hölzel BK, Boucard CC, Wohlschläger AM, Sorg C. Mindfulness is associated with intrinsic functional connectivity between default mode and salience networks. Front Hum Neurosci. 2015;9:461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 72. | Fox KC, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage. 2015;111:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 425] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 73. | Mittner M, Boekel W, Tucker AM, Turner BM, Heathcote A, Forstmann BU. When the brain takes a break: a model-based analysis of mind wandering. J Neurosci. 2014;34:16286-16295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 74. | Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A. 2009;106:8719-8724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1104] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 75. | Denkova E, Nomi JS, Uddin LQ, Jha AP. Dynamic brain network configurations during rest and an attention task with frequent occurrence of mind wandering. Hum Brain Mapp. 2019;40:4564-4576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 76. | Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 4985] [Article Influence: 276.9] [Reference Citation Analysis (0)] |
| 77. | Posner J, Park C, Wang Z. Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychol Rev. 2014;24:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 78. | Chao LL, Knight RT. Contribution of human prefrontal cortex to delay performance. J Cogn Neurosci. 1998;10:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 161] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 79. | Woods DL, Knight RT. Electrophysiologic evidence of increased distractibility after dorsolateral prefrontal lesions. Neurology. 1986;36:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 171] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Konishi M, McLaren DG, Engen H, Smallwood J. Shaped by the Past: The Default Mode Network Supports Cognition that Is Independent of Immediate Perceptual Input. PLoS One. 2015;10:e0132209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 81. | Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5006] [Cited by in RCA: 4767] [Article Influence: 216.7] [Reference Citation Analysis (0)] |
| 82. | Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1977] [Cited by in RCA: 1815] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 83. | Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1197] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 84. | Tang YY, Rothbart MK, Posner MI. Neural correlates of establishing, maintaining, and switching brain states. Trends Cogn Sci. 2012;16:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 85. | Hasenkamp W, Barsalou LW. Effects of meditation experience on functional connectivity of distributed brain networks. Front Hum Neurosci. 2012;6:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 86. | Cotier FA, Zhang R, Lee TMC. A longitudinal study of the effect of short-term meditation training on functional network organization of the aging brain. Sci Rep. 2017;7:598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Godwin CA, Hunter MA, Bezdek MA, Lieberman G, Elkin-Frankston S, Romero VL, Witkiewitz K, Clark VP, Schumacher EH. Functional connectivity within and between intrinsic brain networks correlates with trait mind wandering. Neuropsychologia. 2017;103:140-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 88. | Simon R, Engström M. The default mode network as a biomarker for monitoring the therapeutic effects of meditation. Front Psychol. 2015;6:776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 89. | Doborjeh Z, Doborjeh M, Taylor T, Kasabov N, Wang GY, Siegert R, Sumich A. Spiking Neural Network Modelling Approach Reveals How Mindfulness Training Rewires the Brain. Sci Rep. 2019;9:6367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Hafeman DM, Ostroff AN, Feldman J, Hickey MB, Phillips ML, Creswell D, Birmaher B, Goldstein TR. Mindfulness-based intervention to decrease mood lability in at-risk youth: Preliminary evidence for changes in resting state functional connectivity. J Affect Disord. 2020;276:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 91. | Huang FY, Hsu AL, Chao YP, Shang CM, Tsai JS, Wu CW. Mindfulness-based cognitive therapy on bereavement grief: Alterations of resting-state network connectivity associate with changes of anxiety and mindfulness. Hum Brain Mapp. 2021;42:510-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 92. | Bauer CCC, Rozenkrantz L, Caballero C, Nieto-Castanon A, Scherer E, West MR, Mrazek M, Phillips DT, Gabrieli JDE, Whitfield-Gabrieli S. Mindfulness training preserves sustained attention and resting state anticorrelation between default-mode network and dorsolateral prefrontal cortex: A randomized controlled trial. Hum Brain Mapp. 2020;41:5356-5369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 93. | Black DS, Christodoulou G, Cole S. Mindfulness meditation and gene expression: a hypothesis-generating framework. Curr Opin Psychol. 2019;28:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | Schuman-Olivier Z, Trombka M, Lovas DA, Brewer JA, Vago DR, Gawande R, Dunne JP, Lazar SW, Loucks EB, Fulwiler C. Mindfulness and Behavior Change. Harv Rev Psychiatry. 2020;28:371-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 95. | Venditti S, Verdone L, Reale A, Vetriani V, Caserta M, Zampieri M. Molecules of Silence: Effects of Meditation on Gene Expression and Epigenetics. Front Psychol. 2020;11:1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 96. | Bilevicius E, Smith SD, Kornelsen J. Resting-State Network Functional Connectivity Patterns Associated with the Mindful Attention Awareness Scale. Brain Connect. 2018;8:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 97. | Kim HC, Tegethoff M, Meinlschmidt G, Stalujanis E, Belardi A, Jo S, Lee J, Kim DY, Yoo SS, Lee JH. Mediation analysis of triple networks revealed functional feature of mindfulness from real-time fMRI neurofeedback. Neuroimage. 2019;195:409-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 98. | Tang YY, Posner MI. Tools of the trade: theory and method in mindfulness neuroscience. Soc Cogn Affect Neurosci. 2013;8:118-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 99. | Murakami H, Katsunuma R, Oba K, Terasawa Y, Motomura Y, Mishima K, Moriguchi Y. Neural Networks for Mindfulness and Emotion Suppression. PLoS One. 2015;10:e0128005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 100. | Gu Y, Zhu Y, Brown KW. Mindfulness and Attention Deficit Hyperactivity Disorder: A Neuropsychological Perspective. J Nerv Ment Dis. 2021;209:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |