Published online Jul 19, 2022. doi: 10.5498/wjp.v12.i7.944
Peer-review started: December 22, 2021
First decision: March 13, 2022
Revised: March 28, 2022
Accepted: June 16, 2022
Article in press: June 16, 2022
Published online: July 19, 2022
Processing time: 208 Days and 11 Hours
The rising number of people using methamphetamine leads to an increasing need for treatment options for this patient group. Evidence-based research on the efficacy of treatment programs for methamphetamine users is limited. Due to specific characteristics of methamphetamine users, the question arises whether established treatment methods for individuals using other substances can be effective for the treatment of methamphetamine dependence as well. We hypothesize that there are significant differences between the two groups that may affect the effectiveness of treatment and worsen the prognosis of treatment outcomes for methamphetamine users compared to consumers of other substances.
To investigate potential differences in cognitive functioning and psychopathology between methamphetamine users and other substance users and possible correlations with treatment outcomes.
A total of 110 subjects were recruited for an observational, longitudinal study from a German inpatient addiction treatment center: 55 patients with methamphetamine dependence and 55 patients with dependence of other substances (“OS group”). Both groups were examined at beginning (baseline) and end of treatment (after 6 mo) with regard to treatment retention, craving, cognitive functioning, psychosocial resources, personality traits, depression, and other psychiatric symptoms. Instruments used were Raven’s IQ test, Mannheimer craving scale, cognitrone cognitive test battery, NEO personality factors inventory, Hamilton depression scale, Becks depression inventory, and a symptom checklist. The statistical methods used were χ2-test, t-test and multiple mixed ANOVAs.
A total drop-out rate of 40% (methamphetamine-group: 36.4%; OS-group: 43.6%) was observed without significant differences between groups. At baseline, methamphetamine-group subjects significantly differed from OS-group individuals in terms of a lower intelligence quotient, fewer years of education, slower working speed, and decreased working accuracy, as well as less cannabinoid and cocaine use. Methamphetamine-group subjects further showed a significantly lower score of conscientiousness, depressive, and psychiatric symptoms than subjects from the OS-group. In both groups, a reduction of craving and depressive symptoms and an improvement of working speed and working accuracy was noted after treatment.
There are differences between methamphetamine users and users of other drugs, but not with regard to the effectiveness of treatment in this inpatient setting. There are differences in cognitive function and psychopathology between methamphetamine and other drugs users. The existing treatment options seem to be an effective approach in treating methamphetamine dependence.
Core Tip: There are differences between methamphetamine users and users of other drugs, but not with regard to the effectiveness of treatment in this inpatient setting. The existing treatment options seem to be an effective approach in treating methamphetamine dependence.
- Citation: Behle N, Kamp F, Proebstl L, Hager L, Riebschläger M, Schacht-Jablonowsky M, Hamdorf W, Neumann S, Krause D, Manz K, Franke AG, Koller G, Soyka M. Treatment outcome, cognitive function, and psychopathology in methamphetamine users compared to other substance users. World J Psychiatry 2022; 12(7): 944-957
- URL: https://www.wjgnet.com/2220-3206/full/v12/i7/944.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i7.944
The United Nations estimated that about 27 million people worldwide regularly abuse amphetamine-type stimulants (ATS) in 2018[1]. The rising number of people using methamphetamine has been considered the “next addiction crisis”[2] and causes growing concern[1]. Accordingly, there is a growing need for evidence-based treatment options for methamphetamine users[2,3]. Evidence-based research on the efficacy of treatment programs for methamphetamine users is still limited[4], with no established pharmacotherapy available[2,5]. The question arises whether established treatment methods for individuals using other substances can be effective for the treatment of methamphetamine dependence as well. This question is important since – until a few years ago – methamphetamine use played a minor role in German substance treatment services, and therefore, most methamphetamine users are treated in institutions having a focus on other drugs of abuse, such as alcohol, opioids, amphetamine, or cocaine. However, representative studies comparing the characteristics of methamphetamine users to users of other substances are limited. A study based on expert interviews and focus groups on characteristics of methamphetamine consumers showed that they differ from users of other stimulants with respect to higher levels of dissocial behavioral (e.g., aggressiveness, impulsiveness, egoism, or irritability), as well as emotional instability, unreliability, and other comorbidities[6]. The authors also reported that the therapy of methamphetamine users is substantially affected by their comorbidities and stated, that the provided rehabilitation for methamphetamine users in Germany is inadequate, resulting in a need to adapt the treatment concepts for this group[6]. Another study also showed that methamphetamine use seems to be associated with co-occurring substance use and mental illness[8]. This may be of relevance as reviewed comorbidities were frequently associated with worse treatment outcomes[9]. The available data demonstrate that the rise in methamphetamine use is intimately linked to the ongoing opioid crisis. The concurrent use of opioids and methamphetamines may decrease adherence to short-term residential treatment. Accordingly, effective strategies should be identified to retain individuals who use opioids and methamphetamines concurrently in treatment[10,11]. In addition, there are also data suggesting methamphetamines cause neural damage and persistent forms of cognitive impairment, including deficits in attention, memory, and executive function[12]. These results are in line with other studies also indicating that methamphetamine users may differ from other substance users with respect to cognitive function[13,14]. This may be important in terms of treatment outcome, since for example Bernhardt et al[15] reported correlations between methamphetamine treatment outcome and the recovery of cognitive impairment.
Another study found an association between a low level of perceived social support and methamphetamine dependence[16]. However, the authors also found an association between moderately (and not distinct) pronounced personality factors (agreeableness, neuroticism, extraversion, conscientiousness, and openness) and methamphetamine use[16]. A systematic review of psychological treatments for methamphetamine use disorders states that focusing more on the helping-relationship categories is a key approach for increasing the efficacy of treatments for methamphetamine use[17].
These studies have been mostly of exploratory in nature and were exclusively investigating methamphetamine users without direct comparison to other drug users. In this study, we focus on factors such as cognition, personality traits, comorbidities, psychiatric symptoms, and psychosocial resources and their implication on treatment outcome. Based on limited previous research, one may assume that methamphetamine users have more neuropsychiatric symptoms compared to users of other substances. Specifically, a higher rate of comorbid psychiatric symptoms and disorders, a lower level of cognitive functioning, limited psychosocial resources and lower retention rates in treatment in methamphetamine users can be postulated. This exploratory study focuses on these possible differences in primary methamphetamine users compared to users of other substances. We hypothesize that there are significant differences between the two groups that may affect the effectiveness of treatment and worsen the prognosis of treatment outcomes for methamphetamine users compared to consumers of other substances.
All participants were inpatients at a hospital specialized for treatment of substance use disorders (MEDIAN Klinik Mecklenburg) and were recruited by psychologists and physicians during the first 2 wk to 4 wk after admission. Participation was voluntary. The treatment was set up for 6 mo and the interventions were applied as individual and group therapy, with the main focus on group sessions (five times per week). Table 1 shows details about the treatment concept. Main treatment goals were the analysis of triggers for craving and the development of new behavioral strategies for coping with craving and other substance related problems. The 2-wk initial phase aimed at completion of diagnostics, establishment of self-reflection and motivational support, and defining therapy goals. During the 22-wk core treatment phase, interventions such as psychoeducation, situation and trigger analyses, mindfulness strategies and assertiveness training were applied. The last 2 wk focused on relapse prevention and aftercare. For further details see also Soyka et al[18].
| Therapy phase | Content and therapy frequency | Duration |
| Admission | Checking the entry requirements, e.g., recent drug use | Admission day |
| Entry phase | Diagnostics, self-reflection, strengthen and increasing motivation, defining therapy goals, treatment planning | 2 wk |
| Main phase | Change-, testing and stabilization phase: psychoeducation (2x/wk), mindfulness-based relapse prevention (1x/wk), trigger analysis (1x/wk), individual psychotherapy (50 min/wk), sports (1x/wk), further offers according to the results of diagnostics e.g., nutrition counseling (1x/wk), body therapy (1x/wk), ergotherapy (1x/wk), assertiveness training (1x/wk) | 22 wk |
| Discharge, planning aftercare | Follow-up plan, relapse prevention, arrangement of further care management e.g., contact to job center and clarified housing situation | 2 wk |
Inclusion criteria were a history of methamphetamine abuse or addiction (meeting the respective ICD-10 criteria) for the primary methamphetamine user group and a history of abuse or dependence of other substances for the other substances group (“OS group”). Because polydrug use is very common[19] methamphetamine-group participants were included when having a history of previous use of other substances, but methamphetamine had to be the primary drug of abuse and the main reason for admission to treatment. See Table 2 for information about the history of substance use in both groups.
| Substance class | n | P | |
| MA-group | OS-group | ||
| Alcohol | 16 | 21 | 0.31 |
| Cannabis | 32 | 42 | 0.04 |
| Cocaine | 5 | 19 | 0.001 |
| Hallucinogens | 0 | 1 | 0.3 |
| Opioids | 3 | 7 | 0.18 |
| Sedativa | 2 | 3 | 0.65 |
| Tobacco | 49 | 42 | 0.07 |
| Volatile solvents | 1 | 0 | 0.3 |
| Stimulants | 55 (methamphetamine) | 31 (amphetamine) | - |
Minimum age was 18 years. Exclusion criteria were acute psychotic symptoms, intoxication on test days, and insufficient comprehension of study materials or procedure. Informed written consent was obtained from all participants after a complete and extensive description of the study protocol. The study protocol was approved by the Ethics Committee of the Ludwig-Maximilians-University of Munich. All participants were financially reimbursed with 15 Euro after completion of assessments. Routine urine samples and breath alcohol tests were collected to verify substance use. These tests were part of the usual hospital practice and were conducted by the clinic staff on a sample basis and in case of suspected substance use.
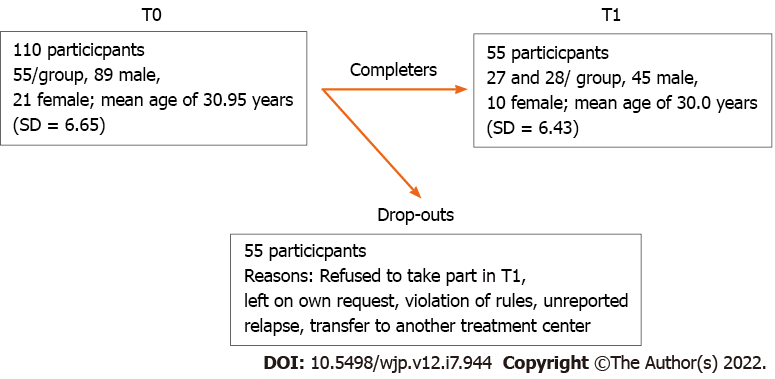
The observational longitudinal study was designed to capture within and between group differences at two time points: “T0” Baseline at the beginning of treatment and “T1” at the end of treatment, after approximately 24 wk. The T1 assessment took place during the last 3 wk before discharge, but the exact time point varied individually. Both surveys were conducted by trained staff. Data were collected between November 2016 and June 2018 for the Methamphetamine-group and between June 2018 and February 2019 for the OS-group. See Figure 1 for details.
The main outcome of interest was the completion of treatment as scheduled (regular discharge). Individuals stopping treatment prematurely (at own request or as a disciplinary decision) were defined as dropouts. A positive urine test result was classified as a non-reported relapse, which led to a disciplinary dismissal.
Further outcomes of interest were differences between methamphetamine- and OS-group and between time points T0 and T1. These differences include craving, cognitive functioning, psychosocial resources, depression, and other psychiatric symptoms, as well as personality traits (only measured at baseline). Table 3 displays the used instruments at the respective assessment.
| Instrument | Description | Assessment |
| Becks Depression Inventory-II (BDI-II) (Hautzinger et al[29], 2006) | 21-question multiple-choice self-report inventory measuring the severity of depression. Raw scores were used for analyses | T0, T1 |
| Cognitrone (Wagner and Karner[30], 2003) | Computer administered Test of cognitive working speed and working accuracy (comparisons of geometrical figures). Scores were standardized into T-values according to test norms | T0, T1 |
| Documentation standards III for the evaluation of the treatment of dependent individuals (German Society for Addiction and Therapy[31], 2001) | Defined items to assess substance use and related factors (e.g., years of substance use, age at use onset, number of withdrawals) | T0 |
| Hamilton Depressive Rating Scale (HAMD)(Hamilton[32], 1960) | Clinician-administered depression assessment scale, containing 17 items of symptoms of depression. Time period: past week. Assessed as a semi structured interview. Raw scores were used for analyses | T0, T1 |
| Inventory of personal psychosocial resources(Küfner et al[33], 2006) | Self-report questionnaire measuring psychosocial resources in the past and at present based on different scales, e.g., relationship, friends, financial and work situation. A total raw score of all scales measuring the present situation was built and used for analyses | T0, T1 |
| Mannheimer Craving Scale (Nakovics et al[34], 2009) | Self-report questionnaire with 12 multiple choice items and 4 additional items measuring Craving within the last 7 d. Raw scores from the main 12 items were used for analyses | T0, T1 |
| NEO-Five-Factor-Inventory (NEO-FFI)(Borkenau and Ostendorf[35], 2008) | Self-report questionnaire with 60 items for the measurement of the so-called “big five” personality traits (neuroticism, extraversion, openness, agreeableness, consciousness). Scores were standardized into T-values according to test norms | T0 |
| Raven's Standard Progressive Matrices(Raven et al[36], 2016) | Nonverbal intelligence test, Computer version. Scores were standardized into IQ values according to test norms | T0 |
| Structured Clinical Interview for DSM-IV Axis I (Wittchen et al[37], 1997) | Diagnostic structured interview to determine the presence of DSM-IV Axis I disorders | T0 |
| Symptom Checklist 90-R (SCL-90R) (Franke[38], 1995) | Self-report questionnaire assessing symptoms of psychopathology on different scales. For this study two scales were use: intensity of depressive symptoms scale and “Positive Symptom Distress Index” (PSDI), a measure of intensity of present symptoms. Scores of both scales were standardized into T-values according to test norms | T0, T1 |
| Wender Utah Rating Scale -short Version (Wursk) (Retz-Junginger et al[39], 2002) | Short version (25 items including 4 control items) of a self-report questionnaire assessing retrogradely childhood symptoms of attention deficit hyperactivity disorder. Raw Scores were built from the 21 core items and used for analyses | T0 |
Continuous variables were summarized by their mean (m) and standard deviation (SD), categorical variables by absolute (n) and relative frequencies (%). Group comparisons were performed using χ²-test (for categorial variables, or in case of small cell numbers, Fisher’s exact test) and t-test (continuous variables). Multiple mixed ANOVAs were calculated to compare mean differences between substance groups taking into account both time points (T0 and T1). Since t-tests and ANOVAs are regarded as robust statistical procedures, both methods were also used for variables potentially deviating from the normality assumption. Univariable logistic regression models were applied to investigate the effect of independent factors on treatment drop-out. Odds ratios (OR) are reported together with their 95% confidence intervals (CI). The significance level was set at P = 0.05 and no P value adjustment for multiple testing was applied in this explorative study. All statistical analyses were conducted in SPSS version 24.
A total of 110 participants (55 in each group, 89 men and 21 women) with a mean age of 30.95 years (SD = 6.65) were included in the first assessment at T0. There were no statistically significant differences in age (30.0 years vs 32.0 years, P = 0.12) or sex distribution (76.4% vs 85.5% males, P = 0.23) between methamphetamine- and OS-groups. Out of this original sample, 18 subjects refused to take part in further assessments after T0 and 55 subjects (27 from methamphetamine, 28 from OS-group) participated again in the second measurement T1 with a mean age of 30.0 years (SD = 6.43). Again, the majority of T1 subjects was male (45 men, 10 women) and there was no significant difference in sex distribution (P = 0.50).
From the baseline sample, 66 subjects (60%) completed the treatment while 44 individuals (40%) dropped-out of treatment. Comparison of the methamphetamine-group and the OS-group revealed no significant difference in drop-out rates (36.4% vs 43.6%, P = 0.44). In addition, there was neither a significant difference in age (P = 0.19) nor in sex distribution (P = 0.84) between drop-outs and completers.
The most common reason for treatment drop-out was at own request (42.2%), followed by violation of institution rules (26.7%), unreported relapse during treatment (24.4%), and transfer to another treatment center (6.7%). There was no significant association in the reasons for drop-out between methamphetamine and OS-group (P = 0.21).
Participants remained in treatment for a mean time of 147 d (SD = 68). There was a trend towards a longer treatment retention in the methamphetamine-group compared to OS-group, but this difference failed to reach statistical significance [159 (SD = 60) vs 135 d (SD = 73), P = 0.07]. The OS group attended a slightly higher mean number of group sessions [OS: 103 (SD = 57); methamphetamine: 87 (SD = 35), P = 0.07], while the methamphetamine-group had a slightly higher mean number of individual therapy sessions [methamphetamine: 27 (SD = 18); OS 22 (SD = 13), P = 0.08]. However, both differences were not statistically significant. A mean treatment duration of 93 d (SD = 57) was found among the patients dropping out of treatment.
Methamphetamine-group subjects had fewer years of education than OS-group subjects (P = 0.048) and showed a significantly lower mean intelligent quotient (Raven`s IQ = 93.7) at baseline than the OS- individuals (IQ = 100.1, P = 0.02, see also Table 4). Methamphetamine-group participants also performed worse on both measures of the cognitive test battery Cognitrone, resulting in a significantly decreased working speed (P = 0.002) and working accuracy (P = 0.03) compared to OS-subjects. Methamphetamine- and OS- subjects showed no significant differences with respect to employment (P = 0.19) or partnership during the last 6 mo prior to admission (P = 0.46).
| MA-group | OS-group | P | |
| n | 55 | 55 | |
| Male | 42 (76.4%) | 47 (85.5%) | 0.23 |
| Age | 30.0 (± 5.3) | 32.0 (± 7.7) | 0.12 |
| Number of withdrawals (n = 48) | 3.0 (± 4.1) | 3.0 (± 4.1) | 0.98 |
| Raven‘s IQ (MA n = 50, OS n = 54) | 93.7 (± 13.5) | 100.1 (± 13.6) | 0.02 |
| Cognitrone working speed (MA n = 53, OS n = 54) | 49.1 (± 8.0) | 54.3 (± 9.0) | 0.002 |
| Cognitrone accuracy (MA n = 53, OS n = 54) | 43.0 (± 8.9) | 47.1 (± 9.8) | 0.03 |
| Personality factors | n = 37 | n = 42 | |
| Neuroticism | 22.8 (± 6.7) | 25.1 (± 9.7) | 0.24 |
| Extraversion | 25.0 (± 6.0) | 25.2 (± 7.5) | 0.89 |
| Openness | 26.3 (± 5.6) | 28.6 (± 6.7) | 0.11 |
| Agreeableness | 26.6 (± 4.2) | 27.9 (± 6.8) | 0.33 |
| Conscientiousness | 29.0 (± 5.6) | 31.9 (± 6.6) | 0.04 |
| BDI-II Score (MA n = 42, OS n = 54) | 13.6 (± 10.8) | 16.8 (± 11.3) | 0.17 |
| HAMD Score (MA n = 46, OS n = 42) | 5.3 (± 4.8) | 8.3 (± 7.9) | 0.04 |
| SCL-PSDI Score (MA n = 39, OS n = 40) | 53.5 (± 11.1) | 59.3 (± 10.1) | 0.02 |
| Wursk Score (MA n = 36, OS n = 40) | n = 3628.6 (± 16.7) | n = 4030.8 (± 15.1) | 0.56 |
| Craving (MA n = 39, OS n = 40) | 13.9 (± 9.5) | 14.2 (± 8.0) | 0.87 |
| Years of education | n = 52 | n = 50 | 0.048 |
| ≤ 9 yr | 35 | 24 | |
| ≥ 10 yr | 17 | 26 | |
| Employment | n = 51 | n = 48 | 0.19 |
| Unemployed | 43 | 33 | |
| Employed | 4 | 7 | |
| Other (e.g., retiree) | 4 | 8 | |
| Ever injected | n = 49 | n = 40 | 0.75 |
| 7 | 4 |
Participants from the methamphetamine-group showed a significantly lower score of the personality trait conscientiousness (measured by the NEO-Five-Factor-Inventory) compared with subjects from the OS-group (P = 0.04). No other personality traits differed significantly between both groups. The OS group showed significantly higher Hamilton Depressive Rating Scale (HAMD) (P = 0.04) and Symptom Checklist (SCL) depression (P = 0.03)but not Beck Depression Inventory-II (BDI- II) (P = 0.17) mean scores at T0 than the methamphetamine-group. The OS-group also had a higher mean score of the SCL “Positive Symptom Distress Index” (PSDI), a measure of intensity of present symptoms, compared to the methamphetamine-group (P = 0.02). There were no statistically significant differences in attention deficit hyperactivity disorder (ADHD) scores (P = 0.56), craving (P = 0.87), or psychosocial resources (P = 0.69) at baseline.
As explained, methamphetamine-group subjects may have had a history of other drug use, but methamphetamine had to be the prior substance. The majority of all subjects also used cannabinoids, but the number of cannabinoid users was significantly higher in the OS-group than in the methamphetamine-group (P = 0.04, see Table 2). The OS-group also included a significantly higher number of individuals that used cocaine (P = 0.001), while there were no differences in the use of other substances. There was no significant difference between groups concerning the number of previous substance abuse treatments (P = 0.98).
Regarding the number of comorbid psychiatric diagnoses (measured by ICD-10), a significantly higher rate of anxiety disorders (P = 0.03) and somatoform disorders (P < 0.0001) was found in methamphetamine-group patients, while there was a higher rate of other psychotic disorders in OS-group participants (P = 0.04, see Table 5).
| MA group, n = 54 | OS group, n = 55 | P | |
| Depression | 11 | 15 | 0.40 |
| Anxiety disorder | 5 | 0 | 0.03 |
| Eating disorder | 0 | 2 | 0.49 |
| Obsessive-compulsive disorder | 0 | 0 | - |
| Posttraumatic stress disorder | 15 | 12 | 0.47 |
| Personality disorder | 11 | 11 | 0.96 |
| ADHD | 6 | 7 | 0.80 |
| Psychotic disorder | 3 | 10 | 0.042 |
| Somatoform disorder | 18 | 0 | < 0.001 |
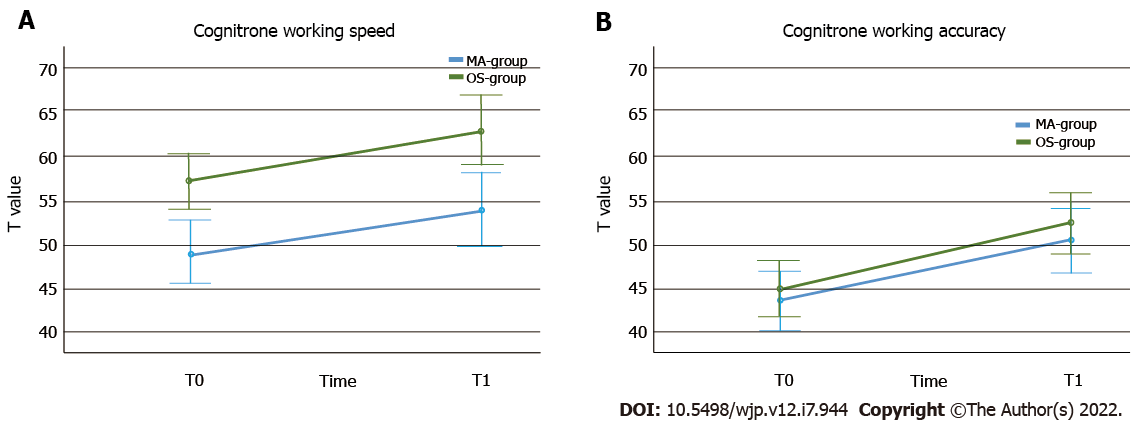
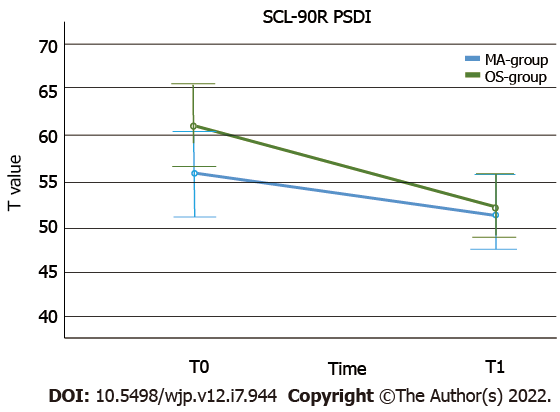
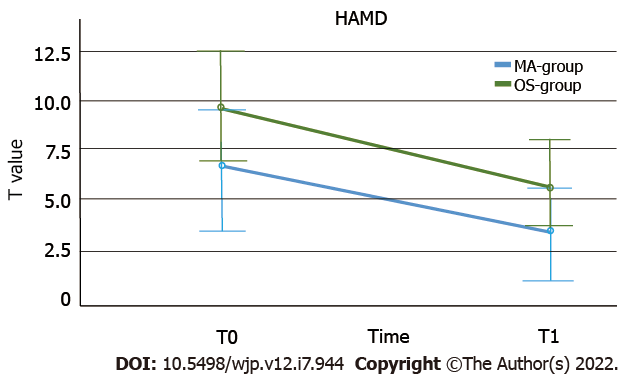
Mixed ANOVAs were used to compare the cognitive functioning over time and between groups. The working speed significantly improved from T0 to T1 in both groups (P < 0.001, see also Table 6) and there was a significant group effect for both measurements, showing a better performance in the OS- than in the methamphetamine group in working speed (P < 0.001, see Figure 2). There was no interaction effect (P = 0.94). Regarding working accuracy, there also was a significant improvement of performance over time in both groups (P < 0.001). The OS-group showed a higher working accuracy at both times, but this effect was not statistically significant (P < 0.43). Again, there was no interaction effect (P < 0.79, see Figure 2). Both groups showed a significant reduction of the intensity of psychiatric burden, as measured by the SCL-90-R PSDI score, over time (P < 0.001). The OS-group showed a greater decrease than the Methamphetamine-group (see Figure 3), but the interaction effect failed to reach statistical significance (P = 0.07). The groups no longer differed significantly in this regard over time (P = 0.29). SCL-90-R depression scores (P < 0.001) and HAMD depression scores (P = 0.001) were significantly decreased over time in both groups. However, taking baseline and T1 assessment together, the difference between the OS- and methamphetamine-groups was no longer significant (SCL depression score: P = 0.09; HAMD: P = 0.09). Again, no interaction effects were found (SCL depression score: P = 0.97; HAMD: P = 0.66, see Figure 4). Analyzing the BDI-II depression scores also revealed a significant reduction of depression scores over time (P < 0.001), but without interaction (P = 0.81) or group effect (P = 0.56). Similar results were seen regarding craving scores with a significant reduction over time (P < 0.001), without interaction (P = 0.94), and without group effect (P = 0.86). We found a significant increase of psychosocial resources over time (P = 0.048), but again, no significant differences between both groups (P = 0.99) and no interaction effect (P = 0.71).
| MA-group | n | OS-group | n | P | ||
| BDI | T0 | 15.31 (± 11.55) | 26 | 16.36 (± 12.39) | 33 | Ptimeb; PgrouP NS; PtimexgrouP NS. |
| T1 | 7.27 (± 7.20) | 8.97 (± 8.98) | ||||
| Cognitrone accuracy | T0 | 43.62 (± 7.84) | 26 | 44.93 (± 9.85) | 28 | Ptimeb; PgrouP NS; PtimexgrouP NS |
| T1 | 50.50 (± 8.63) | 52.54 (± 10.16) | ||||
| Cognitrone speed | T0 | 48.81 (± 7.68) | 26 | 57.18 (± 9.05) | 28 | Ptimeb; PgrouPb;PtimexgrouP NS. |
| T1 | 54.08 (± 10.04) | 62.61 (± 10.88) | ||||
| HAMD | T0 | 6.52 (± 5.36) | 25 | 9.59 (± 9.14) | 27 | Ptimeb; PgrouP NS; PtimexgrouP NS. |
| T1 | 3.60 (± 4.77) | 5.81 (± 5.98) | ||||
| IPR | T0 | 204.43 (± 36.47) | 21 | 201.78 (± 33.84) | 27 | Ptimea; PgrouP NS; PtimexgrouP NS. |
| T1 | 215.48 (± 38.71) | 217.78 (± 54.15) | ||||
| MaCS | T0 | 14.39 (± 9.81) | 23 | 14.59 (± 6.69) | 27 | Ptimeb; PgrouP NS; PtimexgrouP NS. |
| T1 | 8.57 (± 5.71) | 8.96 (± 8.04) | ||||
| SCL 90R Depression Score | T0 | 58.14 (± 9.09) | 21 | 62.70 (± 10.52) | 27 | Ptimeb; PgrouP NS; PtimexgrouP NS. |
| T1 | 50.71 (± 8.19) | 55.19 (± 11.55) | ||||
| SCL 90 R PSDI | T0 | 55.90 (± 10.51) | 21 | 61.26 (± 11.40) | 27 | Ptimeb; PgrouP NS; PtimexgrouP NS |
| T1 | 51.71 (± 8.33) | 52.61 (± 10.66) |
Neuroticism measured at baseline was a significant predictor for treatment drop-out in the whole sample, showing decreasing odds for drop-out with increasing neuroticism scores [OR = 0.93, 95%CI: (0.87, 0.99), P = 0.03]. No other baseline personality variables predicted treatment drop-out. Higher scores in Cognitrone working accuracy, measured at baseline, also significantly predicted a treatment drop-out [OR= 1.05, 95%CI: (1.0, 1.09), P = 0.04], while working speed was not a significant predictor (P = 0.20). Raven’s IQ (P = 0.90), craving at baseline (P = 0.99), and SCL depressive scores (P = 0.10) were also not significant predictors of drop-out.
