Published online Aug 19, 2022. doi: 10.5498/wjp.v12.i8.1031
Peer-review started: March 16, 2022
First decision: June 11, 2022
Revised: June 15, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: August 19, 2022
Processing time: 154 Days and 19.9 Hours
Efficiently detecting Parkinson's disease (PD) with dementia (PDD) as soon as possible is an important issue in geriatric medicine.
To develop a model for predicting PDD based on various neuropsychological tests using data from a nationwide survey conducted by the Korean Centers for Disease Control and Prevention and to present baseline data for the early detection of PDD.
This study comprised 289 patients who were 60 years or older with PD [110 with PDD and 179 Parkinson's Disease-Mild Cognitive Impairment (PD-MCI)]. Regre-ssion with optimal scaling (ROS) was used to identify independent relationships between the neuropsychological test results and PDD.
In the ROS analysis, Korean version of mini mental state ex-amination (MMSE) (KOREAN version of MMSE) (b = -0.52, SE = 0.16) and Hoehn and Yahr staging (b = 0.44, SE = 0.19) were significantly effective models for distinguishing PDD from PD-MCI (P < 0.05), even after adjusting for all of the Parkinson's motor symptom and neuropsychological test results. The optimal number of categories (scaling factors) for KOREAN version of MMSE and Hoehn and Yahr Scale was 10 and 7, respectively.
The results of this study suggest that among the various neuropsychological tests conducted, the optimal classification scores for KOREAN version of MMSE and Hoehn and Yahr Scale could be utilized as an effective screening test for the early discrimination of PDD from PD-MCI.
Core Tip: Although a general linear model can be constructed if all of the variables used in the analysis are numeric, it is difficult to fit the data when the variables are nominal. We build a regression model using the transform variables obtained by iteratively using alternating least squares to compute the optimal scaling. We developed a predictive model to discriminate Parkinson's disease with dementia from Parkinson's Disease-Mild Cognitive Impairment based on the results of nine neuropsychological tests and found that only Korean version of mini mental state examination and Hoehn and Yahr Scale could be successfully employed to this end.
- Citation: Byeon H. Can the prediction model using regression with optimal scale improve the power to predict the Parkinson's dementia? World J Psychiatry 2022; 12(8): 1031-1043
- URL: https://www.wjgnet.com/2220-3206/full/v12/i8/1031.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i8.1031
As the longevity of the South Korean population increases, so does the proportion of advanced-aged individuals[1] along with the incidence of chronic degenerative diseases[1]. For this reason, the importance of prevention and early treatment of degenerative dis-eases in old age should be emphasized. Parkinson's disease (PD) is a representative neurodegenerative disease caused by damaged nerve cells that secrete dopamine in the sub-stantia nigra. However, researchers have paid less attention to PD than dementia or stroke in terms of health science because its incidence rate is only 1% in the older adult population (≥ 65 years old) and its prevalence rate is lower than for dementia or stroke. However, the number of PD patients is steadily increasing in the aged population. The Health Insurance Review and Assessment Service (HIRAS) (2019)[2] reported that the number of patients diagnosed with PD steadily increased from 61565 in 2010 to 100716 in 2018 and predicted that the number of PD patients will double in 2030 compared to 2005 at this rate. In particular, the number of older adults with PD is expected to increase even more in South Korea considering that by 2050, the proportion of the older adult population in South Korea will be 35.9%, the second-highest after Japan (40.1%)[3]. Consequently, the effective early detection of PD is an important topic in the field of geriatric medicine.
PD is a motor disorder comprising a combination of weakness, tremor, and rigidity. However, over the past 20 years, other symptoms including autonomic nerve disorder, affective and sensory disorders such as depressive disorders, and cognitive impairment have been reported[1-7]. Many previous studies[8-10] have reported that 20%-57% of patients develop mild cognitive impairment (MCI) within 5 years from the date of being diagnosed with PD. MCI refers to a state in which cognitive decline is observed without accompanying a decline in activities of daily living (ADL). It is a pre-clinical state of PD with dementia (PDD) and it is an intermediate stage from normal to PDD. Previous follow-up studies also have revealed that approximately 10% to 15% of MCI patients transited to dementia every year[7]. It means that they are highly vulnerable to dementia and it was much higher than the annual dementia incident rate of healthy older adults (65 years or older)[7]. It is the earliest stage of dementia that can be detected in clinical examination, and it is clinically very important because it is possible to maximize the therapeutic effect[7]. Neuropsychological screening battery, cognitive assessment, autonomic function, and other tests have been carried out to objectively assess the clinical status of PD accompanying MCI[11]. However, it is difficult to distinguish MCI from aging or mild dementia only using these screening tests[11]. To make it more challenging, it can be misdiagnosed with progressive supranuclear palsy-parkinsonism (PSP-P) when a patient suffers from PD and cognitive deficit at the same time[12,13].
Compared to the United States and Europe, South Korea currently has insufficient epidemiological data on cognitive impairment in old age. Although community-based studies on PD conducted in South Korea have focused on patients in small and medium-sized cities, prediction models based on a nationwide epidemiological survey have not yet been developed[14-17]. Although a general linear model (GLM) for PD can be constructed if all of the variables used in the analysis are numeric, it is difficult to fit the data when the variables are ordinal or nominal. An alternative method to overcome this limitation is to build a regression model with an optimal scale (optimal regression).
Optimal scaling is based on the prediction theory (also known as the quantification theory) developed by considering how to quantify qualitative variables to enable optimal data analysis rather than simply ranking them and interpreting the results. Optimal scaling has been mainly used in social science fields such as psychology when proving causality is important[18-20]. However, it has only been used in a small number of studies in the cognitive science field. Identifying neuropsychological tests (e.g., cognitive and de-pression tests) and Parkinson’s motor symptom tests that are effective in discriminating PDD from PD-MCI by using regression with optimal scaling (ROS) and checking the optimal classification scores of the tests is clinically meaningful. However, it has only been used in a small number of studies in the cognitive science field. The objectives of the present study were to develop a model for predicting PDD based on various neuropsychological tests using data from a National Biobank of Korea data.
Approval for the study was received from the Distribution Committee (No. KBN-2019-1327) and the Research Ethics Review Committee of the National Biobank of Korea under the Korean Centers for Disease Control and Prevention (No. KBN-2019-005). Epidemiologic data on patients with PD were collected from 14 tertiary care providers nationwide from January to December 2015 under the supervision of the Korean Centers for Disease Control and Prevention. PDD has been designated as idiopathic Parkinson's dis-ease according to the diagnostic criteria of the United Kingdom Parkinson's Disease Society Brain Bank[21]. The diagnostic criteria for probable PDD have been suggested by the Movement Disorder Society Task Force[22]. When causes of cognitive impairment other than PD (e.g., hydrocephalus and vascular Parkinsonism) were found in magnetic resonance imaging scans, the subject was excluded from the study (see Byeon[23] for more details). PD-MCI was diagnosed by neuropsychologists according to the criteria of the International Working Group on MCI[24]. Health surveys were conducted by using computer-assisted personal interviews. We analyzed the PD epidemiologic data comprising demographic information, any family history of PD, health-affecting behaviors (e.g., smoking), disease history (e.g., diabetes), and Parkinson’s motor symptoms (e.g., rigidity) and neuropsychological characteristics (e.g., cognitive level). The variables and their values are reported in Table 1. Thus, data on 289 patients with PD (110 PDD and 179 PD-MCI) who were 60 years or older were used in the study.
| Factors | Measurement | Characteristics |
| Demographic factors | Sex | Male or female |
| Age | 60-74, 75+ | |
| Mainly used hand | Left hand or right hand | |
| Education level | Middle school graduate and below or high school graduate and above | |
| Family history of the disease | Parkinson's disease; Alzheimer's disease | Yes or no; Yes or no |
| Health behaviors | Pack-years (smoking) | Non-smoking, 1-20, 21-40, 41-60, or ≥ 61 pack-years |
| Coffee-drinking | Yes or no | |
| Mean coffee intake per day (cups/d) | No, ≤ 1, 2-3, or ≥ 4 cups | |
| Coffee drinking period (yr) | No, ≤ 5, 6-9, or ≥ 10 | |
| Disease history | Carbon monoxide poisoning | Yes or no |
| Diabetes | Yes or no | |
| Alcoholism | Yes or no | |
| Hyperlipidemia | Yes or no | |
| Traumatic brain injury | Yes or no | |
| Hypertension | Yes or no | |
| Exercise characteristics related to Parkinson's disease relatedmotor signs | Tremor | Yes or no |
| Rigidity | Yes or no | |
| Akinesia/bradykinesia | Yes or no | |
| Postural instability | Yes or no | |
| Late motor complications | Yes or no | |
| Neuropsychological test | K-MoCA | Continuous variable |
| K-MMSE | Continuous variable | |
| Global CDR score | Continuous variable | |
| Sum of boxes in CDR | Continuous variable | |
| Hoehn and Yahr staging | Continuous variable | |
| UPDRS (Total UPDRS score) | Continuous variable | |
| UPDRS (Motor UPDRS score) | Continuous variable | |
| K-IADL | Continuous variable | |
| Schwab and England ADL | Continuous variable |
The label was defined as PDD confirmed by medical diagnosis. To understand the difference in the general characteristics of subjects according to the demographic variables (e.g., age, sex, and education level), medical history (e.g., hypertension), and family history [e.g., PD and Alzheimer's disease (AD)].
Explanatory variables (neuropsychological tests) included scores from the Hoehn and Yahr (H&Y) staging[25], Global Clinical Dementia Rating (CDR)[26], Schwab and England Activities of Daily Living[27], the Korean Instrumental ADL (K-IADL)[28], the Unified PD Rating Scale (UPDRS) total[22], the UPDRS motor[22], the Korean Mini-Mental State Examination (KOREAN version of MMSE)[29], and the Korean-Montreal Cognitive Assessment (K-MoCA)[30]. Hoehn and Yahr Scale[25] is a screening test to determine the stage of PD and is measured by clinicians. The score ranges from 1 to 5, and a higher score indicates that the symptoms of PD are more severe.
CDR[26] is a screening test to determine the stage of dementia and is measured by clinicians. The possible outcomes are 0, 0.5, 1, 2, 3, 4, and 5 points, and a higher score means more severe dementia. Schwab and England ADL[27] is a screening test for physical impairment. It is evaluated by clinicians to measure indices regarding independent performance in the daily activities of PD patients. The score ranges from 0 to 100, and a higher score is interpreted as a lower functional impairment. K-IADL[28] is a cognitive screening test that measures skills and behaviors necessary for social life such as "money management" and "phone use". It consists of eleven items that can score between 0 and 3, and a higher score means higher functional impairment. UPDRS[22] is an overall evaluation scale for the symptoms of PD and consists of four segments (mentation/behavior/mood, ADL, motor examination, and dyskinesia). The test is conducted by a clinician, and a higher score is interpreted as a higher degree of disability. KOREAN version of MMSE[29] is a test for screening cognitive disorders such as dementia and consists of time orientation, spatial orientation, memory registration (input), calculation and attention, memory recall, and language items. The total score is 30 points, and the cut-off score is 24 points. A lower score indicates more severe cognitive impairment. K-MoCA[30] is a test for screening MCI. The total score is 30 points, and people with 22 points and above are interpreted as normal. A lower score is understood as more severe cognitive impairment.
If all the variables (e.g., Independent variables, dependent variables, and confounding variables) used in the analysis are numeric variables (quantitative variables), the GLM can be used. However, if it is an ordinal or nominal variable, it is difficult to use the general linear regression model because these variable types do not meet the assumptions of the regression models and error terms. Therefore, analysis can be con-ducted by deriving an optimized linear regression equation of transformed variables by repeatedly performing optimal scaling based on the alternating least squares method.
It is a way to estimate parameters for the linear relationship between independent and dependent variables using data on each variable. The estimated general linear regression model is presented as follows[19]:
Yi = α + βX_i +ε_i
Yi = dependent variable
Xi = independent variable (Equation 1)
εi = error term
α,β = parameter to estimate
When the assumptions for the error term, such as "the expected value of the error term shall be 0" and "it shall follow a normal distribution and all observations shall have the same variance", parameters are estimated by using the least-squares and other methods to determine the relationship between the independent and dependent variables. The least-squares method is used to obtain parameter estimates (α^ and β^) that minimize the sum-of-squared residuals, where the residual (ε_i) is equal to the difference between the actual observations (Y_i) and the predicted values of the dependent variables ((Y_i)^ ((Y_i)^ =α^+β^ X_i)).
In this study, ROS consisted of three stages. The first is the data transformation stage. After normalizing k categorical indicators for the nth variable by vectorizing them, all of the variables are treated as numeric variables. Subsequently, they are optimized repeatedly by using the calculated categorical quantification values and regression coefficients. The second stage is updating the categorical quantification vector by considering the scale level (i.e., whether the variables are nominal, ordinal, or numeric) and calculating the regression coefficient vector. The third stage is to establish convergence by repeatedly calculating the categorical quantification vector and the regression coefficient vector until they satisfy the predetermined convergence condition[19].
ROS transforms each variable appropriately by considering its scale in the GLM. When dependent variable Y is transformed to θ(Y) and independent variable X to σ(X), the resulting parameters are the intercept and slope of a GLM (linear regression) equation formed by minimizing the sum-of-squares (SSQ) of the error[19] as follows: minSSQ[θ(Y)-βσ(X)] (Equation 2).
The conversion variable has a standardization constraint. Minimizing the SSQ error is achieved by least-squares regressing the transformed variables [e.g., θ(Y), σ1(X1), …, σn(Xn))]. The ROS analysis with standardization constraints is written as

ROS was used to identify the independent relationship between each test and PDD. The analysis results were presented with a regression coefficient, odds ratio, 95% confidence interval (CI), quantification index, and standard error by bootstrap (n = 999). General characteristics of the subjects and the prevalence of PD were analyzed using the Chi-square test.
When independent significance was confirmed in the ROS, the Cochran-Armitage (CA) trend test was used to determine whether the p values had a linear trend based on the reference group as follows[31]:
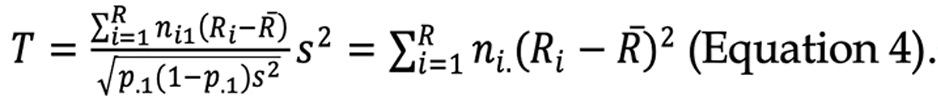
The analysis of ROS was conducted by using CatReg Software version 3.0 (the Data Theory Scaling System Group, Leiden, The Netherlands).
The results of χ2 tests show that age, handed, PD family history, gender, the highest level of education, AD family history, hypertension, traumatic brain injury history, stroke history, carbon monoxide poisoning history, hyperlipidemia, and diabetes were not significantly different between PDD and PD-MCI (Table 2). Therefore, the subjects in this study did not have statistically significant demographic or health differences between the groups.
| Variables | PD-MCI (n = 179) | PDD (n = 110) | P value |
| Age | 0.168 | ||
| 60-74 | 117 (65.0) | 63 (35.0) | |
| 75+ | 62 (56.9) | 47 (43.1) | |
| Sex | 0.550 | ||
| Male | 78 (63.9) | 44 (36.1) | |
| Female | 101 (60.5) | 66 (39.5) | |
| Education level | 0.072 | ||
| Middle school graduate and below | 110 (58.2) | 79 (41.8) | |
| High school graduate and above | 69 (69.0) | 31 (31.0) | |
| Family history of the Parkinson's disease | 0.600 | ||
| No | 144 (64.3) | 80 (35.7) | |
| Yes | 12 (70.6) | 5 (29.4) | |
| Family history of the Alzheimer's disease | 0.285 | ||
| No | 130 (63.4) | 75 (36.6) | |
| Yes | 8 (80.0) | 2 (20.0) | |
| Carbon monoxide poisoning | 0.743 | ||
| No | 158 (62.5) | 95 (37.5) | |
| Yes | 10 (66.7) | 5 (33.3) | |
| Traumatic brain injury | 0.277 | ||
| No | 158 (62.0) | 97 (38.0) | |
| Yes | 10 (76.9) | 3 (23.1) | |
| Diabetes | 0.508 | ||
| No | 144 (64.0) | 81 (36.0) | |
| Yes | 35 (59.3) | 24 (40.7) | |
| Hypertension | 0.304 | ||
| No | 110 (65.5) | 58 (34.5) | |
| Yes | 69 (59.5) | 47 (40.5) | |
| Hyperlipidemia | 0.220 | ||
| No | 155 (61.8) | 96 (38.2) | |
| Yes | 24 (72.7) | 9 (27.3) |
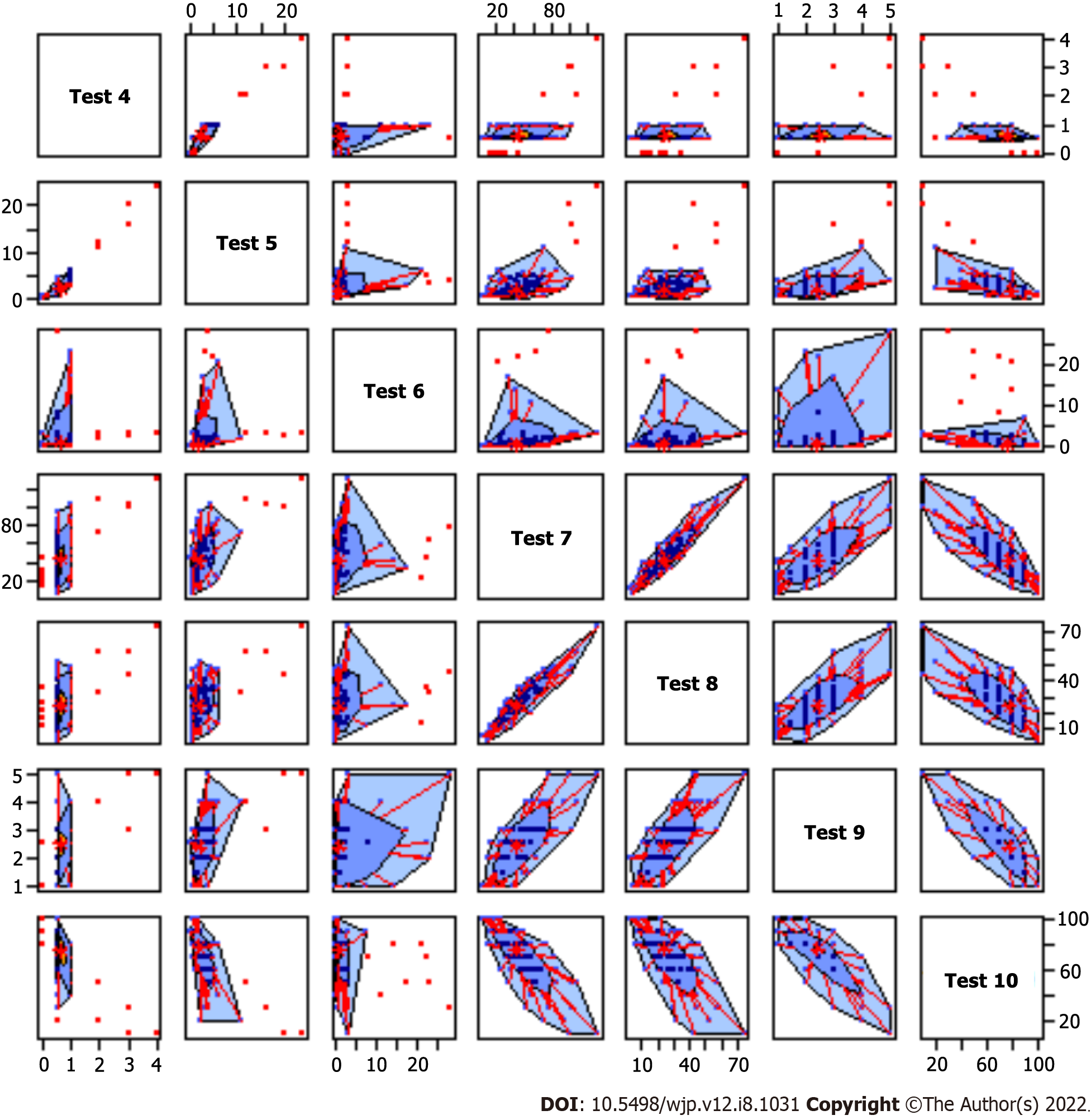
Table 3 reports the data and Figure 1 shows a bag plot for visualizing the spread, location, outliers and skewness.
| Results | K-MMSE | K-MoCA | Global CDR score | Sum of boxes in CDR | K-IADL | Total UPDRS | Motor UPDRS | H&Y staging | ADL |
| Mean | 22.73 | 16.27 | 0.67 | 2.80 | 1.90 | 43.56 | 25.33 | 2.45 | 74.40 |
| Standardized mean error | 0.32 | 0.44 | 0.03 | 0.22 | 0.26 | 2.02 | 0.77 | 0.04 | 1.42 |
| Standard deviation | 5.51 | 6.33 | 0.56 | 3.49 | 4.08 | 23.77 | 12.59 | 0.78 | 18.30 |
| Minimum | 3 | 0 | 0 | 0 | 0 | 0.18 | 2.0 | 1.0 | 10 |
| Maximum | 30 | 27 | 4.0 | 25.0 | 28.0 | 130.00 | 74.0 | 5.0 | 100 |
The neuropsychological test results of PD-MCI and PDD are compared (Table 4). As a result of the independent t-test, KOREAN version of MMSE, K-MoCA, Total UPDRS score, CDR (sum of boxes), K-IADL, Hoehn and Yahr staging, Motor UPDRS score, and Schwab and England ADL were not significantly different between PDD and PD-MCI (P < 0.05).
| Variables | PD-MCI (n = 179) | PDD (n = 110) | P value |
| K-MMSE | 24.3 ± 3.4 | 18.8 ± 5.6 | < 0.001 |
| K-MoCA | 19.4 ± 4.9 | 11.9 ± 5.4 | < 0.001 |
| CDR (sum of boxes) | 1.6 ± 1.4 | 5.1 ± 4.9 | < 0.001 |
| K-IADL | 1.3 ± 2.9 | 3.0 ± 5.4 | 0.001 |
| UPDRS (Total UPDRS score) | 36.4 ± 17.9 | 56.1 ± 27.2 | < 0.001 |
| UPDRS (Motor UPDRS score) | 22.6 ± 10.1 | 29.4 ± 14.6 | < 0.001 |
| H&Y staging | 2.2 ± 0.6 | 2.7 ± 0.8 | 0.001 |
| Schwab and England ADL | 80.0 ± 14.4 | 65.6 ± 19.8 | < 0.001 |
The analysis results of ROS are summarized in Table 5. Hoehn and Yahr Scale (b = 0.44, SE = 0.19) and KOREAN version of MMSE (b = -0.52, SE = 0.16) were significantly effective for distinguishing PDD from PD-MCI even after adjusting for all of test results (P < 0.05). The regression model was adjusted for demographic factors, family disease history, health-affecting behaviors, dis-ease history, Parkinson’s motor symptoms, and neuropsychological test.