The present study found differences between methamphetamine and other drug users in terms of cognitive function, psychiatric comorbidities, and personality traits, but not regarding treatment outcome and retention. The latter finding suggests that despite the encountered differences between methamphetamine users and other drug users, methamphetamine users do not perform worse than other drug users in currently provided treatments. This result raises the question if there is need for new and specialized treatment options for methamphetamine users. For example, patients may have reported methamphetamine related situations or consequences when reflecting their use patterns and for example possible relapse situations. Previously, in another longitudinal study, we compared the methamphetamine group from this study with another methamphetamine user group that received a more stimulant specific treatment[20]. We found no differences in treatment retention or long-term relapse rates between both groups, which supports the hypothesis that methamphetamine users may not benefit automatically from a more stimulant specific treatment. Study results reveal that a high number of methamphetamine users use other substances, too. These patients may benefit from existing treatments.
Interestingly, the present study revealed a trend (although not statistically significant) towards longer treatment duration of approximately 20 d in the methamphetamine group, which may indicate that methamphetamine users may have a greater benefit from the investigated treatment. However, with regards to all other treatment outcome measures, we did not find any relevant interaction, which suggests that both groups overall benefited from treatment. For example, both groups showed a reduction of craving, depression scores and overall psychiatric burden (measured by SCL-90R) and an improvement in working speed and working accuracy, as well as an increase of psychosocial resources at the end of the treatment compared to its initiation. Therefore, it can be concluded that a current “treatment as usual” inpatient addiction program is helpful for methamphetamine users and users of other substances, and that both user groups do not differ from each other in their response to the treatment.
Nevertheless, this study did reveal differences between methamphetamine users and other substance users; for example, differences were found between the two groups with respect to cognitive function. Neurotoxic effects of metamphetamine use are well established[2]. As we hypothesized, metham-phetamine users had significantly lower baseline intelligence quotient, slower working speed, and decreased working accuracy compared to users of other drugs. This finding confirms results from other studies indicating that methamphetamine use can impair cognitive functions[13,14]. However, years of school education were fewer in the methamphetamine-group, raising the question of whether impaired cognitive function in the methamphetamine-group is a reason for, or rather a consequence of, methamphetamine use. Unfortunately, there are no longitudinal data to further explore this point. A previous study failed to show improvement of cognitive impulsivity deficits in metamphetamine users after short term abstinence of 6 wk[21]. Furthermore, the performance of the methamphetamine user group was still in the average range, when applying the test norms (t-values), and we had no matched control group without drug users to clarify the differences between both groups. Interestingly, and contrary to our hypothesis, higher scores in working accuracy at baseline were associated with a higher likelihood for treatment drop-out. Other studies that have examined ADHD patients have found lower accuracy scores as significant predictors of drop out and mild cognitive deficits, which is in contrast to the results of this study[22]. Furthermore, we did not find an effect of working speed and IQ on treatment retention, which makes it difficult to generalize the impact of cognitive performance on drop-out rates.
Again, as assumed, methamphetamine-patients had a higher rate of comorbid anxiety and somatoform disorders. But contrary to this result, OS- group participants showed a higher rate of psychotic disorders, and there were no differences between both groups in terms of other comorbidities. Therefore, different substance use patterns may be associated with different comorbidities, but not in this study.
Another unexpected result was the negative association between neuroticism and treatment drop-out which found that the higher the score for neuroticism, the lower the odds of treatment drop-out. Other studies conclude, contrary to our results, that emotional instability and high neuroticism scores are risk factors for relapse, at least in alcohol users[23]. Treatment dropouts in a program for cocaine addiction showed a higher score on histrionic and antisocial scales compared to completers[24]. Since it can be assumed that histrionic, as well as antisocial personality traits, tend to be associated with higher neuroticism, this result is also not consistent with our finding. We are not aware of any studies that specifically examined neuroticism as a predictor of addiction treatment dropout.
Our study has several limitations. For example, we did not correct the analyses for multiple testing, as this study was designed to generate hypotheses for future research on possible differences between methamphetamine- and OS patients.
Furthermore, in the group that used other substances, amphetamine use was not an exclusion criterion. Even though the two substances are very similar, it has been suggested that metham-phetamine has a stronger effect on the dopamine transporter mediated cell physiology than methamphetamine; therefore, the latter has a higher addictive potential[25].
Beyond that, the reported treatment effects are limited to the sample of treatment completers. Regarding the therapeutic outcome of the drop-out patients, there were no available data for T1, and therefore, the treatment effects for the drop-out sample remain unclear. In particular, there is not enough information on patients who stopped treatment at their own request. The present study showed that the average time patients spend in treatment before they dropped out is still quite high (around 3 mo). It remains unclear why they did not continue the treatment. Future investigations covering the whole treatment process may help gaining further information on characteristics of later drop-outs with focus on craving, treatment satisfaction and value of therapeutic relationship[26-28].
There are differences between methamphetamine users and users of other drugs, but not with regard to the overall effectiveness of a 6-mo inpatient addiction treatment. Both groups showed a reduction in psychiatric symptoms over time and improved cognitive function after treatment. Methamphetamine users, therefore, seem to benefit from existing, stimulant nonspecific treatment options in a similar way than other drug users do.
Over the last years the misuse of methamphetamine has risen, leading to an increased need for treatment options for this group of patients. To date, it remains elusive whether treatment programs for methamphetamine users are effective. One question arises whether established treatment methods for individuals using other substances can effectively target individuals with methamphetamine dependence.
The present study aims to investigate the potential differences in cognitive functioning and psychopathology between methamphetamine users and other substance users and possible correlations with treatment outcomes.
In order to provide effective therapy for the subgroup of methamphetamine users, differences to the group of other substance abusers need to be identified.
For this observational longitudinal study from a German inpatient addiction treatment center a total of 110 subjects were recruited. Of those, 55 patients had methamphetamine dependence and 55 patients had dependence of other substances (“OS group”). Both groups were examined at beginning (baseline) and end of treatment (after 6 mo) with regard to treatment retention, craving, cognitive functioning, psychosocial resources, personality traits, depression, and other psychiatric symptoms. Instruments used were Raven’s IQ test, Mannheimer craving scale, Cognitrone cognitive test battery, NEO personality factors inventory, Hamilton depression scale, Becks depression inventory and symptom checklist. The statistical methods used were χ²-tests, t-tests, and multiple mixed ANOVAs.
Over the period of 6 mo, a total drop-out rate of 40% (methamphetamine-group: 36.4%; OS-group: 43.6%) was observed without significant differences between groups. At baseline, methamphetamine-group subjects significantly differed from OS-group individuals in terms of a lower intelligence quotient, fewer years of education, slower working speed and lower working accuracy as well as less cannabinoid and cocaine use. Methamphetamine-group subjects further showed a significantly lower score of conscientiousness, depressive, and psychiatric symptoms than subjects from the OS-group. In both groups a reduction of craving and depressive symptoms and an improvement of working speed and working accuracy were noted after treatment.
The existing treatment options for substance abuse seem to be an effective approach in treating methamphetamine dependence.
Future studies should investigate specific programs that aim to improve cognitive function and psychopathology in methamphetamine dependent patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Glumac S, Croatia; Stoyanov D, Bulgaria A-Editor: Liu X, China S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | World Drug Report 2021. United Nations publication, Sales No. E.21.. |
| 2. | Paulus MP, Stewart JL. Neurobiology, Clinical Presentation, and Treatment of Methamphetamine Use Disorder: A Review. JAMA Psychiatry. 2020;77:959-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 3. | Hamdorf W, Susemihl I, Schacht-Jablonowsky M. Katamneseergebnisse der Entwöhnungsbehandlung bei apmhetaminabhängigen Patienten. Sucht Aktuell 2015; 2: 43-46.. |
| 4. | Die Drogenbeauftragte, Bundesministerium für Gesundheit (BMG), Bundesärztekammer (BÄK), Deutsche Gesellchaft für Psychiatrie und Psychotherapie, Psychosomatik und Nervenheilkunde (DGPPN). S3-Leitlinie „Methamphetamin-bezogene Störungen“ – Leitlinienreport, 1. Auflage. Version 1. 2016.. |
| 5. | Chan B, Freeman M, Kondo K, Ayers C, Montgomery J, Paynter R, Kansagara D. Pharmacotherapy for methamphetamine/amphetamine use disorder-a systematic review and meta-analysis. Addiction. 2019;114:2122-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (2)] |
| 6. | Hoffmann L, Buchenauer L, Schumann N, Schröder SL, Martin O, Richter M. Improving Rehabilitative Care of Methamphetamine Users in Germany: The Expert's Perspective. Qual Health Res. 2019;29:248-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Petzold J, Weber B, Bassett TR, Bauer M, Bernhardt N, Groß C, Hasler H, Schützwohl M, Pilhatsch M. Effectiveness of the First German-Language Group Psychotherapy Manual to Accompany Short-Term Treatment in Methamphetamine Dependence. Front Psychiatry. 2020;11:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Jones CM, Compton WM, Mustaquim D. Patterns and Characteristics of Methamphetamine Use Among Adults - United States, 2015-2018. MMWR Morb Mortal Wkly Rep. 2020;69:317-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 9. | Kuitunen-Paul S, Roessner V, Basedow LA, Golub Y. Beyond the tip of the iceberg: A narrative review to identify research gaps on comorbid psychiatric disorders in adolescents with methamphetamine use disorder or chronic methamphetamine use. Subst Abus. 2021;42:13-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 10. | Jones CM, Houry D, Han B, Baldwin G, Vivolo-Kantor A, Compton WM. Methamphetamine use in the United States: epidemiological update and implications for prevention, treatment, and harm reduction. Ann N Y Acad Sci. 2022;1508:3-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 11. | Ware OD, Manuel JI, Huhn AS. Adults With Opioid and Methamphetamine Co-use Have Lower Odds of Completing Short-Term Residential Treatment Than Other Opioid Co-use Groups: A Retrospective Health Services Study. Front Psychiatry. 2021;12:784229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci. 2006;31:301-313. [PubMed] |