| Test | b | SE by boost 1 | df | F | P value |
| K-MMSE | -0.522 | 0.168 | 2 | 9.684 | < 0.001 |
| KMoCA | -0.206 | 0.238 | 3 | 0.750 | 0.527 |
| CDR (Global CDR score) | 0.127 | 0.269 | 1 | 0.222 | 0.639 |
| CDR (sum of boxes) | -0.271 | 0.412 | 3 | 0.431 | 0.732 |
| K-IADL | 0.237 | 0.224 | 2 | 1.119 | 0.334 |
| UPDRS (Total UPDRS score) | 0.433 | 0.444 | 3 | 0.949 | 0.423 |
| UPDRS (Motor UPDRS score) | -0.338 | 0.330 | 3 | 1.045 | 0.380 |
| H&Y staging | 0.440 | 0.197 | 3 | 5.008 | 0.004 |
| Schwab and England ADL | 0.353 | 0.333 | 2 | 1.123 | 0.333 |
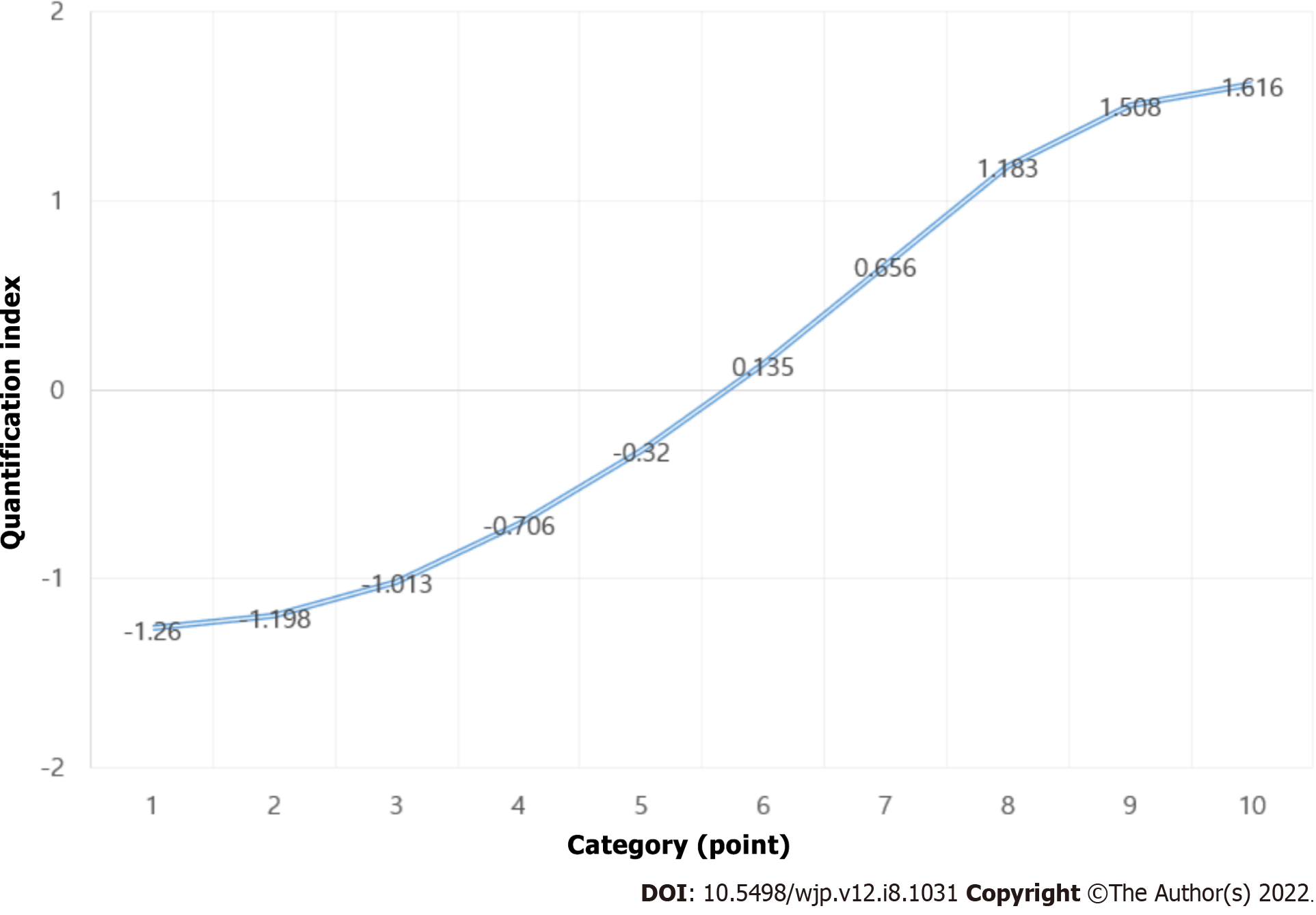
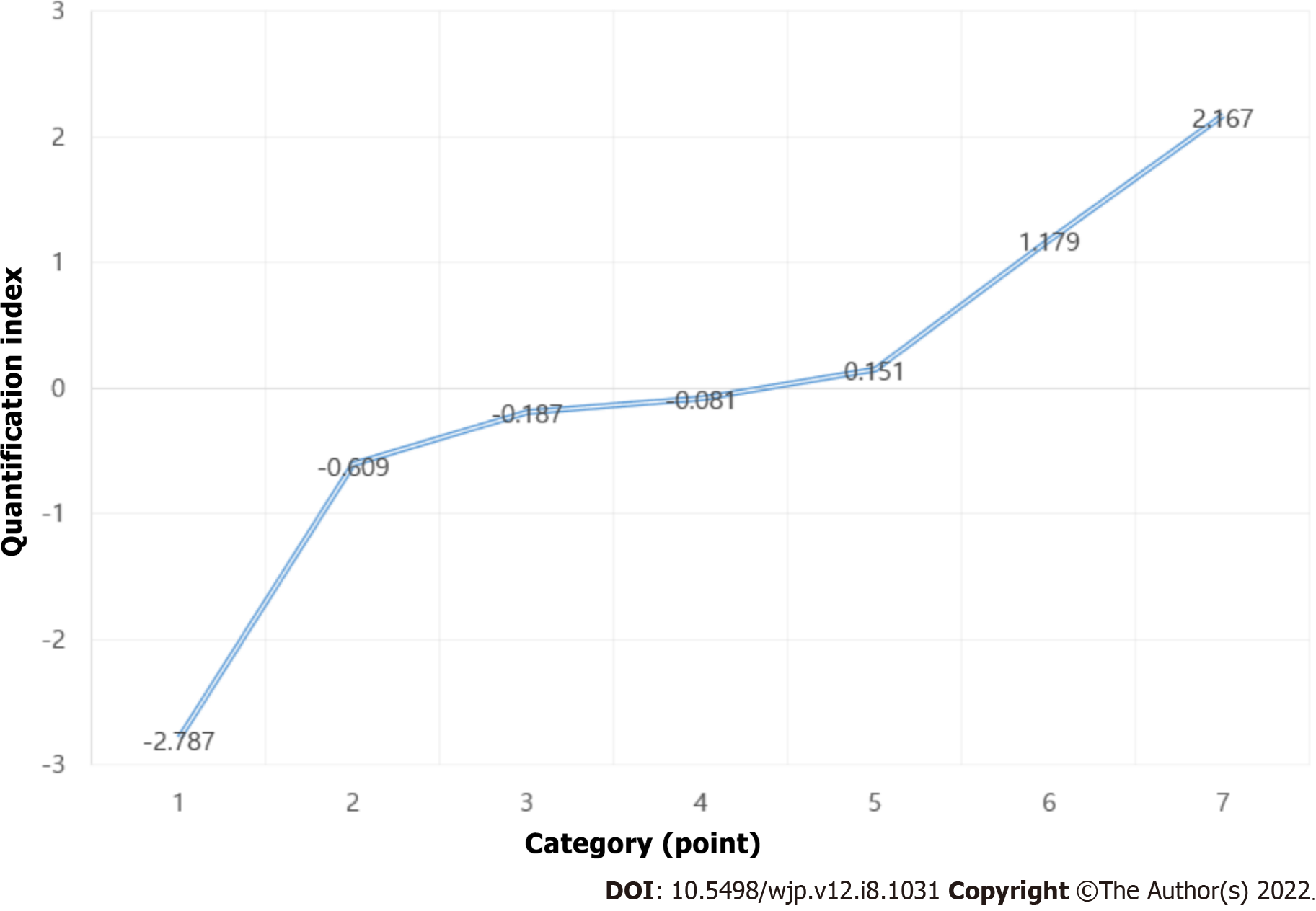
Quantification scores for KOREAN version of MMSE and Hoehn and Yahr Scale are reported in Tables 6 and 7, respectively, and presented in Figures 2 and 3, respectively. The results show that the optimal number of categories (scaling factors) for KOREAN version of MMSE and Hoehn and Yahr Scale was 10 and 7, respectively. The odds ratios (ORs) and 95%CIs for the optimal categories of KOREAN version of MMSE and Hoehn and Yahr Scale are reported in Table 8. When distinguishing PDD from PD-MCI, PD-MCI patients who had 23 or 24 points for KOREAN version of MMSE had a 4.5-fold higher risk of PDD than those who had 25 or higher. Moreover, those who scored 21 or 22, 19 or 20, 15 to 18, and 3 to 14 points had a 2.7-fold, 13.3-fold, 22.4-fold, and 55-fold higher risk of developing PDD, respectively, than those who had 25 or higher. The results of the CA Trend test show a significant relationship (P for Trend < 0.001) between the increase in OR and the KOREAN version of MMSE score (optimal categories score).
| Category (point) | Quantification index |
| 3-14 | -1.260 |
| 15-18 | -1.198 |
| 19-20 | -1.013 |
| 21-22 | -.706 |
| 23-24 | -.320 |
| 25 | 0.135 |
| 26 | 0.656 |
| 27 | 1.183 |
| 28 | 1.508 |
| 29-30 | 1.616 |
| Category (point) | Quantification index |
| 1.0 | -2.787 |
| 1.5 | -0.609 |
| 2.0 | -0.187 |
| 2.5 | -0.081 |
| 3.0 | 0.151 |
| 4.0 | 1.179 |
| 5.0 | 2.167 |