| 13. | Potvin S, Pelletier J, Grot S, Hébert C, Barr AM, Lecomte T. Cognitive deficits in individuals with methamphetamine use disorder: A meta-analysis. Addict Behav. 2018;80:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 227] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 14. | Proebstl L, Kamp F, Koller G, Soyka M. Cognitive Deficits in Methamphetamine Users: How Strong is The Evidence? Pharmacopsychiatry. 2018;51:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Bernhardt N, Petzold J, Groß C, Scheck A, Pooseh S, Mayer-Pelinski R, Zimmermann US, Smolka MN, Pilhatsch M. Neurocognitive Dysfunctions and Their Therapeutic Modulation in Patients With Methamphetamine Dependence: A Pilot Study. Front Psychiatry. 2020;11:581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 16. | Jalali A, Shabrandi B, Jalali R, Salari N. Methamphetamine Abusers' Personality Traits and its Relational with Spiritual Well-being and Perceived Social Support. Curr Drug Res Rev. 2019;11:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Phukao D. Systematic Review of Psychological Treatments for Methamphetamine. International Journal of Innovation, Creativity and Change 2021. Volume 15, Issue 4.. |
| 18. | Soyka M, Koller G, Proebstl L, Kamp F, Franke A, Schmidt P, Baumgärtner G, Schacht-Jablonowsky M, Sievert A, Straif M, Hamdorf W. [Prevalence and Therapy of Crystal Methamphetamine Dependence]. Fortschr Neurol Psychiatr. 2017;85:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Crummy EA, O'Neal TJ, Baskin BM, Ferguson SM. One Is Not Enough: Understanding and Modeling Polysubstance Use. Front Neurosci. 2020;14:569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 20. | Kamp F, Proebstl L, Hager L, Schreiber A, Riebschläger M, Neumann S, Straif M, Schacht-Jablonowsky M, Manz K, Soyka M, Koller G. Effectiveness of methamphetamine abuse treatment: Predictors of treatment completion and comparison of two residential treatment programs. Drug Alcohol Depend. 2019;201:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 21. | Fitzpatrick RE, Robinson AH, Rubenis AJ, Lubman DI, Verdejo-Garcia A. Lack of longitudinal changes in cognition in individuals with methamphetamine use disorder during the first 6 wk after commencing treatment. Am J Drug Alcohol Abuse. 2021;47:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | van Emmerik-van Oortmerssen K, Blankers M, Vedel E, Kramer F, Goudriaan AE, van den Brink W, Schoevers RA. Prediction of drop-out and outcome in integrated cognitive behavioral therapy for ADHD and SUD: Results from a randomized clinical trial. Addict Behav. 2020;103:106228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Bottlender M, Soyka M. Impact of different personality dimensions (NEO Five-Factor Inventory) on the outcome of alcohol-dependent patients 6 and 12 mo after treatment. Psychiatry Res. 2005;136:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Fernandez-Montalvo J, & López-Goñi J J. Comparison of completers and dropouts in psychological treatment for cocaine addiction. Addiction Research & Theory 2010; 18(4), 433-44. [DOI] [Full Text] |
| 25. | Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, De Felice LJ, Khoshbouei H. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284:2978-2989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Meier PS, Donmall MC, McElduff P, Barrowclough C, Heller RF. The role of the early therapeutic alliance in predicting drug treatment dropout. Drug Alcohol Depend. 2006;83:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Kelly SM, O'Grady KE, Brown BS, Mitchell SG, Schwartz RP. The role of patient satisfaction in methadone treatment. Am J Drug Alcohol Abuse. 2010;36:150-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Brorson HH, Ajo Arnevik E, Rand-Hendriksen K, Duckert F. Drop-out from addiction treatment: a systematic review of risk factors. Clin Psychol Rev. 2013;33:1010-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 379] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 29. | Hautzinger M, Keller F, Kühner C. Beck Depressions-Inventar (BDI-II). artcourt Test Services, Frankfurt 2006.. |
| 30. | Wagner M, Karner T. Manual Cognitrone. Schuhfried, Mödling 2003.. |
| 31. | German Society for Addiction Research and Therapy. Dokumentationsstandards III für die Evaluation der Behandlung von Abhängigen: [Documentation standards III for the evaluation of addiction treatment] 47. SUCHT. 2001;3-94. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21041] [Cited by in RCA: 22872] [Article Influence: 351.9] [Reference Citation Analysis (0)] |
| 33. | Küfner H, Coenen M, Indlekofer W. PREDI – Psychosoziale essourcenorientierte Diagnostik: Ein Problem- Und Lösungsorientierter Ansatz, ersion 3.0. Pabst Science Publ, Lengerich 2006.. |
| 34. | Nakovics H, Diehl A, Geiselhart H, Mann K. [Development and validation of an overall instrument to measure craving across multiple substances: the Mannheimer Craving Scale (MaCS)]. Psychiatr Prax. 2009;36:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Borkenau P, Ostendorf F. NEO-FFI: NEO-Fünf-Faktoren-Inventar Nach Costa und McCrae: Manual, 2nd ed. Hogrefe, Göttingen, 2008.. |
| 36. | Raven J, Raven C, Court JH. Manual SPM Raven’s Standard Progressive atrices: Version 33- Revision 1. Schuhfried, Mödling 2016.. |
| 37. | Wittchen HU, Wunderlich U, Gruschwitz S, Zaudig M. SKID I. Strukturiertes Klinisches Interview Für DSM-IV. Achse I: Psychische Störungen. Interviewheft und Beurteilungsheft.: Eine Deutschsprachige, Erweiterte Bearbeitung Der Amerikanischen Originalversion Des SKID I. Hogrefe, Göttingen 1997.. |
| 38. | Franke GH. SCL-90-R: Die Symptom-Checkliste von Derogatis: Deutsche Version. Beltz Test, Göttingen 1995; 21-28. |
| 39. | Retz-Junginger P, Retz W, Blocher D, Weijers HG, Trott GE, Wender PH, Rössler M. [Wender Utah rating scale. The short-version for the assessment of the attention-deficit hyperactivity disorder in adults]. Nervenarzt. 2002;73:830-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 251] [Article Influence: 10.9] [Reference Citation Analysis (0)] |