| Optimal classification scores | B | SE | Wald | P value | OR (95%CI) |
| K-MMSE 25+ (Ref) | 69.856 | < 0.01 | |||
| 23-24 | 1.499 | 0.473 | 10.035 | 0.002 | 4.478 (1.77-11.32) |
| 21-22 | 2.731 | 0.494 | 30.522 | < 0.01 | 15.345 (5.82-40.43) |
| 19-20 | 2.587 | 0.549 | 22.195 | < 0.01 | 13.294 (4.53-39.00) |
| 15-18 | 3.111 | 0.505 | 37.937 | < 0.01 | 22.441 (8.33-60.39) |
| 3-14 | 4.008 | 0.799 | 25.185 | < 0.01 | 55.020 (11.50-263.19) |
| H&Y staging 1.0-2.5 (Ref) | |||||
| 3.0-5.0 | 1.110 | 0.350 | 10.079 | 0.001 | 3.035 (1.52-6.02) |
In this study, KOREAN version of MMSE and Hoehn and Yahr Scale could independently differentiate PDD from PD-MCI even after adjusting for all of the PD’s test results. Moreover, when the ROS (optimal classification scores) were calculated, the increase in OR according to all of the categories showed a significant proportional trend.
It is not easy to accurately detect and diagnose PSP-P by identifying the pattern of PD-MCI in PDD by using neuropsychological tests[26]. First, it is difficult to determine whether dementia is the cause of a patient's cognitive impairment symptoms[27] because patients with PD often take a variety of medications (e.g., anticholinergics, amantadine, anxiolytics, and sedatives) and can experience temporary cognitive decline or confusion (easily mistaken for dementia) as side effects of the medications[32]. Second, cognitive impairment can occur temporarily due to endocrine imbalance due to depression, electrolyte imbalance, and/or dehydration; systemic diseases; or infection[22]. Third, even if dementia is diagnosed, it is necessary to effectively differentiate it from other types of irreversible dementia such as Alzheimer's disease or, especially, dementia with Lewy bodies[22]. Hence, it is necessary to develop predictive models that can more efficiently discriminate PDD from PD-MCI as well as other types of dementia while simultaneously considering the results of several neuropsychological tests related to cognitive impairment.
Nevertheless, in most of the previous studies, evaluating the predictive performance for PDD was conducted by comparing individual diagnostic performances in terms of accuracy and reliability[30,31,33,34]. The results of the present study suggest that among the various neuropsychological tests examined, the optimal classification scores by MMSE-K and Hoehn and Yahr Scale show that these two tests could be utilized for effective early discrimination of PDD from PD-MCI. Moreover, they could be used to clinically determine whether PD-MCI patients will develop PDD or whether existing PDD patients are getting worse. Conducting these tests when a PD-MCI patient visits the hospital (or Public Health Center) for the first time provides baseline information and carrying them out sequentially at regular visits can be used to recognize clinically meaningful changes.
Although it is very important to efficiently distinguish PDD from other diseases showing symptoms of PD as soon as possible, PD can only be accurately diagnosed through pathological examination with autopsy[6]. Dopamine transporter imaging has been reported as an effective test for diagnosing PDD at an early stage[35], but it is too expensive to be used as a screening test in the primary care setting. As a result, it is diagnosed through an interview on the symptoms of a patient and an examination of a specialist along with a cognitive screening test such as KOREAN version of MMSE in the clinical practice.
However, Rizzo et al[36] reported that the misdiagnosis rate of dyskinesis was at least 20% even for neurologists with extensive experience in dyskinesias. Therefore, to accurately diagnose PD-MCI, a specialist must have a broad perspective to comprehensively consider the symptoms of a patient (e.g., resting tremor, bradypragia, postural changes, and gait abnormalities), living environment, presence of trauma, lifestyle, and occupation as well as the results of cognitive screening tests. Particularly, since cognitive issues and dyskinesias (e.g., bradypragia, resting tremor, and ankylosis) are slowly progressive cardinal symptoms, clinicians are more likely to rely on experience and the judgment of inexperienced clinicians may have low reliability.
It is believed that the analysis indices of this study can offer a range of information regarding the cognitive characteristics of the patient because they provide the optimal criteria for the screening test to distinguish PDD from PD-MCI. In particular, the optimal scale for early detection of PDD proposed in this study is inexpensive, unlike dopamine transporter imaging and other methods, which have been proposed as efficient tests for early diagnosis of PDD but have limitations as screening tests due to space and cost. Moreover, the proposed scale can be utilized as a screening test simply in the primary medical setting without spatial restrictions. Consequently, it is believed that clinical application will be easy. Additional longitudinal studies are required to prove the effectiveness of the optimal scale for distinguishing PDD from PD-MCI proposed in this study.
This study had several limitations. First, although we used secondary data from a national survey, it is difficult to generalize the results of the study because the number of subjects was small due to the difficulties in diagnosing PD-MCI, which is not yet being actively screened for in PD patients. Second, we included patients taking medications such as dopaminergic drugs to treat PD, which can cause behavioral symptoms such as visual hallucinations that could influence the neuropsychological examination. Future studies are required to develop a model that can predict PDD from PD-MCI quickly while considering the administration of dopaminergic medication for PD. Third, the results of this study cannot be interpreted as a causal relationship because it was conducted using secondary data and the PD with Dementia Epidemiologic Data, the source data of this study, was designed as a cross-sectional survey. Further longitudinal studies are needed to prove the causality of the results of this study. Fourth, the diagnosis of PSP-P was not distinguished in this study. Since the cognitive deficits in PD patients can be caused by PSP-P as well as PD-MIC, future studies are needed to exclude PSP-P in analysis.
We developed a predictive model to discriminate PDD from PD-MCI based on the results of nine neuropsychological tests and found that only KOREAN version of MMSE and Hoehn and Yahr Scale could be successfully employed to this end. For most efficiently discriminating PDD from PD-MCI, the optimal scaling factors for KOREAN version of MMSE and Hoehn and Yahr Scale were 10 and 7, respectively. We believe that our optimal scaling approach can be used to detect PDD in the early stages. Further longitudinal studies are required to confirm the performance of neuropsychological tests such as KOREAN version of MMSE and MoCA in predicting the progression of PD-MCI to PDD.
It has been reported that Parkinson's disease (PD) with dementia (PDD) occurs frequently in people with PD.
The effective early detection of PD is an important topic in the field of geriatric medicine.
The aims of the present study were to develop a model for early detection of PDD based on neuropsychological testing.
Data on 289 patients with PD [110 PDD and 179 Parkinson's Disease-Mild Cognitive Impairment (PD-MCI)] who were 60 years or older were used in the study. Regression with optimal scaling was used to identify independent relationships between the screening test results and PDD.
The Korean version of mini mental state examination (MMSE) (KOREAN version of MMSE) (b = -0.52, SE = 0.16) and Hoehn and Yahr scale (b = 0.44, SE = 0.19) were significantly effective models for distinguishing PDD from PD-MCI (P < 0.05), even after adjusting for all of the test results.
The optimal number of categories (scaling factors) for KOREAN version of MMSE and Hoehn and Yahr Scale was 10 and 7, respectively.
We believe that our optimal scaling approach can be used to detect PDD in the early stages.
The authors wish to thank the Korea CDC that provided the raw data for analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dabbakuti JRKKK, India; Kujawski S, Poland S-Editor: Gao CC L-Editor: A P-Editor: Chen YX
| 1. | Jay R, Jung SB, Park BH, Jeong BC, Seo SI, Jeon SS, Lee HM, Choi HY, Jeon HG. Compensatory structural and functional adaptation after radical nephrectomy for renal cell carcinoma according to preoperative stage of chronic kidney disease. Choi DK, Jung SB, Park BH, Jeong BC, Seo SI, Jeon SS, Lee HM, Choi HY, Jeon HG.J Urol. 2015 Oct;194(4):910-5. [Epub 2015 Apr 28]. doi: 10.1016/j.juro.2015.04.093. Urol Oncol. 2017;35:118-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Health Insurance Review and Assessment Service. Regarding the role of the Health Insurance Review and Assessment Service that needs to be strengthened as the health care environment changes rapidly. HIRA Research. 2021;1:98-102. [DOI] [Full Text] |
| 3. | Statistics Korea. Population projections for Korea (2017~2067), Statistics Korea: Daejeon, 2019. |
| 4. | Gonzalez-Latapi P, Bayram E, Litvan I, Marras C. Cognitive Impairment in Parkinson's Disease: Epidemiology, Clinical Profile, Protective and Risk Factors. Behav Sci (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Rosca EC, Simu M. Parkinson's Disease-Cognitive Rating Scale for Evaluating Cognitive Impairment in Parkinson's Disease: A Systematic Review. Brain Sci. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Vasconcellos LF, Pereira JS. Parkinson's disease dementia: Diagnostic criteria and risk factor review. J Clin Exp Neuropsychol. 2015;37:988-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Mov Disord. 2005;20:1255-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 575] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 8. | Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130:1787-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 662] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 9. | Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord. 2006;21:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 392] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 10. | Caviness JN, Driver-Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, Evidente VG, Shill HA, Adler CH. Defining mild cognitive impairment in Parkinson's disease. Mov Disord. 2007;22:1272-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 11. | Byeon H. Application of Machine Learning Technique to Distinguish Parkinson's Disease Dementia and Alzheimer's Dementia: Predictive Power of Parkinson's Disease-Related Non-Motor Symptoms and Neuropsychological Profile. J Pers Med. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Necpál J, Borsek M, Jeleňová B. "Parkinson's disease" on the way to progressive supranuclear palsy: a review on PSP-parkinsonism. Neurol Sci. 2021;42:4927-4936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Alster P, Madetko N, Koziorowski D, Friedman A. Progressive Supranuclear Palsy-Parkinsonism Predominant (PSP-P)-A Clinical Challenge at the Boundaries of PSP and Parkinson's Disease (PD). Front Neurol. 2020;11:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Pigott K, Rick J, Xie SX, Hurtig H, Chen-Plotkin A, Duda JE, Morley JF, Chahine LM, Dahodwala N, Akhtar RS, Siderowf A, Trojanowski JQ, Weintraub D. Longitudinal study of normal cognition in Parkinson disease. Neurology. 2015;85:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 15. | Martinez-Martin P, Rodriguez-Blazquez C, Forjaz MJ, Frades-Payo B, Agüera-Ortiz L, Weintraub D, Riesco A, Kurtis MM, Chaudhuri KR. Neuropsychiatric symptoms and caregiver's burden in Parkinson's disease. Parkinsonism Relat Disord. 2015;21:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 16. | Sun C, Armstrong MJ. Treatment of Parkinson's Disease with Cognitive Impairment: Current Approaches and Future Directions. Behav Sci (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Byeon H. Predicting the Severity of Parkinson's Disease Dementia by Assessing the Neuropsychiatric Symptoms with an SVM Regression Model. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Dusseldorp E, Meulman JJ. Prediction in medicine by integrating regression trees into regression analysis with optimal scaling. Methods Inf Med. 2001;40:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Cleophas TJ, Zwinderman AH. Optimal scaling and automatic linear regression. Regression Analysis in Medical Research. Springer, Cham, 2018: 255-266. |
| 20. | Zhang D, Zhang C, Jiang P. Optimal scaling regression analysis for impact factors of SCI papers output. Zhonghua Yixue Keyan Guanli Zazhi. 2018;465-469. |
| 21. | Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7288] [Cited by in RCA: 7973] [Article Influence: 241.6] [Reference Citation Analysis (0)] |
| 22. | Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18:738-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1147] [Cited by in RCA: 1275] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 23. | Byeon H. Exploring the Predictors of Rapid Eye Movement Sleep Behavior Disorder for Parkinson's Disease Patients Using Classifier Ensemble. Healthcare (Basel). 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Bäckman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3006] [Cited by in RCA: 3467] [Article Influence: 165.1] [Reference Citation Analysis (0)] |
| 25. | Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8028] [Cited by in RCA: 8397] [Article Influence: 144.8] [Reference Citation Analysis (1)] |
| 26. | Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Yoon SJ, Yoo KH, Ha CK, Han IW. Estimating the validity of the Korean version of expanded Clinical Dementia Rating (CDR) scale. J Korean Neurol Assoc. 2001;19:585-591. [DOI] [Full Text] |
| 27. | Gillingham FJ, Donaldson MC. Schwab and England activities of daily living. Third symposium of Parkinson’s disease. E&S Livingstone: Edinburgh, Scotland, 1969. |
| 28. | Kang SJ, Choi SH, Lee BH, Kwon JC, Na DL, Han SH. The reliability and validity of the Korean Instrumental Activities of Daily Living (K-IADL). J Korean Neurol Assoc. 2002;20:8-14. [DOI] [Full Text] |
| 29. | Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15:300-308. |
| 30. | Kang Y, Park J, Yu KH, Lee BC. The validity of the Korean-Montreal Cognitive Assessment (K-MoCA) as a screening test for both MCI and VCI. Conference Abstract: The 20th Annual Rotman Research Institute Conference. The frontal lobes, 2010: 10. |
| 31. | Wang DZ, Wang C, Shen CF, Zhang Y, Zhang H, Song GD, Xue XD, Xu ZL, Zhang S, Jiang GH. [Comparison of application of Cochran-Armitage trend test and linear regression analysis for rate trend analysis in epidemiology study]. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38:684-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Chin J, Park J, Yang SJ, Yeom J, Ahn Y, Baek MJ, Ryu HJ, Lee BH, Han NE, Ryu KH, Kang Y. Re-standardization of the Korean-Instrumental Activities of Daily Living (K-IADL): Clinical Usefulness for Various Neurodegenerative Diseases. Dement Neurocogn Disord. 2018;17:11-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Van der Kooij AJ. Prediction accuracy and stability of regression with optimal scaling transformations. M.D. Thesis, Leiden University, Leiden. 2007. Available from: https://scholarlypublications.universiteitleiden.nl/access/item%3A2889259/view. |
| 34. | Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, Dickson D, Duyckaerts C, Cummings J, Gauthier S, Korczyn A, Lees A, Levy R, Litvan I, Mizuno Y, McKeith IG, Olanow CW, Poewe W, Sampaio C, Tolosa E, Emre M. Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 858] [Cited by in RCA: 775] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 35. | Marshall V, Grosset D. Role of dopamine transporter imaging in routine clinical practice. Mov Disord. 2003;18:1415-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis. Neurology. 2016;86:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 509] [Article Influence: 56.6] [Reference Citation Analysis (0)] |